Systematic Design of Polypyrrole/Carbon Fiber Electrodes for Efficient Flexible Fiber-Type Solid-State Supercapacitors
Abstract
1. Introduction
2. Experiments
2.1. Fabrication of Polypyrrole on Carbon Fiber (CF@PPy)
2.2. Assembly of Flexible Fiber-Type Solid-State Supercapacitors
2.3. Measurements and Characterizations
3. Result and Discussion
3.1. Effects of Electropolymerized Electrolyte on Electrochemical Performance of CF@PPy
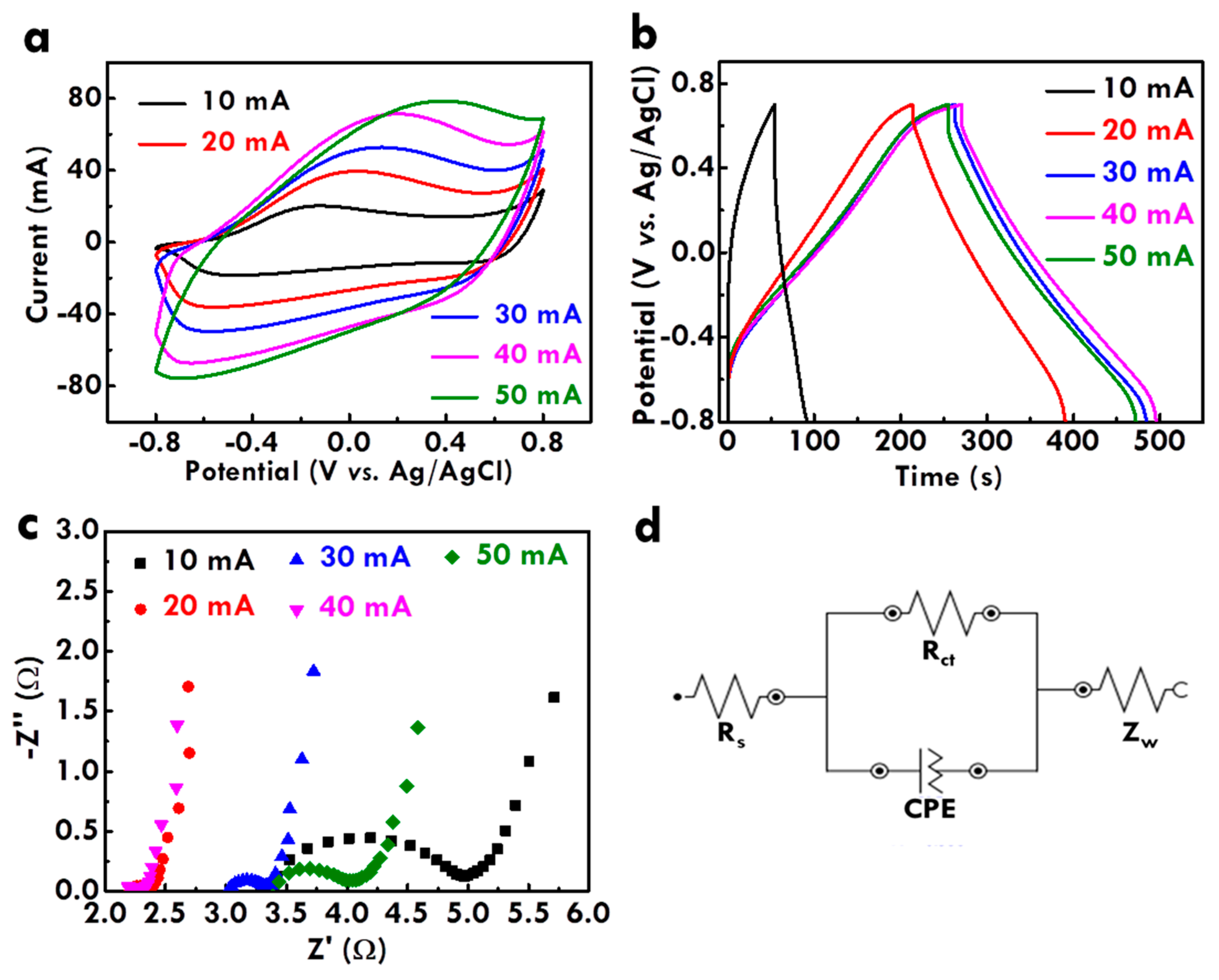
3.2. Effect of the Applied Current for Electropolymerization on Physical and Electrochemical Properties of CF@PPy
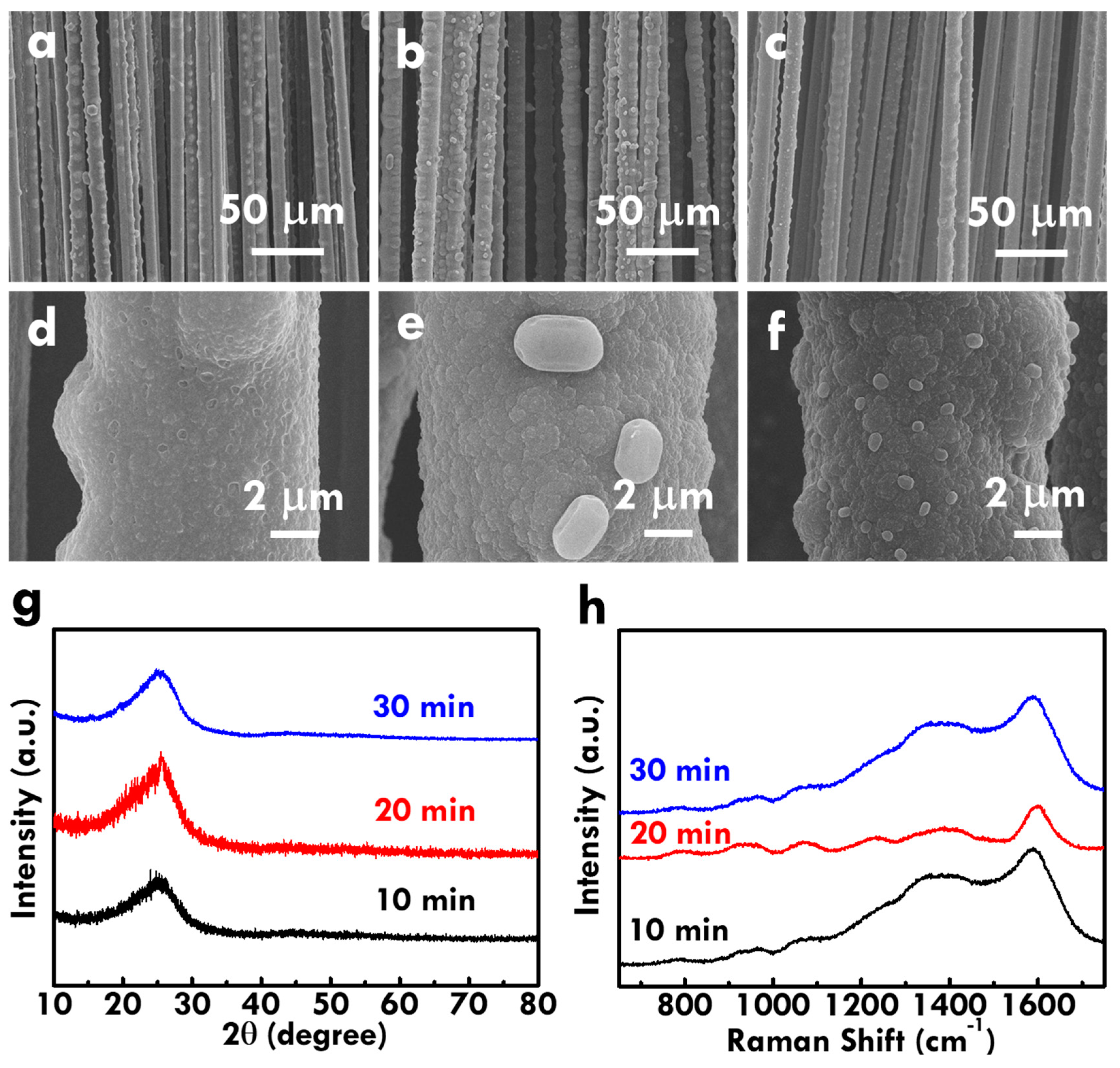
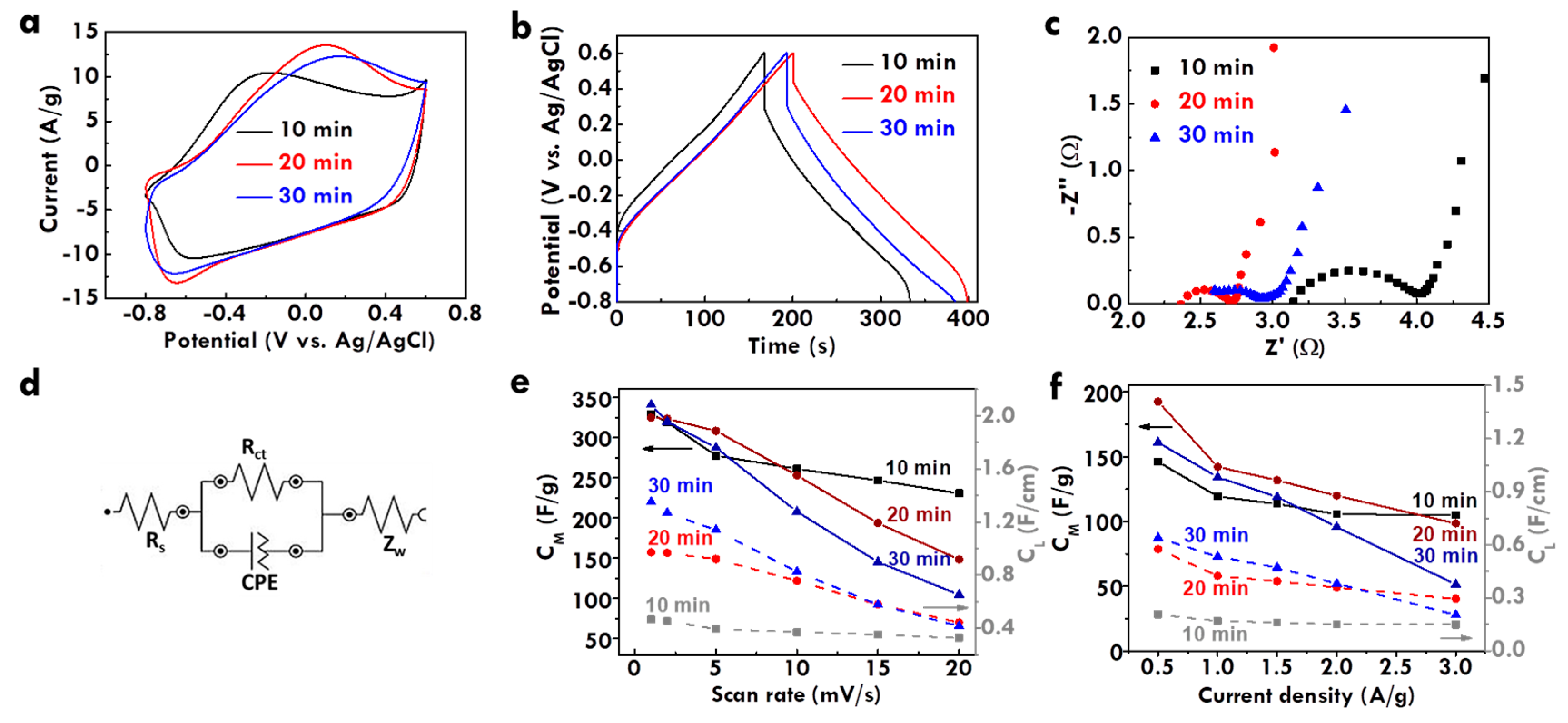
3.3. Effect of Electropolymerization Time on the Physical and Electrochemical Features of CF@PPy
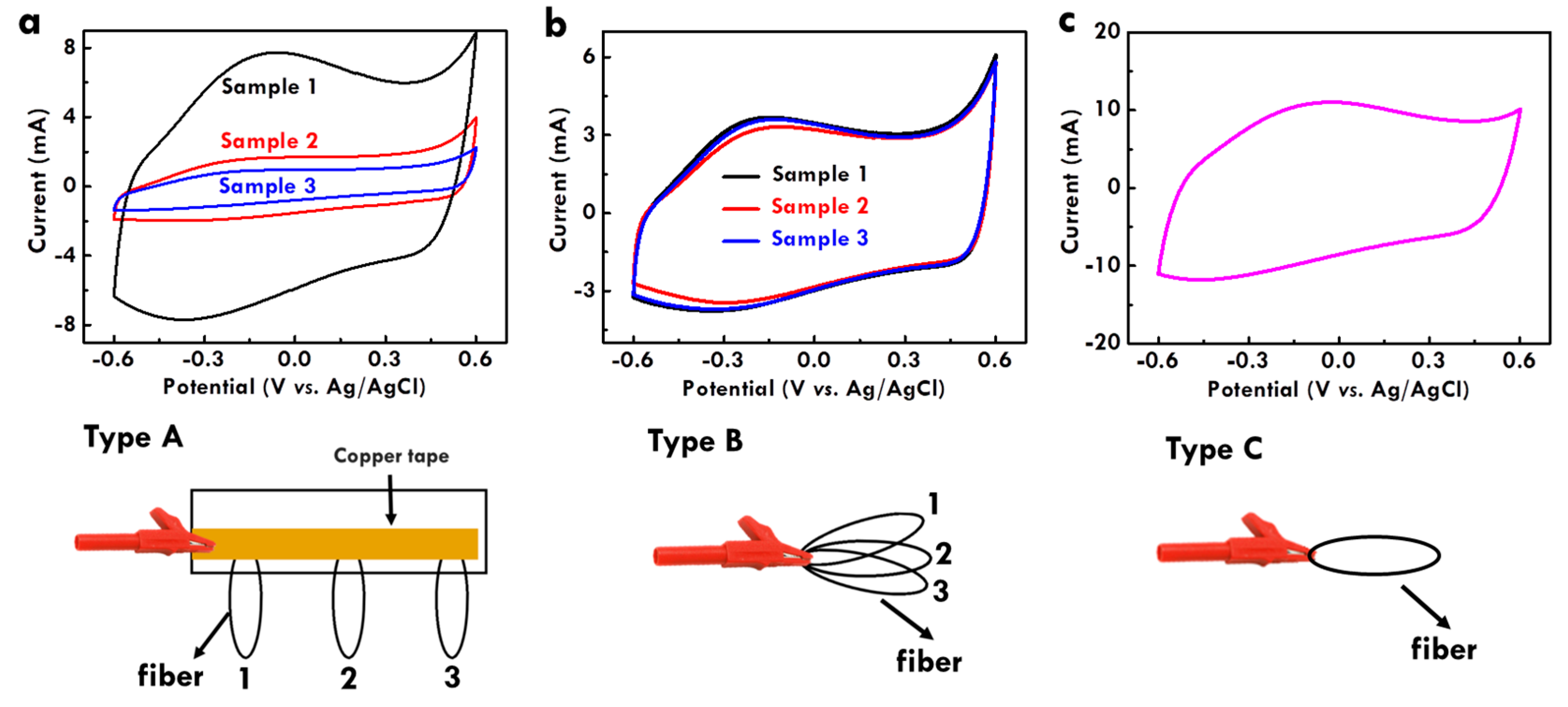
3.4. Effects of Electropolymerization Systems on the Electrochemical Performance of CF@PPy
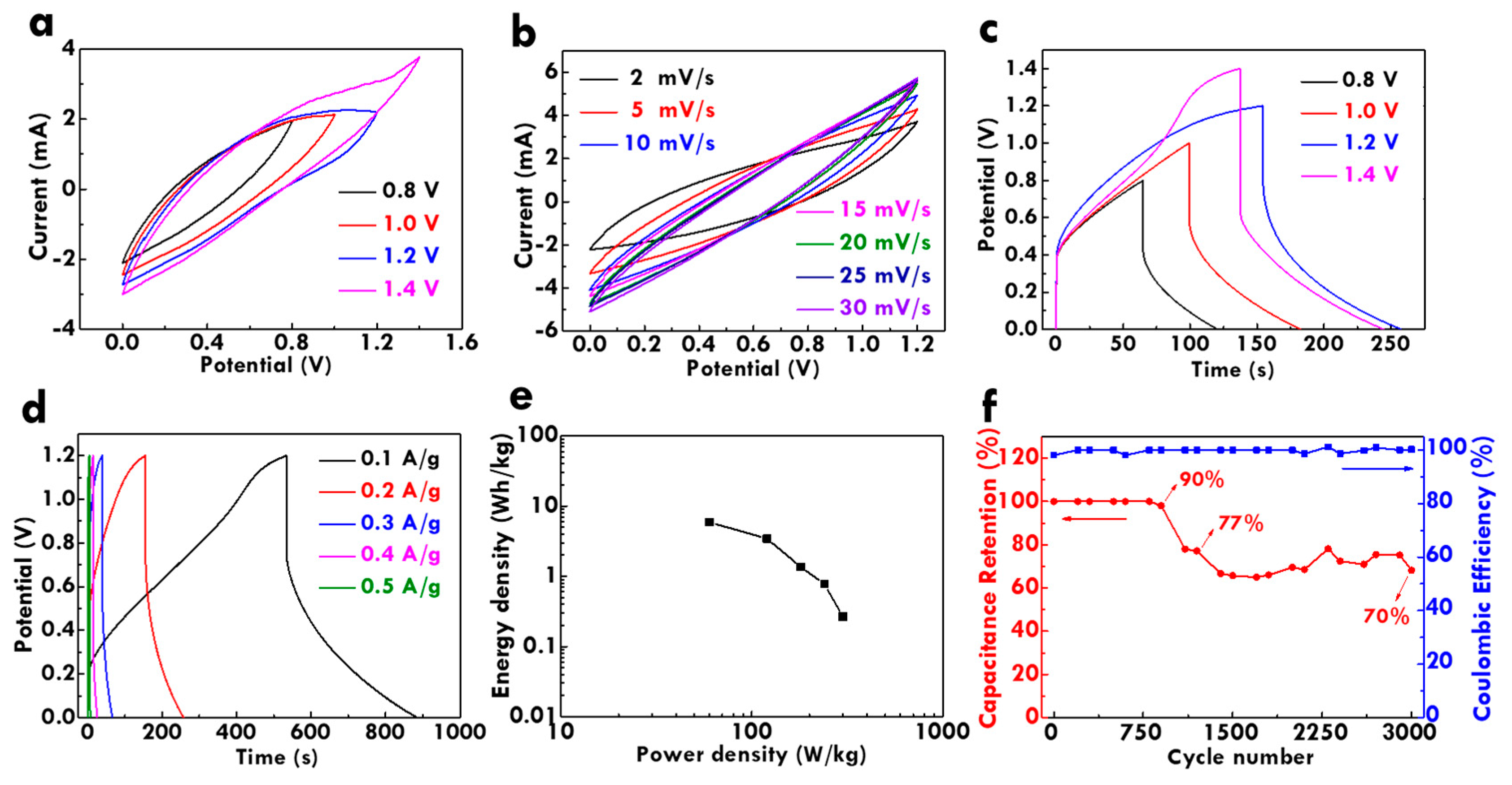
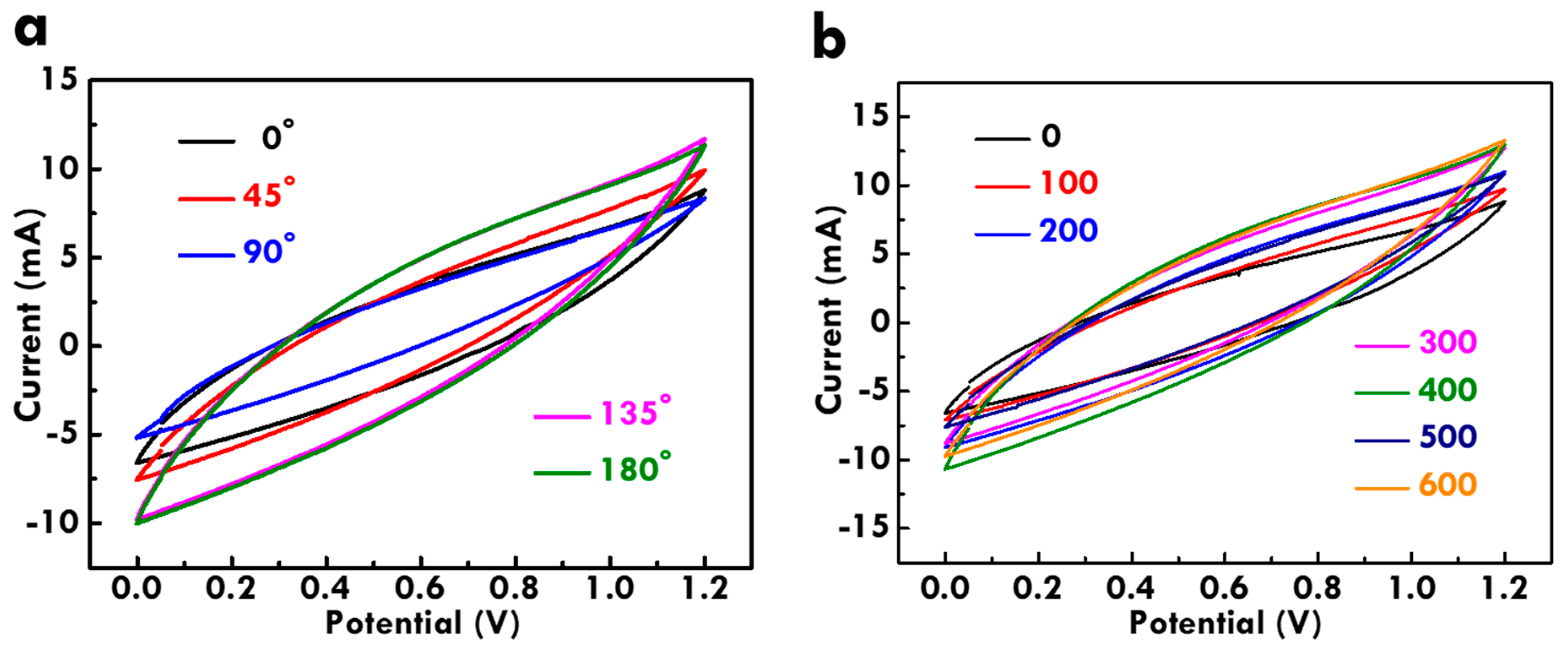
3.5. Electrochemical Performance Analysis of the FSC with CF@PPy electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Y.; Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef]
- An, B.W.; Shin, J.H.; Kim, S.-Y.; Kim, J.; Ji, S.; Park, J.; Lee, Y.; Jang, j.; Park, Y.-G.; Cho, E.; et al. Smart Sensor Systems for Wearable Electronic Devices. Polymers 2017, 9, 303. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, B.; Chen, Y.; Feng, X. Breathable and Stretchable Temperature Sensors Inspired by Skin. Sci. Rep. 2015, 5, 11505. [Google Scholar]
- Senthilkumar, S.T.; Wang, Y.; Huang, H. Advances and prospects of fiber supercapacitors. J. Mater. Chem. A 2015, 3, 20863–20879. [Google Scholar] [CrossRef]
- Kim, B.K.; Sy, S.; Yu, A.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Conversion. In Handbook of Clean Energy Systems; Wiley: New York, NY, USA, 2015. [Google Scholar]
- Wu, Z.; Li, L.; Yan, J.M.; Zhang, X.B. Materials Design and System Construction for Conventional and New-Concept Supercapacitors. Adv. Sci. 2017, 4, 1600382. [Google Scholar]
- Rani, J.R.; Thangavel, R.; Oh, S.-I.; Lee, Y.S.; Jang, J.-H. An ultra-high-energy density supercapacitor; fabrication based on thiol-functionalized graphene oxide scrolls. Nanomaterials 2019, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Z.; Liang, Y.; Yang, Y.; An, N.; Li, Z.; Wu, H. Growth of 3D SnO2 nanosheets on carbon cloth as a binder-free electrode for supercapacitors. J. Mater. Chem. A 2015, 3, 15057–15067. [Google Scholar] [CrossRef]
- Nakayama, M.; Komine, K.; Inohara, D. Eletrochemical Processing of Commercial Carbon Cloth for Supercapacitor Applications. ECS Trans. 2017, 75, 31–39. [Google Scholar] [CrossRef]
- Wang, S.; Pei, B.; Zhao, X.; Dryfe, R.A.W. Highly porous graphene on carbon cloth as advanced electrodes for flexible all-solid-state supercapacitors. Nano Energy 2013, 2, 530–536. [Google Scholar] [CrossRef]
- Purkait, T.; Singh, G.; Kumar, D.; Singh, M.; Dey, R.S. High-performance flexible supercapacitors based on electrochemically tailored three-dimensional reduced graphene oxide networks. Sci. Rep. 2018, 8, 640. [Google Scholar]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef] [PubMed]
- Dogru, I.B.; Durukan, M.B.; Turel, O.; Unalan, H.E. Flexible supercapacitor electrodes with vertically aligned carbon nanotubes grown on aluminum foils. Prog. Nat. Sci. 2016, 232–236. [Google Scholar]
- Wang, Y.; Ding, Y.; Guo, X.; Yu, G. Conductive polymers for stretchable supercapacitors. Nano Res. 2019, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Yang, K.; Fan, X.; Tong, Y.; Zhang, Z.; Lu, X.; Mai, K.; Ni, Q.; Zhang, M.; et al. Ultrahigh energy fiber-shaped supercapacitors based on porous hollow conductive polymer composite fiber electrodes. J. Mater. Chem. A 2018, 6, 12250–12258. [Google Scholar] [CrossRef]
- Shown, I.; Ganguly, A.; Chen, L.-C.; Chen, K.-H. Conducting polymer-based flexible supercapacitor. Energ. Sci. Eng. 2015, 3, 2–26. [Google Scholar]
- Dong, L.; Xu, C.; Li, Y.; Huang, Z.-H.; Kang, F.; Yang, Q.-H.; Zhao, X. Flexible electrodes and supercapacitors for wearable energy storage: A review by category. J. Mater. Chem. A 2016, 4, 4659–4685. [Google Scholar] [CrossRef]
- Liu, Q.; Zang, L.; Yang, C.; Wei, C.; Qiu, J.; Liu, C.; Xu, X. A Flexible and Knittable Fiber Supercapacitor for Wearable Energy Storage with High Energy Density and Mechanical Robustness. J. Electrochem. Soc. 2018, 165, A1515–A1522. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Cui, L.; Chen, N.; Qu, L. Preparation and supercapacitor performance of assembled graphene fiber and foam. Prog. Nat. Sci. 2016, 26, 212–220. [Google Scholar]
- Ren, J.; Xu, Q.; Li, Y.-G. Flexible fiber-shaped energy storage devices: Principles, progress, applications and challenges. Flex. Print. Elec. 2018, 3, 013001. [Google Scholar] [CrossRef]
- Chen, X.; Paul, R.; Dai, L. Carbon-based supercapacitors for efficient energy storage. Nat. Sci. Rev. 2017, 4, 453–489. [Google Scholar]
- Liu, T.; Zhang, F.; Song, Y.; Li, Y. Revitalizing carbon supercapacitor electrodes with hierarchical porous structures. J. Mater. Chem. A 2017, 5, 17705–17733. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Ruoff, Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Snook, G.A.; Kao, P.; Best, A. Conducting-Polymer-Based Supercapacitor Devices and Electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Kinlen, P.J.; Mbugua, J.; Kim, Y.-G.; Jung, J.-H.; Viswanathan, S. Supercapacitors using n and p-Type Conductive Polymers Exhibiting Metallic Conductivity. ECS Trans. 2010, 25, 157–162. [Google Scholar]
- Sharma, V.; Singh, I.; Chandra, A. Hollow nanostructures of metal oxides as next generation electrode materials for supercapacitors. Sci. Rep. 2018, 8, 1307. [Google Scholar]
- Yang, C.; Sun, M.; Lu, H. Asymmetric All-Metal-Oxide Supercapacitor with Superb Cycle Performance. Chem. Eur. J. 2018, 24, 6169–6177. [Google Scholar] [CrossRef]
- Rui, X.; Tan, H.; Yan, Q. Nanostructured metal sulfides for energy storage. Nanoscale 2014, 6, 9889–9924. [Google Scholar] [CrossRef]
- Abu-thabit, N.; Hamdy Makhlouf, A.S. Smart Textile Supercapacitors Coated with Conducting Polymers for Energy Storage Applications. Indus. Appl. Intel. Polym. Coat. 2016, 437–477. [Google Scholar]
- Meng, C.; Liu, C.; Chen, L.; Hu, C.; Fan, S. Highly Flexible and All-Solid-State Paperlike Polymer Supercapacitors. Nano Lett. 2010, 10, 4025–4031. [Google Scholar] [CrossRef]
- Chen, L.; Li, D.; Chen, L.; Si, P.; Feng, J.; Zhang, L.; Li, Y.; Lou, J.; Ci, L. Core-shell structured carbon nanofibers yarn@polypyrrole@graphene for high performance all-solid-state fiber supercapacitors. Carbon 2018, 138, 264–270. [Google Scholar] [CrossRef]
- Guo, F.M.; Xu, R.Q.; Cui, X.; Zhang, L.; Wang, K.L.; Yao, Y.W.; Wei, J.Q. High performance of stretchable carbon nanotube–polypyrrole fiber supercapacitors under dynamic deformation and temperature variation. J. Mater. Chem. A 2016, 4, 9311–9318. [Google Scholar] [CrossRef]
- Sun, J.; Huang, Y.; Fu, C.; Wang, Z.; Huang, Y.; Zhu, M.; Zhi, C.; Hu, H. High-performance stretchable yarn supercapacitor based on PPy@CNTs@urethane elastic fiber core spun yarn. Nano Energy 2016, 27, 230–237. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Mohammed Modawe Aldris Edris, N.; Kulandaivalu, S.; Abdul Rahman, N.; Sulaiman, Y. Supercapacitor with superior electrochemical properties derived from symmetrical manganese oxide-carbon fiber coated with polypyrrole. Int. J. Hydrogen Energy 2018, 43, 17328–17337. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Nanostructured MnO₂ as Electrode Materials for Energy Storage. Nanomaterials 2017, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Magu, T.O.; Agobi, A.U.; Hitler, L.; Dass, P.M. A Review on Conducting Polymers-Based Composites for Energy Storage Application. J. Chem. Rev. 2019, 1, 19–34. [Google Scholar]
- Fong, K.D.; Wang, T.; Smoukov, S.K. Multidimensional performance optimization of conducting polymer-based supercapacitor electrodes. Sust. Energ. Fuels 2017, 1, 1857–1874. [Google Scholar]
- Pieta, P.; Obraztsov, I.; D’Souza, F.; Kutner, W. Composites of Conducting Polymers and Various Carbon Nanostructures for Electrochemical Supercapacitors. ECS J. Solid State Sci. Technol. 2013, 2, M3120–M3134. [Google Scholar] [CrossRef]
- Isah, S. Advanced Materials for Energy Storage Devices. Asian J. Nanosci. Mater. 2018, 1, 90–103. [Google Scholar]
- Elnahrawy, A.; Haroun, A.; Hamadneh, I.; Al-Dujaili, A. Conducting Cellulose/TiO2 composites by in Situ Polymerization of Pyrrole. Carbohydr. Polym. 2017, 168, 182–190. [Google Scholar]
- Hameed, A.S.; Reddy, M.V.; Chowdari, B.V.R.; Vittal, J.J. Preparation of rGO-wrapped magnetite nanocomposites and their energy storage properties. RSC Adv. 2014, 4, 64142–64150. [Google Scholar] [CrossRef]
- Huang, W.; Hao, Q.; Lei, W.; Wu, L.; Xia, X. Polypyrrole-hemin-reduce graphene oxide: Rapid synthesis and enhanced electrocatalytic activity towards the reduction of hydrogen peroxide. Mater. Res. Express 2014, 1, 4. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, J.; Xu, Q.; Chen, Z.; Yang, D.; Wang, B.; Jiang, Z. Biomass-derived three-dimensional porous N-doped carbonaceous aerogel for efficient supercapacitor electrodes. RSC Adv. 2014, 45, 23412–23419. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Park, H.S.; Lee, J.Y. Electrochemical deposition of conductive and adhesive polypyrrole-dopamine films. Sci. Rep. 2016, 6, 30475. [Google Scholar]
- Wang, Y.; Wang, A.; Yang, P.; Hu, W.; Guo, X.; Zhang, J.; Li, C.; Zhang, C. A Polypyrrole Hybrid Material Self-Assembled with Porphyrin: Facial Synthesis and Enhanced Optical Limiting Properties. J. Chem. Eng. Mater. Sci. 2017, 5, 26–43. [Google Scholar] [CrossRef]
- Chen, L.; Chen, L.; Ai, Q.; Li, D.; Si, P.; Feng, J.; Zhang, L.; Li, Y.; Lou, J.; Ci, L. Flexible all-solid-state supercapacitors based on freestanding, binder-free carbon nanofibers@polypyrrole@ graphene film. Chem. Eng. J. 2018, 334, 184–190. [Google Scholar]


| Materials | Specific Capacitance | Capacitance Retention@Cycle Number | Ref. |
|---|---|---|---|
| Electrode (Electrolyte = KCl) | |||
| CF@PPy@rGO | 117.5 F/g@25 mV/s | -- | [39] |
| CF@MnO2@PPy | 315.8 F/g@25 mV/s | 82.46%@2000 cycles | [39] |
| CF@PPy | 202.0 F/g@20 mV/s | 90%@2500 cycles | [47] |
| CF@PPy | 244.9 F/g a @20 mV/s | -- | TW |
| Full Devices (Electrolyte = PVA/H3PO4) | |||
| CF@PPy@rGO | 188 F/g@2 mV/s | 76%@5000 cycles | [47] |
| CNY@PPy@rGO | 111.5 mF/cm@2 mV/s | 86%@10000 cycles | [36] |
| UEF@CNT@PPy | 67 mF/cm2@5 mV/s | -- | [38] |
| CF@CNT@PPy | 3.5 mF/cm@2 mV/s | 92%@5000 cycles | [37] |
| CF@PPy | 796.7 mF/g@2 mV/s 30 F/g@0.2 A/g | 70%@3000 cycles | TW |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, Y.-S.; Lin, L.-Y. Systematic Design of Polypyrrole/Carbon Fiber Electrodes for Efficient Flexible Fiber-Type Solid-State Supercapacitors. Nanomaterials 2020, 10, 248. https://doi.org/10.3390/nano10020248
Sung Y-S, Lin L-Y. Systematic Design of Polypyrrole/Carbon Fiber Electrodes for Efficient Flexible Fiber-Type Solid-State Supercapacitors. Nanomaterials. 2020; 10(2):248. https://doi.org/10.3390/nano10020248
Chicago/Turabian StyleSung, Yu-Shun, and Lu-Yin Lin. 2020. "Systematic Design of Polypyrrole/Carbon Fiber Electrodes for Efficient Flexible Fiber-Type Solid-State Supercapacitors" Nanomaterials 10, no. 2: 248. https://doi.org/10.3390/nano10020248
APA StyleSung, Y.-S., & Lin, L.-Y. (2020). Systematic Design of Polypyrrole/Carbon Fiber Electrodes for Efficient Flexible Fiber-Type Solid-State Supercapacitors. Nanomaterials, 10(2), 248. https://doi.org/10.3390/nano10020248




