Ag as Cocatalyst and Electron-Hole Medium in CeO2 QDs/Ag/Ag2Se Z-scheme Heterojunction Enhanced the Photo-Electrocatalytic Properties of the Photoelectrode
Abstract
1. Introduction
2. Materials and Methods
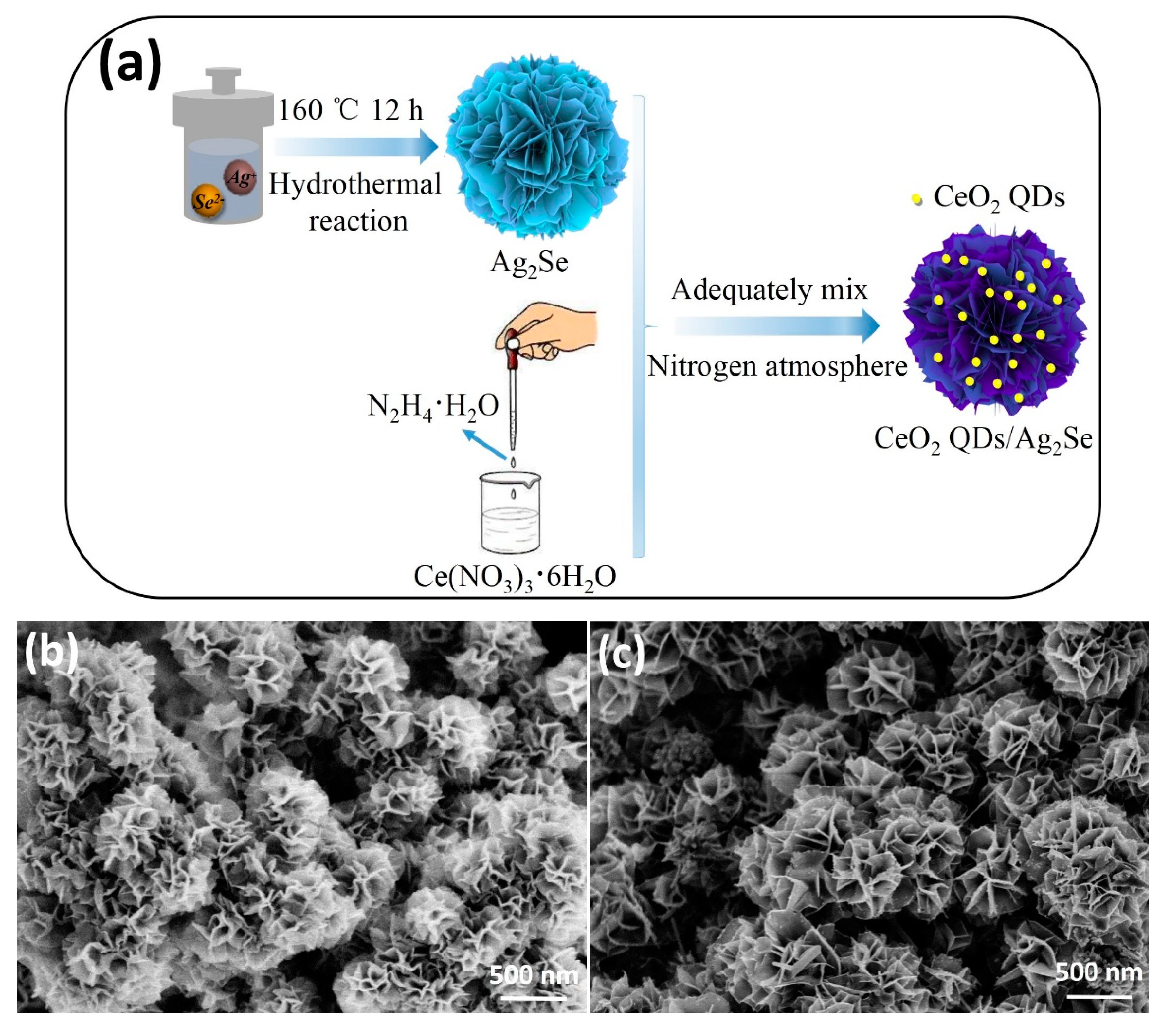
2.1. Preparation of Ag2Se Nanoflowers
2.2. Formation of CeO2 QDs/Ag2Se Z-Scheme Nanoheterojunction
2.3. Characterization
2.4. Photocatalytic Activity Evaluation
2.5. Electrocatalytic and Photoelectron Catalytic Activity Assessment
3. Results and Discussion
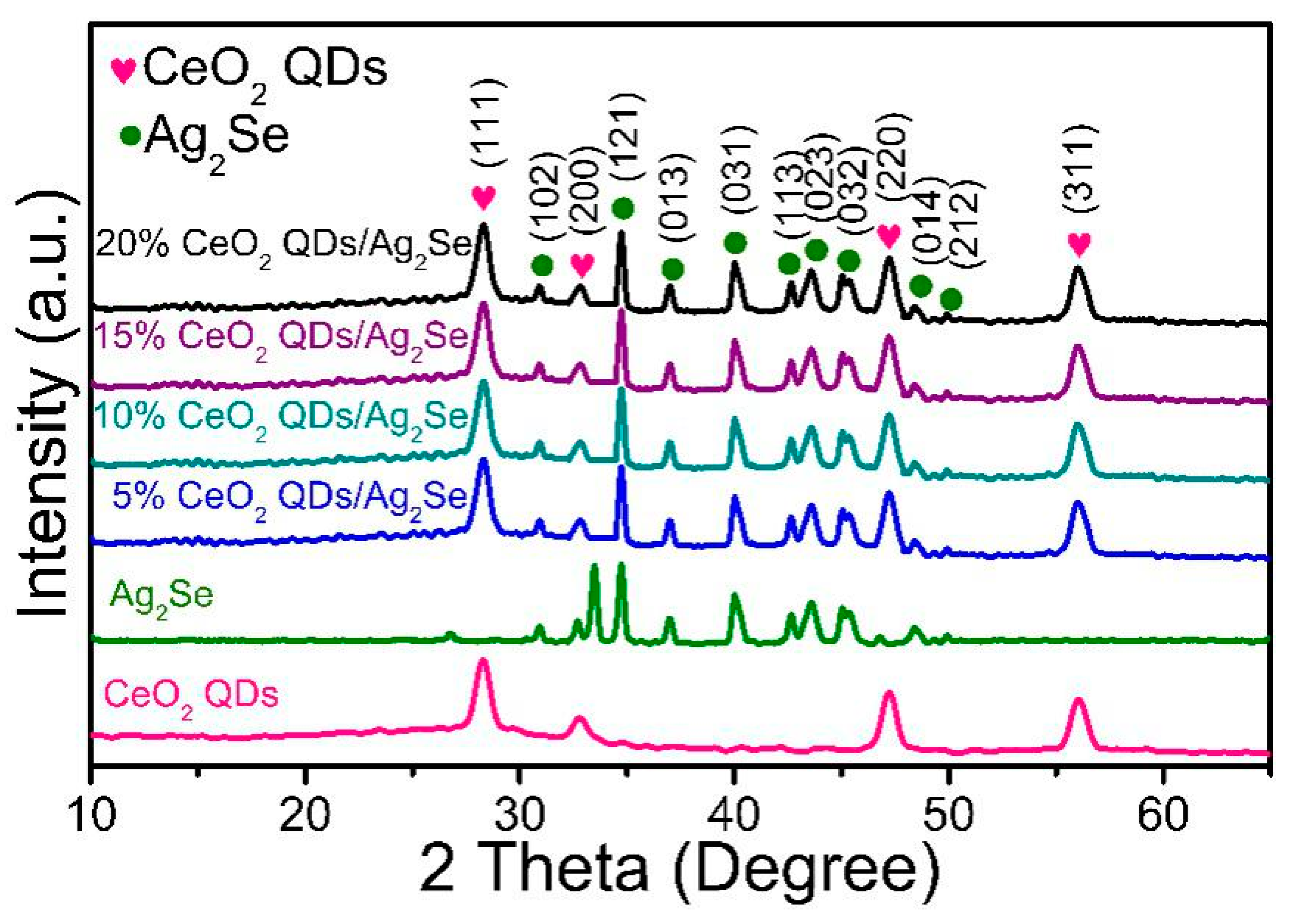
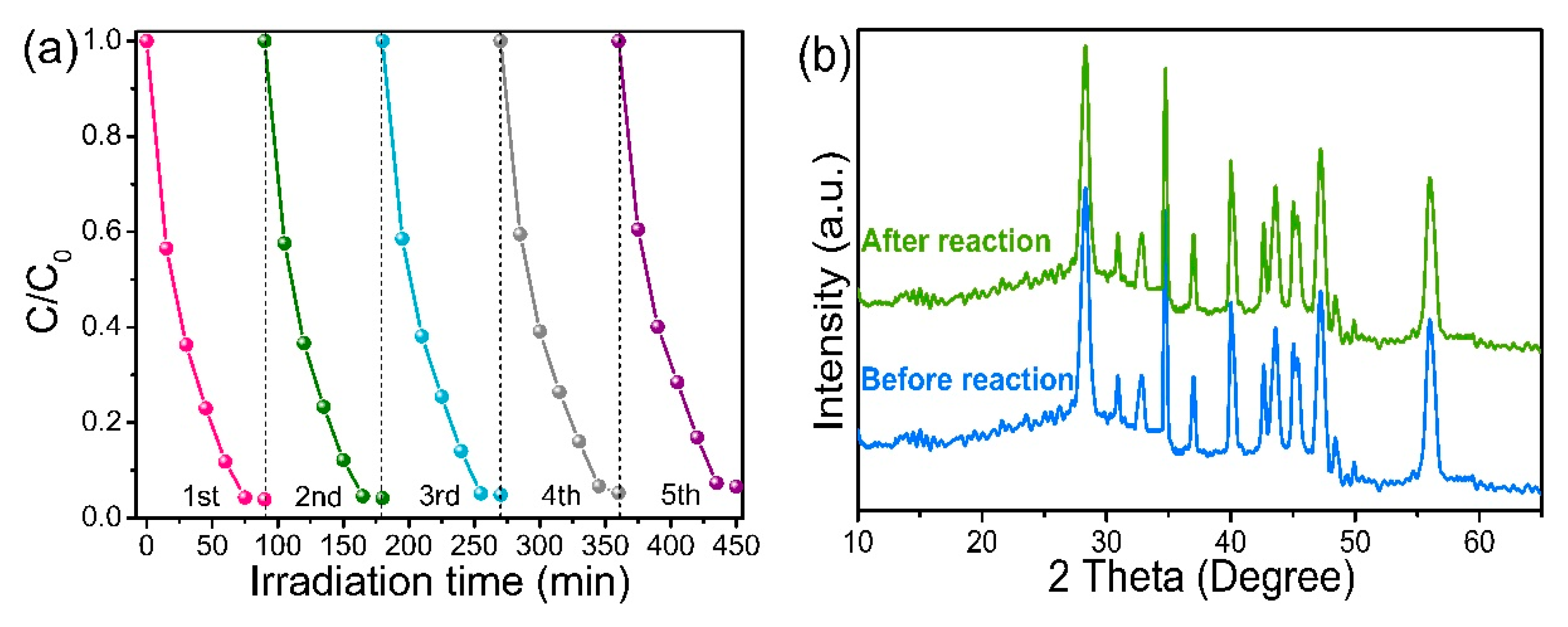
3.1. Phase Analysis
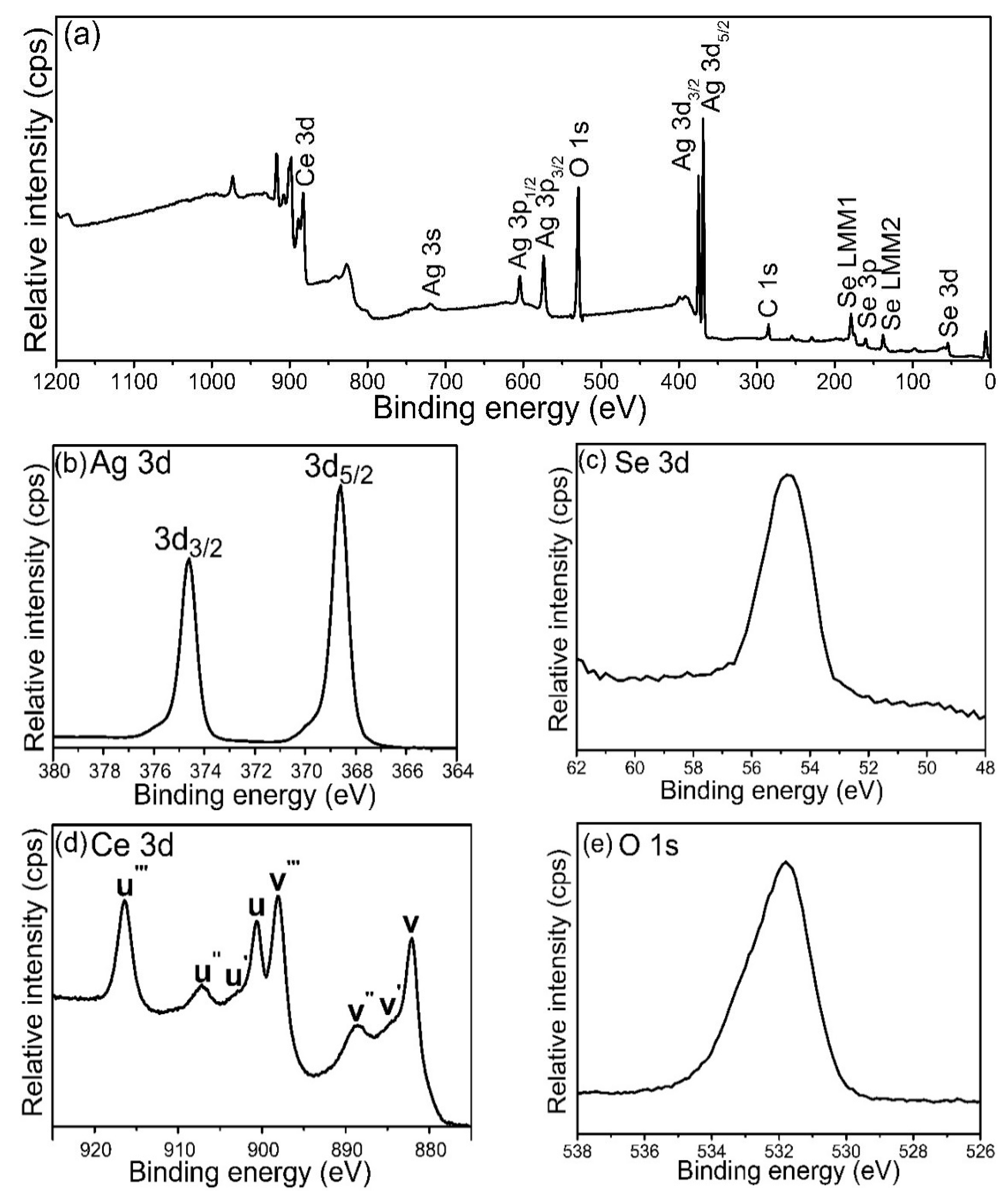
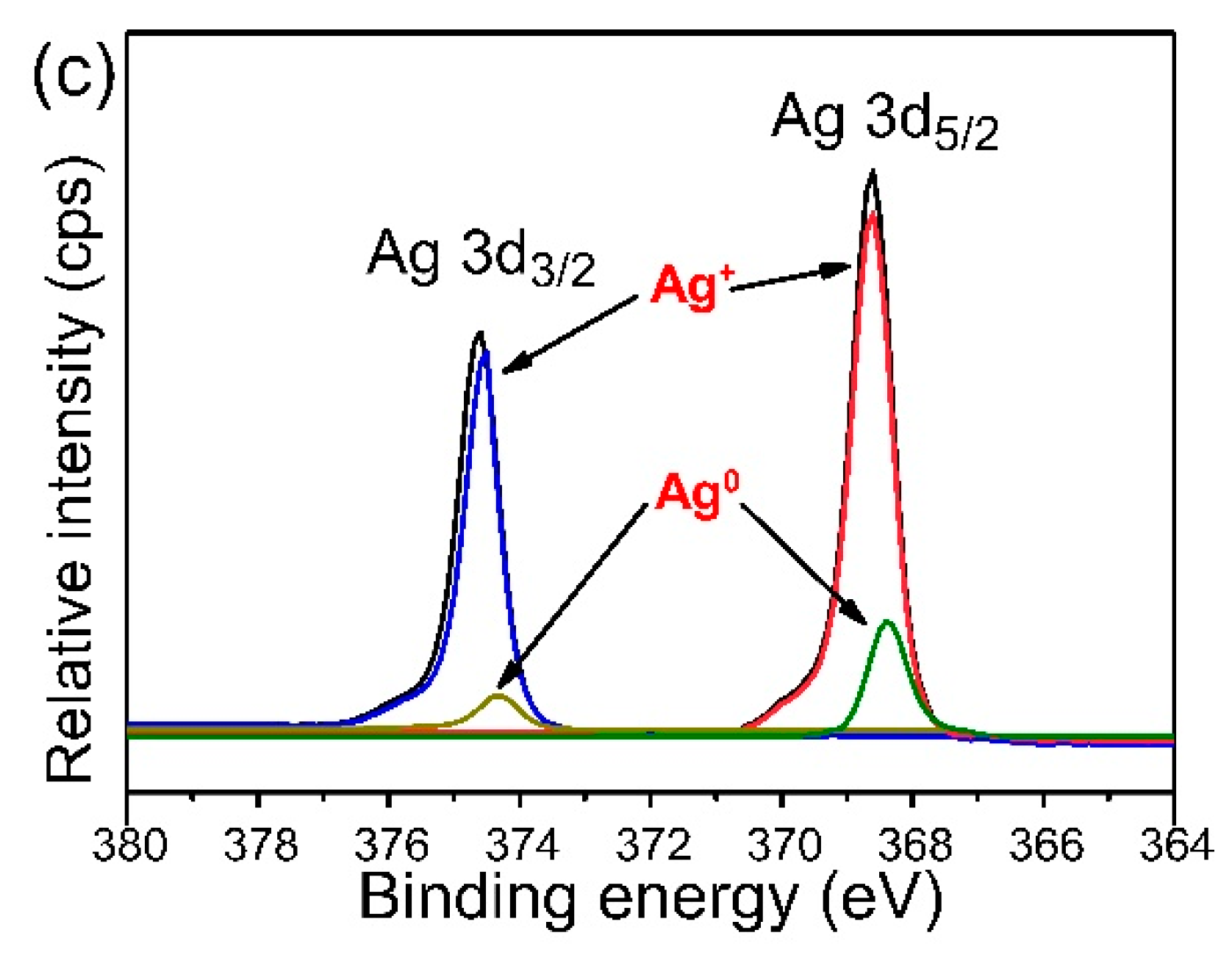
3.2. XPS
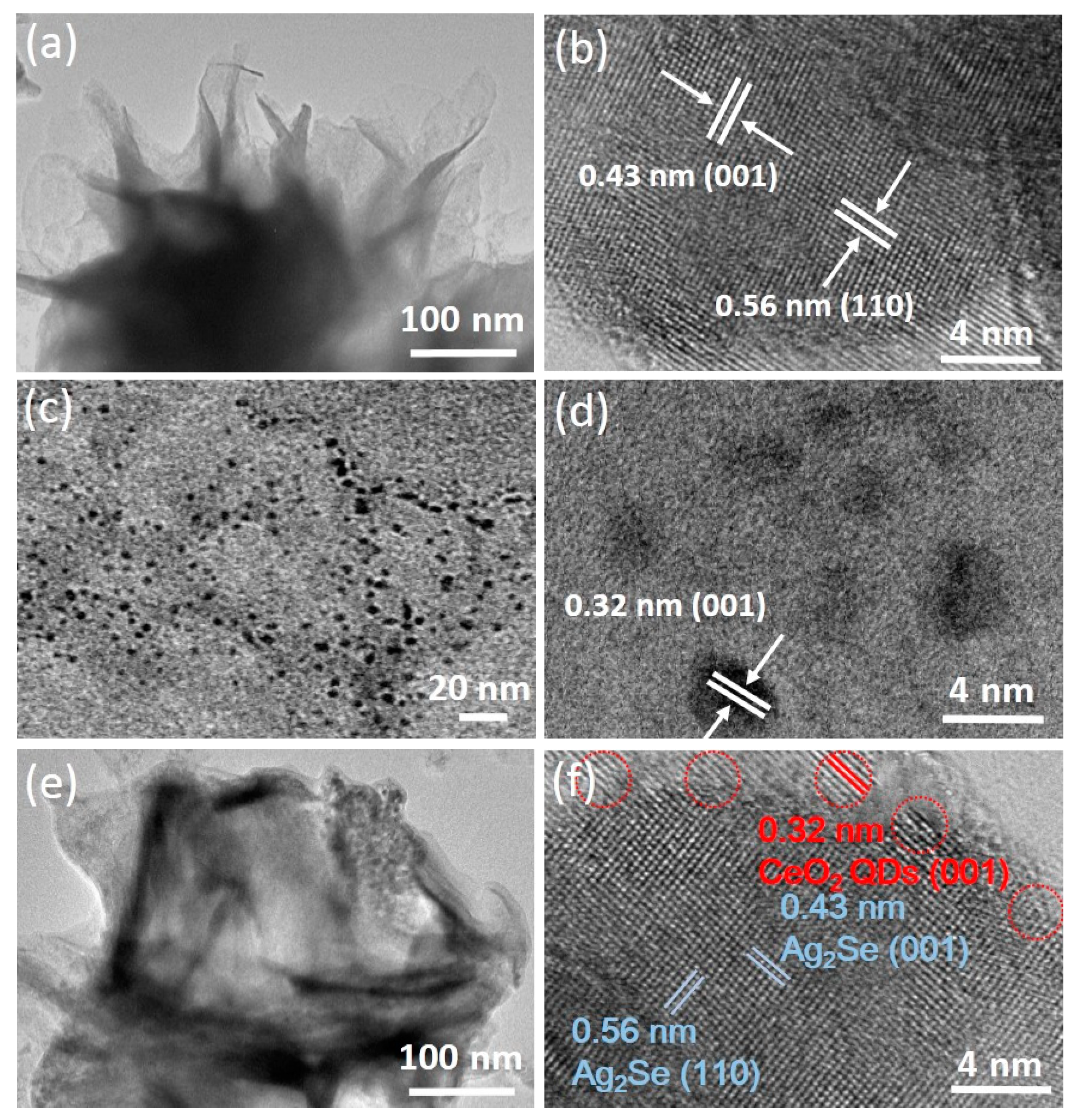
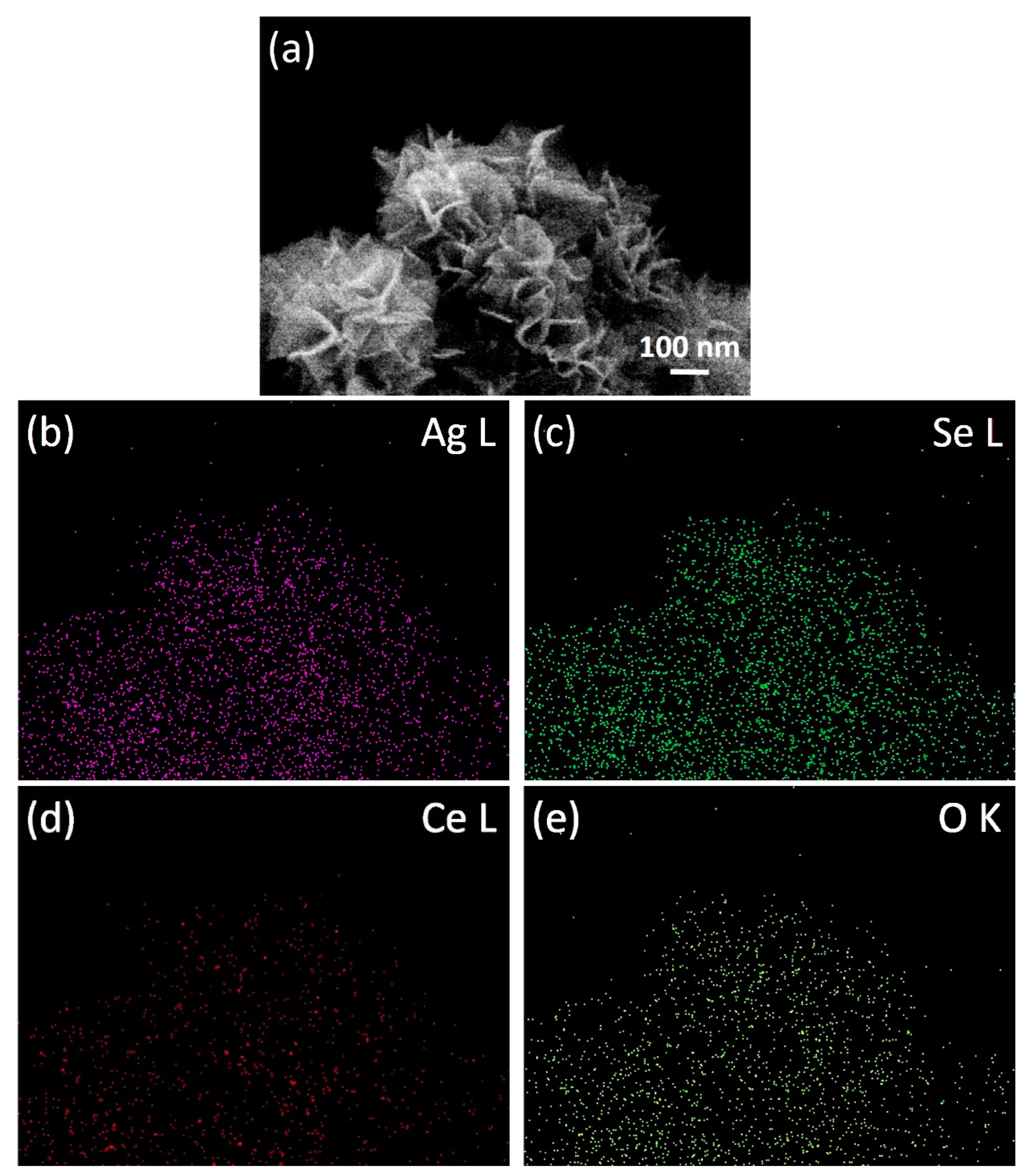
3.3. Morphological Examination and Reaction Mechanism
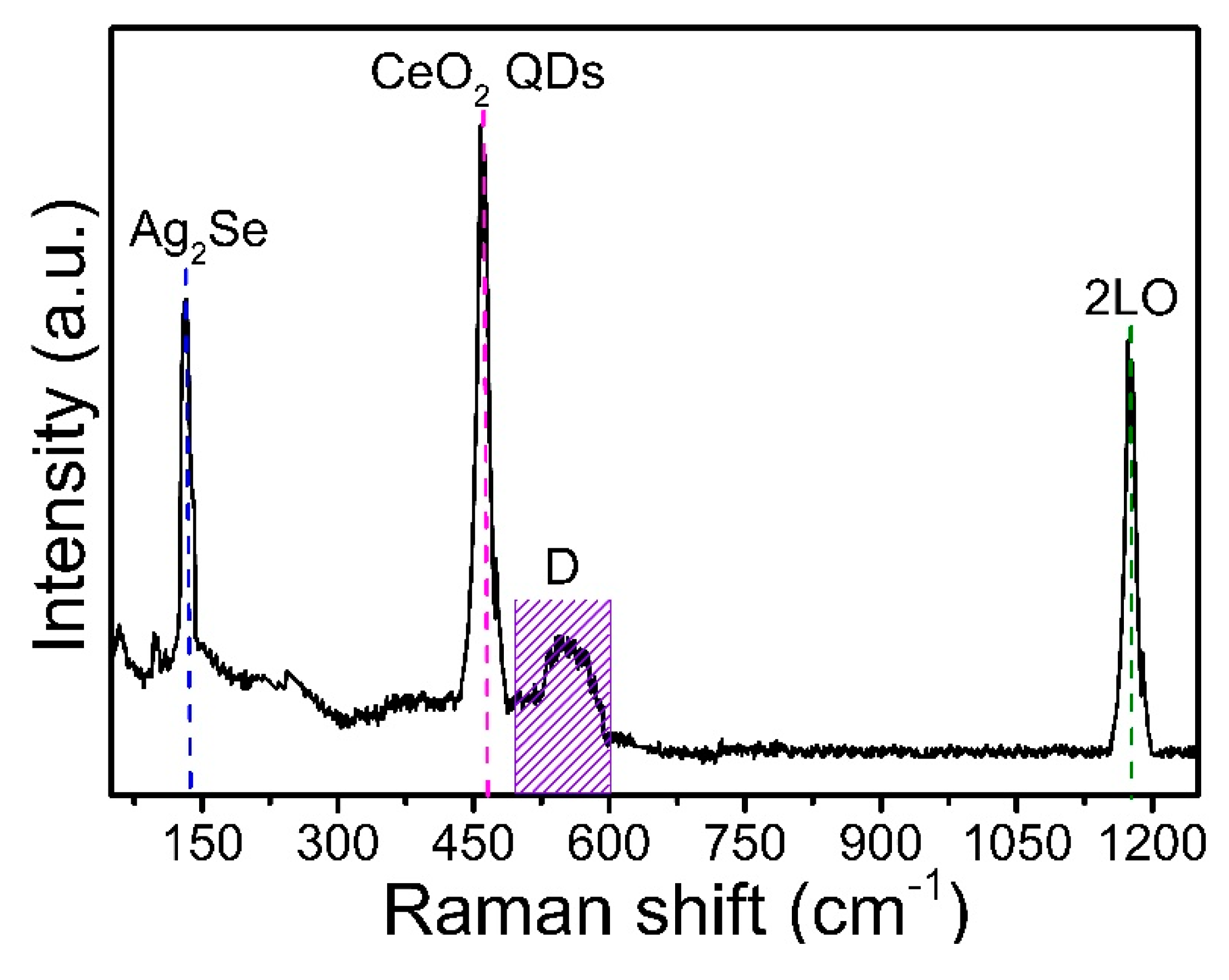
3.4. Raman Scattering
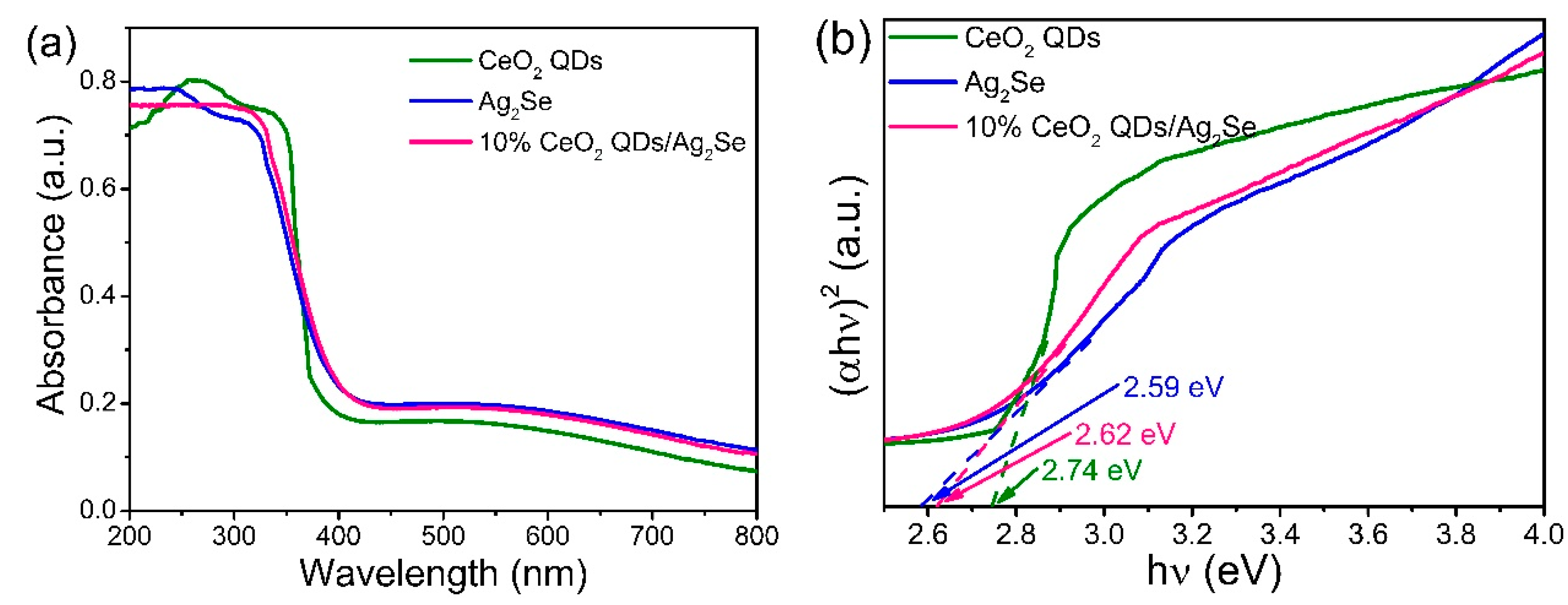
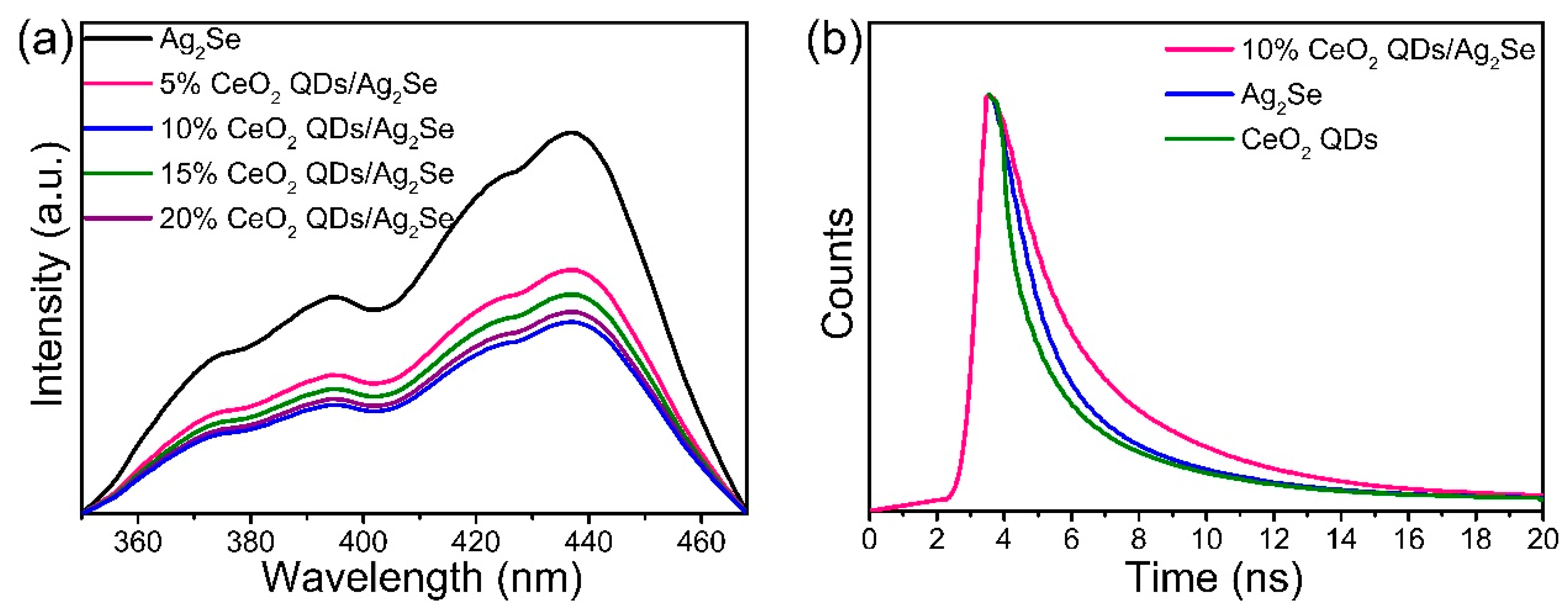
3.5. Optical Properties
3.6. Photocatalytic Characteristics Under Visible Light Illumination
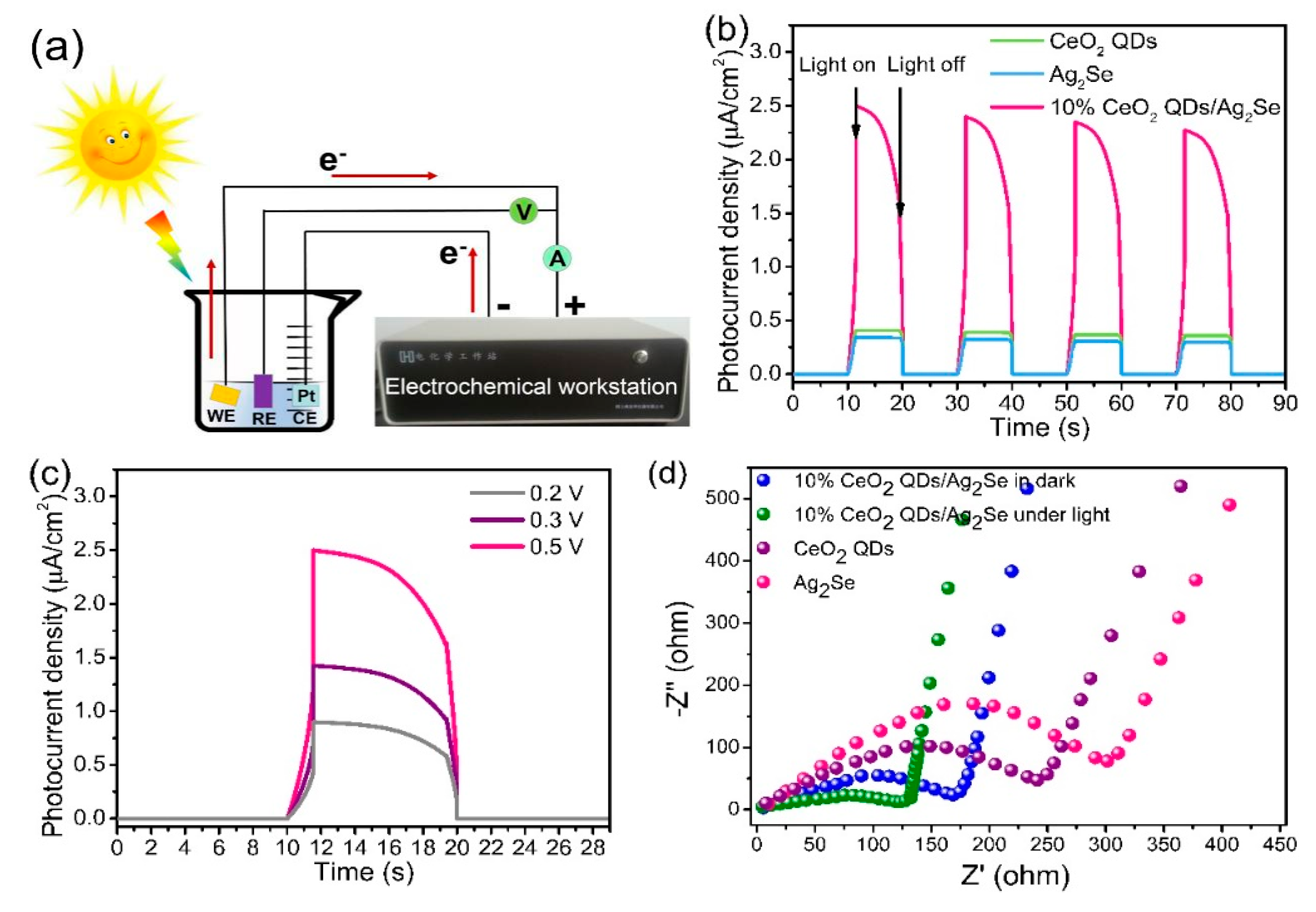
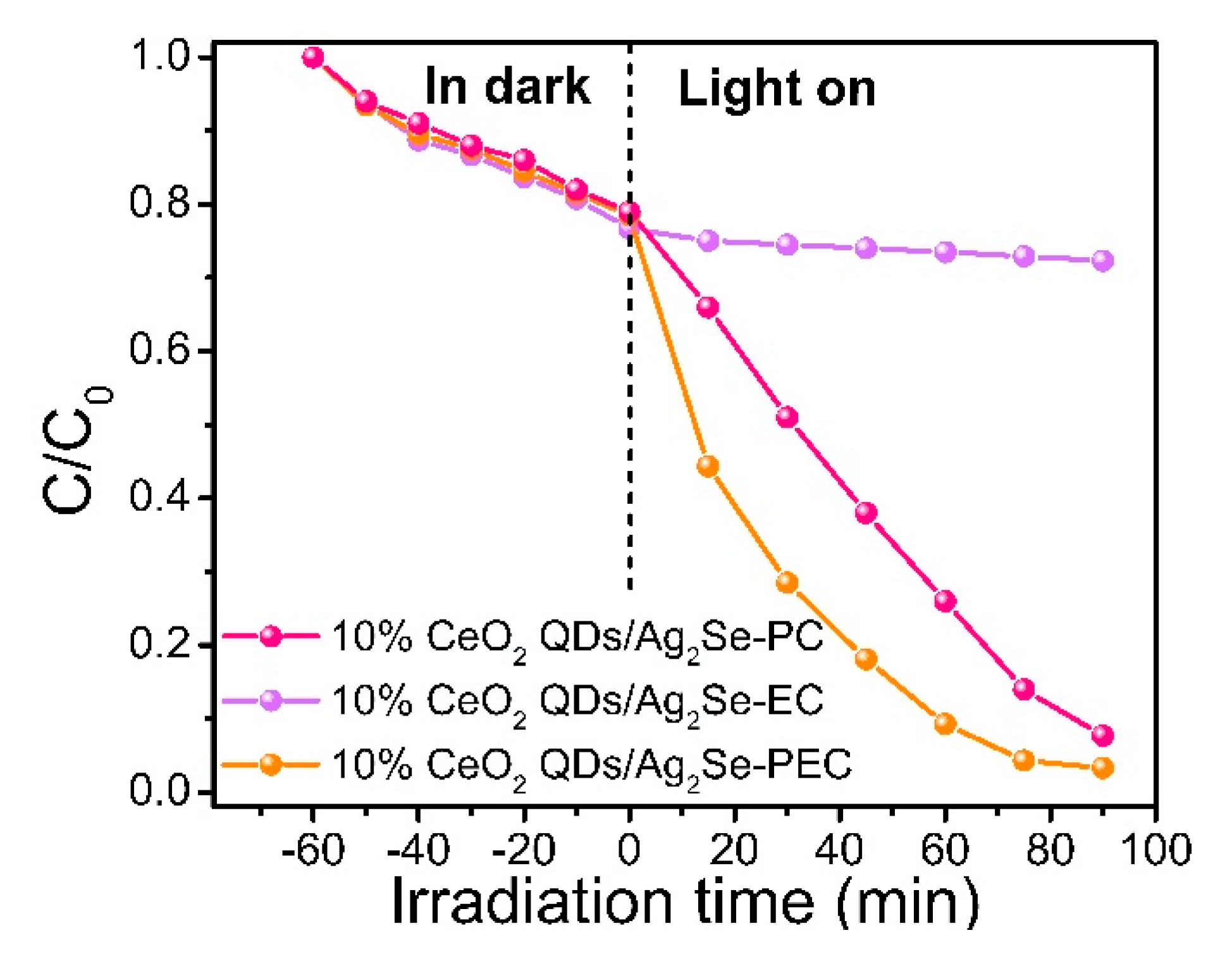
3.7. Photoelectrocatalytic Properties of CeO2 QDs/Ag2Se
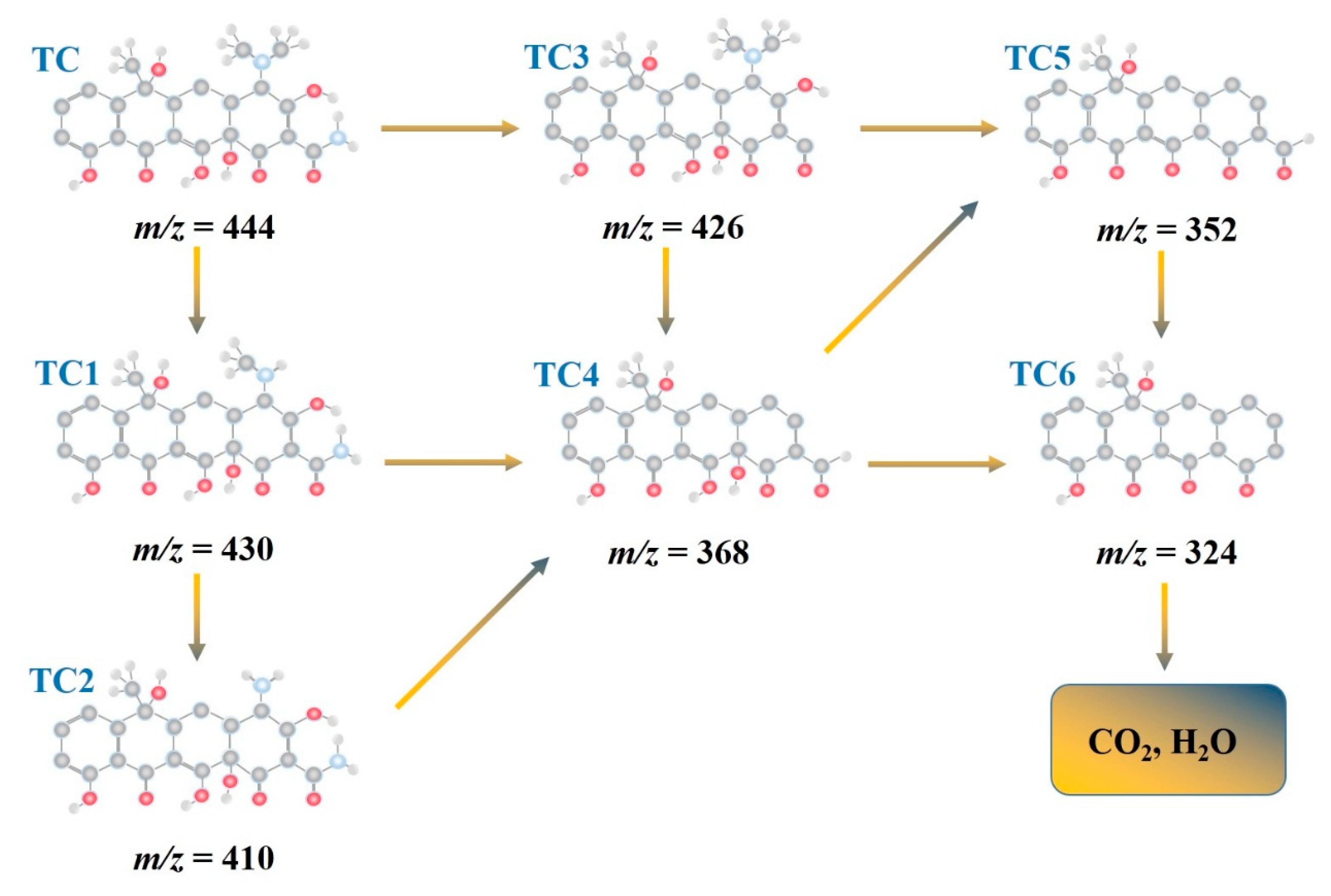
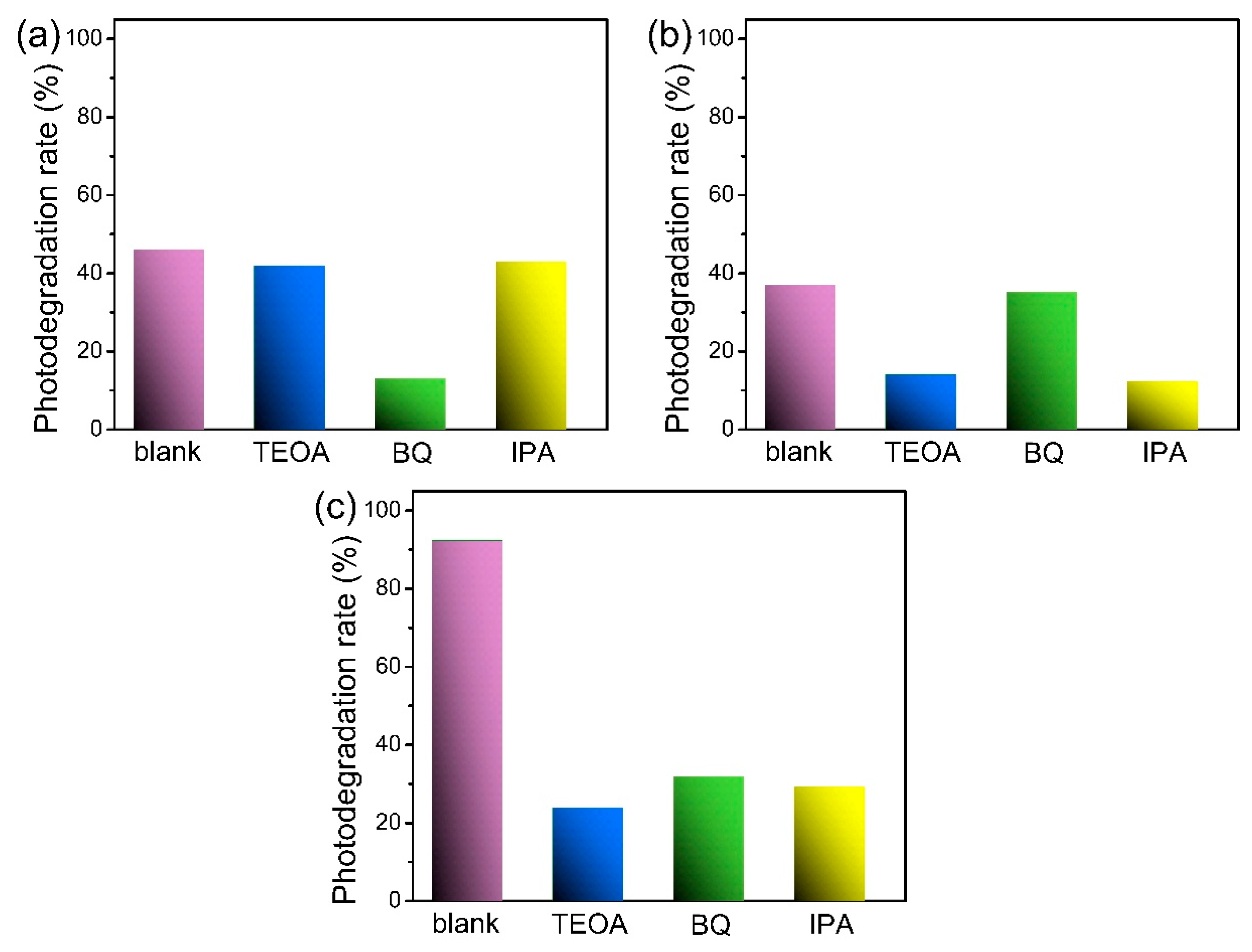
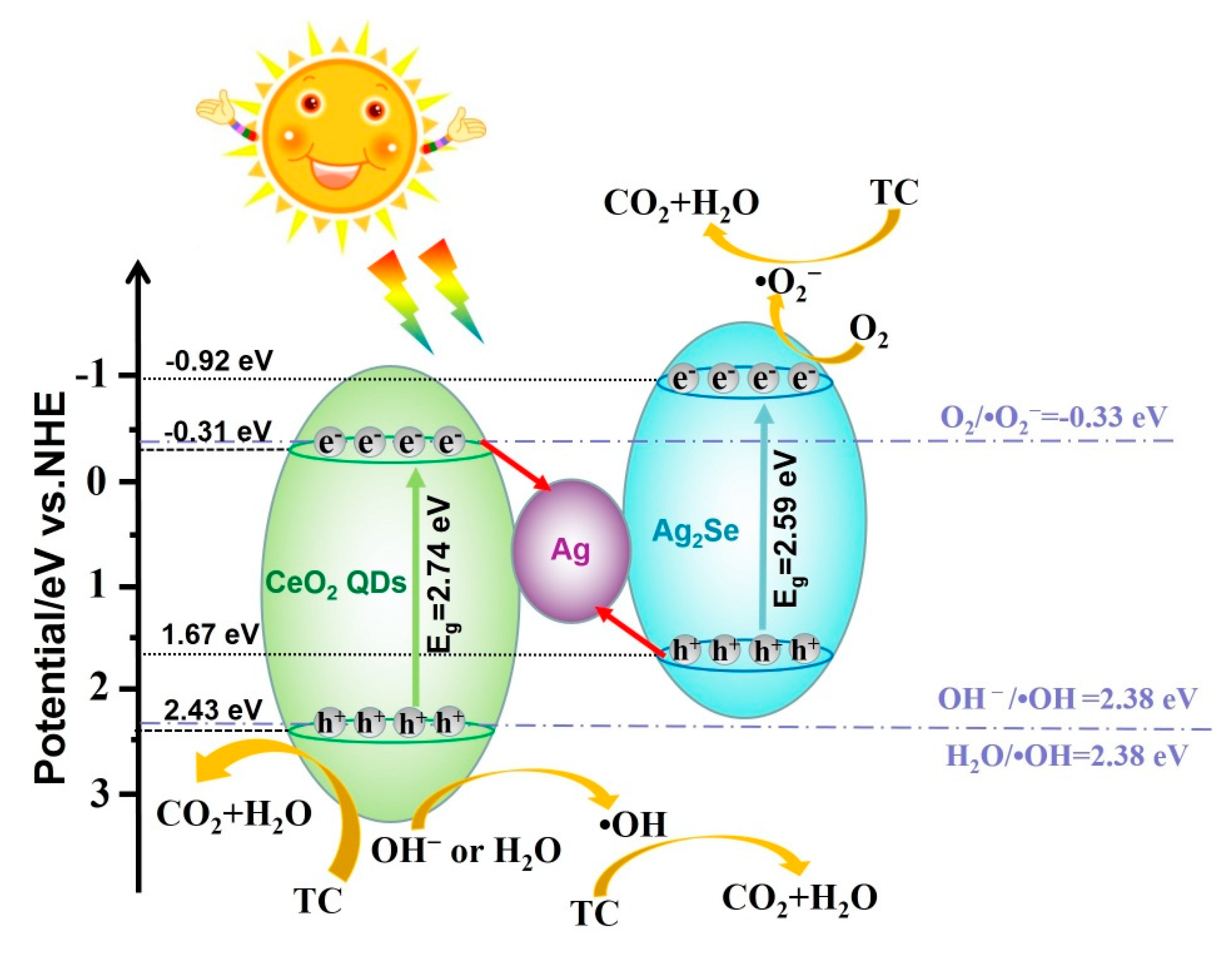
3.8. Photocatalytic Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ravikumar, K.V.G.; Sudakaran, S.V.; Ravichandran, K.; Pulimi, M.; Natarajan, C.; Mukherjee, A. Green synthesis of NiFe nano particles using Punica granatum peel extract for tetracycline removal. J. Clean. Prod. 2019, 210, 767–776. [Google Scholar] [CrossRef]
- Sandin, G.; Peters, G.M. Environmental impact of textile reuse and recycling-A review. J. Clean. Prod. 2018, 184, 353–365. [Google Scholar] [CrossRef]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): a review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, M.; Wu, M.; Li, Z.; Liu, X. Occurrences and regional distributions of 20 antibiotics in water bodies during groundwater recharge. Sci. Total Environ. 2015, 518, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xiong, Z.; Lai, B. Effect of initial pH on the tetracycline (TC) removal by zerovalentiron: adsorption, oxidation and reduction. Chem. Eng. J. 2018, 343, 492–499. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Fu, Y.; Dionysiou, D.D. Degradation kinetics and mechanism of oxytetracycline by hydroxyl radical-based advanced oxidation processes. Chem. Eng. J. 2016, 284, 1317–1327. [Google Scholar] [CrossRef]
- Huang, L.; Liu, G.; Dong, G.; Wu, X.; Wang, C.; Liu, Y. Reaction mechanism of zerovalentiron coupling with microbe to degrade tetracycline in permeable reactivebarrier (PRB). Chem. Eng. J. 2017, 316, 525–533. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Ma, Y.L.; Yang, J.; Wang, L.Q.; Lv, J.M.; Ren, C.J. Aqueous tetracycline degradation by H2O2 alone: removal and transformation pathway. Chem. Eng. J. 2017, 307, 15–23. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wan, J.; Zhang, C.; Cheng, M.; Qin, X.; Xue, W. Stabilized nanoscale zerovalent iron mediated cadmium accumulation and oxidative damage of boehmeria nivea (L.) gaudich cultivated in cadmium contaminated sediments. Environ. Sci. Technol. 2017, 51, 11308–11316. [Google Scholar] [CrossRef]
- Huo, Y.; Wang, Z.; Zhang, J.; Liang, C.; Dai, K. Ag SPR-promoted 2D porous g-C3N4/Ag2MoO4 composites for enhanced photocatalytic performance towards methylene blue degradation. Appl. Surf. Sci. 2018, 459, 271–280. [Google Scholar] [CrossRef]
- Liu, R.D.; Li, H.; Duan, L.B.; Shen, H.; Zhang, Q.; Zhao, X.R. The synergistic effect of graphene oxide and silver vacancy in Ag3PO4-based photocatalysts for rhodamine B degradation under visible light. Appl. Surf. Sci. 2018, 462, 263–269. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, J.; Wang, Z.; Cheng, B.; Dai, K.; Ho, W. Direct Z-scheme porous g-C3N4/BiOI heterojunction for enhanced visible-light photocatalytic activity. J. Alloys Compd. 2018, 766, 841–850. [Google Scholar] [CrossRef]
- Wang, H.L.; Xu, L.J.; Liu, C.L.; Jiang, Z.; Feng, Q.; Wu, T.Z.; Wang, R.Q. A novel magnetic photocatalyst Bi3O4Cl/SrFe12O19: Fabrication, characterization and its photocatalytic activity. Ceram. Int. 2020, 46, 460–467. [Google Scholar] [CrossRef]
- Jia, Y.L.; Lin, Y.H.; Ma, Y.; Shi, W.B. Fabrication of hollow Bi2MoO6 nanorods with efficient photocatalytic performance. Mater. Lett. 2019, 234, 83–86. [Google Scholar] [CrossRef]
- Xue, Y.T.; Wu, Z.S.; He, X.F.; Yang, X.; Chen, X.Q.; Gao, Z.Z. Constructing a Z-scheme heterojunction of egg-like core@shell CdS@TiO2 photocatalyst via a facile reflux method for enhanced photocatalytic performance. Nanomaterials 2019, 9, 222. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.G.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Zhang, T.T.; Lei, W.Y.; Liu, P.; Rodriguez, J.A.; Yu, J.G.; Qi, Y.; Liu, G.; Liu, M.H. Organic pollutant photodecomposition by Ag/KNbO3 nanocomposites: a combined experimental and theoretical study. J. Phys. Chem. C 2016, 120, 2777–2786. [Google Scholar] [CrossRef]
- Song, S.Q.; Cheng, B.; Wu, N.S.; Meng, A.Y.; Cao, S.W.; Yu, J.G. Structure effect of graphene on the photocatalytic performance of plasmonic Ag/Ag2CO3-rGO for photocatalytic elimination of pollutants. Appl. Catal. B 2016, 181, 71–78. [Google Scholar] [CrossRef]
- Shezad, N.; Maafa, I.M.; Johair, K.; Hafeez, A.; Akhter, P.; Shabir, M.; Raza, A.; Anjum, H.; Hussain, M.; Tahir, M. Carbon nanotubes incorporated Z-Scheme assembly of AgBr/TiO2 for photocatalytic hydrogen production under visible light irradiations. Nanomaterials 2019, 9, 1767. [Google Scholar] [CrossRef]
- Tian, Q.Y.; Yao, W.J.; Wu, Z.H.; Liu, J.; Liu, L.; Wu, W.; Jiang, C.Z. Full-spectrum-activated Z-scheme photocatalysts based on NaYF4: Yb3+/Er3+, TiO2 and Ag6Si2O7. J. Mater. Chem. A 2017, 5, 23566–23576. [Google Scholar] [CrossRef]
- Jin, J.; Yu, J.G.; Guo, D.P.; Cui, C.; Ho, W.A. Hierarchical Z-scheme CdS-WO3 photocatalyst with enhanced CO2 reduction activity. Small 2015, 11, 5262–5271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Y.; Lai, C.; Xu, P.; Zeng, G.M.; Huang, D.L.; Li, Z.H.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; et al. Rational design of carbon-doped carbon nitride/Bi12O17Cl2 composites: A promising candidate photocatalyst for boosting visible-light-driven photocatalytic degradation of tetracycline. ACS Sustain. Chem. Eng. 2018, 6, 6941–6949. [Google Scholar] [CrossRef]
- Li, B.S.; Lai, C.; Xu, P.; Zeng, G.M.; Huang, D.L.; Qin, L.; Yi, H.; Cheng, M.; Wang, L.L.; Huang, F.L.; et al. Facile synthesis of bismuth oxyhalogen-based Z-scheme photocatalyst for visible-light-driven pollutant removal: Kinetics, degradation pathways and mechanism. J. Clean. Prod. 2019, 225, 898–912. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, Z.; Zhu, Y. Systematic research on Ag2X (X = O, S, Se, Te) as visible and near-infrared light driven photocatalysts and effects of their electronic structures. Appl. Surf. Sci. 2018, 427, 1202–1216. [Google Scholar] [CrossRef]
- Tian, J.; Sang, Y.H.; Zhao, Z.H.; Zhou, W.J.; Wang, D.Z.; Kang, X.L.; Liu, H.; Wang, J.Y.; Chen, S.W.; Cai, H.Q.; et al. Enhanced photocatalytic performances of CeO2/TiO2 nanobelt heterostructures. Small 2013, 9, 3864–3872. [Google Scholar] [CrossRef]
- Skorodumova, N.V.; Simak, S.I.; Lundqvist, B.I.; Abrikosov, I.A.; Johansson, B. Quantum origin of the oxygen storage capability of ceria. Phys. Rev. Lett. 2002, 89, 166601–166604. [Google Scholar] [CrossRef]
- Carey, G.H.; Abdelhady, A.L.; Ning, Z.; Thon, S.M.; Bakr, O.M.; Sargent, E.H. Colloidal quantum dot solar cells. Chem. Rev. 2015, 115, 12732–12763. [Google Scholar] [CrossRef]
- Ye, M.Y.; Zhao, Z.H.; Hu, Z.F.; Liu, L.Q.; Ji, H.M.; Shen, Z.R.; Ma, T.Y. 0D/2D heterojunctions of vanadate quantum dots/graphitic carbon nitride nanosheets for enhanced visible-light-driven photocatalysis. Angew. Chem. Int. Ed. 2017, 56, 8407–8411. [Google Scholar] [CrossRef]
- Imagawa, H.; Suda, A.; Yamamura, K.; Sun, S. Monodisperse CeO2 nanoparticles and their oxygen storage and release properties. J. Phys. Chem. C 2011, 115, 1740–1745. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray photoelectron spectroscopy. Phyiosical Electronics Division 190; Perkin-Elmer Corporation: Eden Prairie, Minnesota, USA, 1979. [Google Scholar]
- Zou, W.X.; Shao, Y.; Pu, Y.; Luo, Y.D.; 7 Sun, J.F.; Ma, K.L.; Tang, C.J.; Gao, F.; Dong, L. Enhanced visible light photocatalytic hydrogen evolution via cubic CeO2 hybridized g-C3N4 composite. Appl. Catal. B 2017, 218, 51–59. [Google Scholar] [CrossRef]
- Naumov, P.; Barkalov, O.; Mirhosseini, H. Atomic and electronic structures evolution of the narrow band gap semiconductor Ag2Se under high pressure. J. Phys-Condens. Mat. 2016, 28, 385801. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Kumar, S.; Ojha, A.K.; Patrice, D.; Yadav, B.S.; Materny, A. One-step in situ synthesis of CeO2 nanoparticles grown on reduced graphene oxide as an excellent fluorescent and photocatalyst material under sunlight irradiation. Phys. Chem. Chem. Phys. 2016, 18, 11157–11167. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Che, H.N.; Liu, C.B.; Che, G.B. Facile fabrication of g-C3N4 QDs/BiVO4 Z-scheme heterojunction towards enhancing photodegradation activity under visible light. J. Taiwan. Inst. Chem. E 2019, 95, 669–681. [Google Scholar] [CrossRef]
- Wetchakun, N.; Chaiwichain, S.; Inceesungvorn, B.; Pingmuang, K.; Phanichphant, S.; Minett, A.I.; Chen, J. BiVO4/CeO2 nanocomposites with high visible-light-induced photocatalytic activity. ACS Appl. Mater. Inter. 2012, 4, 3718–3723. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, Y.; Lu, F.; Qin, J.; Wei, F.; Xu, C.; Wang, S. Magnetic ZnFe2O4-C3N4 hybrid for photocatalytic degradation of aqueous organic pollutants by visible light. Ind. Eng. Chem. Res. 2014, 53, 17294–17302. [Google Scholar] [CrossRef]
- Liang, C.; Niu, C.G.; Wen, X.J.; Yang, S.F.; Shen, M.C.; Zeng, G.M. Effective removal of colourless pollutants and organic dyes by Ag@AgCl nanoparticle-modified CaSn(OH)6 composite under visible light irradiation. New J. Chem. 2017, 41, 5334–5346. [Google Scholar] [CrossRef]
- Wen, X.J.; Niu, C.G.; Zhang, L.; Zeng, G.M. Fabrication of SnO2 nanopaticles/BiOI n-p heterostructure for wider spectrum visible-light photocatalytic degradation of antibiotic oxytetracycline hydrochloride. ACS Sustain. Chem. Eng. 2017, 5, 5134–5147. [Google Scholar] [CrossRef]
- Wei, X.; Feng, H.; Li, L.; Gong, J.; Jiang, K.; Xue, S.; Chu, P. Synthesis of tetragonal prismatic γ-In2Se3 nanostructures with predominantly {110} facets and photocatalytic degradation of tetracycline. Appl. Catal. B 2020, 260, 118218–118226. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Feng, H.; Wei, X.; Jiang, K.; Xue, S.; Chu, P.K. Ag as Cocatalyst and Electron-Hole Medium in CeO2 QDs/Ag/Ag2Se Z-scheme Heterojunction Enhanced the Photo-Electrocatalytic Properties of the Photoelectrode. Nanomaterials 2020, 10, 253. https://doi.org/10.3390/nano10020253
Li L, Feng H, Wei X, Jiang K, Xue S, Chu PK. Ag as Cocatalyst and Electron-Hole Medium in CeO2 QDs/Ag/Ag2Se Z-scheme Heterojunction Enhanced the Photo-Electrocatalytic Properties of the Photoelectrode. Nanomaterials. 2020; 10(2):253. https://doi.org/10.3390/nano10020253
Chicago/Turabian StyleLi, Lingwei, Hange Feng, Xiaofan Wei, Kun Jiang, Shaolin Xue, and Paul K. Chu. 2020. "Ag as Cocatalyst and Electron-Hole Medium in CeO2 QDs/Ag/Ag2Se Z-scheme Heterojunction Enhanced the Photo-Electrocatalytic Properties of the Photoelectrode" Nanomaterials 10, no. 2: 253. https://doi.org/10.3390/nano10020253
APA StyleLi, L., Feng, H., Wei, X., Jiang, K., Xue, S., & Chu, P. K. (2020). Ag as Cocatalyst and Electron-Hole Medium in CeO2 QDs/Ag/Ag2Se Z-scheme Heterojunction Enhanced the Photo-Electrocatalytic Properties of the Photoelectrode. Nanomaterials, 10(2), 253. https://doi.org/10.3390/nano10020253





