A Review of Applications Using Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes
Abstract
1. Introduction
2. Feature of Nanocellulose and Carbon Nanotubes
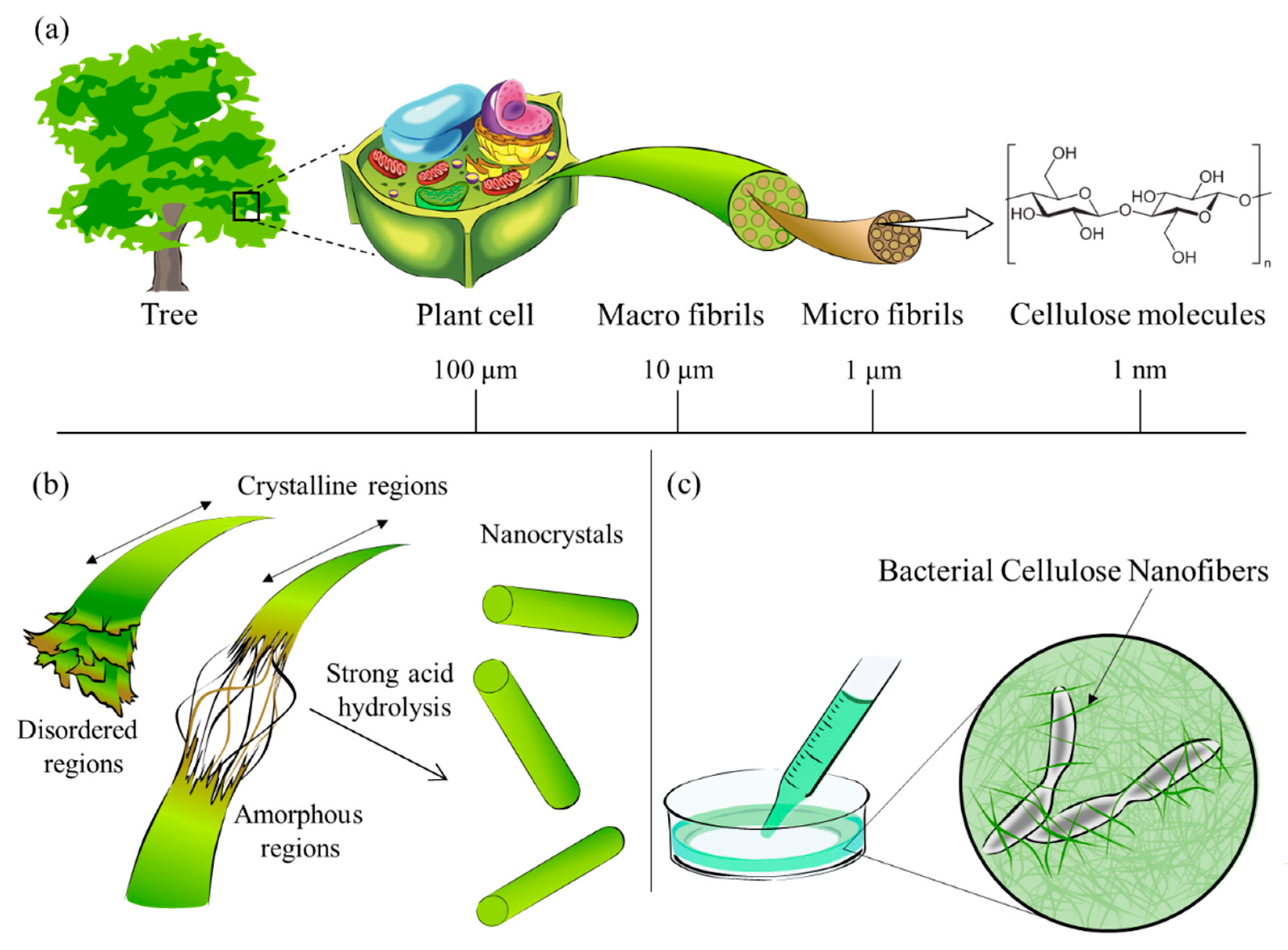
2.1. Nanocellulose
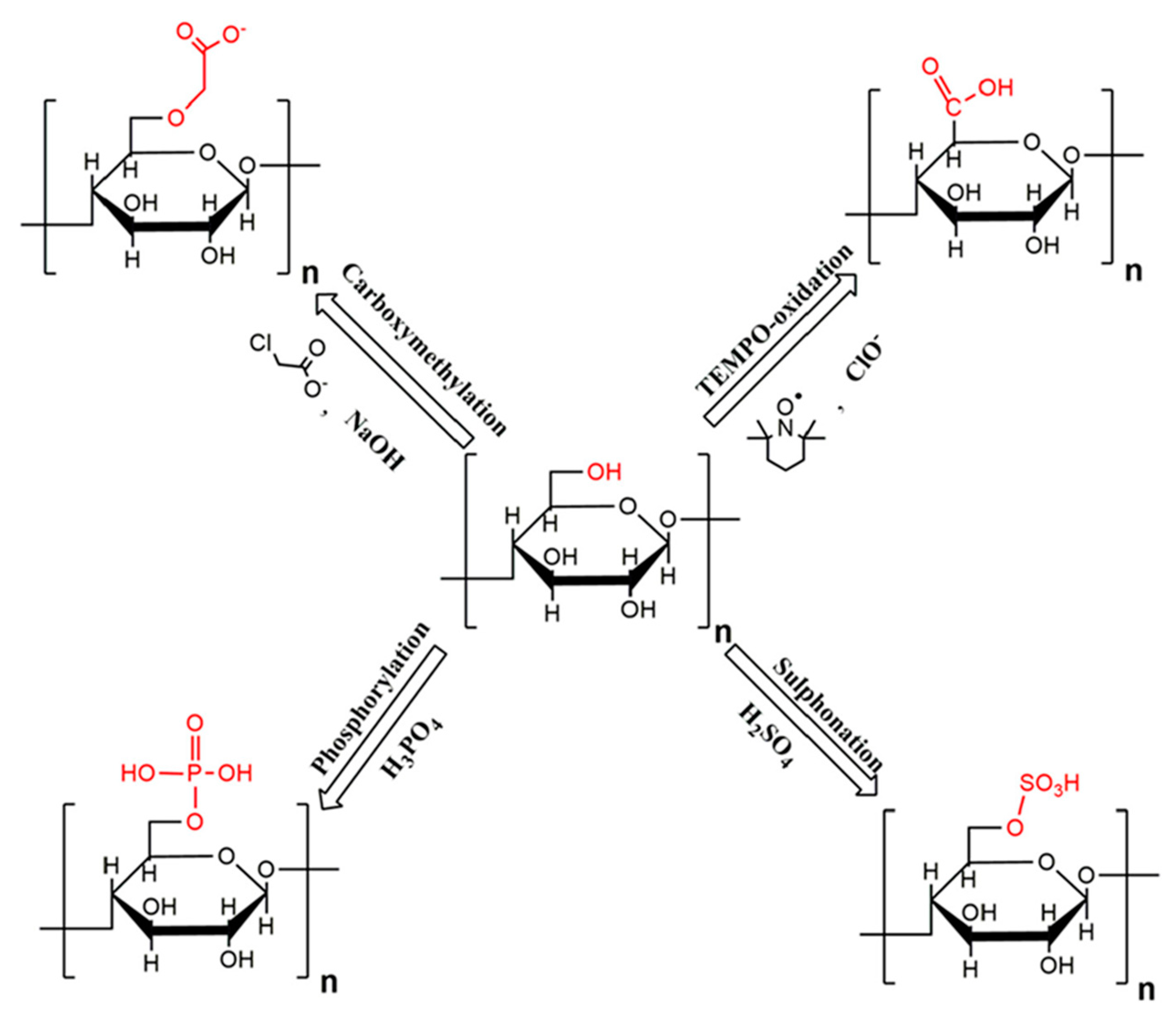
2.2. Surface Modification of Nanocellulose
2.3. Application of Nanocellulose
2.4. Carbon nanotubes
2.5. Application of Carbon Nanotubes
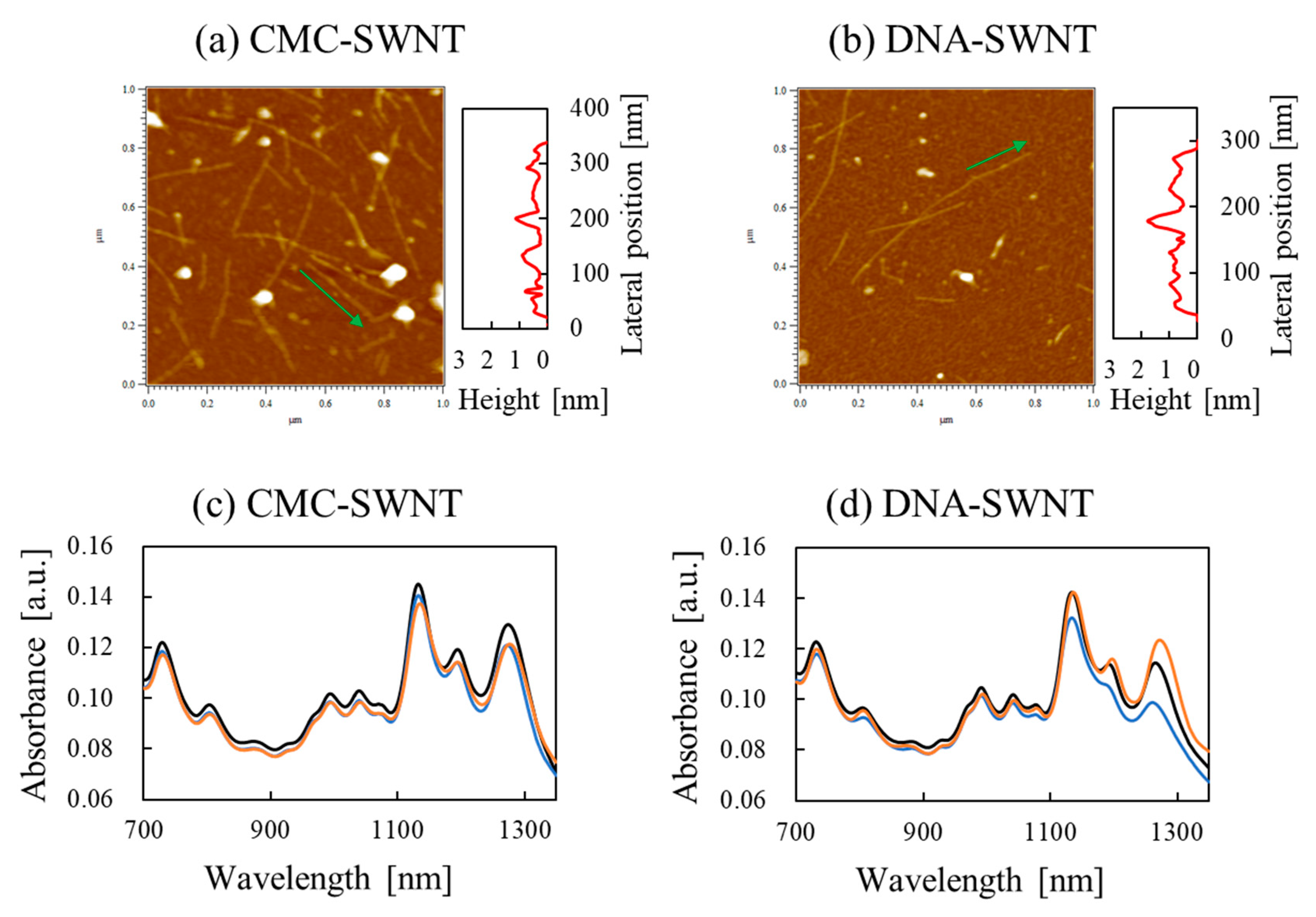
3. Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes
3.1. Composites
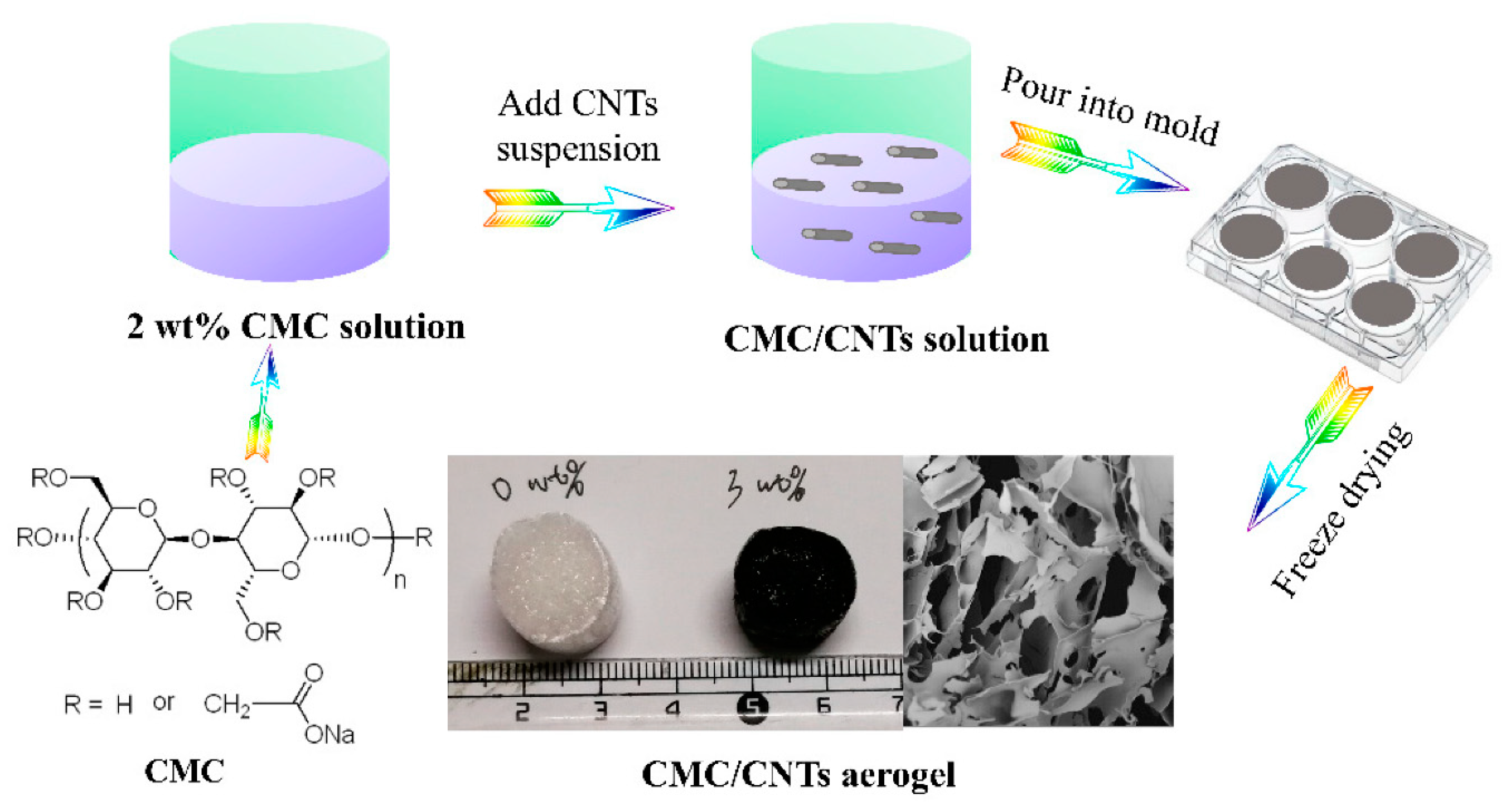
3.2. Aerogels
3.3. Smart Paper and Film
3.4. Fibers
4. Mixed Materials of Bacterial Nanocellulose and Carbon Nanotubes
5. Prospects for mixed materials
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Casas, C.L.; Li, W. A review of application of carbon nanotubes for lithium ion battery anode material. J. Power Sources 2012, 208, 74–85. [Google Scholar] [CrossRef]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Thostenson, E.T.; Chou, T.J. Sensors and actuators based on carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2008, 68, 1227–1249. [Google Scholar] [CrossRef]
- Park, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef]
- Hartschuh, A.; Pedrosa, H.N.; Peterson, J.; Huang, L.; Anger, P.; Qian, H.; Meixner, A.J.; Steiner, M.; Novotny, L.; Krauss, T.D. Single Carbon Nanotube Optical Spectroscopy. ChemPhysChem 2005, 6, 577–582. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, S. Polymer/Carbon Nanotube Nano Composite Fibers—A Review. ACS Appl. Mater. Interfaces 2014, 6, 6069–6087. [Google Scholar] [CrossRef]
- Lee, J.; Mahendra, S.; Alvarez, P.J.J. Nanomaterials in the Construction Industry: A Review of Their Applications and Environmental Health and Safety Considerations. ACS Nano 2010, 4, 3580–3590. [Google Scholar] [CrossRef]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Yu, L.; Shearer, C.; Shapter, J. Recent Development of Carbon Nanotube Transparent Conductive Films. Chem. Rev. 2016, 116, 13413–13453. [Google Scholar] [CrossRef]
- Muhulet, A.; Miculescu, F.; Voicu, S.I.; Schütt, F.; Thakur, V.K.; Mishra, Y.K. Fundamentals and scopes of doped carbon nanotubes towards energy and biosensing applications. Mater. Today Energy 2018, 9, 154–186. [Google Scholar] [CrossRef]
- Ronald, F.G.; Emmanuel, O.A.; Yuan, F.W. Vibration of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2007, 67, 1–28. [Google Scholar]
- Obreja, V.V.N. On the performance of supercapacitors with electrodes based on carbon nanotubes and carbon activated material—A review. Phys. E Low Dimens. Sys. Nanostructures 2008, 40, 2596–2605. [Google Scholar] [CrossRef]
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; Shan AH, A.; Mian, S.A.; Ali, G. An Overview of the Recent Progress in the Synthesis and Applications of Carbon Nanotube. J. Carbon Res. 2019, 5, 3. [Google Scholar] [CrossRef]
- Green, A.A.; Duch, M.C.; Hersam, M.C. Isolation of single-walled carbon nanotube enantiomers by density differentiation. Nano Res. 2009, 2, 69–77. [Google Scholar] [CrossRef]
- Liu, H.; Nishida, D.; Tanaka, T.; Kataura, H.C. Large-scale single-chirality separation of single-wall carbon nanotubes by simple gel chromatography. Nat. Commun. 2011, 2, 309. [Google Scholar] [CrossRef]
- Bejoy, T.; Midhun, C.R.; Athira, K.B.; Rubiyah, M.H.; Jithin, J.A.; MooresGlenna, L.D.; Clément, S. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar]
- Kaushik, M.; Putaux, J.L.; Fraschini, C.; Chauve, G.; Moores, A. Transmission Electron Microscopy for the Characterization of Cellulose Nanocrystals. Transm. Electron Microsc. Theory Appl. 2015. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.R.N.; Taiwo, O.F.A.; Hassan, T.M.; Haafiz, M.K.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Processing and characterization of natural cellulose fibers/thermoset polymer composites. Carbohydr. Polym. 2014, 109, 102–117. [Google Scholar] [CrossRef]
- Filipe, V.F.; Ivanei, F.P.; Sivoney, F.S.; Lucia, H.I.M.; Liliane, M.F.L. Polymer Composites Reinforced with Natural Fibers and Nanocellulose in the Automotive Industry: A Short Review. J. Compos. Sci. 2019, 3, 51. [Google Scholar]
- Wang, Q.; Yao, Q.; Liu, J.; Sun, J.; Zhu, Q.; Chen, H. Processing nanocellulose to bulk materials: A review. Cellulose 2019, 26, 7585–7617. [Google Scholar] [CrossRef]
- Mao, J.; Abushammala, H.; Brown, N.; Laborie, M.P. Comparative Assessment of Methods for Producing Cellulose I Nanocrystals from Cellulosic Sources; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2017; Volume 1251, pp. 19–53. [Google Scholar]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171. [Google Scholar] [CrossRef]
- Kiziltas, A.; Erbas Kiziltas, E.; Boran, S.; Gardner, D.J. Micro-and nanocellulose composites for automotive applications. In Proceedings of the SPE Automotive Composites Conference and Exhibition (ACCE), Novi, MI, USA, 11–13 September 2013. [Google Scholar]
- Abushammala, H.; Mao, J. A Review of the Surface Modification of Cellulose and Nanocellulose Using Aliphatic and Aromatic Mono- and Di-Isocyanates. Molecules 2019, 24, 2782. [Google Scholar] [CrossRef]
- Joseph, P.V.; Joseph, K.; Thomas, S. Effect of Processing Variables on the Mechanical Properties of Sisal-Fiber-Reinforced Polypropylene Composites. Compos. Sci. Technol. 1999, 59, 1625–1640. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Gassan, J. Composites Reinforced with Cellulose based Fibers. Polym. Sci. 1999, 24, 221–274. [Google Scholar]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Boday, D.J.; Weder, C.; Foster, E.J. Cellulose Nanocrystal Driven Crystallization of Poly(D,L-Lactide) and Improvement of the Thermomechanical Properties. J. Appl. Poly. Sci. 2015, 132, 41607. [Google Scholar] [CrossRef]
- Usov, I.; Nyström, G.; Adamcik, J.; Handschin, S.; Schütz, C.; Fall, A.; Bergström, L.; Mezzenga, R. Understanding Nanocellulose Chirality and Structure–Properties Relationship at the Single Fibril Level. Nat. Commun. 2015, 6, 7564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, N.; Zhang, X.; Xu, L.; Wu, J.; Yu, J.; He, J.; Zhang, J. All-Cellulose Nanocomposites Reinforced with in Situ Retained Cellulose Nanocrystals during Selective Dissolution of Cellulose in an Ionic Liquid. ACS Sustain. Chem. Eng. 2016, 4, 4417–4423. [Google Scholar] [CrossRef]
- Nishino, T.; Matsuda, I.; Hirao, K. All-Cellulose Composite. Macromolecules 2004, 37, 7683–7687. [Google Scholar] [CrossRef]
- Ye, C.; Malak, S.T.; Hu, K.; Wu, W.; Tsukruk, V.V. Cellulose Nanocrystal Microcapsules as Tunable Cages for Nano- and Microparticles. ACS Nano 2015, 9, 10887–10895. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, D.; Yao, F.; Wu, Q. Fabrication and Properties of Transparent Polymethylmethacrylate/ Cellulose Nanocrystals Composites. Bioresour. Technol. 2010, 101, 5685–5692. [Google Scholar] [CrossRef]
- Tanpichai, S.; Quero, F.; Nogi, M.; Yano, H.; Youn, R.; Lindstrom, T.; Sampson, W.W.; Eichhorn, S. Effective Young’s Modulus of Bacterial and Microfibrillated Cellulose Fibrils in Fibrous Networks. Biomacromolecules 2012, 13, 1340–1349. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface Modification of Cellulose Nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S. Steric Stabilization of a Cellulose Microcrystal Suspension by Poly (ethylene glycol) Grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Roux, D.; Nishiyama, Y. Rheological Properties of Microfibrillar Suspension of TEMPO-Oxidized Pulp. Cellulose 2008, 15, 425–433. [Google Scholar] [CrossRef]
- Moon, D.; Tsukahara, K.; Sagisaka, M.; Tahara, K. Effect of Cellulose Nanofibers Composites in Automotive Components on Greenhouse Gas Emissions. J. Jap. Inst. Energy 2016, 95, 648–652. [Google Scholar] [CrossRef]
- Himeno, N.; Miyashiro, D.; Takara, Y.; Sato, Y.; Wakabayashi, Y.; Nagao, Y.; Ebisawa, H.; Shibahata, Y. A Study of Vehicle Body Stiffness and Steering Stability (1st Report). In Proceedings of the JSAE Annual Congress, Pacifico Yokohama, Japan, 18–26 May 2017. [Google Scholar]
- Miyashiro, D.; Nakamura, J.; Himeno, N.; Sato, Y.; Wakabayashi, Y.; Nagao, Y.; Ebisawa, H.; Shibahata, Y. A Study of Vehicle Body Stiffness and Steering Stability (2nd Report). In Proceedings of the JSAE Annual Congress, Pacifico Yokohama, Japan, 23–25 May 2018. [Google Scholar]
- Koronis, G.; Silva, A.; Fontul, M. Green composites: A review of adequate materials for automotive applications. Compos. Part B Eng. 2013, 44, 120–127. [Google Scholar] [CrossRef]
- Parajuli, R. Carbon Footprint Analysis of Upm Formi Used in Biofore Concept Car; Metropolia Ammattikorkeakoulu (Metropolia University of Applied Sciences): Helsinki, Finland, 2014. [Google Scholar]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603. [Google Scholar] [CrossRef]
- Peng, B.; Locascio, M.; Zapol, P.; Li, S.; Mielke, S.L.; Schatz, G.C.; Espinosa, H.D. Measurements of near-ultimate strength for multiwalled carbon nanotubes and irradiation-induced crosslinking improvements. Nat. Nanotechol. 2008, 3, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Santiago, E.; Rongsie, X.; Martin, F.; Richard, C.; David, H.; Junwei, Y.; John, R. Measurement of area density of vertically aligned carbon nanotube forests by the weight-gain method. J. Appl. Phys. 2013, 113, 144309. [Google Scholar]
- Krishnan, A.; Dujardin, E.; Ebbesen, T.W.; Yianilos, P.N.; Treacy, M.M.J. Young’s modulus of single-walled nanotubes. Phys. Rev. B 1998, 58, 14013. [Google Scholar] [CrossRef]
- Filleter, T.; Bernal, R.; Li, S.; Espinosa, H.D. Ultrahigh Strength and Stiffness in Cross-Linked Hierarchical Carbon Nanotube Bundles. Adv. Mater. 2011, 23, 2855–2860. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal Transport Measurements of Individual Multiwalled Nanotube. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef]
- Pop, E.; Mann, Q.; Wang, Q.; Goodson, K.; Dai, H. Thermal Conductance of an Individual Single-Wall Carbon Nanotube above Room Temperature. Nano Lett. 2006, 6, 96–100. [Google Scholar] [CrossRef]
- Deng, L.; Young, R.J.; Kinloch, I.A.; Sun, R.; Zhang, G.; Noe, L.; Monthioux, M. Coefficient of thermal expansion of carbon nanotubes measured by Raman spectroscopy. Appl. Phys. Lett. 2014, 104, 051907. [Google Scholar] [CrossRef]
- An, K.H.; Jeon, K.J.; Kim, W.S.; Park, Y.S.; Lim, S.C.; Bae, D.J.; Lee, Y.H. Characterization of Supercapacitors Using Singlewalled Carbon Nanotube Electrodes. J. Korean Phys. Soc. 2001, 39, 511–517. [Google Scholar]
- Ye, J.S.; Liu, X.; Cui, H.F.; Zhang, W.D.; Sheu, F.S.; Lim, T.M. Electrochemical oxidation of multi-walled carbon nanotubes and its application to electrochemical double layer capacitors. Electrochem. Commun. 2005, 7, 249–255. [Google Scholar] [CrossRef]
- Hata, K.; Fukuda, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef]
- Umemura, K.; Izumi, K.; Oura, S. Hybrids of Nucleic Acids and Carbon Nanotubes for Nanobiotechnology: A review. Nanomaterials 2015, 5, 321–350. [Google Scholar] [CrossRef]
- Zheng, M.; Jagota, A.; Semke, E.D.; Diner, B.A.; McLean, R.S.; Lustig, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003, 2, 338–342. [Google Scholar] [CrossRef]
- Nakashima, N.; Okuzono, S.; Murakami, H.; Nakai, T.; Yoshikawa, K. DNA dissolves single-walled carbon nanotubes in water. Chem. Lett. 2003, 32, 456–457. [Google Scholar] [CrossRef]
- Andrews, J.B.; Cardenas, J.A.; Lim, C.J.; Noyce, S.G.; Mullett, J.; Franklin, A.D. Fully Printed and Flexible Carbon Nanotube Transistors for Pressure Sensing in Automobile Tires. IEEE Sens. J. 2018, 18, 1180–1189. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Eshwaran, S.B.; Stöckelhuber, K.W.; Wießner, S.; Pötschke, P.; Das, A. Strong Strain Sensing Performance of Natural Rubber Nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 4860–4872. [Google Scholar] [CrossRef]
- Cheng, Q.; Tang, J.; Ma, J.; Zhang, H.; Shinya, N.; Qin, L.C. Graphene and carbon nanotube composite electrodes for supercapacitors with ultra-high energy density. Phys. Chem. Chem. Phys. 2011, 13, 17615–17624. [Google Scholar] [CrossRef]
- Gui, X.; Wei, J.; Wang, K.; Cao, A.; Zhu, H.; Jia, Y.; Shu, Q.; Wu, D. Carbon Nanotube Sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Tsunoda, K.; Yajima, H.; Ishii, T. Dispersion and Purification of Single-Wall Carbon Nanotubes Using Carboxymethylcellulose. Jpn. J. Appl. Phys. 2004, 43, 3636–3639. [Google Scholar] [CrossRef]
- Minami, N.; Kim, Y.; Miyashita, K.; Kazaoui, S.; Nalini, B. Cellulose derivatives as excellent dispersants for single-wall carbon nanotubes as demonstrated by absorption and photoluminescence spectroscopy. Appl. Phys. Lett. 2006, 88, 093123. [Google Scholar] [CrossRef]
- Matsukawa, Y.; Ohura, S.; Umemura, K. Differences in the response of the near-infrared absorbance spectra of single-walled carbon nanotubes; Effects of chirality and wrapping polymers. Colloids Surf. B Biointerfaces 2018, 172, 684–689. [Google Scholar] [CrossRef]
- Zhao, E.H.; Ergul, B.; Zhao, W. Caffeine’s Antioxidant Potency Optically Sensed with Double-Stranded DNA-Encased Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2015, 119, 4068–4075. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Q.; Su, l.; Yan, Y.; Zhang, J.; Mao, L. Bright Fluorescence from Individual Single-Walled Carbon Nanotubes. Nano Lett. 2011, 14, 1636–1640. [Google Scholar]
- Oura, S.; Ito, M.; Homma, Y.; Nii, D.; Homma, Y.; Umemura, K. Biomolecular recognition ability of RecA proteins for DNA onsingle-walled carbon nanotubes. Colloids Surf. B Biointerfaces 2015, 126, 496–501. [Google Scholar] [CrossRef]
- Riou, I.; Bertoncini, P.; Bizot, H.; Mevellec, J.Y.; Buléon, A.; Chauvet, O. Carboxymethylcellulose/Single Walled Carbon Nanotube Complexes. J. Nanosci. Nanotechnol. 2009, 9, 6176–6180. [Google Scholar] [CrossRef]
- Im, J.; Sterner, E.S.; Swager, T.M. Integrated Gas Sensing System of SWCNT and Cellulose Polymer Concentrator for Benzene, Toluene, and Xylenes. Sensors 2016, 16, 183. [Google Scholar] [CrossRef]
- Chen, Y.; Mun, S.C.; Kim, J.M.A. Wide Range Conductometric pH Sensor Made with Titanium Dioxide/Multiwall Carbon Nanotube/Cellulose Hybrid Nanocomposite. IEEE Sens. J. 2013, 11, 4157–4162. [Google Scholar] [CrossRef]
- Shao, D.; Jiang, Z.; Wang, X.; Li, J.; Meng, Y. Plasma Induced Grafting Carboxymethyl Cellulose on Multiwalled Carbon Nanotubes for the Removal of UO22+ from Aqueous Solution. J. Phys. Chem. B 2009, 113, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Shao, Z.; Wang, X.; Zhang, Y.; Wang, W.; Wang, F. Cellulose nanofibers/multi-walled carbon nanotube nanohybrid aerogel for all-solid-state flexible supercapacitors. RSC Adv. 2013, 3, 15058. [Google Scholar] [CrossRef]
- Zheng, Q.; Javadi, A.; Sabo, R.; Cai, Z.; Gong, S. Polyvinyl alcohol (PVA)–Cellulose nanofibril (CNF)–Multiwalled carbon nanotube (MWCNT) hybrid organicaerogels with superior mechanical properties. RSC Adv. 2013, 3, 20816. [Google Scholar] [CrossRef]
- Yang, C.; Chen, C.; Pan, Y.; Li, S.; Wang, F.; Li, J.; Li, N.; Li, X.; Zhang, Y.; Li, D. Flexible highly specific capacitance aerogel electrodes based on cellulose nanofibers, carbon nanotubes and polyaniline. Electrochim. Acta. 2015, 182, 264–271. [Google Scholar] [CrossRef]
- Li, J.; Jiang, X.; Tan, S.; Chen, P.; Zhou, H.; Xu, Z. Directional preparation of superhydrophobic magnetic CNF/PVA/MWCNT carbon aerogel. IET Nanobiotechnol. 2019, 13, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cai, Z.; Ma, Z.; Gong, S. Cellulose Nanofibril/Reduced Graphene Oxide/Carbon Nanotube Hybrid Aerogels for Highly Flexible and All-Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3263–3271. [Google Scholar] [CrossRef]
- Solodyankin, M.A.; Obraztsova, E.D.; Lobach, A.S.; Chernov, A.I.; Tausenev, A.V.; Konov, V.I.; Dianov, E.M. Mode-locked 1.93 microm thulium fiber laser with a carbon nanotube absorber. Opt. Lett. 2008, 33, 1336–1338. [Google Scholar] [CrossRef]
- Gnanaseelan, M.; Chen, Y.; Tan, S.; Luo, J.; Krause, B.; Pionteck, J.; Pötschkea, P.; Qi, H. Cellulose-carbon nanotube composite aerogels as novel thermoelectric materials. Compos. Sci. Technol. 2018, 163, 133–140. [Google Scholar] [CrossRef]
- Long, L.; Li, F.; Shu, M.; Zhang, C.; Weng, Y. Fabrication and Application of Carboxymethyl Cellulose-Carbon Nanotube Aerogels. Materials 2019, 12, 1867. [Google Scholar] [CrossRef]
- Hajian, A.; Fu, Q.; Berglund, L.A. Recyclable and superelastic aerogels based on carbon nanotubes and carboxymethyl cellulose. Compos. Sci. Technol. 2018, 159, 1–10. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, X.; Tan, S.; Wu, W.; Shi, J.; Zhou, H.; Chen, P. Preparation and characterisation of CNF/MWCNT carbon aerogel as efficient adsorbents. IET Nanobiotechnol. 2018, 12, 500–504. [Google Scholar] [CrossRef]
- Wang, M.; Anoshkin, I.V.; Nasibulin, A.G.; Korhonen, T.J.; Seitsonen, J.; Pere, J.; Kauppinen, E.I.; Ras, R.H.A.; Ikkala, O. Modifying Native Nanocellulose Aerogels with Carbon Nanotubes for Mechanoresponsive Conductivity and Pressure Sensing. Adv. Mater. 2013, 25, 2428–2432. [Google Scholar] [CrossRef] [PubMed]
- Fugetsu, B.; Sano, E.; Sunada, M.; Sambongi, Y.; Shibuya, T.; Wang, X.; Hiraki, T. Electrical conductivity and electromagnetic interference shielding efficiency of carbon nanotube/cellulose composite paper. Carbon 2008, 46, 1256–1258. [Google Scholar] [CrossRef]
- Imai, M.; Akiyama, K.; Tanaka, T.; Sano, E. Highly strong and conductive carbon nanotube/cellulose composite paper. Compos. Sci. Technol. 2010, 70, 1564–1570. [Google Scholar] [CrossRef]
- Moilanen, P.; Luukkainen, M.; Jekkonen, J.; Kangas, V. EMI shielding effects of carbon nanotube cellulose nanocomposite. In Proceedings of the IEEE International Symposium on Electromagnetic Compatibility, Convention Center, Fort Lauderdale, FL, USA, 25–30 July 2010. [Google Scholar]
- Han, J.M.; Kim, B.; Li, J.; Meyyappan, M. Carbon Nanotube Based Humidity Sensor on Cellulose Paper. J. Phys. Chem. C 2012, 116, 22094–22097. [Google Scholar] [CrossRef]
- Han, J.W.; Kim, B.; Li, J.; Meyyappan, M. A carbon nanotube based ammonia sensor on cellulose paper. RSC Adv. 2014, 4, 549. [Google Scholar] [CrossRef]
- Yun, S.; Kim, J. Multi-walled carbon nanotubes–cellulose paper for a chemical vapor sensor. Sens. Actuators B Chem. 2010, 150, 308–313. [Google Scholar] [CrossRef]
- Dichiara, A.B.; Song, A.; Goodman, S.M.; He, D.; Bai, J. Smart papers comprising carbon nanotubes and cellulose microfibers for multifunctional sensing applications. J. Mater. Chem. A 2017, 5, 20161–20169. [Google Scholar] [CrossRef]
- Koga, H.; Saito, T.; Kitaoka, T.; Nogi, M.; Suganuma, K.; Isogai, A. Transparent, Conductive, and Printable Composites Consisting of TEMPO-Oxidized Nanocellulose and Carbon Nanotube. Biomacromolecules 2013, 14, 1160–1165. [Google Scholar] [CrossRef]
- Hamedi, M.M.; Hajian, A.; Fall, A.B.; Håkansson, K.; Salajkova, M.; Lundell, F.; Wågberg, L.; Berglund, L.A. Highly Conducting, Strong Nanocomposites Based on Nanocellulose-Assisted Aqueous Dispersions of Single-Wall Carbon Nanotubes. ACS Nano 2014, 8, 2467–2476. [Google Scholar] [CrossRef]
- Ito, M.; Yajima, H.; Homma, Y. Strain effect of cellulose-wrapped single-walled carbon nanotubes measured by photoluminescence and Raman scattering spectroscopy. Jpn. J. Appl. Phys. 2016, 55, 075101. [Google Scholar] [CrossRef]
- Yun, S.; Jang, S.D.; Yun, G.Y.; Kim, J.H.; Kim, J. Paper transistor made with covalently bonded multiwalled carbon nanotube and cellulose. Appl. Phys. Lett. 2009, 95, 104102. [Google Scholar] [CrossRef]
- Salajkova, M.; Valentini, L.; Zhou, Q.; Berglund, L.A. Tough nanopaper structures based on cellulose nanofibers and carbon nanotubes. Compos. Sci. Technol. 2013, 87, 103–110. [Google Scholar] [CrossRef]
- Koga, H.; Nogi, M.; Komoda, N.; Nge, T.T.; Sugahara, T.; Suganuma, K. Uniformly connected conductive networks on cellulose nanofiber paper for transparent paper electronics. NPG Asia Mater. 2014, 6, e93. [Google Scholar] [CrossRef]
- Son, Y.R.; Park, S.J. Green preparation and characterization of graphene oxide/carbon nanotubesloaded carboxymethyl cellulose nanocomposites. Sci. Rep. 2018, 8, 17601. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Karabuluta, E.; Pernevik, E.; Enokssona, P.; Gatenholma, P. Tailor-made conductive inks from cellulose nanofibrils for 3D printing of neural guidelines. Carbohydr. Polym. 2018, 189, 22–30. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, B.; Li, J.; Meyyappan, M. Carbon nanotube ink for writing on cellulose paper. Mater. Res. Bull. 2014, 50, 249–253. [Google Scholar] [CrossRef]
- Cao, S.; Feng, X.; Song, Y.; Xue, X.; Liu, H.; Miao, M.; Fang, J.; Shi, L. Integrated Fast Assembly of Free-Standing Lithium Titanate/Carbon Nanotube/Cellulose Nanofiber Hybrid Network Film as Flexible Paper-Electrode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 10695–10701. [Google Scholar] [CrossRef]
- Nguyen, H.K.; Bae, J.; Hur, J.; Pak, S.J.; Park, M.S. Tailoring of Aqueous-Based Carbon Nanotube–Nanocellulose Films as Self-Standing Flexible Anodes for Lithium-Ion Storage. Nanomaterials 2019, 9, 655. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.G.; Zhang, Z.N.; Wu, J.; Zhang, J.; He, J.S. Regenerated-Cellulose/Multiwalled-ultiwalledd-Cellulose/Flexible Anodes for Lithium-Ion Storage. -Ion Storagelectrode for Lithium-Ion Batterie-3-methylimidazolium Chloride. Adv. Mater. 2007, 19, 698–704. [Google Scholar] [CrossRef]
- Deng, L.; Young, R.J.; Kinloch, J.A.; Abdelkader, A.M.; Holmes, S.M.; Haro-Del Rio DA, D.; Eichhorn, S.J. Supercapacitance from Cellulose and Carbon Nanotube Nanocomposite Fibers. ACS Appl. Mater. Interfaces 2013, 5, 9983–9990. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, M.; Miao, J.; Simmons, T.J.; Lee, J.W.; Doherty, T.V.; Dordick, J.S.; Linhardt, R.J. Conductive Cable Fibers with Insulating Surface Prepared by Coaxial Electrospinning of Multiwalled Nanotubes and Cellulose. Biomacromolecules. 2010, 11, 2440–2445. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hsieh, Y.L. Multiwalled Carbon Nanotube (MWCNT) Reinforced Cellulose Fibers by Electrospinning. ACS Appl. Mater. Interfaces 2010, 2, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, H.; Wang, Y.; Ray, U.; Zhu, S.; Dai, J.; Chen, C.; Fu, K.; Jang, S.H.; Henderson, D.; et al. Cellulose-Nanofiber-Enabled 3D Printing of a Carbon-Nanotube Microfiber Network. Small 2017, 1, 1700222. [Google Scholar] [CrossRef]
- Wan, Z.; Chen, C.; Meng, T.; Mojtaba, M.; Teng, Y.; Li, D. Multifunctional Wet-Spun Filaments through Robust Nanocellulose Networks Wrapping to Single-Walled Carbon Nanotubes. ACS Appl. Mater. Interfaces 2019, 11, 45. [Google Scholar] [CrossRef]
- Miyashiro, D.; Akiyama, N.; Wakayama, J.; Kunioka, Y.; Yamada, T. Radial stability of the actomyosin filament latice in isolated skeletal myofibrils studied using atomicforce microscopy. J. Physiol. Sci. 2013, 63, 299–310. [Google Scholar] [CrossRef]
- Miyashiro, D.; Ohsuki, M.; Shimamoto, Y.; Wakayama, J.; Kunioka, Y.; Kobayashi, T.; Ishiwata, S.; Yamada, T. Radial stiffness characteristics of the overlap regions of sarcomeres in isolated skeletal myofibrils in pre-force generating stat. Biophys. Physico. 2017, 14, 207–220. [Google Scholar] [CrossRef][Green Version]
- Niu, Q.; Gao, K.; Shao, Z. Cellulose nanofiber/single-walled carbon nanotube hybrid non-woven macrofiber mats as novel wearable supercapacitors with excellent stability, tailorability and reliability. Nanoscale 2014, 6, 4083–4088. [Google Scholar] [CrossRef]
- Jung, R.; Kim, H.; Kim, Y.; Kwon, S.M.; Lee, H.S.; Jin, H.J. Electrically conductive transparent papers using multiwalled carbon nanotubes. J. Polym. Sci. B Polym. Phys. 2008, 46, 1235–1242. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Chao, L.i.; Liang, H.W.; Chen, J.F.; Yu, S.H. Ultralight, Flexible, and Fire-Resistant Carbon Nanofiber Aerogels from Bacterial Cellulose. Angew. Chem. Int. Ed. 2013, 52, 2925–2929. [Google Scholar] [CrossRef]
- Yoon, S.H.; Jin, H.J.; Kook, M.C.; Pyun, Y.R. Electrically Conductive Bacterial Cellulose by Incorporation of Carbon Nanotubes. Biomacromolecules 2006, 74, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Chen, S.; Wang, H.; Wang, B.; Wang, C.; Jiang, J. Cellulose synthesized by Acetobacter xylinum in the presence of multi-walled carbon nanotubes. Carbohydr. Res. 2008, 343, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Qiu, X.; Yung, P.T. MWCNTs-like protection layer formation on bacterial cellulose bundles as a potential material for suspended resonator. In Proceedings of the 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014. [Google Scholar]
- Kang, Y.J.; Chun, S.J.; Lee, S.S.; Kim, B.Y.; Kim, J.H.; Chung, H.; Lee, S.Y.; Kim, W. All-Solid-State Flexible Supercapacitors Fabricated with Bacterial Nanocellulose Papers, Carbon Nanotubes, and Triblock-Copolymer Ion Gels. ACS Nano 2012, 6, 6400–6406. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.Q.; Yuen, J.; Slaughter, G. Carbon Nanotube-Cellulose Pellicle for Glucose Biofuel Cell. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Honolulu, HI, USA, 17–21 July 2018. [Google Scholar]
- Lv, P.; Feng, Q.; Wang, Q.; Li, G.; Li, D.; Wei, Q. Biosynthesis of Bacterial Cellulose/Carboxylic Multi-Walled Carbon Nanotubes for Enzymatic Biofuel Cell Application. Materials 2016, 9, 183. [Google Scholar] [CrossRef]
- Carbon Nanotubes (CNT) Market by Type (Single, Multi Walled), Method (Chemical Vapor Deposition, Catalytic Chemical Vapor Deposition, High Pressure Carbon Monoxide), Application (Electronics, Chemical, Batteries, Energy, Medical)—Global Forecast to 2023. MARKET AND MARKETS 2018, OCT, CH3951. Available online: https://www.marketsandmarkets.com/Market-Reports/carbon-nanotubes-139.html (accessed on 4 December 2019).
- Nanocellulose Market by Type (Microfibrillated Cellulose, Cellulose Nanocrystals), Application (Pulp& Paper, Composites & Packaging, Biomedical & Pharmaceuticals, Electronics & Sensors), and Region (Europe, North America, APAC)—Global Forecast to 2023. MARKET AND MARKETS 2019, OCT, CH3320. Available online: https://www.marketsandmarkets.com/Market-Reports/nano-cellulose-market-56392090.html (accessed on 4 December 2019).
- Glouia, Y.; Chaabouni, Y.; Oudiani, A.E.; Maatoug, I.; Msahli, S. Finite element analysis of mechanical response of cellulosic fiber-reinforced composites. Int. J. Adv. Manuf. Technol. 2019, 103, 4671–4680. [Google Scholar] [CrossRef]
- Bergenstrahle, W.M.; Brady, J.W. Overview of computer modeling of cellulose. Methods Mol. Biol. 2012, 908, 11–22. [Google Scholar]
- Jebali, A.; Ardakani, S.A.Y.; Sedighi, N.; Hekmatimoghaddam, S. Nanocellulose conjugated with retinoic acid: Its capability to adsorb aflatoxin B1. Cellulose 2015, 22, 363–372. [Google Scholar] [CrossRef]
- Johnson, R.R.; Johnson, A.T.C.; Klein, M.L. Probing the Structure of DNA-Carbon Nanotube Hybrids with Molecular Dynamics. Nano Lett. 2008, 8, 69–75. [Google Scholar] [CrossRef]
- Johnson, R.R.; Kohlmeyer, A.; Johnson, A.T.C.; Klein, M.L. Free Energy Landscape of a DNA−Carbon Nanotube Hybrid Using Replica Exchange Molecular Dynamics. Nano Lett. 2009, 9, 537–541. [Google Scholar] [CrossRef]
- Mayo, M.L.; Chen, Z.Q.; Kilina, S.V. Computational Studies of Nucleotide Selectivity in DNA-Carbon Nanotube Hybrids. J. Phys. Chem. Lett. 2012, 3, 2790–2797. [Google Scholar] [CrossRef]
- Arroyo, M.; Belytschko, T. Finite element methods for the non-linear mechanics of crystalline sheets and nanotubes. Int. J. Numer. Methods Eng. 2004, 59, 419–456. [Google Scholar] [CrossRef]
- Kumar, A.; Mukherjee, S.; Paci, J.T.; Chandraseker, K.; Schatz, G.C. A rod model for three dimensional deformations of single-walled carbon nanotubes. Int. J. Solid Struct. 2011, 48, 2849–2858. [Google Scholar] [CrossRef]
- Tserpes, K.I.; Papanikos, P. Finite element modeling of single-walled carbon nanotubes. Compos. Part B Eng. 2005, 36, 468–477. [Google Scholar] [CrossRef]
- Xiaoxing, L.; Zhong, H. Mechanical property evaluation of single-walled carbon nanotubes by finite element modeling. Compos. Part B Eng. 2012, 43, 1902–1913. [Google Scholar]
- Miyashiro, D.; Taira, H.; Umemura, K. Vibration analysis of single-stranded DNA-wrapped single-walled carbon nanotubes using finite element method. Compos. Part B Eng. 2019, 173, 106896. [Google Scholar] [CrossRef]
- Huang, J.; Rodrigue, D. Analysis of multiaxial properties of carbon nanotubes/polypropylene and nanocrystalline cellulose/polypropylene composites. Polym. Compos. 2014, 37, 7875–7880. [Google Scholar] [CrossRef]
- Huang, J.; Rodrigue, D. Comparison of the mechanical properties between carbon nanotube and nanocrystalline cellulose polypropylene based nano-composites. Mater. Des. 2015, 65, 974–982. [Google Scholar] [CrossRef]
- Ozkan, M.; Karakoc, A.; Borghei, M.; Wiklund, J.; Rojas, O.J.; Paltakari, J. Machine Learning assisted design of tailor-made nanocellulose films: A combination of experimental and computational studies. Polym. Compos. 2019, 40, 4013–4022. [Google Scholar] [CrossRef]
- Ozkan, M.; Borghei, M.; Karakoc, A.; Rojas, O.J.; Paltakari, J. Films based on crosslinked TEMPO-oxidized cellulose and predictive analysis via machine learning. Sci. Rep. 2018, 8, 4748. [Google Scholar] [CrossRef]
- Gernand, J.M.; Casman, E.A. Machine Learning for Nanomaterial Toxicity Risk Assessment. IEEE Intell. Syst. 2014, 29, 84–88. [Google Scholar] [CrossRef]
- Durruthy, M.G.; Alberici, L.C.; Curti, C.; Naal, Z.; Sawazaki, D.A.; Naya JM, V.; Diaz, H.G.; Munteanu, C.R. Experimental–Computational Study of Carbon Nanotube Effects on Mitochondrial Respiration: In Silico Nano-QSPR Machine Learning Models Based on New Raman Spectra Transform with Markov–Shannon Entropy Invariants. J. Chem. Inf. Model. 2017, 57, 1029–1044. [Google Scholar] [CrossRef] [PubMed]
- Alred, J.M.; Bets, K.V.; Xie, Y.; Yakobson, B.I. Machine learning electron density in sulfur crosslinked carbon nanotubes. Compos. Sci. Technol. 2018, 166, 3–9. [Google Scholar] [CrossRef]
- González-Durruthy, M.; Monserrat, J.M.; Rasulev, B.; Casañola-Martín, G.M.; Barreiro Sorrivas, J.M.; Paraíso-Medina, S.; Maojo, V.; González-Díaz, H.; Pazos, A.; Munteanu, C.R. Carbon Nanotubes’ Effect on Mitochondrial Oxygen Flux Dynamics: Polarography Experimental Study and Machine Learning Models using Star Graph Trace Invariants of Raman Spectra. Nanomaterials 2017, 7, 386. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, M.; Jagota, A. Learning to predict single-wall carbon nanotube-recognition DNA sequences. NPJ Comput. Mater. 2019, 5, 3. [Google Scholar] [CrossRef]
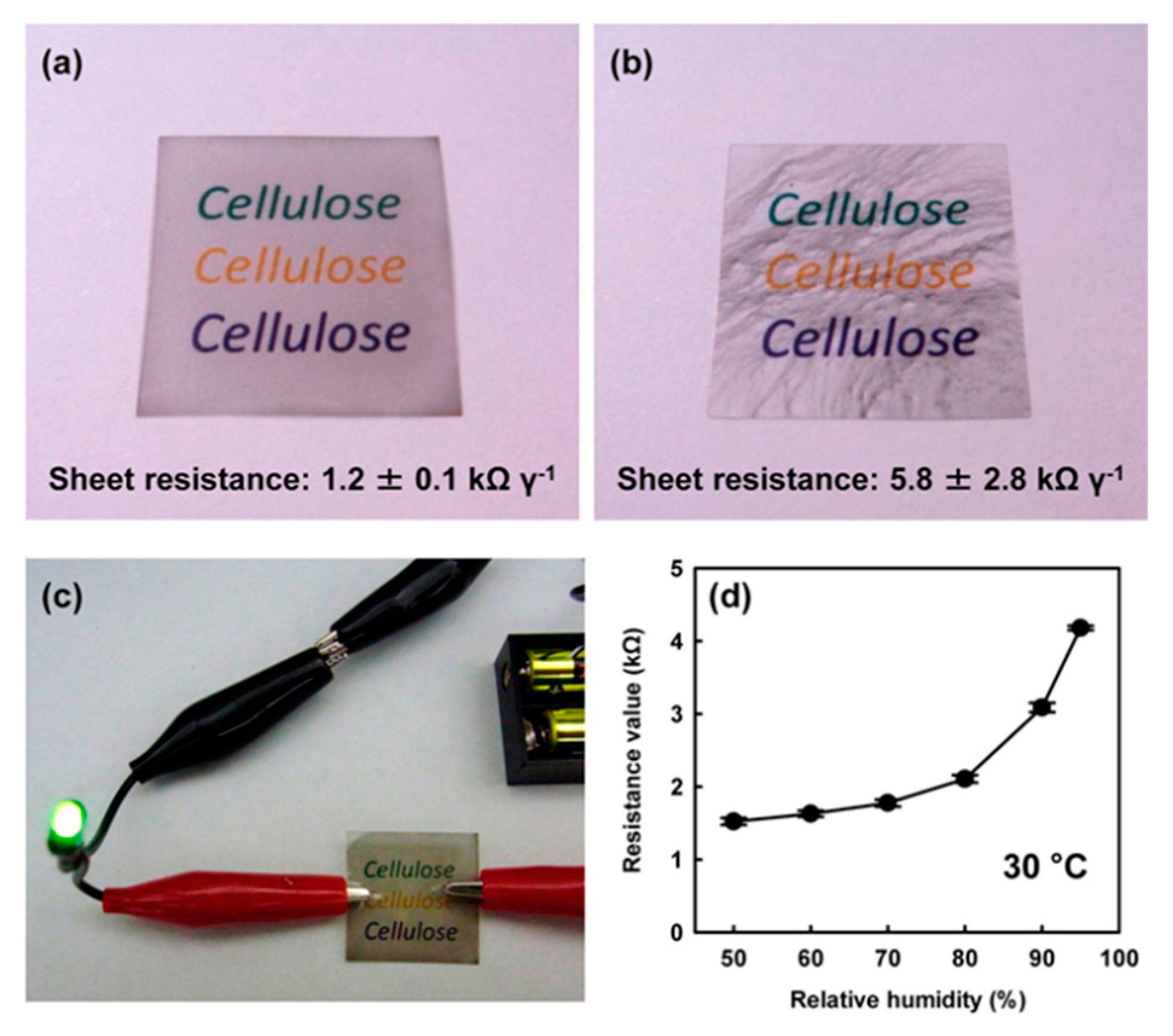
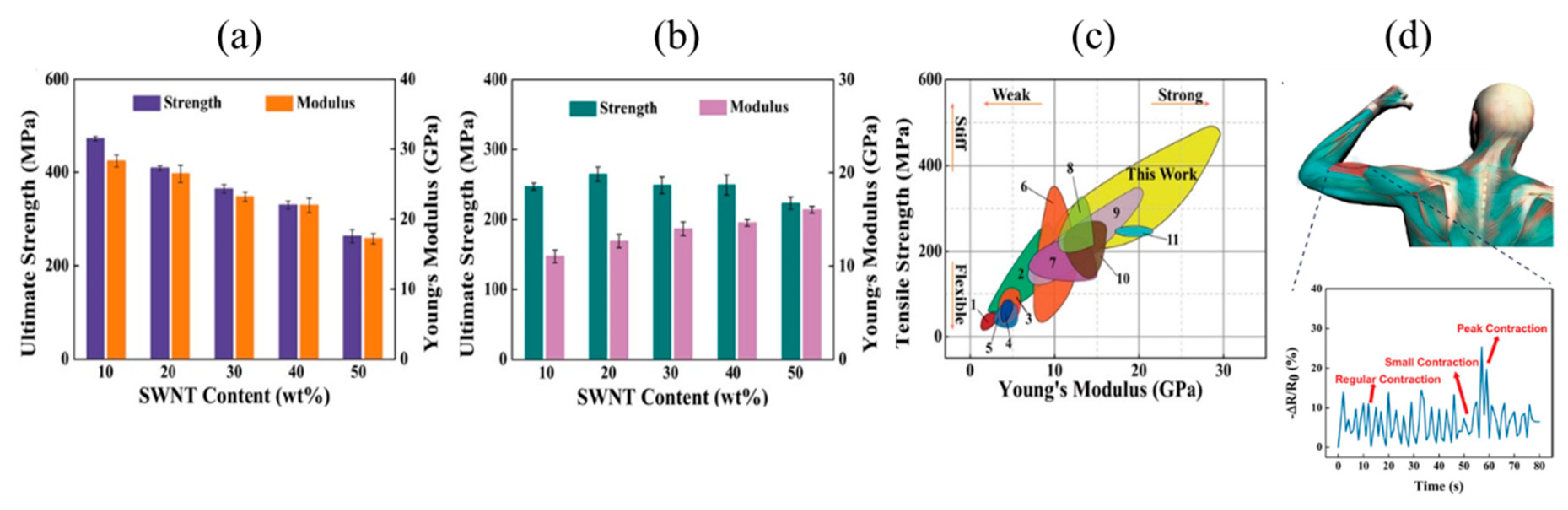
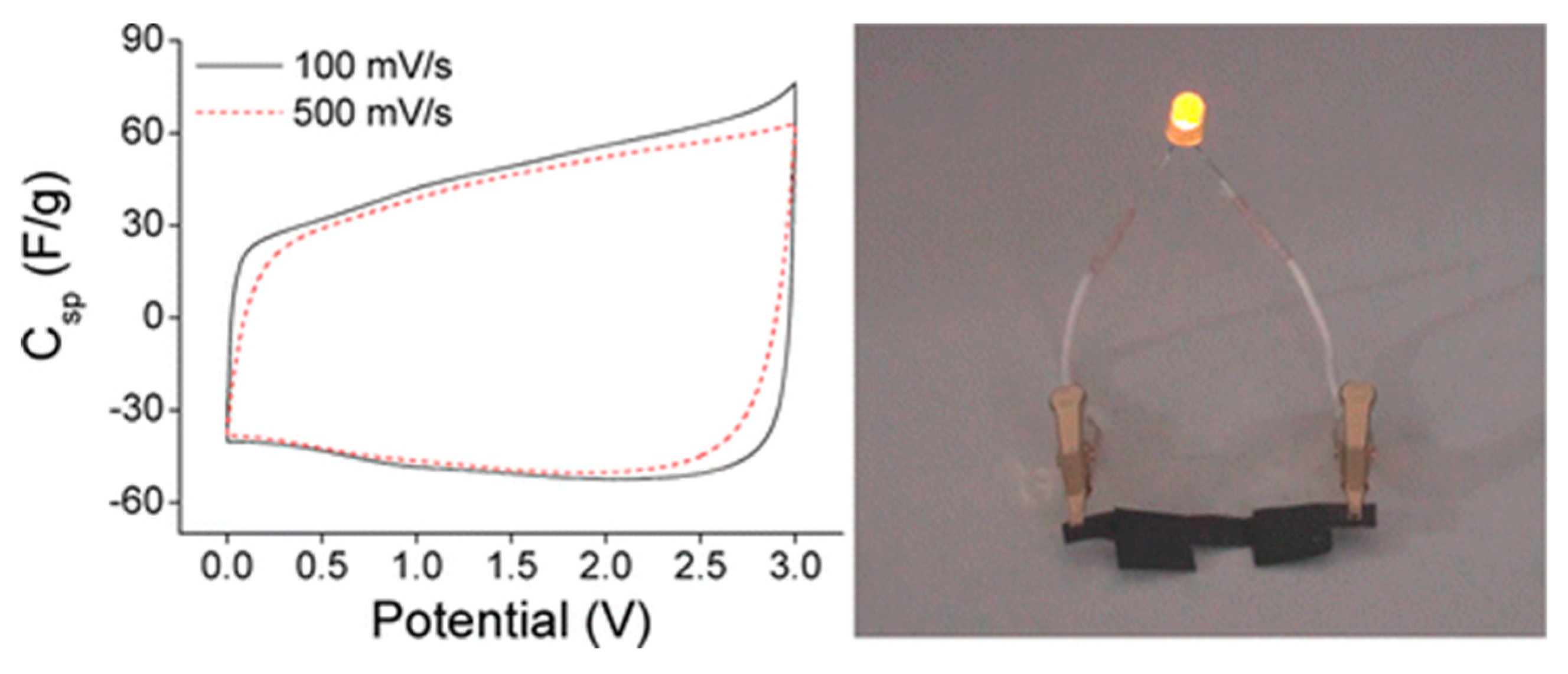

| Tensile Strength [GPa] | Young’s Modulus [GPa] | |
|---|---|---|
| Cotton | 0.3–0.7 | 5.0–10.9 |
| Wool | 0.1–0.2 | 2.3–3.4 |
| Silk | 0.3–0.5 | 7.3–11.2 |
| Flex | 0.3–0.9 | 24.0 |
| Jute | 0.3–0.7 | 43.8 |
| Sisa | 0.4–0.6 | – |
| Ramie | 0.3–0.8 | 53.4 |
| CNC | CNF | BNC | Ref. | SWNT | MWNT | Ref. | |
|---|---|---|---|---|---|---|---|
| Width [nm] | 3–50 | 4–100 | 20–140 | [33,40] | 0.5–10 | 5–100 | [49,53] |
| Length [nm] | 100–500 | 5000– | 5000– | [33,40] | 10–1000 | 100– | [49,53] |
| Young’s modulus [GPa] | 20–50 | 0.5–10 | 79–88 | [31,34,40] | 1000 | 70–950 | [51,52,53] |
| Tensile strength [GPa] | 9 | 0.1–1.0 | 21 | [31,35,40] | 13–53 | 11–150 | [51,52,53] |
| Mass density [g/cm3] | 1.5–1.6 | 1.3–1.4 | 1.1 | [38,40] | 1.3–1.5 | 1.8–2.0 | [50] |
| Thermo conductivity [W/mK] | – | – | – | – | 3500 | 3000 | [54,55] |
| Coefficient of Thermal expansion [ppm/K] | 0.1 | [36] | 20.0 | [56] | |||
| Capacitance [F/g] | – | – | – | – | 180 | 32.7 | [57,58] |
| Remarks | Transparency thermal stability (±260 °C) [37] Thixotropic | ChiralityHigh dispersibility | Wide range ofdimensions | – | |||
| Type | Materials | Purpose | Ref. | ||
|---|---|---|---|---|---|
| Cellulose | Carbon Nanotube | Other | |||
| Composites | Cellulose | SWNT | – | Detection of benzene, toluene, and xylene (BTX) vapors | [74] |
| Composites | Cellulose | MWNT | TiO2 | pH sensor | [75] |
| Composites | CMC | MWNT | – | Removal sensor of UO22+ | [76] |
| Aerogels | CNF | MWNT | – | Flexible supercapacitors | [77] |
| Aerogels | CNF | MWNT | PVA | Application for insulation and structural parts | [78] |
| Aerogels | CNF | MWNT | PANI | Supercapacitance | [79] |
| Aerogels | CNF | MWNT | PVA | Adsorption material for organic solvent, oil | [80] |
| Aerogels | CNF | CNT | RGO | Super capacitor electrolyte | [81] |
| Aerogels | CMC | CNT | – | Conductive aerogels | [85] |
| Aerogels | CNF | MWNT | – | Sensor for detecting the conductivity and pressure | [87] |
| Aerogels | BNC | CNT | – | Adsorption material for organic solvent, oil | [116] |
| Aerogels | BNC | CNT | GA | Enzyme biofuel cell | [122] |
| Paper | Cellulose paper | CNT | – | EMI shielding | [88] |
| Paper | CNF | CNT | – | EMI shielding | [89] |
| Paper | CNC | CNT | – | EMI shielding | [90] |
| Paper | Cellulose paper | CNT | – | Humidity sensor | [91] |
| Paper | Cellulose paper | CNT | – | Ammonia sensor | [92] |
| Paper | Cellulose paper | MWNT | – | Chemical vapor sensor | [93] |
| paper | Cellulose paper | CNT | – | Smart paper for portable electronics and sensing | [94] |
| paper | CNF | CNT | – | Conductive paper | [99] |
| paper | Cellulose paper | SWNT | – | Transparent, Conductive paper | [100] |
| Paper | CNF | CNT | – | conductive inks for 3D printing of scaffolding | [102] |
| Paper | Cellulose paper | CNT | – | chemical sensor | [103] |
| Paper | CNF | CNT | LTO | Lithium ion battery (LIB) electrode | [104] |
| Paper | BNC | MWNT | – | Translucent conductive paper | [115] |
| Film | TOCNs | CNT | – | Highly conductive and printable nanocomposites | [95] |
| Film | CNF | SWNT | – | Translucent conductive film | [96] |
| Film | CMC | SWNT | – | Translucent conductive film | [97] |
| Film | CMC | CNT | GO | Reinforced film | [101] |
| Film | CNF | CNT | – | Lithium ion battery anode | [105] |
| Film | BNC | MWNT | – | Conductive polymer film | [117] |
| Film | BNC | CNT | – | Flexible supercapacitor | [120] |
| Film | BNC | MWNT | – | Flexible supercapacitor for biofuel cell | [121] |
| Fiber | CNF | MWNT | – | Super capacitor electrode | [107] |
| Fiber | CNF | MWNT | – | Conductive fiber mat | [108] |
| Fiber | CNF | MWNT | – | Reinforced fiber | [109] |
| Fiber | TEMPO-CNF | MWNT | – | Wearable electronic devices | [110] |
| Fiber | CNF | SWNT | – | Flexible strain and mass sensor | [111] |
| Fiber | CNF | SWNT | – | Wearable supercapacitor | [114] |
| Fiber | BNC | MWNT | – | Resonator for biochemical sensing | [119] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyashiro, D.; Hamano, R.; Umemura, K. A Review of Applications Using Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes. Nanomaterials 2020, 10, 186. https://doi.org/10.3390/nano10020186
Miyashiro D, Hamano R, Umemura K. A Review of Applications Using Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes. Nanomaterials. 2020; 10(2):186. https://doi.org/10.3390/nano10020186
Chicago/Turabian StyleMiyashiro, Daisuke, Ryo Hamano, and Kazuo Umemura. 2020. "A Review of Applications Using Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes" Nanomaterials 10, no. 2: 186. https://doi.org/10.3390/nano10020186
APA StyleMiyashiro, D., Hamano, R., & Umemura, K. (2020). A Review of Applications Using Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes. Nanomaterials, 10(2), 186. https://doi.org/10.3390/nano10020186




