Effects of 5-Ammonium Valeric Acid Iodide as Additive on Methyl Ammonium Lead Iodide Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effect of AVA Additive and Substrate on the MAPI Layers Properties
3.2. Effect of AVA Additive and Cell Structure on the Current-Voltage Curves and Performances
3.3. Effect of Light Intensity at Voc
3.4. Effect of Applied Potential on Impedance Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Veldhuis, S.A.; Boix, P.P.; Yantara, N.; Li, M.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G. Perovskite materials for light-emitting diodes and lasers. Adv. Mater. 2016, 28, 6804–6834. [Google Scholar] [CrossRef]
- Birowosuto, M.D.; Cortecchia, D.; Drozdowski, W.; Brylew, K.; Lachmanski, W.; Bruno, A.; Soci, C. X-ray Scintillation in lead halide perovskite crystals. Sci. Rep. 2016, 6, 37254. [Google Scholar] [CrossRef]
- Wang, H.; Kim, D.H. Perovskite-based photodetectors: materials and devices. Chem. Soc. Rev. 2017, 46, 5204–5236. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead iodide perovskite Ssnsitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Barboux, P.; Pauporté, T. Electrochemical design of nanostructured ZnO charge carrier layers for efficient solid-state perovskite-sensitized solar cells. Adv. Energy Mater. 2014, 4, 1400932. [Google Scholar] [CrossRef]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Juárez-Pérez, E.J.; Mora-Seró, I.; Viana, B.; Pauporté, T. Fast and Low temperature growth of electron transport layers for efficient perovskite solar cells. J. Mater. Chem. A 2015, 3, 4909–4915. [Google Scholar] [CrossRef]
- Zhang, J.; Pauporté, T. Effects of oxide contact layer on the preparation and properties of CH3NH3PbI3 for perovskite solar cell application. J. Phys. Chem. C 2015, 119, 14919–14928. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, X.R.; Wang, K.; Wu, C.C.; Yang, R.X.; Hou, Y.C.; Jiang, Y.Y.; Liu, S.Z.; Priya, S. Stable efficiency exceeding 20.6% for inverted perovskite solar cells through polymer-optimized PCBM electron-transport layers. NanoLett 2019, 19, 3313–3320. [Google Scholar] [CrossRef]
- Tu, Y.G.; Xu, G.N.; Yang, X.Y.; Zhang, Y.F.; Li, Z.J.; Su, R.; Luo, D.Y.; Yang, W.Q.; Miao, Y.; Cai, R.; et al. Mixed-cation perovskite solar cells in space. Sci. China-Phys. Mech. Astron. 2019, 62, 974221. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, A.; Mercier, N.; Allain, M.; Dittmer, J.; Fernandez, V.; Pauporté, T. Lead-and iodide-deficient (CH3NH3)PbI3 (d-MAPI): the bridge between 2D and 3D hybrid perovskites. Angew. Chem. Int. Ed. 2017, 56, 16067–16072. [Google Scholar] [CrossRef]
- Wang, P.; Shao, Z.; Ulfa, M.; Pauporté, T. Insights into the hole blocking layer effect on the perovskite solar cell performance and impedance response. J. Phys. Chem. C 2017, 121, 9131–9141. [Google Scholar] [CrossRef]
- Wang, P.; Ulfa, M.; Pauporté, T. Effects of perovskite monovalent cation composition on the high and low frequency impedance response of efficient solar cells. J. Phys. Chem. C 2018, 122, 1973–1981. [Google Scholar] [CrossRef]
- Turren-Cruz, S.H.; Hagfeldt, A.; Saliba, M. Methylammonium-free, high-performance, and stable perovskite solar cells on a planar architecture. Science 2018, 362, 449–453. [Google Scholar] [CrossRef]
- Zhu, T.; Zheng, D.; Rager, N.; Pauporté, T. Actual organic cations composition determination in perovskite thin films. Application to formamidinium lead iodide stabilization for high efficiency solar cell. Sol. RRL 2020, 2000348. [Google Scholar] [CrossRef]
- Zhu, T.; Zheng, D.; Liu, J.; Coolen, L.; Pauporté, T. Electrical response of high efficiency and stable solar cells based on MACl mediated grown FA0.94MA0.06PbI3 perovskite. ACS Appl. Mater. Interfaces 2020, 12, 37197–37207. [Google Scholar] [CrossRef]
- NREL Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 4 November 2020).
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y.; et al. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Grancini, G.; Roldan-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M.; et al. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 15684. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, Z.; Mei, A.; Jiang, Y.; Hou, X.; Wang, Q.; Du, K.; Rong, Y.; Zhou, Y.; Xu, G.; et al. Improved performance of printable perovskite solar cells with bifunctional conjugated organic molecule. Adv. Mater. 2018, 30, 1705786. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, N.; Sitaaraman, S.R.; Pounraj, P.; Govindaraj, R.; Senthil Pandian, M.; Ramasamy, P. Fabrication of hole-transport-free perovskite solar cells using 5-ammonium valeric acid iodide as additive and carbon as counter electrode. Mater. Lett. 2019, 236, 706–709. [Google Scholar] [CrossRef]
- Papadatosa, D.; Sygkridoua, D.; Stathatos, E. Carbon-based, novel triple cation mesoscopic perovskite solar cellfabricated entirely under ambient air conditions. Mater. Lett. 2020, 268, 127621. [Google Scholar] [CrossRef]
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, 8235. [Google Scholar] [CrossRef]
- Xu, M.; Ji, W.; Sheng, Y.; Wu, Y.; Cheng, H.; Meng, J.; Yan, Z.; Xu, J.; Mei, A.; Hu, Y.; et al. Efficient triple-mesoscopic perovskite solar mini-modules fabricated with slot-die coating. Nano Energy 2020, 74, 104842. [Google Scholar] [CrossRef]
- Mei, A.; Sheng, Y.; Ming, Y.; Hu, Y.; Rong, Y.; Zhang, W.; Luo, S.; Na, G.; Tian, C.; Hou, X.; et al. Stabilizing perovskite solar cells to IEC61215:2016 standards with over 9,000-h operational tracking. Joule 2020, 4, 1–15. [Google Scholar] [CrossRef]
- Lin, C.-T.; De Rossi, F.; Kim, J.; Baker, J.; Ngiam, J.; Xu, B.; Pont, S.; Aristidou, N.; Haque, S.A.; Watson, T.; et al. Evidence for surface defect passivation as the origin of the remarkable photostability of unencapsulated perovskite solar cells employing aminovaleric acid as a processing additive. J. Mater. Chem. A 2019, 7, 3006–3011. [Google Scholar] [CrossRef]
- Péan, E.V.; De Castro, C.S.; Dimitrov, S.; De Rossi, F.; Meroni, S.; Baker, J.; Watson, T.; Davies, M.L. Investigating the superoxide formation and stability in mesoporous carbon perovskite solar cells with an aminovaleric acid additive. Adv. Funct. Mater. 2020, 30, 1909839. [Google Scholar] [CrossRef]
- Kayesh, M.E.; Matsuishi, K.; Kaneko, R.; Kazaoui, S.; Lee, J.-J.; Noda, T.; Islam, A. Coadditive engineering with 5‑ammonium valeric acid iodide for efficient and stable Sn perovskite solar cells. ACS Energy Lett. 2019, 4, 278–284. [Google Scholar] [CrossRef]
- Wei, N.; Chen, Y.; Miao, Y.; Zhang, T.; Wang, X.; Wei, H.; Zhao, Y. 5‑Ammonium Valeric Acid Iodide to Stabilize MAPbI3 via a Mixed-Cation Perovskite with Reduced Dimension. J. Phys. Chem. Lett. 2020, 11, 8170–8176. [Google Scholar] [CrossRef] [PubMed]
- Tress, W.; Yavari, M.; Domanski, K.; Yadav, P.; Niesen, B.; Baena, J.P.C.; Hagfeldt, A.; Graetzel, M. Interpretation and evolution of open-circuit voltage, recombination, ideality factor and subgap defect states during reversible light-soaking and irreversible degradation of perovskite solar cells. Energy Environ. Sci. 2018, 11, 151–165. [Google Scholar] [CrossRef]
- Ulfa, M.; Wang, P.; Zhang, J.; Liu, J.; Daney de Marcillac, W.; Coolen, L.; Peralta, S.; Pauporté, T. Charge injection and electrical response in low temperature SnO2-based efficient perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 10, 35118–35128. [Google Scholar] [CrossRef]
- Ulfa, M.; Zhu, T.; Goubard, F.; Pauporté, T. Molecular versus polymeric hole transporting materials for perovskite solar cell application. J. Mater. Chem. A 2018, 6, 13350–13358. [Google Scholar] [CrossRef]
- Zhu, T.; Su, J.; Labat, F.; Ciofini, I.; Pauporté, T. Interfacial engineering through chloride-functionalized self-assembled monolayer for high efficiency perovskite solar cells. ACS Appl. Mater Interfaces 2020, 12, 744–752. [Google Scholar] [CrossRef]
- Ulfa, M.; Wang, P.; Shao, Z.; Viana, B.; Pauporté, T. Oxide hole blocking selective contacts in perovskite solar cells. Proc. SPIE 2018, 10533, 105332R1–105332R10. [Google Scholar]
- Ku, Z.; Rong, Y.; Xu, M.; Liu, T.; Han, H. Full printable processed mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells with carbon counter electrode. Sci. Rep. 2013, 3, 3132. [Google Scholar] [CrossRef]
- Pitarch-Tena, D.; Ngo, T.T.; Vallés-Pelarda, M.; Pauporté, T.; Mora-Seró, I. Impedance spectroscopy measurements in perovskite solar cells. device stability during the measurement and noise reduction. ACS Energy Lett. 2018, 3, 1044–1048. [Google Scholar] [CrossRef]
- Lee, H.; Gaiaschi, S.; Chapon, P.; Marronnier, A.; Lee, H.; Vanel, J.C.; Tondelier, D.; Bourée, J.-E.; Bonnassieux, Y.; Geffroy, B. Direct Experimental Evidence of Halide Ionic Migration under Bias in CH3NH3PbI3−xClx‑Based Perovskite Solar Cells Using GD-OES Analysis. ACS Energy Lett. 2017, 2, 943–949. [Google Scholar] [CrossRef]
- Zheng, D.; Zhu, T.; Pauporté, T. Using Monovalent- to Trivalent-Cation Hybrid Perovskites for Producing High-Efficiency Solar Cells: Electrical Response, Impedance, and Stability. ACS Appl. Energy Mater. 2020, 3, 10349–10361. [Google Scholar] [CrossRef]
- Leguy, A.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible Hydration of CH3NH3PbI3 in Films, Single Crystals, and Solar Cells. Chem. Mater. 2015, 27, 3397–3407. [Google Scholar] [CrossRef]
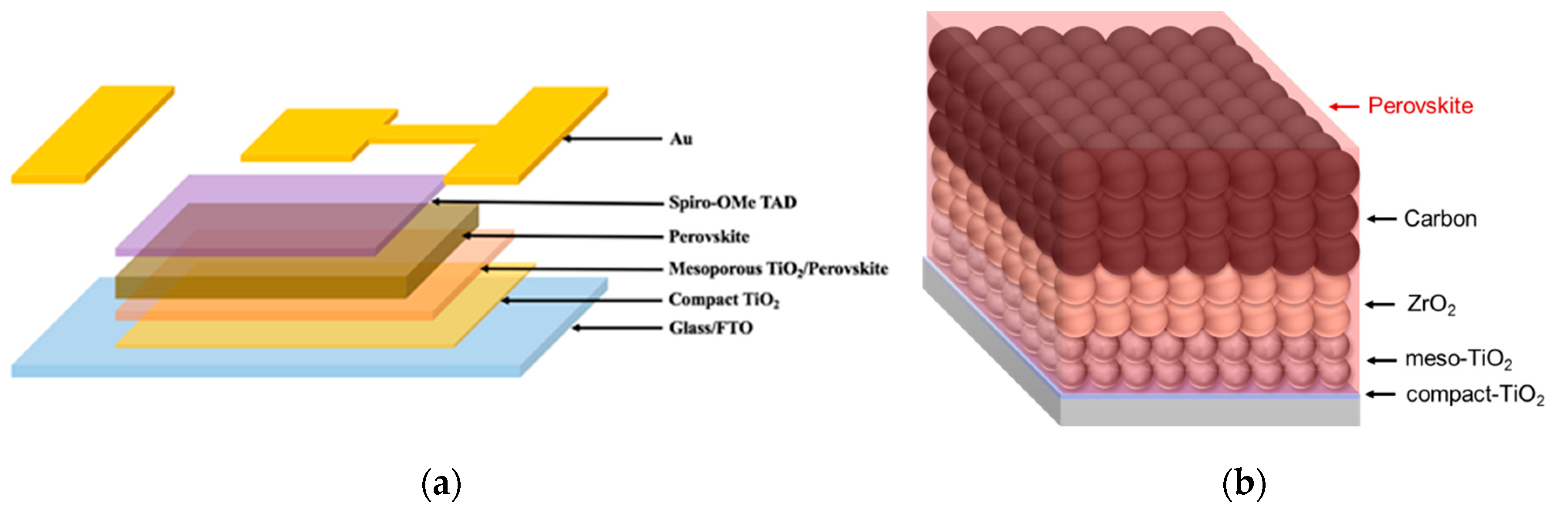
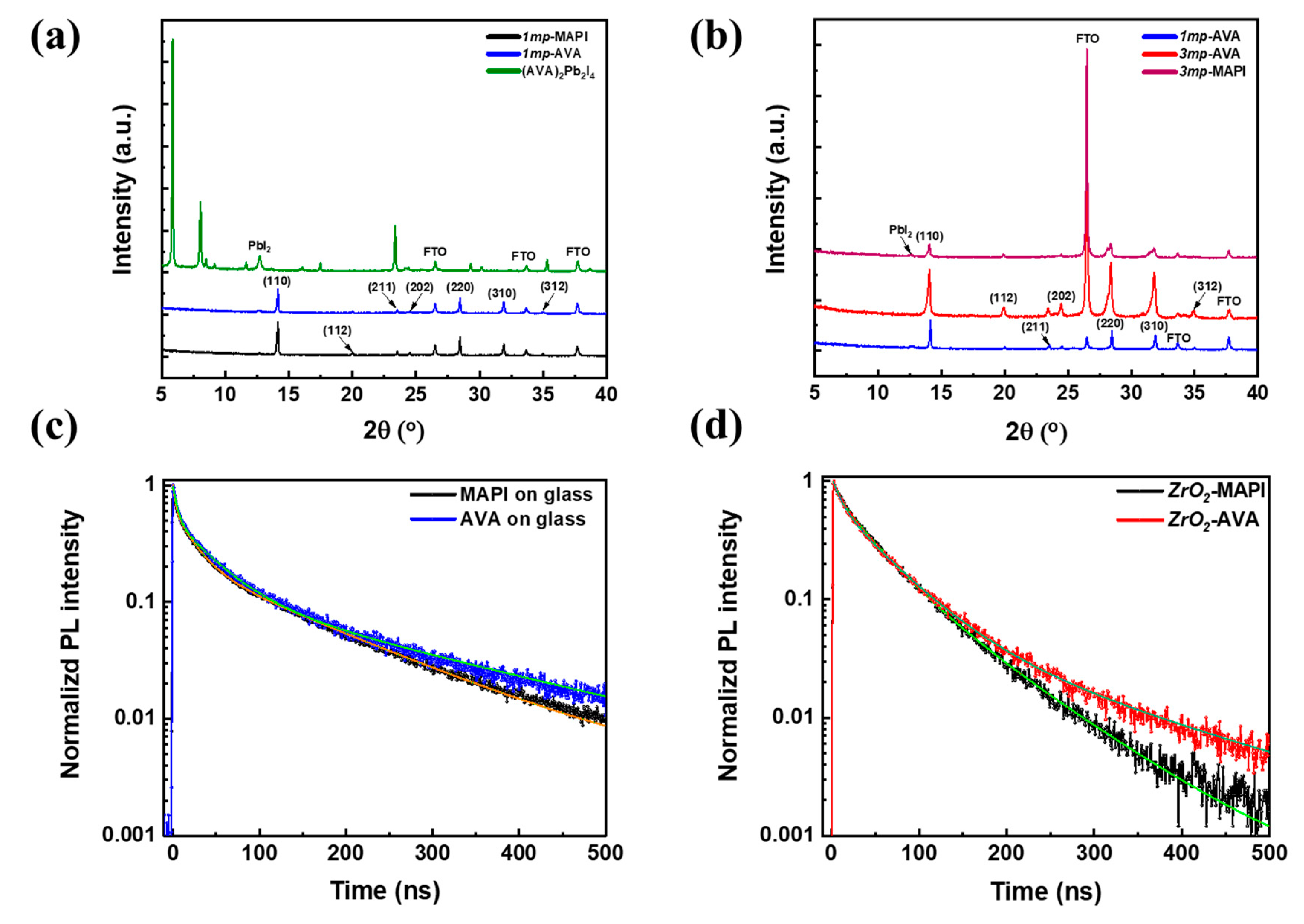
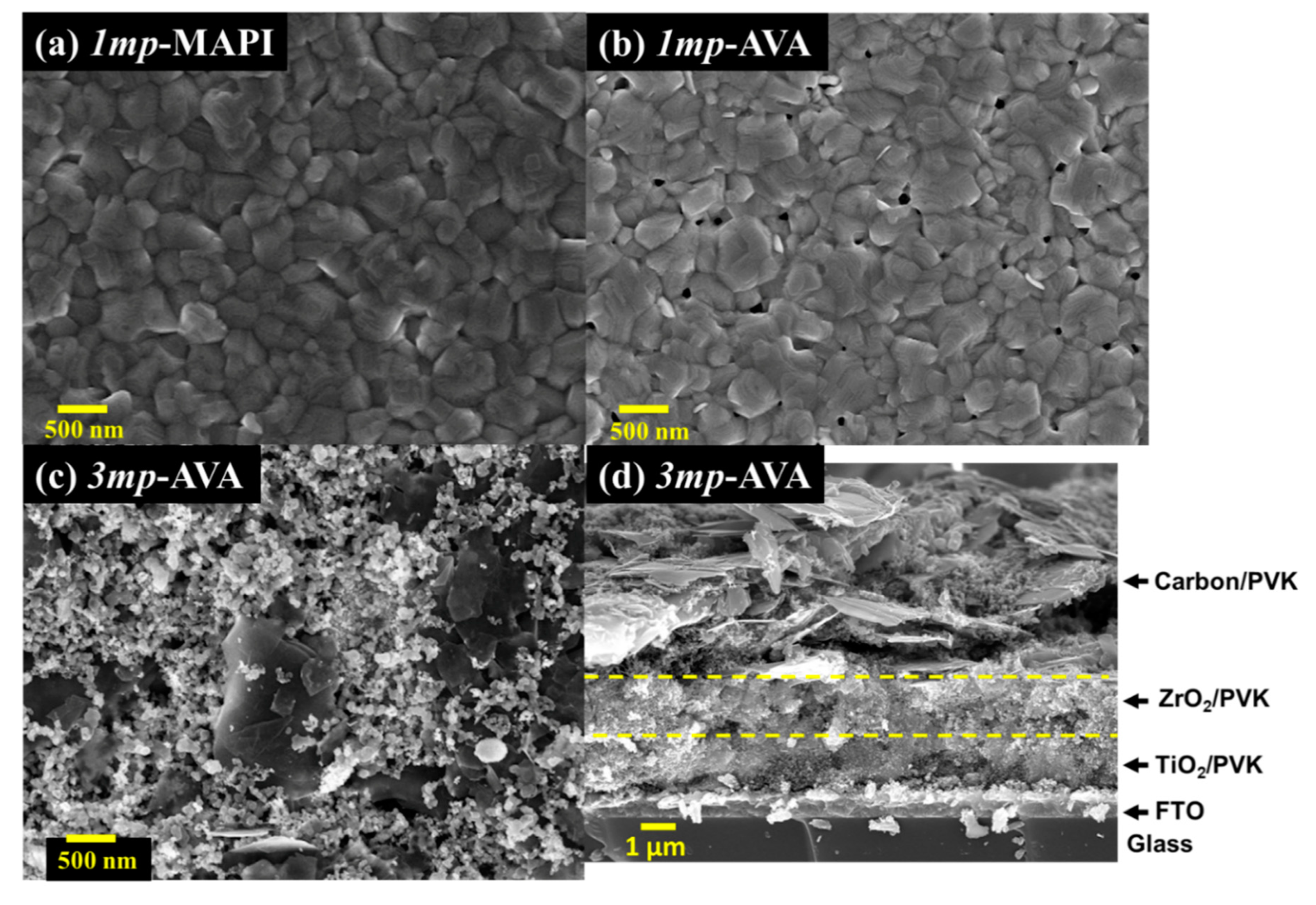
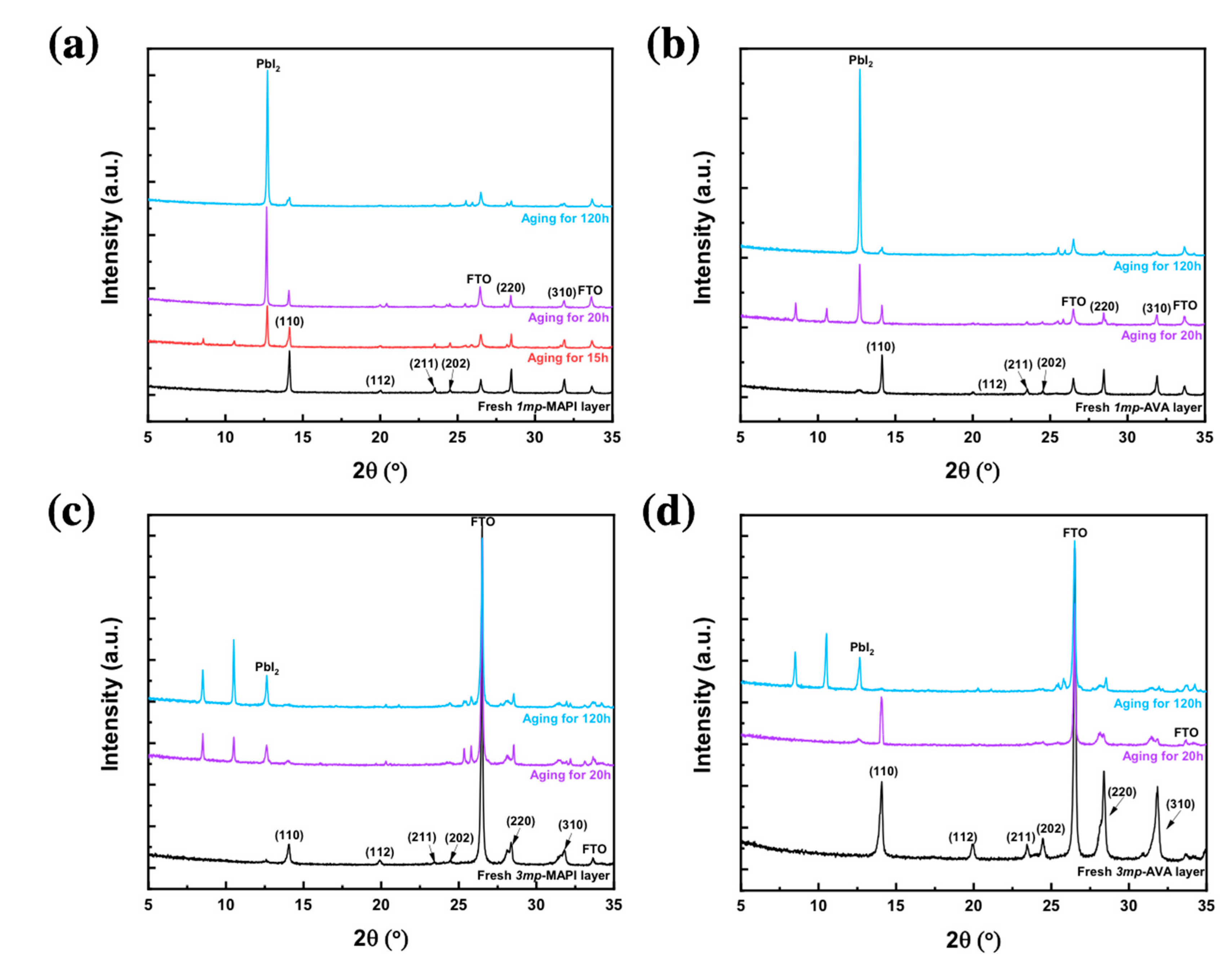
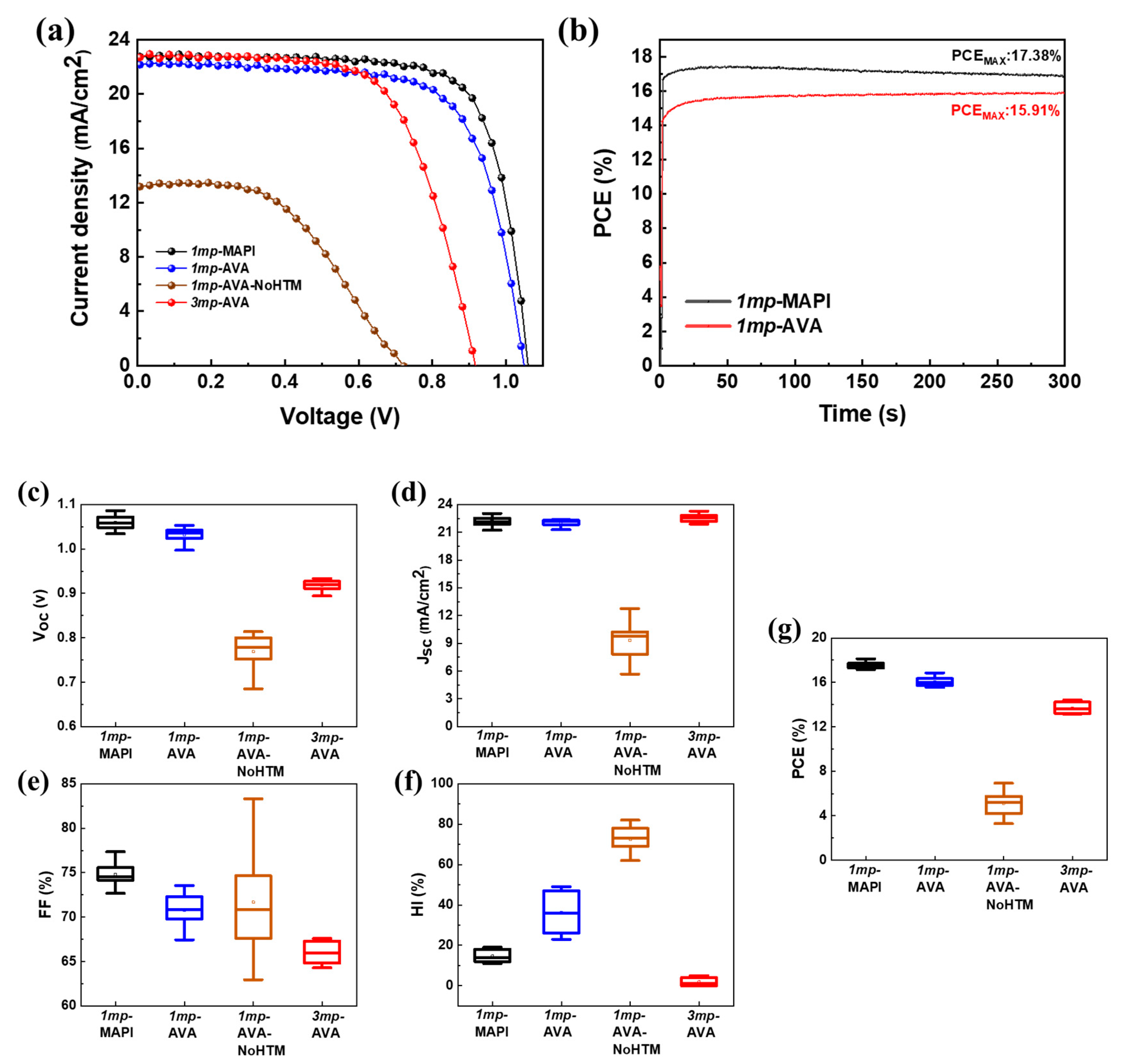
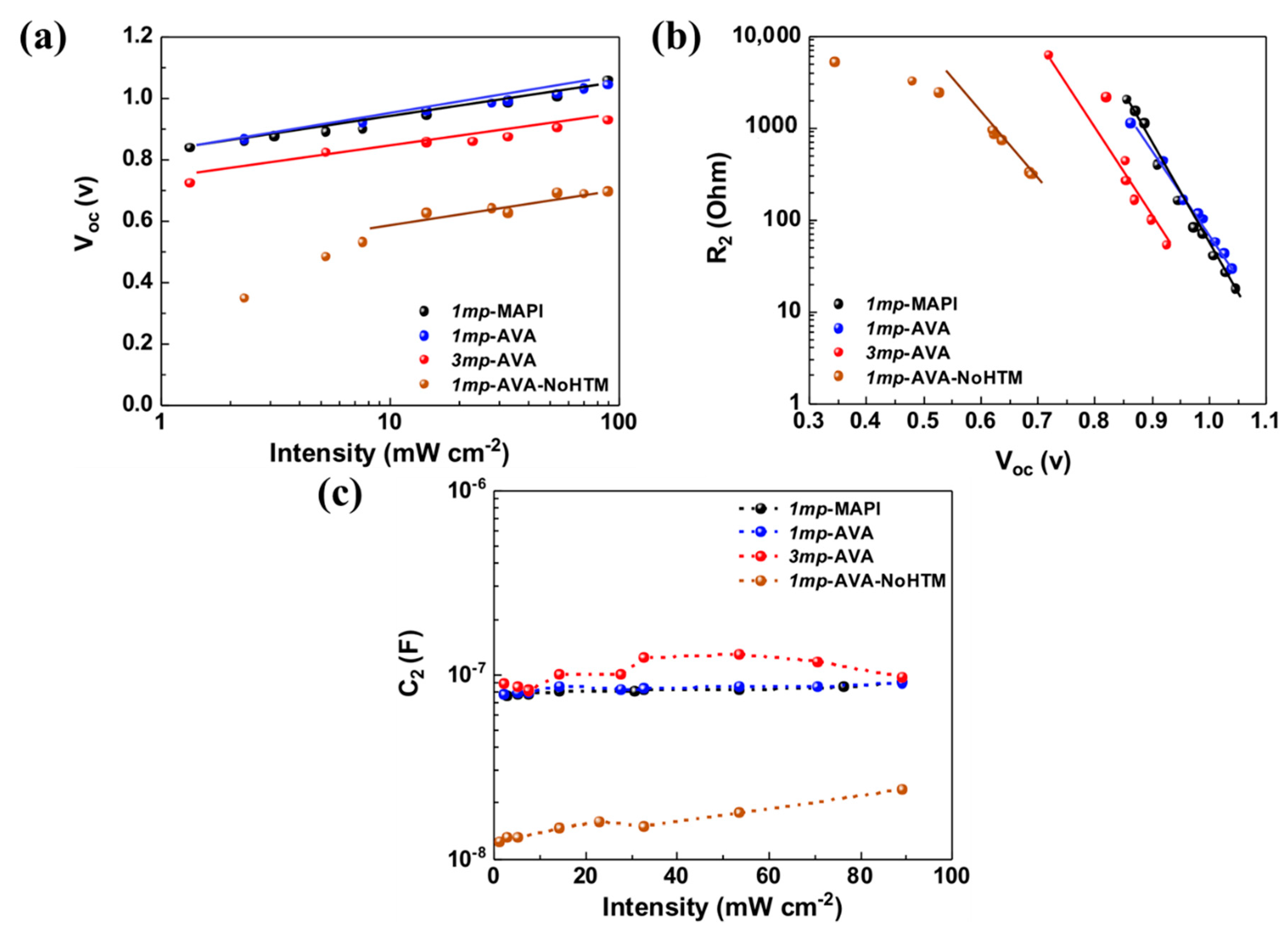
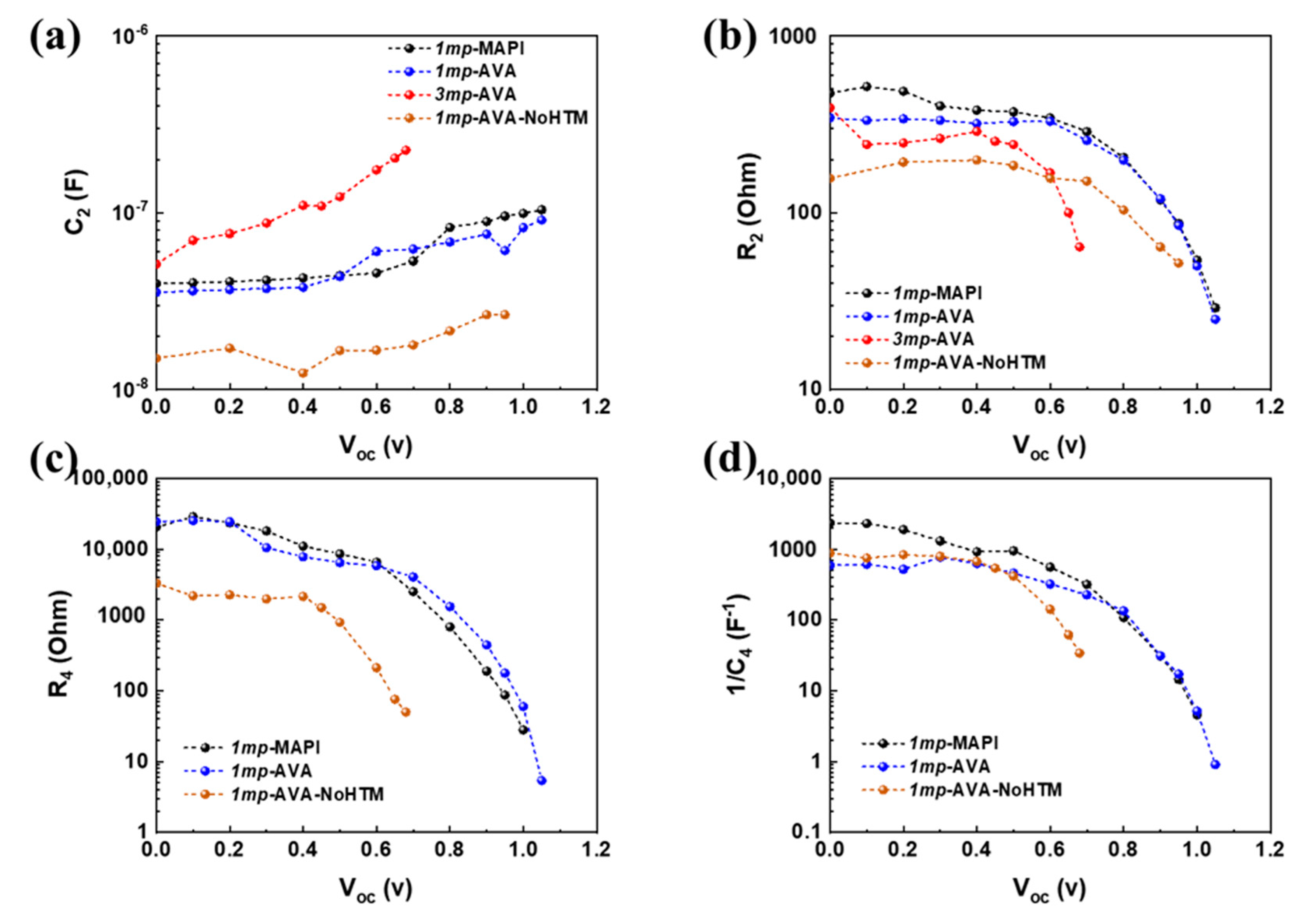
| Cell Structure | PVK | Scan Direction | Voc (V) | Jsc (mA cm−2) | FF a | PCE (%) | HI b |
|---|---|---|---|---|---|---|---|
| 1mp | MAPI | Reverse | 1.058 | 22.78 | 75.03 | 18.09 | 0.12 |
| Forward | 1.044 | 22.70 | 66.91 | 15.86 | |||
| 1mp | AVA | Reverse | 1.040 | 22.05 | 73.54 | 16.86 | 0.31 |
| Forward | 1.017 | 21.94 | 51.87 | 11.58 | |||
| 1mp | AVA-NoHTM | Reverse | 0.805 | 12.75 | 67.62 | 6.94 | 0.73 |
| Forward | 0.790 | 6.71 | 35.56 | 1.89 | |||
| 3mp | AVA | Reverse | 0.932 | 22.88 | 67.62 | 14.41 | 0.00 |
| Forward | 0.929 | 22.86 | 68.23 | 14.49 |
| nID | n’ID | |
|---|---|---|
| 1mp-MAPI | 1.99 | 1.56 |
| 1mp-AVA | 1.89 | 1.92 |
| 1mp AVA-NoHTM a | 3.50 | 3.44 |
| 3mp-AVA | 1.82 | 1.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, D.; Tong, C.; Zhu, T.; Rong, Y.; Pauporté, T. Effects of 5-Ammonium Valeric Acid Iodide as Additive on Methyl Ammonium Lead Iodide Perovskite Solar Cells. Nanomaterials 2020, 10, 2512. https://doi.org/10.3390/nano10122512
Zheng D, Tong C, Zhu T, Rong Y, Pauporté T. Effects of 5-Ammonium Valeric Acid Iodide as Additive on Methyl Ammonium Lead Iodide Perovskite Solar Cells. Nanomaterials. 2020; 10(12):2512. https://doi.org/10.3390/nano10122512
Chicago/Turabian StyleZheng, Daming, Changheng Tong, Tao Zhu, Yaoguang Rong, and Thierry Pauporté. 2020. "Effects of 5-Ammonium Valeric Acid Iodide as Additive on Methyl Ammonium Lead Iodide Perovskite Solar Cells" Nanomaterials 10, no. 12: 2512. https://doi.org/10.3390/nano10122512
APA StyleZheng, D., Tong, C., Zhu, T., Rong, Y., & Pauporté, T. (2020). Effects of 5-Ammonium Valeric Acid Iodide as Additive on Methyl Ammonium Lead Iodide Perovskite Solar Cells. Nanomaterials, 10(12), 2512. https://doi.org/10.3390/nano10122512





