A Guided Walk through the World of Mesoporous Bioactive Glasses (MBGs): Fundamentals, Processing, and Applications
Abstract
1. Introduction
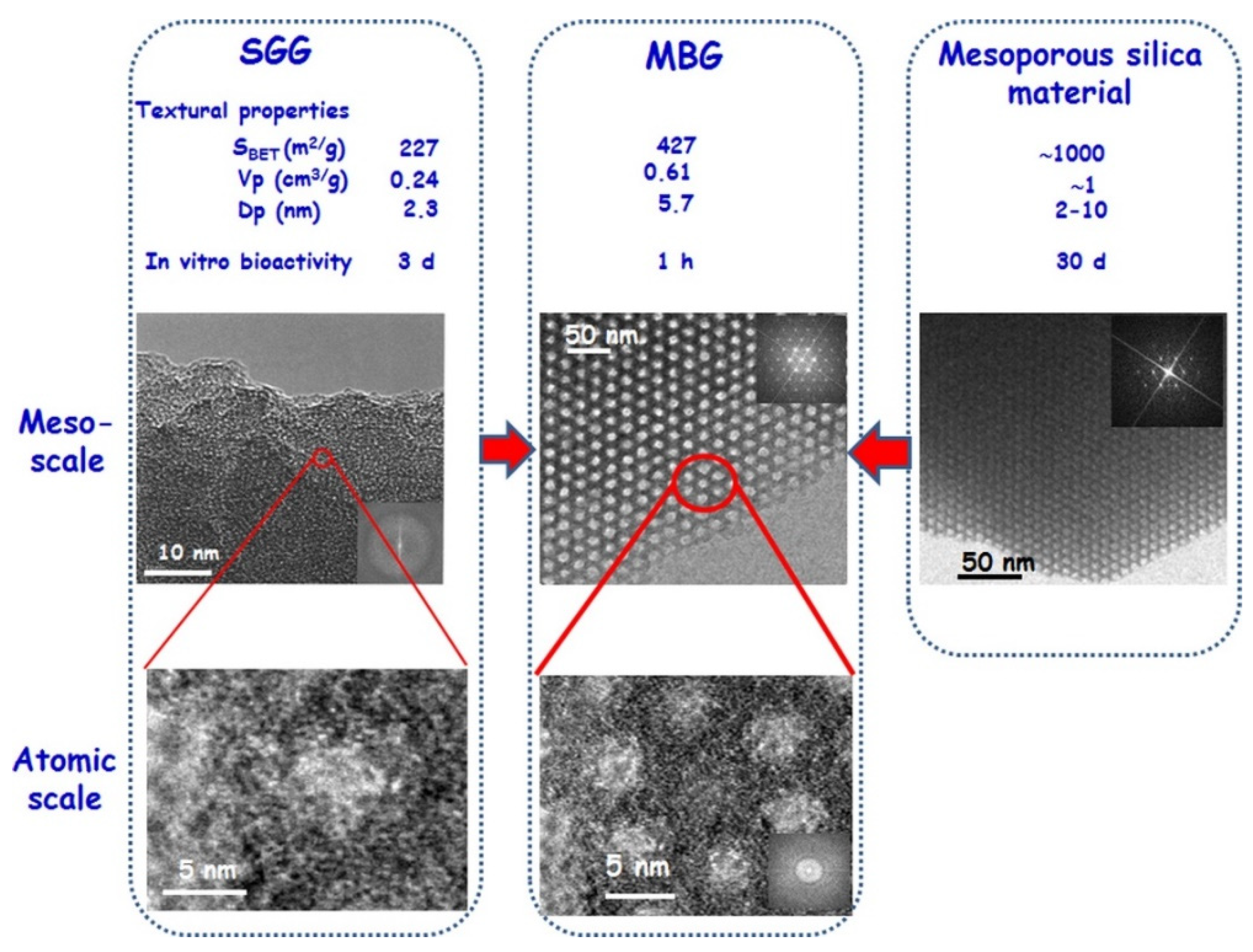
2. Properties of MBGs—A Short Overview
- High pore volume (about 1 cm3/g [10]);
- Ultrahigh specific surface area (above 100 m2/g [22]), which impressively accelerates the apatite-forming kinetics;
- Ordered meso-structure;
- Tunable and narrow pore size distribution (2–30 nm [10]), which can be finely controlled at the synthesis stage (e.g., by varying the type of surfactant);
- Lower synthesis temperatures;
- Relatively easy powder technology production;
- Improved homogeneity and purity of the final products;
- Wider range of bioactive compositions even with high amount of SiO2 (up to 90 mol.%);
- Presence of tunable mesoporosity;
- Ability to form hierarchical scaffolds with multiscale porosity (from macro- to meso-range) [27].
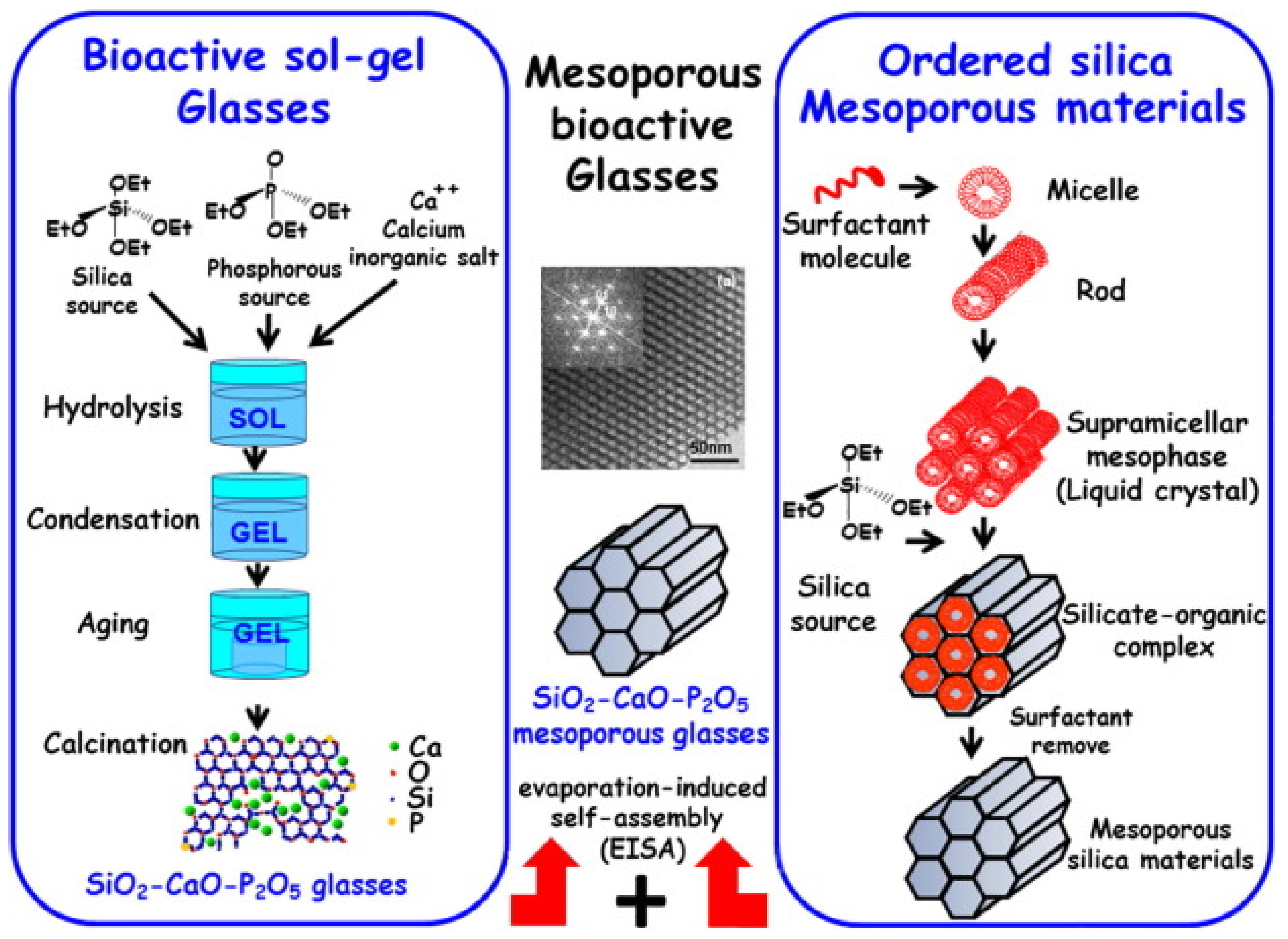
3. Synthesis of MBGs
3.1. Hydrothermal Method
- Mixing surfactants in a solvent, typically water, at high temperature (up to 130 °C);
- Addition of silicate precursors into the solution; hydrolysis of precursors occurs during this step through the action of an acid or base catalyst;
- Formation of a sol composed by silicate oligomers;
- Condensation of a gel due to interactions between silicate oligomers and surfactants and precipitation of mesoporous silicate;
- Hydrothermal treatment, such as cooling down to room temperature, leads to sudden precipitation (solidification) of an ordered meso-structure;
- Filtering, washing, and drying of resulted mesoporous materials;
- Surfactant removal and consolidation by calcination to obtain the final mesoporous products [36].
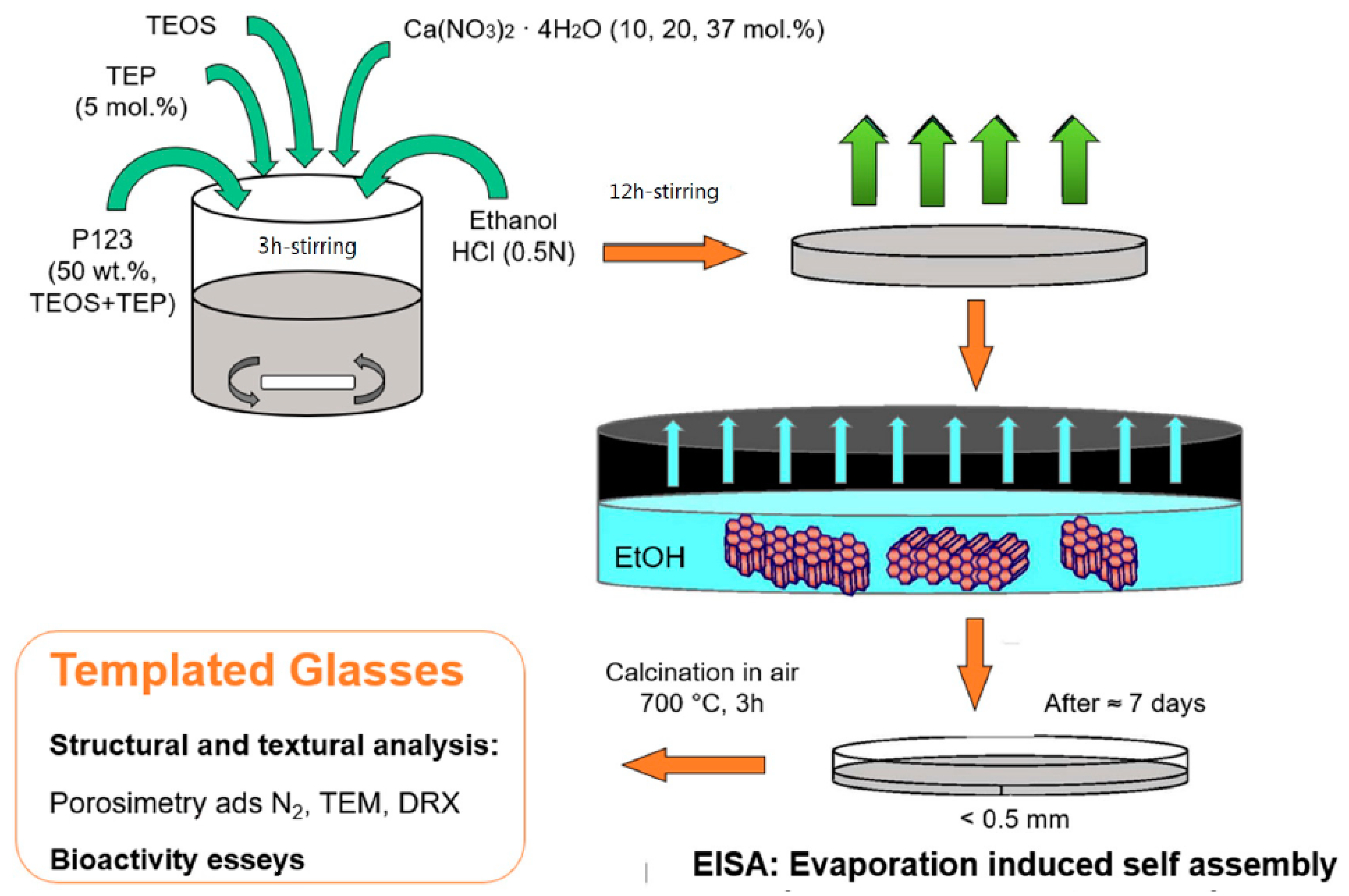
3.2. Evaporation-Induced Self-Assembly (EISA)
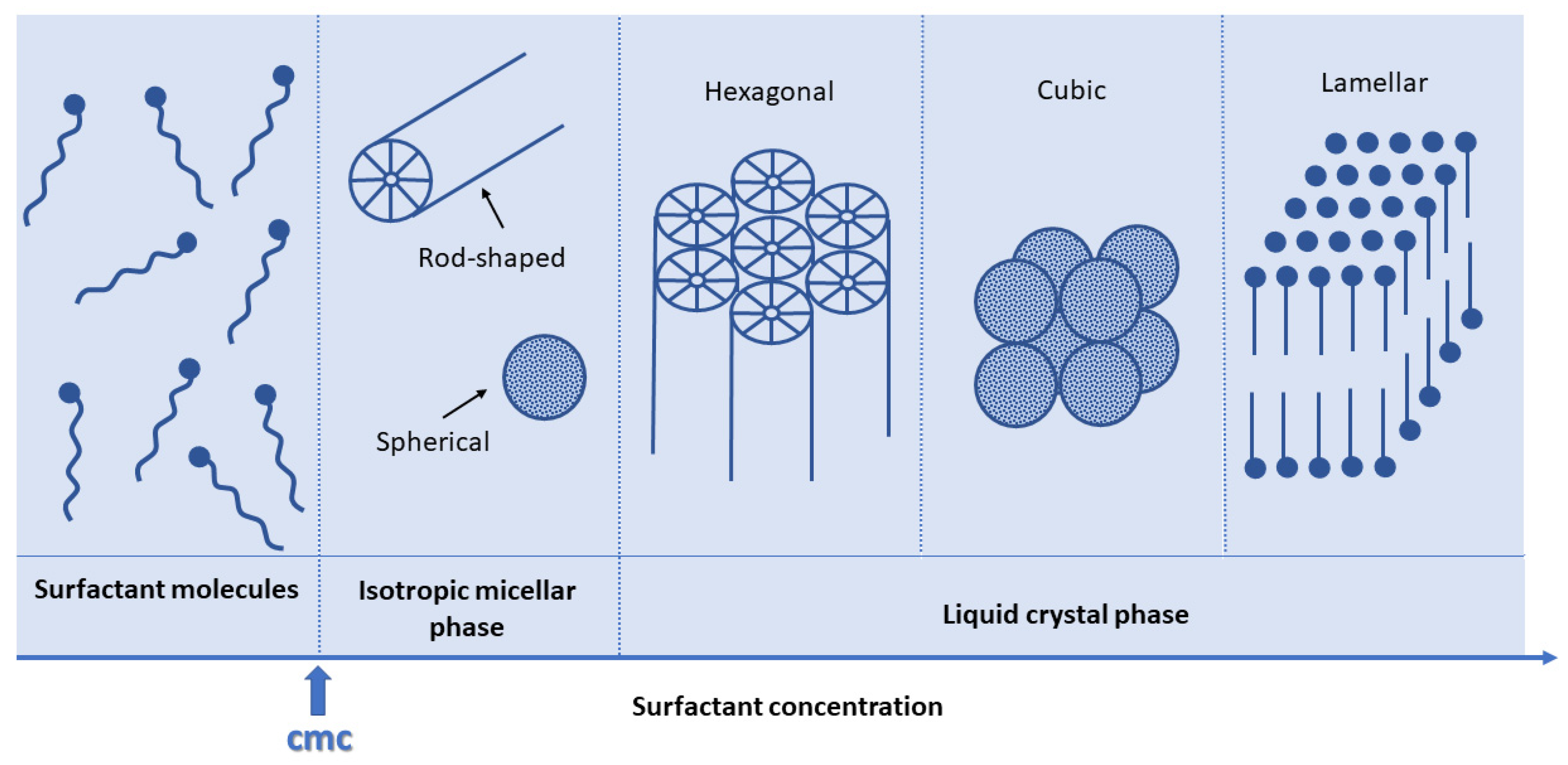
3.2.1. Behavior of Surfactant Molecules in an Aqueous Solution
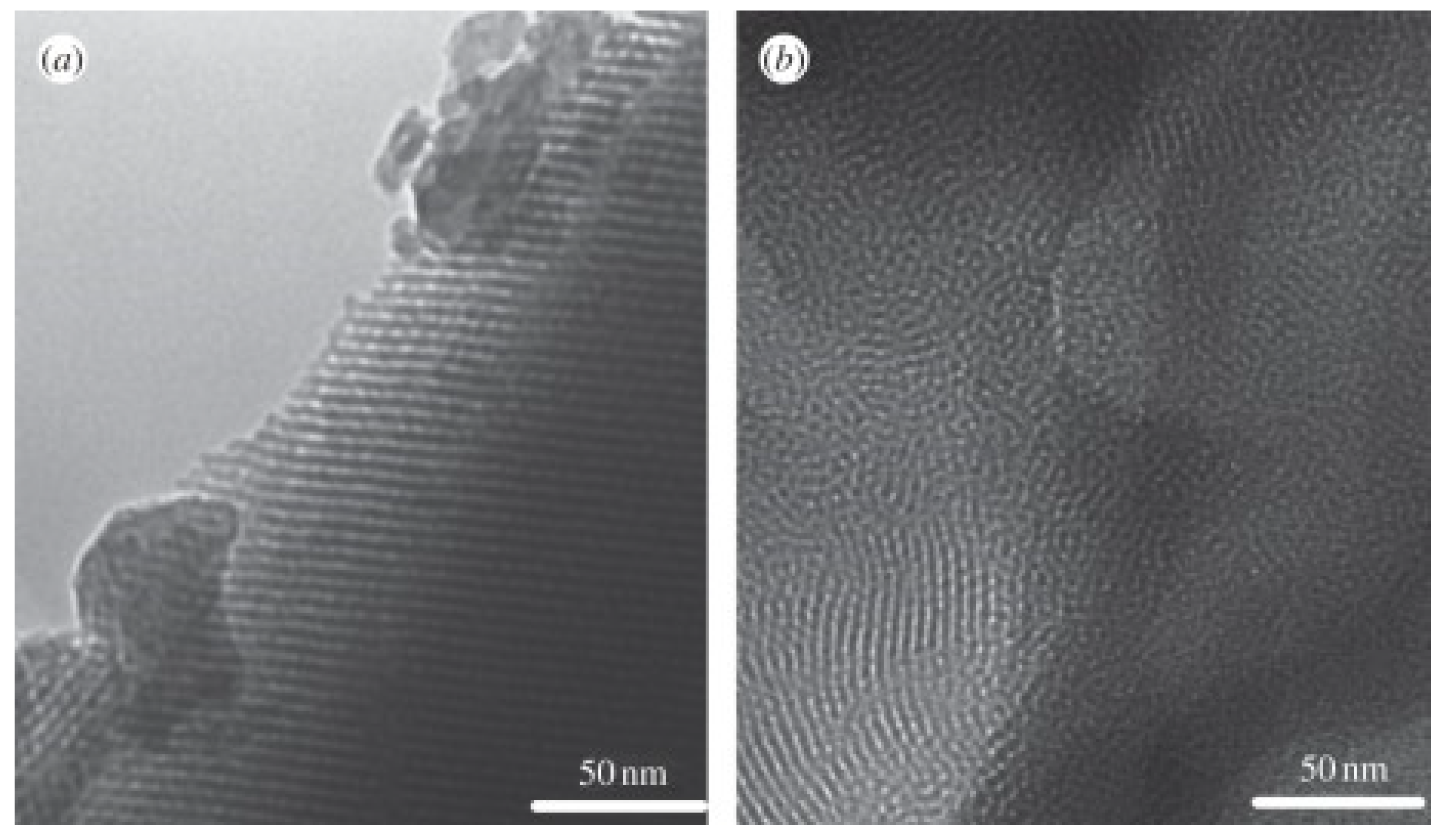
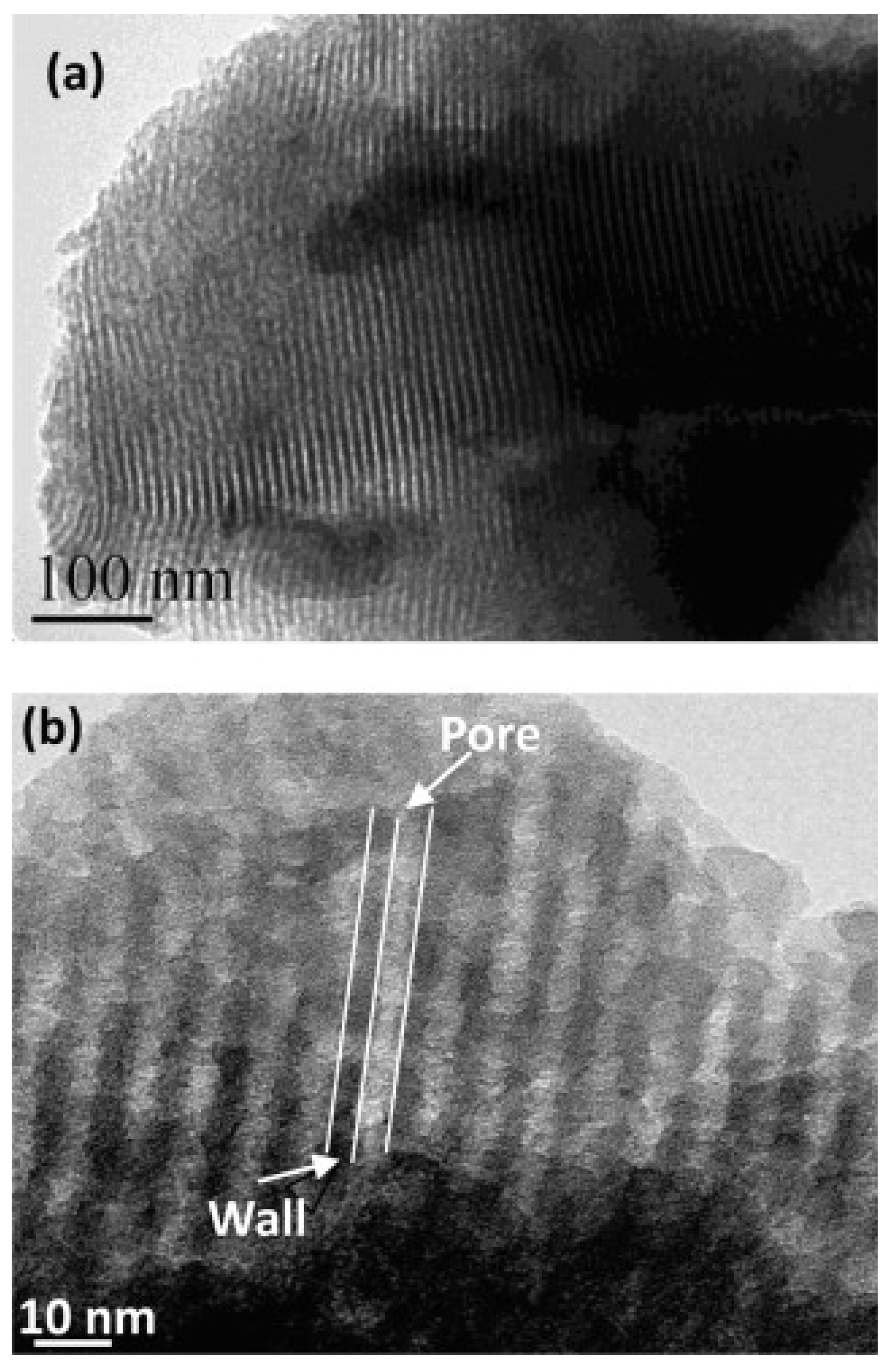
3.2.2. EISA Process in MBG Synthesis
4. Effects of Surfactants and Composition
4.1. Brief Overview of Surfactants Used in the Production of Mesoporous Materials
4.1.1. CTAB
4.1.2. Pluronics®
4.2. Surfactant Molecules as Structure-Directing Agents in MBG Synthesis
4.3. Composition–Mesopore Structure Relationship in MBGs
4.3.1. Role of Calcium Oxide
4.3.2. Role of Phosphorous Oxide
4.3.3. Role of Dopants
4.3.4. The Challenge of Multicomponent Mesoporous Systems
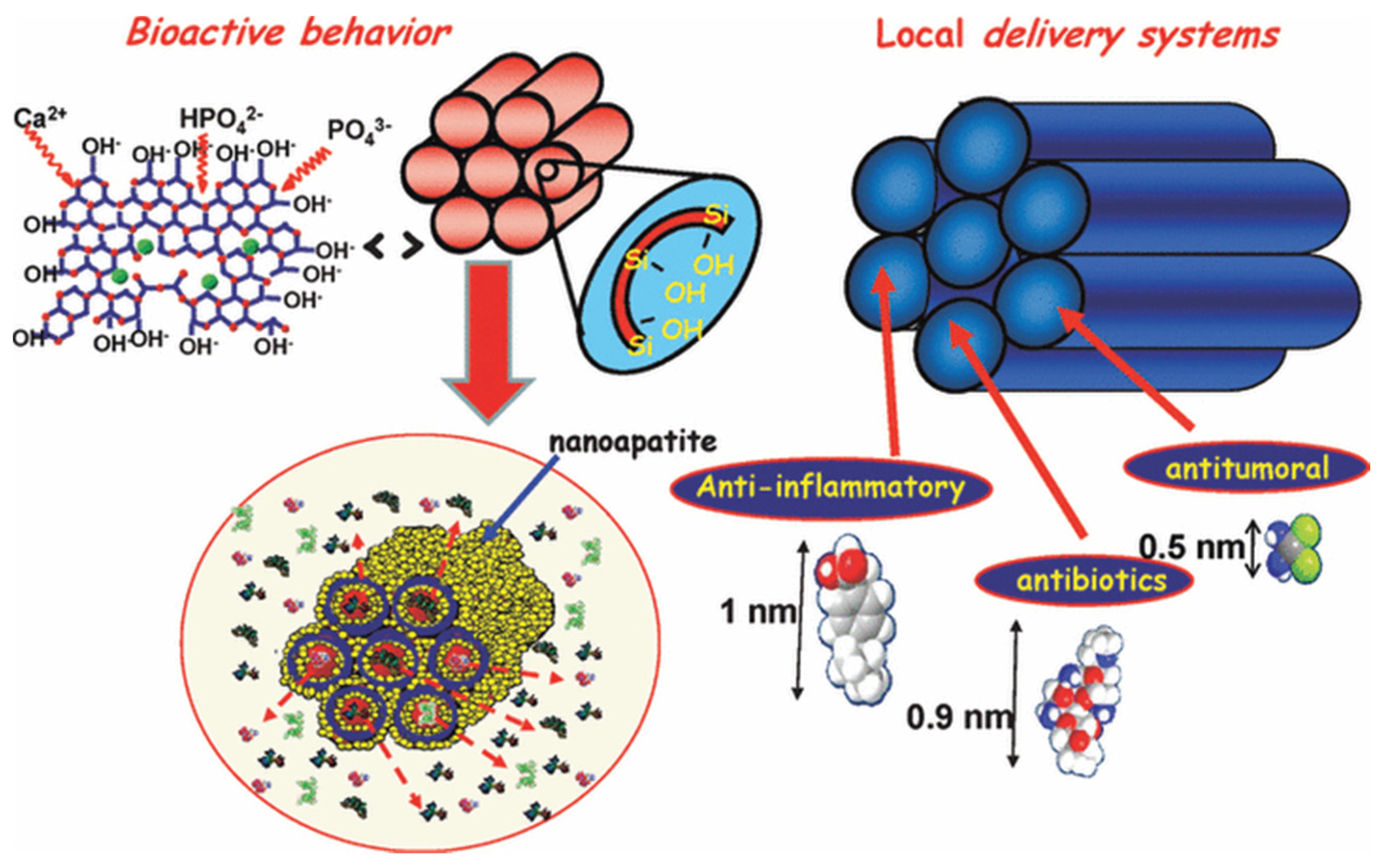
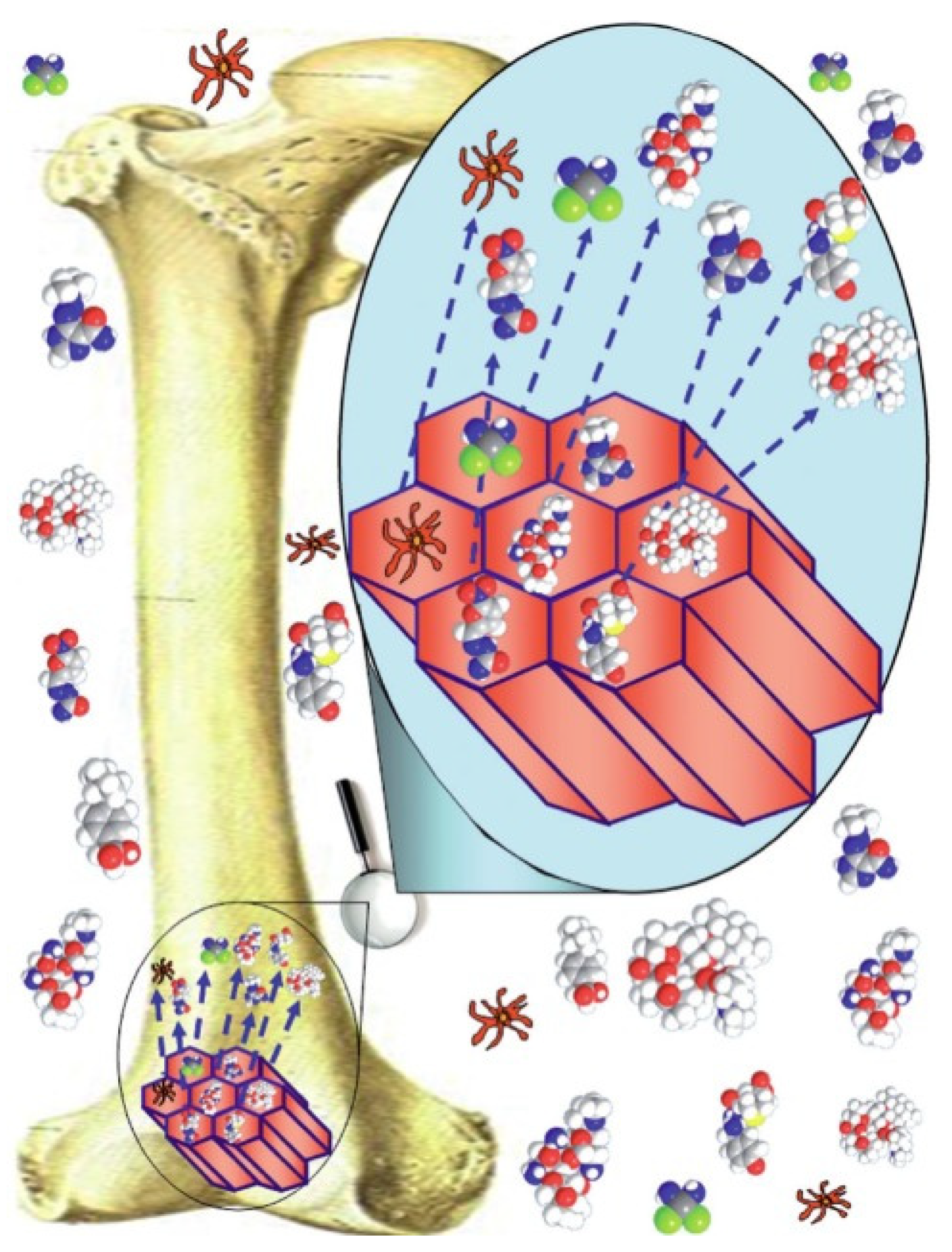
5. Biomedical Applications of MBGs
5.1. Bone Regeneration
5.2. Drug Delivery Systems
5.3. MBGs as Multifunctional Platforms for Tissue Repair
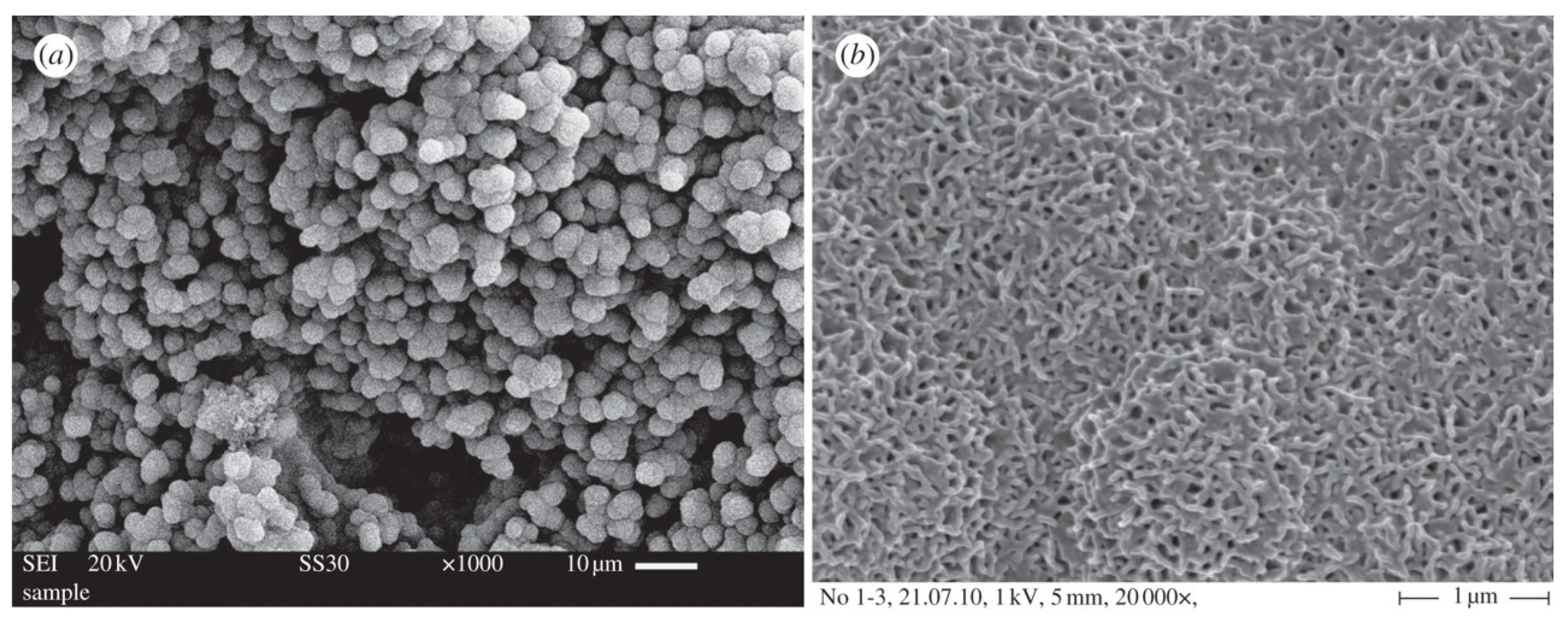
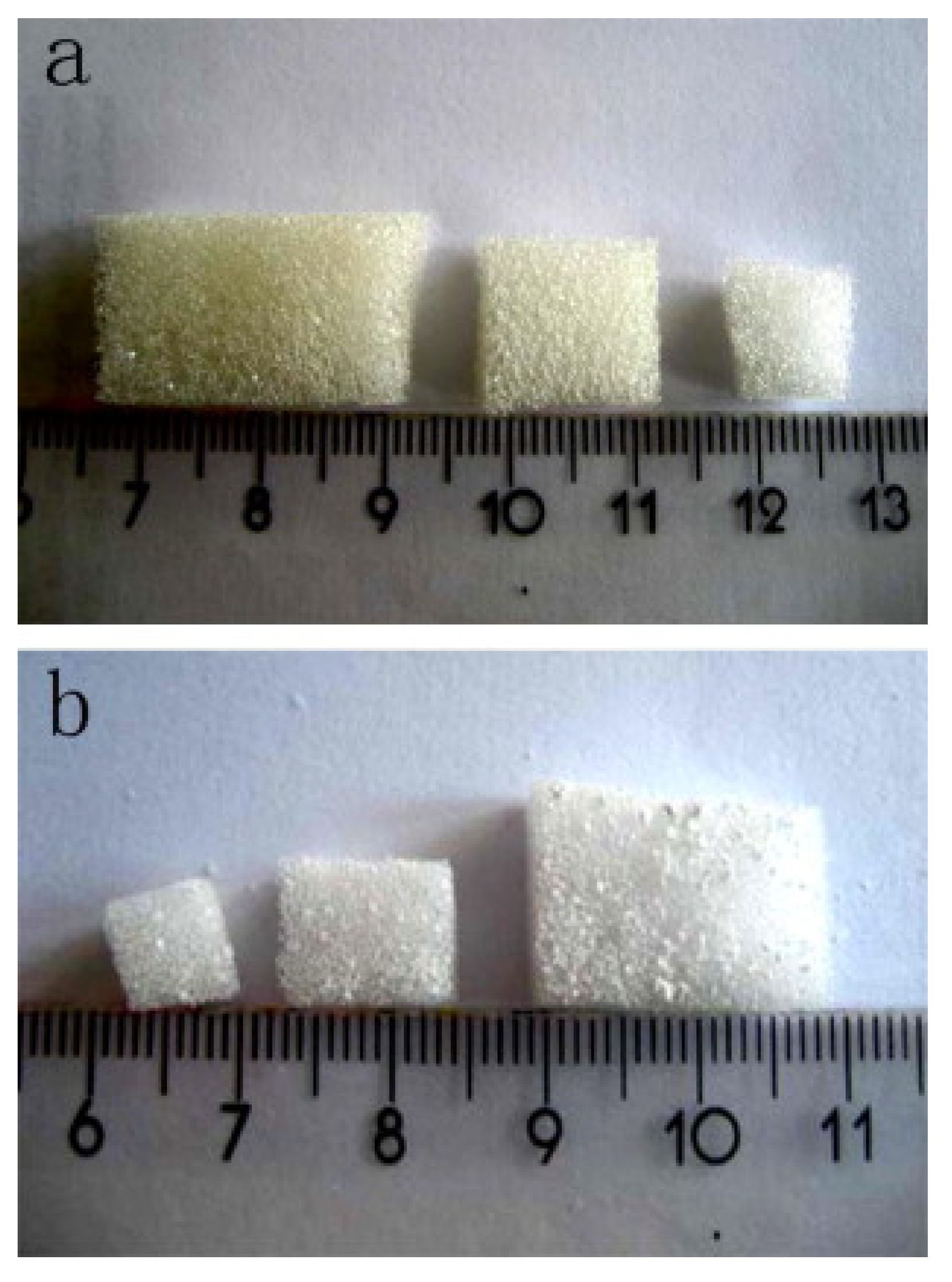
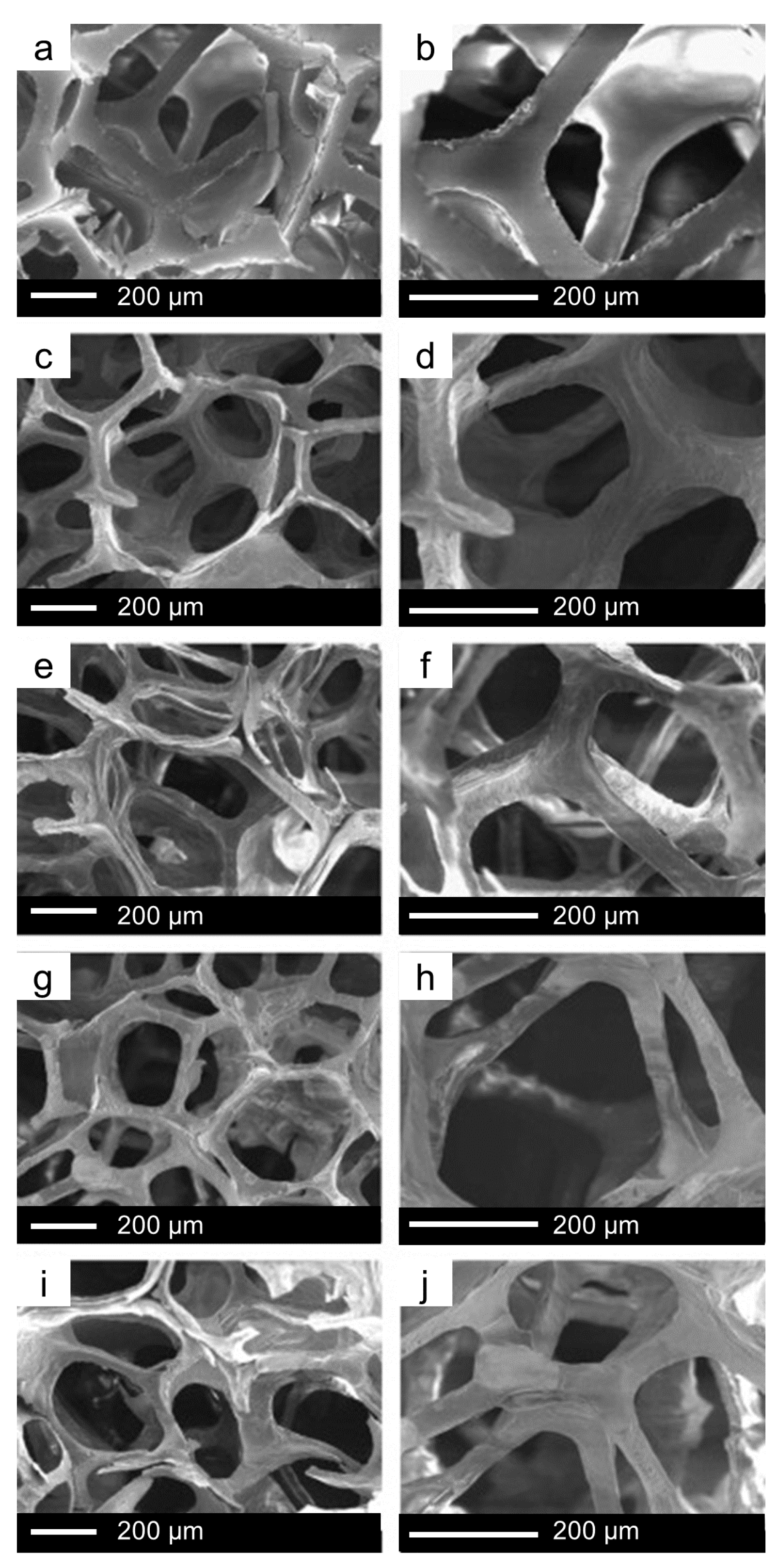
6. The Last Frontier: Hierarchical MBG Scaffolds
6.1. Fabrication of MBG Scaffolds: From Macro- to Meso-Scale… and Back
6.2. The Potential of MBG Scaffolds for Advanced Therapies
7. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Nandi, P.; Mahato, S.K.; Kundu, A.; Mukherjee, B. Doped Bioactive Glass Materials in Bone Regeneration. In Advanced Techniques in Bone Regeneration; InTechOpen: Rijeka, Croatia, 2016; pp. 275–329. [Google Scholar]
- Vallet-Regí, M.; Ragel, C.V.; Salinas, A.J. Glasses with Medical Applications. Eur. J. Inorg. Chem. 2003, 2003, 1029–1042. [Google Scholar] [CrossRef]
- Li, R.; Clark, A.E.; Hench, L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991. [Google Scholar] [CrossRef] [PubMed]
- Ylänen, H.O. Clinical application of bioactive glasses. In Bioceramics and their Clinical Applications; Woodhead Publishing Limited: Cambridge, UK, 2008. [Google Scholar]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Miola, M.; Verné, E. Bioactive sol-gel glasses: Processing, properties, and applications. Int. J. Appl. Ceram. Technol. 2018, 15, 841–860. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef] [PubMed]
- López-Noriega, A.; Arcos, D.; Izquierdo-Barba, I.; Sakamoto, Y.; Terasaki, O.; Vallet-Regí, M. Ordered mesoporous bioactive glasses for bone tissue regeneration. Chem. Mater. 2006, 18, 3137–3144. [Google Scholar] [CrossRef]
- Zhao, L.; Yan, X.; Zhou, X.; Zhou, L.; Wang, H.; Tang, J.; Yu, C. Mesoporous bioactive glasses for controlled drug release. Microporous Mesoporous Mater. 2008, 109, 210–215. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for the treatment of complex bone diseases: Bone cancer, bone infection and osteoporosis. Pharmaceutics 2020, 12, 83. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; Del Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Zhao, X.S.; Lu, G.Q.; Millar, G.J. Advances in mesoporous molecular sieve MCM-41. Ind. Eng. Chem. Res. 1996, 35, 2075–2090. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Izquierdo-Barba, I.; Ramila, A.; Pérez-Pariente, J.; Babonneau, F.; González-Calbet, J.M. Phosphorous-doped MCM-41 as bioactive material. Solid State Sci. 2005, 7, 233–237. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Sol–gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef] [PubMed]
- Hamadouche, M.; Meunier, A.; Greenspan, D.C.; Blanchat, C.; Zhong, J.P.; La Torre, G.P.; Sedel, L. Long-term in vivo bioactivity and degradability of bulk sol-gel bioactive glasses. J. Biomed. Mater. Res. 2001, 54, 560–566. [Google Scholar] [CrossRef]
- Horcajada, P.; Rámila, A.; Boulahya, K.; González-Calbet, J.; Vallet-Regí, M. Bioactivity in ordered mesoporous materials. Solid State Sci. 2004, 6, 1295–1300. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Ruiz-González, L.; Doadrio, J.C.; González-Calbet, J.M.; Vallet-Regí, M. Tissue regeneration: A new property of mesoporous materials. Solid State Sci. 2005, 7, 983–989. [Google Scholar] [CrossRef]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- Yan, X.; Huang, X.; Yu, C.; Deng, H.; Wang, Y.; Zhang, Z.; Qiao, S.; Lu, G.; Zhao, D. The in-vitro bioactivity of mesoporous bioactive glasses. Biomaterials 2006, 27, 3396–3403. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Nanostructured mesoporous silica matrices in nanomedicine. J. Intern. Med. 2010, 267, 22–43. [Google Scholar] [CrossRef]
- Hench, L.L. Bioactive Ceramics: Theory and Clinical Applications. In Bioceramics, Proceedings of the 7th International Symposium on Ceramics in Medicine, Turku, Finland, July 1994; Butterworth-Heinemann Ltd.: Oxford, UK, 1994; Volume VII. [Google Scholar]
- Baino, F. Bioactive glasses—When glass science and technology meet regenerative medicine. Ceram. Int. 2018, 44, 14953–14966. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ruiz-González, L.; Izquierdo-Barba, I.; González-Calbet, J.M. Revisiting silica based ordered mesoporous materials: Medical applications. J. Mater. Chem. 2006, 16, 26–31. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous bioactive glasses: Relevance of their porous structure compared to that of classical bioglasses. Biomed. Glasses 2015, 1, 140–150. [Google Scholar] [CrossRef]
- Pereira, M.M.; Clark, A.E.; Hench, L.L. Effect of Texture on the Rate of Hydroxyapatite Formation on Gel-Silica Surface. J. Am. Ceram. Soc. 1995, 78, 2463–2468. [Google Scholar] [CrossRef]
- Kumar, A.; Murugavel, S.; Aditya, A.; Boccaccini, A.R. Mesoporous 45S5 bioactive glass: Synthesis, in vitro dissolution and biomineralization behavior. J. Mater. Chem. B 2017, 5, 8786–8798. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Montazerian, M.; Hamzehlou, S.; Kim, H.W.; Baino, F. Mesoporous bioactive glasses: Promising platforms for antibacterial strategies. Acta Biomater. 2018, 81, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kleitz, F.; Schmidt, W.; Schuth, F. Calcination behavior of different surfactant-templated mesostructured silica materials. Microporous Mesoporous Mater. 2003, 65, 1–29. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, X.; Jing, X.; Fan, H.; Guo, B.; Gu, Z.; Zhang, X. Preparation, Bioactivity, and Drug Release of Hierarchical Nanoporous Bioactive Glass Ultrathin Fibers. Adv. Mater. 2010, 22, 754–758. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Ke, X.; Xie, Y.; Zhu, H.Y.; Crawford, R. Bioactive mesopore-glass microspheres with controllable protein-delivery properties by biomimetic surface modification. J. Biomed. Mater. Res. Part A 2010, 95, 476–485. [Google Scholar] [CrossRef]
- Arcos, D.; Vila, M.; López-Noriega, A.; Rossignol, F.; Champion, E.; Oliveira, F.; Vallet-Regí, M. Mesoporous bioactive glasses: Mechanical reinforcement by means of a biomimetic process. Acta Biomater. 2011, 7, 2952–2959. [Google Scholar] [CrossRef]
- Lei, B.; Chen, X.; Wang, Y.; Zhao, N.; Du, C.; Zhang, L. Acetic acid derived mesoporous bioactive glasses with an enhanced in vitro bioactivity. J. Non-Cryst. Solids 2009, 355, 2583–2587. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J. Well-ordered mesoporous bioactive glasses (MBG): A promising bioactive drug delivery system. J. Control Release 2006, 110, 522–530. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J. Preparation, in vitro bioactivity and drug release property of well-ordered mesoporous 58S bioactive glass. J. Non-Cryst. Solids 2008, 354, 1338–1341. [Google Scholar] [CrossRef]
- Yun, H.; Kim, S.; Hyun, Y. Preparation of bioactive glass ceramic beads with hierarchical pore structure using polymer self-assembly technique. Mater. Chem. Phys. 2009, 115, 670–676. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D. On the Controllable Soft-Templating Approach to Mesoporous Silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. ChemInform Abstract: Evaporation-Induced Self-Assembly: Nanostructures Made Easy. ChemInform 2010, 30, 579–585. [Google Scholar] [CrossRef]
- Holmqvist, P.; Alexandridis, P.; Lindman, B. Modification of the Microstructure in Block Copolymer–Water–‘Oil’ Systems by Varying the Copolymer Composition and the ‘Oil’ Type: Small-Angle X-ray Scattering and Deuterium-NMR Investigation. J. Phys. Chem. B 1998, 102, 1149–1158. [Google Scholar] [CrossRef]
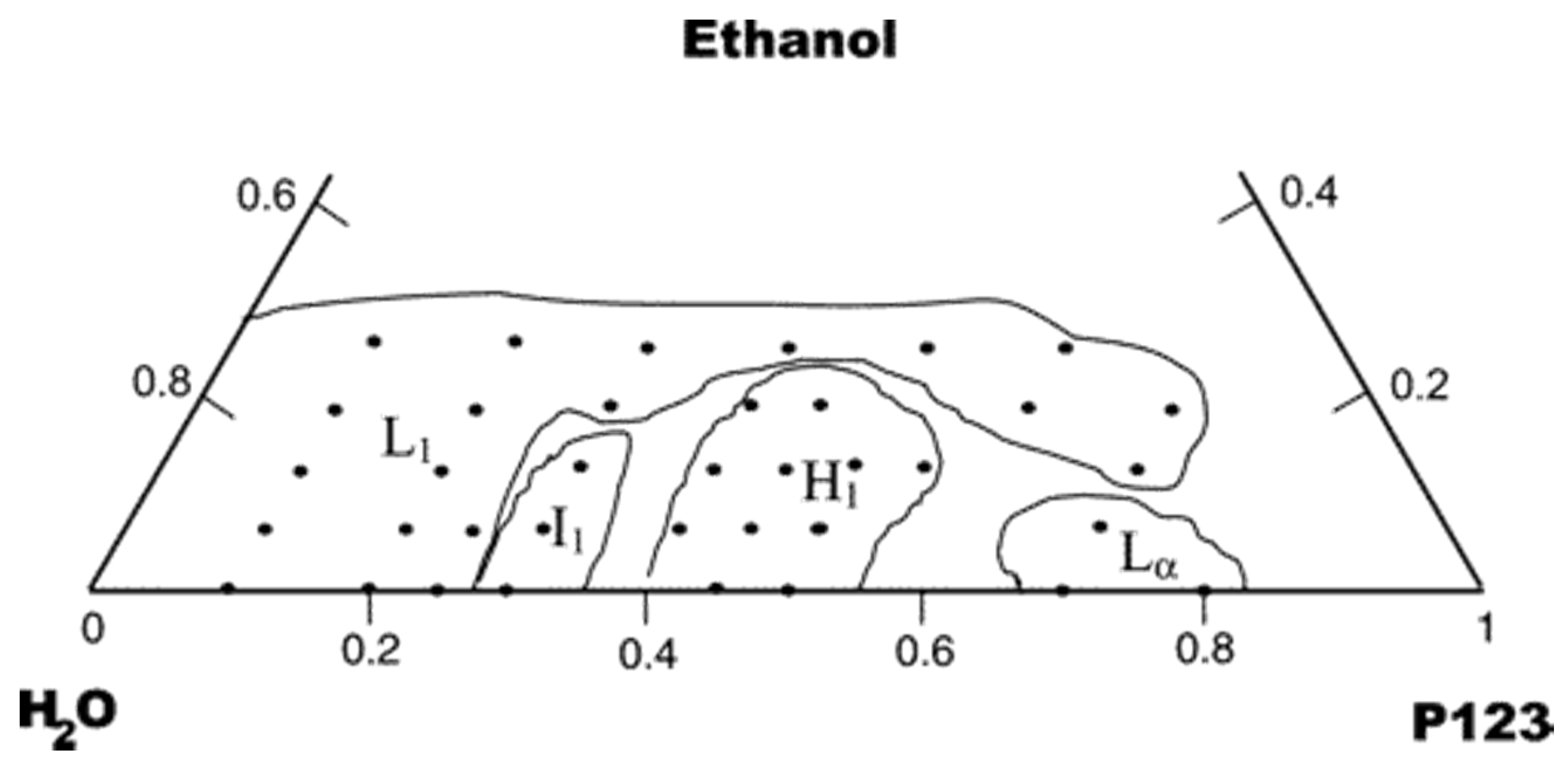
- Soni, S.S.; Brotons, G.; Bellour, M.; Narayanan, T.; Gibaud, A. Quantitative SAXS analysis of the P123/water/ethanol ternary phase diagram. J. Phys. Chem. B 2006, 110, 15157–15165. [Google Scholar] [CrossRef]
- Elgh, B.; Yuan, N.; Cho, H.S.; Magerl, D.; Philipp, M.; Roth, S.V.; Yoon, K.B.; Müller-Buschbaum, P.; Terasaki, O.; Palmqvist, A.E.C. Controlling morphology, mesoporosity, crystallinity, and photocatalytic activity of ordered mesoporous TiO2 films prepared at low temperature. APL Mater. 2014, 2, 113313. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Lee, S.; Pfefferle, L.D.; Haller, G.L. Surfactant chain length effect on the hexagonal-to-cubic phase transition in mesoporous silica synthesis. Microporous Mesoporous Mater. 2012, 147, 242–251. [Google Scholar] [CrossRef]
- Salinas, A.J.; Arcos, D. Tailoring the Structure of Bioactive Glasses: From the Nanoscale to Macroporous Scaffolds. Int. J. Appl. Glass Sci. 2016, 7, 195–205. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, C.; Hou, C.; Zhang, H.; Zhang, H.; Zhang, Q.; Guo, Z. Cetyl trimethyl ammonium bromide (CTAB) micellar templates directed synthesis of water-dispersible polyaniline rhombic plates with excellent processability and flow-induced color variation. Polymer 2017, 117, 30–36. [Google Scholar] [CrossRef]
- Ii, R.E.M.; Biradar, A.V.; Duncan, C.T.; Schiff, E.A.; Asefa, T. Solvent-washable polymer templated synthesis of mesoporous materials and solid-acid nanocatalysts in one-pot. Chem. Commun. 2009, 6203, 6201. [Google Scholar] [CrossRef]
- Fu, Q.; Saiz, E.; Tomsia, A.P. Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration. Acta Biomater. 2011, 7, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Landers, R.; Hübner, U.; Schmelzeisen, R.; Mülhaupt, R. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials 2002, 23, 4437–4447. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control Release 2008, 130, 98–106. [Google Scholar] [CrossRef]
- List, P. Pluronic ® F-127; Molecular Probes, Inc.: Eugene, OR, USA, 2001. [Google Scholar]
- Shih, C.C.; Chien, C.-S.; Kung, J.-C.; Chen, J.C.; Chang, S.-S.; Lu, P.-S.; Shih, C.-J. Effect of surfactant concentration on characteristics of mesoporous bioactive glass prepared by evaporation induced self-assembly process. Appl. Surf. Sci. 2013, 264, 105–110. [Google Scholar] [CrossRef]
- Arcos, D.; Loópez-Noriega, A.; Ruiz-Hernaández, E.; Terasaki, O.; Vallet-Regí, M. Ordered mesoporous microspheres for bone grafting and drug delivery. Chem. Mater. 2009, 21, 1000–1009. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Loo, S.C.J.; Chen, Y.Z.; Boey, F.Y.C.; Ma, J. In situ SAXRD study of sol-gel induced well-ordered mesoporous bioglasses for drug delivery. J. Biomed. Mater. Res. Part A 2008, 85, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Mortera, B.R.; Baino, F.; Croce, G.; Fiorilli, S.; Vitale-Brovarone, C.; Verné, E.; Onida, B. Monodisperse Mesoporous Silica Spheres Inside a Bioactive Macroporous Glass—Ceramic Scaffold. Adv. Eng. Mater. 2010, 12, B256–B259. [Google Scholar] [CrossRef]
- Wu, C.; Fan, W.; Zhu, Y.; Gelinsky, M.; Chang, J.; Cuniberti, G.; Albrecht, V.; Friis, T.; Xiao, Y. Multifunctional magnetic mesoporous bioactive glass scaffolds with a hierarchical pore structure. Acta Biomater. 2011, 7, 3563–3572. [Google Scholar] [CrossRef]
- Wu, X.; Wei, J.; Lu, X.; Lv, Y.; Chen, F.; Zhang, Y.; Liu, C. Chemical characteristics and hemostatic performances of ordered mesoporous calcium-doped silica xerogels. Biomed. Mater. 2010, 5, 035006. [Google Scholar] [CrossRef]
- Garcia, A.M.; Izquierdo-Barba, I.; Colilla, M.; De Laorden, C.L.; Vallet-Regí, M. Preparation of 3-D scaffolds in the SiO2–P2O5 system with tailored hierarchical meso-macroporosity. Acta Biomater. 2011, 7, 1265–1273. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, C.; Ramaswamy, Y.; Kockrick, E.; Simon, P.; Kaskel, S.; Zreiqat, H. Preparation, characterization and in vitro bioactivity of mesoporous bioactive glasses (MBGs) scaffolds for bone tissue engineering. Microporous Mesoporous Mater. 2008, 112, 494–503. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Hua, Z.; Shi, J. One-pot synthesis of magnetic and mesoporous bioactive glass composites and their sustained drug release property. Acta Mater. 2008, 56, 3260–3265. [Google Scholar] [CrossRef]
- Salinas, A.J.; Shruti, S.; Malavasi, G.; Menabue, L.; Vallet-Regí, M. Substitutions of cerium, gallium and zinc in ordered mesoporous bioactive glasses. Acta Biomater. 2011, 7, 3452–3458. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Y.; Wu, C.; Fang, Y.; Yang, J.; Wang, S. The effect of zirconium incorporation on the physiochemical and biological properties of mesoporous bioactive glasses scaffolds. Microporous Mesoporous Mater. 2011, 143, 311–319. [Google Scholar] [CrossRef]
- Wu, C.; Miron, R.; Sculean, A.; Kaskel, S.; Doert, T.; Schulze, R.; Zhang, Y. Proliferation, differentiation and gene expression of osteoblasts in boron-containing associated with dexamethasone deliver from mesoporous bioactive glass scaffolds. Biomaterials 2011, 32, 7068–7078. [Google Scholar] [CrossRef]
- Fiume, E.; Migneco, C.; Verné, E.; Baino, F. Comparison between Bioactive Sol-Gel and Melt-Derived Glasses/Glass-Ceramics Based on the Multicomponent SiO2–P2O5–CaO–MgO–Na2O–K2O System. Materials 2020, 13, 540. [Google Scholar] [CrossRef]
- Shoaib, M.; Saeed, A.; Rahman, M.S.U.; Naseer, M.M. Mesoporous nano-bioglass designed for the release of imatinib and in vitro inhibitory effects on cancer cells. Mater. Sci. Eng. C 2017, 77, 725–730. [Google Scholar] [CrossRef]
- Afzal, M.A.F. A Review on Pluronic Block Copolymer Micelles: Structure and Dynamics. 2013. Available online: https://www.acsu.buffalo.edu/~m27/images/Pluronics_structure_dynamics_review.pdf (accessed on 20 December 2020).
- Gunawidjaja, P.N.; Lo, A.Y.H.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Stevensson, B.; Grins, J.; Vallet-Regí, M.; Edén, M. Biomimetic Apatite Mineralization Mechanisms of Mesoporous Bioactive Glasses as Probed by Multinuclear 31P, 29Si, 23Na and 13C Solid-State NMR. J. Phys. Chem. C 2010, 114, 19345–19356. [Google Scholar] [CrossRef]
- He, H.; Liang, Q.; Shin, M.C.; Lee, K.; Gong, J.; Ye, J.; Liu, Q.; Wang, J.; Yang, V. Significance and strategies in developing delivery systems for bio-macromolecular drugs. Front. Chem. Sci. Eng. 2013, 7, 496–507. [Google Scholar] [CrossRef]
- Defoort, J.P.; Nardelli, B.; Huang, W.; Ho, D.D.; Tam, J.P. Macromolecular assemblage in the design of a synthetic AIDS vaccine. Proc. Natl. Acad. Sci. USA 1992, 89, 3879–3883. [Google Scholar] [CrossRef]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef] [PubMed]
- Gibson, U.E.M.; Heid, C.A.; Williams, P.M. A novel method for real time quantitative RT-PCR. Genome Res. 1996, 6, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control Release 2014, 193, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M. Ordered mesoporous materials in the context of drug delivery systems and bone tissue engineering. Chem. Eur. J. 2006, 12, 5934–5943. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2011, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, Y.; Zhu, Y.; Friis, T.; Xiao, Y. Structure—Property relationships of silk-modified mesoporous bioglass scaffolds. Biomaterials 2010, 31, 3429–3438. [Google Scholar] [CrossRef]
- Zhu, Y.; Kaskel, S. Comparison of the in vitro bioactivity and drug release property of mesoporous bioactive glasses (MBGs) and bioactive glasses (BGs) scaffolds. Microporous Mesoporous Mater. 2009, 118, 176–182. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, J.; Qu, F.; Jiang, J.; Jiang, P. In Vitro Hydroxyapatite-Forming Ability and Antimicrobial Properties of Mesoporous Bioactive Glasses Doped with Ti/Ag. J. Nanomater. 2013, 2013, 786420. [Google Scholar] [CrossRef]
- Han, P.; Wu, C.; Chang, J.; Xiao, Y. The cementogenic differentiation of periodontal ligament cells via the activation of Wnt/β-catenin signalling pathway by Li+ ions released from bioactive scaffolds. Biomaterials 2012, 33, 6370–6379. [Google Scholar] [CrossRef]
- Fredholm, Y.C.; Karpukhina, N.; Law, R.V.; Hill, R.G. Strontium containing bioactive glasses: Glass structure and physical properties. J. Non-Cryst. Solids 2010, 356, 2546–2551. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Ito, A.; Sogo, Y. Synthesis and characterization of hierarchically macroporous and mesoporous CaO–MO–SiO2–P2O5 (M = Mg, Zn, Sr) bioactive glass scaffolds. Acta Biomater. 2011, 7, 3638–3644. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiume, E. 3D Printing of Hierarchical Scaffolds Based on Mesoporous Bioactive Glasses (MBGs)—Fundamentals and Applications. Materials 2020, 13, 1688. [Google Scholar] [CrossRef]
- Yun, H.S.; Kim, S.E.; Hyun, Y.T.; Heo, S.J.; Shin, J.W. Hierarchically mesoporous-macroporous bioactive glasses scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 374–380. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Chen, H.; Jiang, P.; Dong, X.; Shi, J. Hierarchically Porous Bioactive Glass Scaffolds Synthesized with a PUF and P123 Cotemplated Approach. Chem. Mater. 2007, 19, 4322–4326. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Ma, J.; Xiang, D.; Lin, H.; Li, X.; Bian, C.; Lin, H. Synthesis of hierarchical porous bioactive glasses for bone tissue regeneration. IET Nanobiotechnol. 2014, 8, 216–221. [Google Scholar] [CrossRef]
- Han, X.; Li, X.; Lin, H.; Ma, J.; Chen, X.; Bian, C.; Wu, X.; Qu, F. Hierarchical meso-macroporous bioglass for bone tissue engineering. J. Sol-Gel Sci. Technol. 2014, 70, 33–39. [Google Scholar] [CrossRef]
- Yun, H.-S.; Kim, S.-E.; Hyeon, Y. Design and Preparation of Bioactive Glasses with Hierarchical Pore Networks. Chem. Commun. 2007, 21, 2139–2141. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Barberi, J.; Kargozar, S.; Marchi, J.; Massera, J.; Verné, E. Processing methods for making porous bioactive glass-based scaffolds—A state-of-the-art review. Int. J. Appl. Ceram. Technol. 2019, 16, 1762–1796. [Google Scholar] [CrossRef]
- Yun, H.-S.; Kim, S.-E.; Park, E. Bioactive glass–poly(ε-caprolactone) composite scaffolds with 3 dimensionally hierarchical pore networks. Mater. Sci. Eng. C 2011, 31, 198–205. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiume, E.; Miola, M.; Leone, F.; Onida, B.; Laviano, F.; Gerbaldo, R.; Verné, E. Fe-Doped Sol-Gel Glasses and Glass-Ceramics for Magnetic Hyperthermia. Materials 2018, 11, 173. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Jacquemin, M.; Page, S.J.; Li, S.; Bertazzo, S.; Stevens, M.M.; Hanna, J.V.; Jones, J.R. Lithium-silicate sol–gel bioactive glass and the effect of lithium precursor on structure-property relationships. J. Sol-Gel Sci. Technol. 2017, 81, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.S.; Gentile, P.; Pires, R.A.; Reis, R.L.; Hatton, P.V. Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue. Acta Biomater. 2017, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Vulpoi, A.; Baia, L.; Simon, S.; Simon, V. Silver effect on the structure of SiO2–CaO–P2O5 ternary system. Mater. Sci. Eng. C 2012, 32, 178–183. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, C.; Zhang, F.; Feng, X.; Li, J.; Liu, T.; Chen, J.; Zhang, J. Preparation and antibacterial property of silver-containing mesoporous 58S bioactive glass. Mater. Sci. Eng. C 2014, 42, 22–30. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Büttner, T.; Maçon, A.L.B.; Jones, J.R.; Fey, T.; De Ligny, D.; Greil, P.; Chevalier, J.; Malchere, A.; Boccaccini, A.R. Development and characterization of lithium-releasing silicate bioactive glasses and their scaffolds for bone repair. J. Non-Cryst. Solids 2016, 432, 65–72. [Google Scholar] [CrossRef]
- Kavitha, R.J.; Subha, B.; Shanmugam, S.; Ravichandran, K. Synthesis and Invitro Characterisation of Lithium Doped Bioactive Glass through Quick Alkali Sol-Gel Method. Int. J. Innov. Res. Sci. Eng. 2014, 3, 2347–3207. [Google Scholar]
- Khan, P.K.; Mahato, A.; Kundu, B.; Nandi, S.K.; Mukherjee, P.; Datta, S.; Sarkar, S.; Mukherjee, J.; Nath, S.; Balla, V.K.; et al. Influence of single and binary doping of strontium and lithium on in vivo biological properties of bioactive glass scaffolds. Sci. Rep. 2016, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zreiqat, H.; Evans, P.; Howlett, C.R. Effect of surface chemical modification of bioceramic on phenotype of human bone-derived cells. J. Biomed. Mater. Res. 1999, 44, 389–396. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Strobel, L.A.; Kneser, U.; Boccaccini, A.R. Zinc-containing bioactive glasses for bone regeneration, dental and orthopedic applications. Biomed. Glasses 2015, 1, 51–69. [Google Scholar] [CrossRef]
- Hu, G.F. Copper stimulates proliferation of human endothelial cells under culture. J. Cell. Biochem. 1998, 69, 326–335. [Google Scholar] [CrossRef]
- Yamasaki, K.; Hagiwara, H. Excess iron inhibits osteoblast metabolism. Toxicol. Lett. 2009, 191, 211–215. [Google Scholar] [CrossRef]
- Vallet-Regì, M.; Ruiz-Hernàndez, E. Bioceramics: From bone regeneration to cancer nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef]
- Deilmann, L.; Winter, O.; Cerrutti, B.; Bradtmüller, H.; Herzig, C.; Limbeck, A.; Lahayne, O.; Hellmich, C.; Eckert, H.; Eder, D. Effect of boron incorporation on the bioactivity, structure, and mechanical properties of ordered mesoporous bioactive glasses. J. Mater. Chem. B 2020, 8, 1456–1465. [Google Scholar] [CrossRef]
- Wray, P. ‘Cotton candy’ that heals? Am. Ceram. Soc. Bull. 2011, 90, 25–29. [Google Scholar]

| Structure-Directing Agent | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| P123 | 300–350 | 0.4–0.49 | 4.3–4.6 |
| 278–400 | 0.54–0.73 | 6.5–6.9 | |
| 250–350 | 0.4–0.5 | 5 | |
| 438–466 | 3.5–3.7 | ||
| 450–480 | 0.63–0.73 | 5.37–6.43 | |
| 499 | 0.7 | 6.1 | |
| F127 | 520 | 0.51 | 5.4 |
| 228–300 | 0.36–0.42 | 5.0–7.1 | |
| 152–310 | 0.235–0.356 | 4.2–5.0 | |
| CTAB | 1040 | 1.54 | 1.82–2.2 |
| 443 | 0.57 | 2.9 | |
| P123 + CTAB | 552–618 | 0.69–1.08 | 4.1–6.2 |
| MBGs with Different Compositions (mol.%) | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | References |
|---|---|---|---|---|
| 100Si | 490 | 3.6 | [54] | |
| 95Si5Ca | 467 | 3.7 | ||
| 90Si10Ca | 438 | 3.5 | ||
| 100Si | 310 | 0.356 | 4.2 | [55] |
| 97.5Si2.5P (TEP) | 270 | 0.308 | 4.4 | |
| 97.5Si2.5P (H3PO4) | 152 | 0.235 | 4.8 | |
| 80Si15Ca5P | 351 | 0.49 | 4.6 | [18] |
| 70Si15Ca5P | 319 | 0.49 | 4.6 | |
| 60Si15Ca5P | 310 | 0.43 | 4.3 | |
| 100Si | 384 | 0.4 | 4.9 | [56] |
| 90Si5Ca5P | 330 | 0.35 | 4.9 | |
| 80Si15Ca5P | 351 | 0.36 | 4.8 | |
| 70Si25Ca5P | 303 | 0.33 | 4.8 | |
| 80Si10Ca5P5Fe | 260 | 0.26 | 3.5 | [57] |
| 80Si5Ca5P10Fe | 334 | 0.3 | 3.6 | |
| 80Si0Ca5P15Fe | 367 | 0.36 | 3.7 | |
| 80Si15Ca5P | 342 | 0.38 | 3.62 | [34] |
| 80Si10Ca5P5Mg | 274 | 0.35 | 3.31 | |
| 80Si10Ca5P5Zn | 175 | 0.23 | 3.33 | |
| 80Si10Ca5P5Cu | 237 | 0.31 | 3.66 | |
| 80Si10Ca5P5Sr | 247 | 0.31 | 3.66 | |
| 80Si15Ca5P | 515 | 0.58 | 4.7 | [58] |
| 76.5Si15Ca5P3.5Ce | 397 | 0.38 | 2.9 | |
| 76.5Si15Ca5P3.5Ga | 335 | 0.31 | 3.8 | |
| 80Si15Ca5P | 317 | 0.37 | 4.1 | [59] |
| 80Si10Ca5P5Zr | 287 | 0.32 | 3.7 | |
| 80Si5Ca5P10Zr | 278 | 0.33 | 4.1 | |
| 80Si5P15Zr | 277 | 0.27 | 3.4 | |
| 80Si15Ca5P | 265 | 0.33 | 5.29 | [60] |
| 75Si15Ca5P5B | 234 | 0.24 | 5.28 | |
| 70Si15Ca5P10B | 194 | 0.21 | 5.09 |
| Samples | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | Drug Loading (%) |
|---|---|---|---|---|
| MBG | 334.4 | 0.348 | 4.8 | - |
| MBG-Gen | 208.9 | 0.216 | 4.4 | 12.33 |
| BG | 86.7 | 0.099 | - | - |
| BG-Gen | 53.1 | 0.081 | - | 5.03 |
| Therapeutic Ions Released from MBG | Concentration Level (mg/L) | Functional Properties | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Osteogenesis | Cementogenesis | Angiogenesis | Antibacterial Effect | ||||
| Monovalent | Ag+ | 0.014 | ✓ | [75] | |||
| Li+ | ˂17.28 | ✓ | ✓ | [76] | |||
| Divalent | Sr2+ | ˂22 | ✓ | [77] | |||
| Zn2+ | ˂0.75 | ✓ | [78] | ||||
| Mg2+ | ˂100 | ✓ | ✓ | [78] | |||
| Cu2+ | ˂152 | ✓ | ✓ | [79] | |||
| Co2+ | ˂25 | ✓ | [69] | ||||
| Trivalent | B3+ | <50 | ✓ | [69] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migneco, C.; Fiume, E.; Verné, E.; Baino, F. A Guided Walk through the World of Mesoporous Bioactive Glasses (MBGs): Fundamentals, Processing, and Applications. Nanomaterials 2020, 10, 2571. https://doi.org/10.3390/nano10122571
Migneco C, Fiume E, Verné E, Baino F. A Guided Walk through the World of Mesoporous Bioactive Glasses (MBGs): Fundamentals, Processing, and Applications. Nanomaterials. 2020; 10(12):2571. https://doi.org/10.3390/nano10122571
Chicago/Turabian StyleMigneco, Carla, Elisa Fiume, Enrica Verné, and Francesco Baino. 2020. "A Guided Walk through the World of Mesoporous Bioactive Glasses (MBGs): Fundamentals, Processing, and Applications" Nanomaterials 10, no. 12: 2571. https://doi.org/10.3390/nano10122571
APA StyleMigneco, C., Fiume, E., Verné, E., & Baino, F. (2020). A Guided Walk through the World of Mesoporous Bioactive Glasses (MBGs): Fundamentals, Processing, and Applications. Nanomaterials, 10(12), 2571. https://doi.org/10.3390/nano10122571







