Combined Effect of Active Packaging of Polyethylene Filled with a Nano-Carrier of Salicylate and Modified Atmosphere to Improve the Shelf Life of Fresh Blueberries
Abstract
1. Introduction
2. Materials and Methods
2.1. Active Packaging Material Preparation
2.2. Active Packaging Material Characterization
2.3. Blueberries Packaging and Storage
2.4. Blueberries Postharvest Quality Evaluation
2.4.1. Chemicals and Reagents
2.4.2. Respiration Rate and Weight Loss
2.4.3. Sensory Evaluation and Color
2.4.4. Firmness, Total Soluble Solids, Titratable Acidity, and pH
2.4.5. Total Phenols, Antioxidant Activity, and Carotenoids
2.4.6. Microbiological Analysis
2.4.7. Statistical Analysis
3. Results
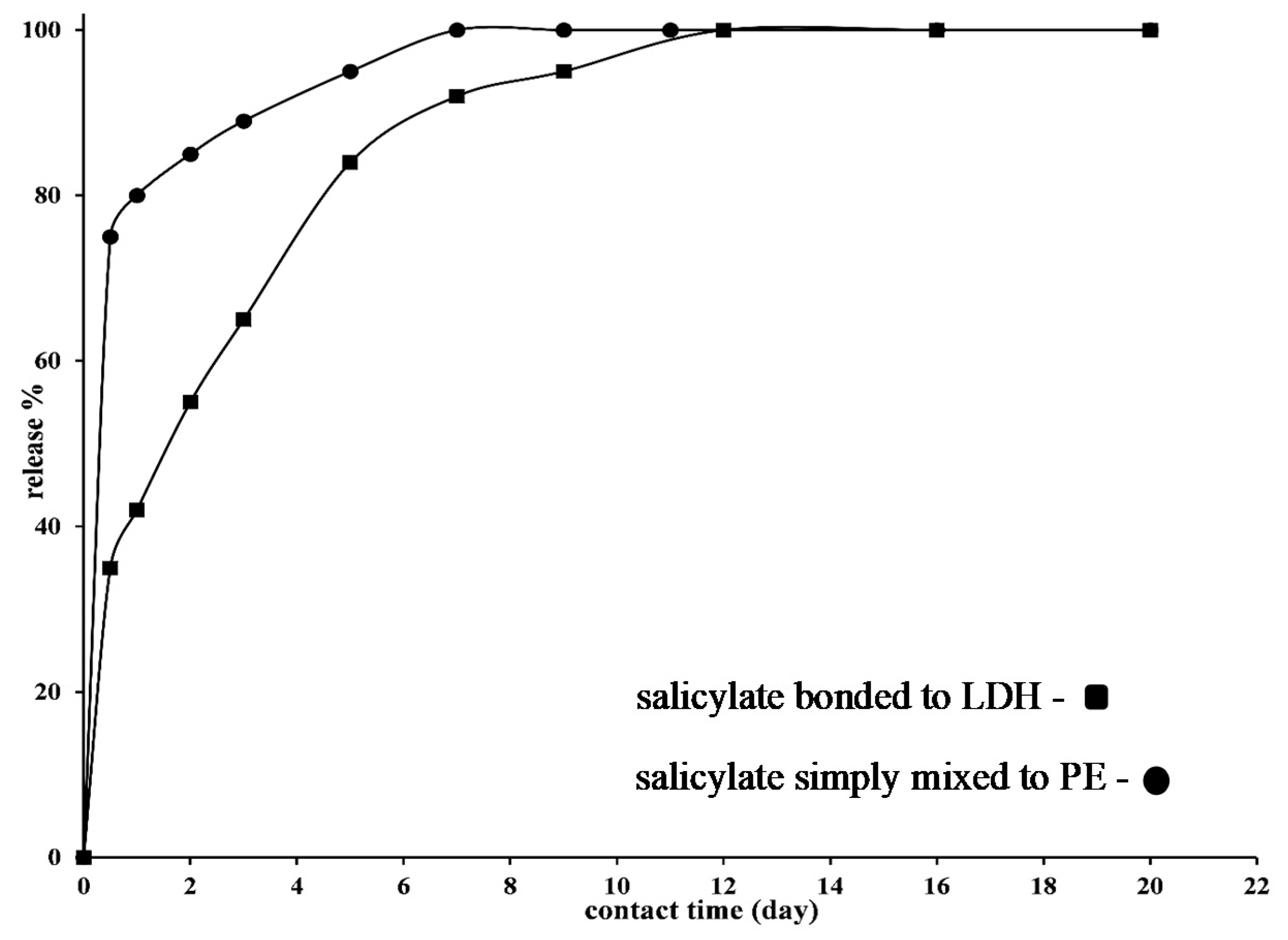
3.1. Packaging Material Characterization
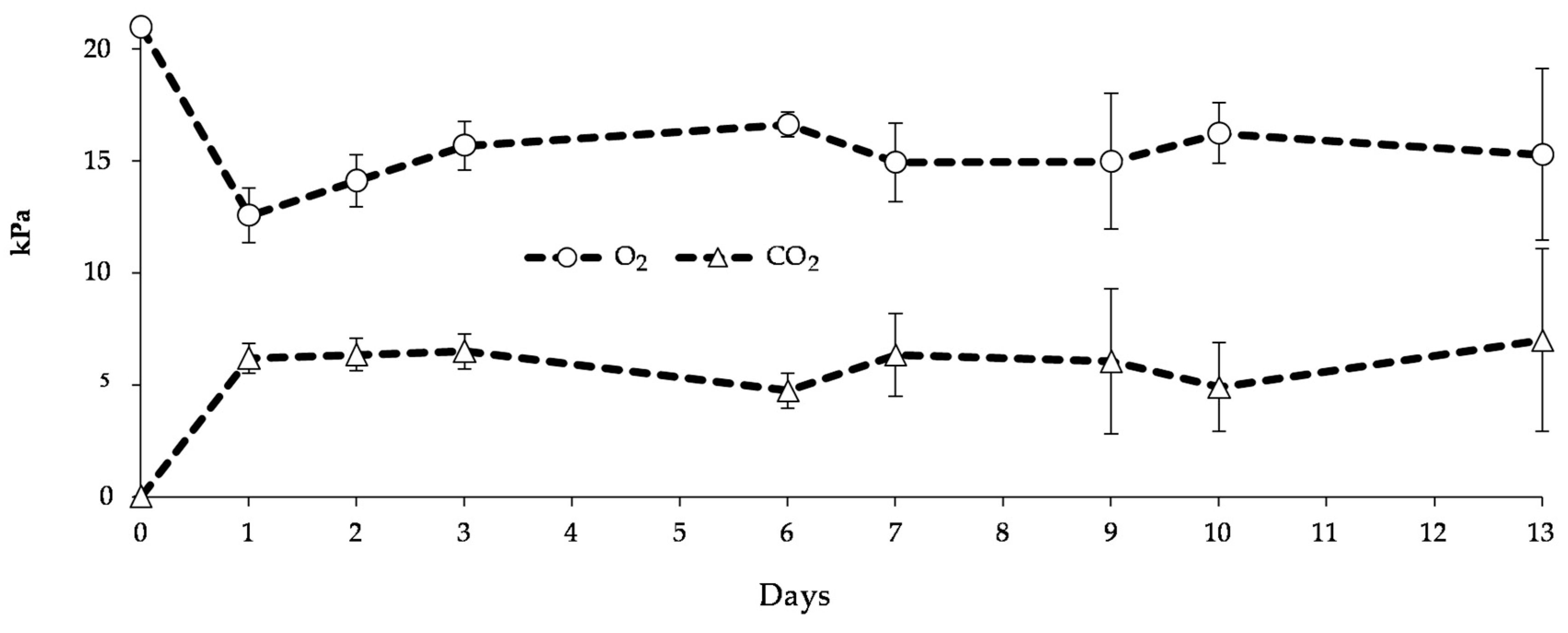
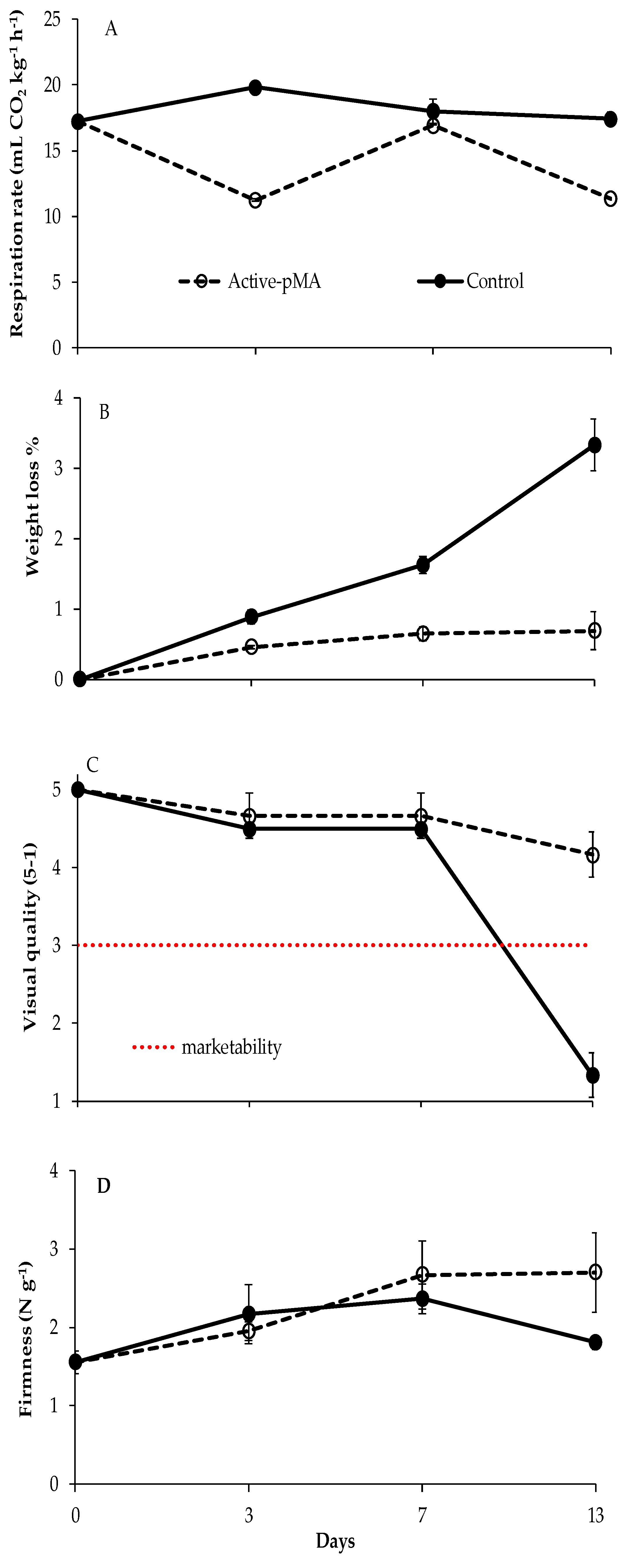
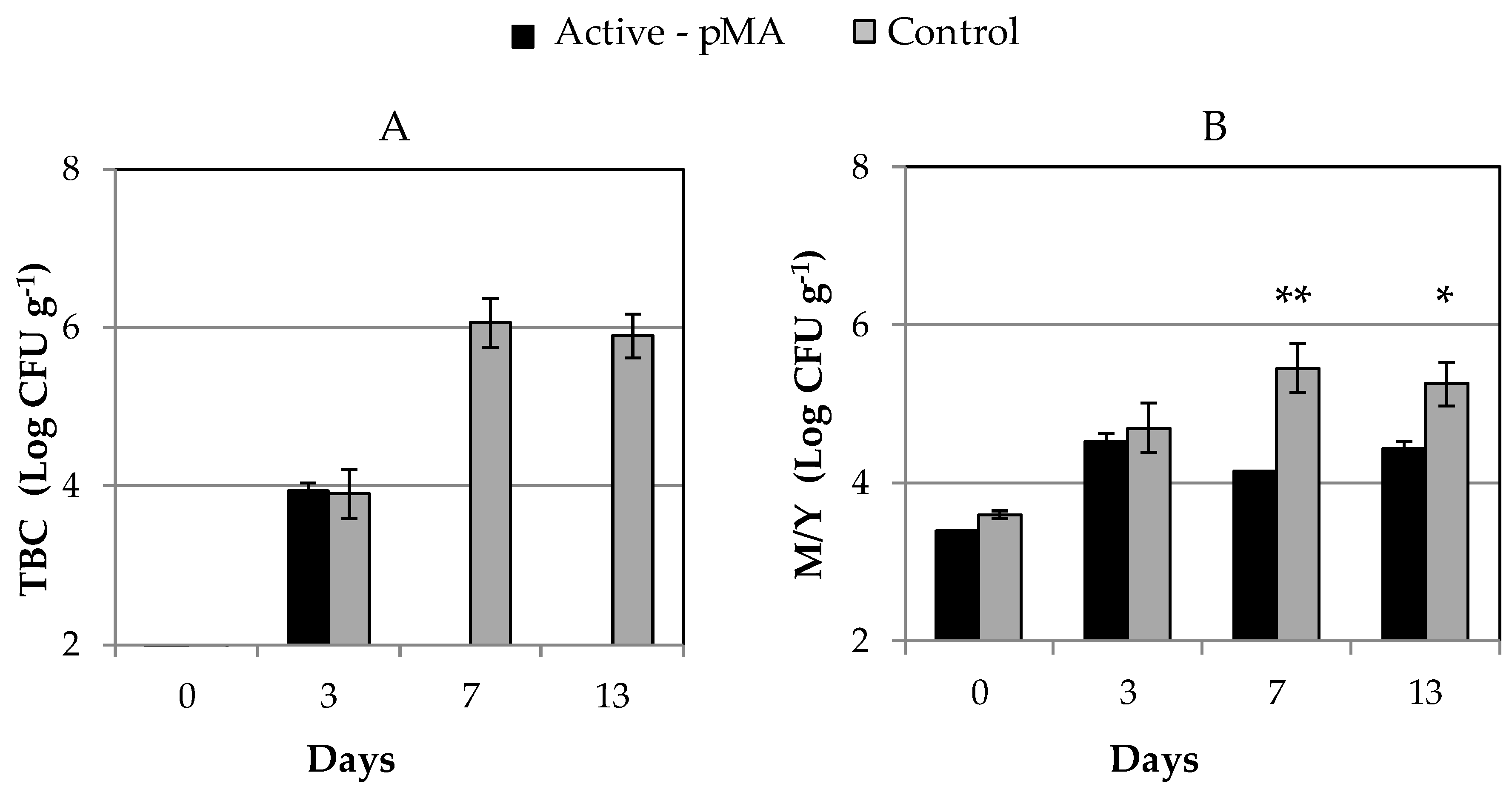
3.2. Effect of Active-pMA on Postharvest Quality Parameters of Blueberries
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gough, R.E. The Highbush Blueberry and Its Management, 1st ed.; Food Products Press, Haworth Press, Inc.: Bringhamton, NY, USA, 1994. [Google Scholar]
- Lyrene, P. Development of highbush blueberry cultivars adapted to Florida. J. Am. Pomol. Soc. 2002, 56, 79. [Google Scholar]
- Mazzoni, L.; Balducci, F.; Di Vittori, L.; Scalzo, J.; Capocasa, F.; Zhong, C.F.; Forbes-Hernandez, T.Y.; Giampieri, F.; Battino, M.; Mezzetti, B. Yield and nutritional quality of highbush blueberry genotypes trialled in a Mediterranean hot summer climate. J. Sci. Food Agric. 2020, 100, 3675–3686. [Google Scholar] [CrossRef]
- Giovanelli, G.; Buratti, S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009, 112, 903–908. [Google Scholar] [CrossRef]
- Chu, W.; Gao, H.; Chen, H.; Fang, X.; Zheng, Y. Effects of cuticular wax on the postharvest quality of blueberry fruit. Food Chem. 2018, 239, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D.; Mikulic-Petkovsek, M. Changes in berry quality of northern highbush blueberry (Vaccinium corymbosum L.) during the harvest season. Turk. J. Agric. For. 2016, 40, 855–864. [Google Scholar] [CrossRef]
- Hancock, J.; Callow, P.; Serçe, S.; Hanson, E.; Beaudry, R. Effect of cultivar, controlled atmosphere storage, and fruit ripeness on the long-term storage of highbush blueberries. HortTechnology 2008, 18, 199–205. [Google Scholar] [CrossRef]
- Paniagua, A.C.; East, A.R.; Heyes, J.A. Interaction of temperature control deficiencies and atmosphere conditions during blueberry storage on quality outcomes. Postharvest Biol. Technol. 2014, 95, 50–59. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Howard, L. Blueberry fruit response to postharvest application of ultraviolet radiation. Postharvest Biol. Technol. 2008, 47, 280–285. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active packaging applications for food. Compr. Rev. Food Sci. 2018, 17, 165–199. [Google Scholar] [CrossRef]
- Huynh, N.K.; Wilson, M.D.; Eyles, A.; Stanley, R.A. Recent advances in postharvest technologies to extend the shelf life of blueberries (Vaccinium sp.), raspberries (Rubusidaeus L.) and blackberries (Rubus sp.). J. Berry Res. 2019, 9, 687–707. [Google Scholar] [CrossRef]
- Hussein, Z.; Caleb, O.J.; Opara, U.L. Perforation-mediated modified atmosphere packaging of fresh and minimally processed produce—A review. Food Packag. Shelf Life 2015, 6, 7–20. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.; Tsantili, E.; Plotto, A.; Sun, X.; Wang, L.; Kafkaletou, M.; Wang, Z.; Narciso, J.; Zhao, W.; et al. Modified humidity clamshells to reduce moisture loss and extend storage life of small fruits. Food Packag. Shelf Life 2019, 22, 100376. [Google Scholar] [CrossRef]
- Mukama, M.; Ambaw, A.; Opara, U.L. Advances in design and performance evaluation of fresh fruit ventilated distribution packaging: A review. Food Packag. Shelf Life 2020, 24, 100472. [Google Scholar] [CrossRef]
- Bugatti, V.; Vertuccio, L.; Zuppardi, F.; Vittoria, V.; Gorrasi, G. PET and active coating based on a LDH nanofiller hosting p-hydroxybenzoate and food-grade zeolites: Evaluation of antimicrobial activity of packaging and shelf life of red meat. Nanomaterials 2019, 9, 1727. [Google Scholar] [CrossRef] [PubMed]
- Sanyang, M.L.; Ilyas, R.A.; Sapuan, S.M.; Jumaidin, R. Sugar palm starch-based composites for packaging applications. In Bionanocomposites for Packaging Applications; Jawaid, M., Swain, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 125–147. [Google Scholar]
- Gorrasi, G.; Bugatti, V. Mechanical dispersion of layered double hydroxides hosting active molecules in polyethylene: Analysis of structure and physical properties. Appl. Clay Sci. 2016, 132, 2–6. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V. Edible bio-nano-hybrid coatings for food protection based on pectins and LDH-salicylate: Preparation and analysis of physical properties. LWT 2016, 69, 139–145. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Official Journal of the European Union. Available online: https://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:012:0001:0089:EN:PDF (accessed on 28 November 2013).
- Frunza, M.; Lisa, G.; Popa, M.; Miron, N.; Nistor, D. Thermogravimetric analysis of layered double hydroxides with chloramphenicol and salicylate in the interlayer space. J. Therm. Anal. Calorim. 2008, 93, 373–379. [Google Scholar] [CrossRef]
- Kader, A.A. Methods of gas mixing, sampling, and analysis. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California: Berkley, CA, USA, 1992; pp. 93–95. [Google Scholar]
- Pace, B.; Capotorto, I.; Cefola, M.; Minasi, P.; Montemurro, N.; Carbone, V. Evaluation of quality, phenolic and carotenoid composition of fresh-cut purple Polignano carrots stored in modified atmosphere. J. Food Compost. Anal. 2020, 86, 103363. [Google Scholar] [CrossRef]
- Tudela, J.A.; Marín, A.; Garrido, Y.; Cantwell, M.; Medina-Martínez, M.S.; Gil, M.I. Off-odour development in modified atmosphere packaged baby spinach is an unresolved problem. Postharvest Biol. Technol. 2013, 75, 75–85. [Google Scholar] [CrossRef]
- Cefola, M.; Carbone, V.; Minasi, P.; Pace, B. Phenolic profiles and postharvest quality changes of fresh-cut radicchio (Cichorium intybus L.): Nutrient value in fresh vs. stored leaves. J. Food Compost. Anal. 2016, 51, 76–84. [Google Scholar] [CrossRef]
- Pace, B.; Cardinali, A.; D’Antuono, I.; Serio, F.; Cefola, M. Relationship between quality parameters and the overall appearance in lettuce during storage. Int. J. Food Sci. Technol. 2014, 1, 19. [Google Scholar]
- Cefola, M.; Pace, B.; Buttaro, D.; Santamaria, P.; Serio, F. Postharvest evaluation of soilless-grown table grape during storage in modified atmosphere. J. Sci. Food Agric. 2011, 91, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- International Organization for Standardization (ISO). Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Microorganisms—Colony Count Technique at 30 °C. Method Number 4833. 2003. Available online: https://www.iso.org/standard/34524.html (accessed on 20 September 2020).
- International Organization for Standardization (ISO). Microbiology of Food and Animal Feeding Stuffs Horizontal Method for the Enumeration of Coliform Colony Count Technique. Method Number 4832. 2006. Available online: https://www.iso.org/standard/38282.html (accessed on 20 September 2020).
- International Organization for Standardization (ISO). Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Yeasts and Moulds. Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. Method Number 21527-1. 2008. Available online: https://www.iso.org/standard/38275.html (accessed on 20 September 2020).
- Bugatti, V.; Bernardo, P.; Clarizia, G.; Viscusi, G.; Vertuccio, L.; Gorrasi, G. Ball milling to produce composites based of natural clinoptilolite as a carrier of salicylate in Bio-Based PA11. Polymers 2019, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- UNI EN 1186-1:2003. Materiali ed articoli in contatto con gli alimenti—Materie plastiche—Guida per la scelta delle condizioni e dei metodi di prova per la migrazione globale. 2003. Available online: https://img.21food.cn/img/biaozhun/20090815/187/11183625.pdf (accessed on 20 September 2020).
- UNI EN 1186-9:2003. Materiali ed articoli in contatto con gli alimenti—Materie plastiche—Metodi di prova della migrazione globale in simulanti alimentari acquosi mediante riempimento di un contenitore. 2003. Available online: http://store.uni.com/catalogo/uni-en-1186-9-2003 (accessed on 20 September 2020).
- Alsmairat, N.; Contreras, C.; Hancock, J.; Callow, P.; Beaudry, R. Use of combinations of commercially relevant O2 and CO2 partial pressures to evaluate the sensitivity of nine highbush blueberry fruit cultivars to controlled atmospheres. HortScience 2011, 46, 74–79. [Google Scholar] [CrossRef]
- Beaudry, R.M.; Moggia, C.E.; Retamales, J.B.; Hancock, J.F. Quality of ‘Ivanhoe’ and ‘Bluecrop’ blueberry fruit transported by air and sea from Chile to North America. HortScience 1998, 33, 313–317. [Google Scholar]
- Cantín, C.M.; Minas, I.S.; Goulas, V.; Jiménez, M.; Manganaris, G.A.; Michailides, T.J.; Crisosto, C.H. Sulfur dioxide fumigation alone or in combination with CO2-enriched atmosphere extends the market life of highbush blueberry fruit. Postharvest Biol. Technol. 2012, 67, 84–91. [Google Scholar] [CrossRef]
- Schotsmans, W.; Molan, A.; MacKay, B. Controlled atmosphere storage of rabbiteye blueberries enhances postharvest quality aspects. Postharvest Biol. Technol. 2007, 44, 277–285. [Google Scholar] [CrossRef]
- Shafiee, M.; Taghavi, T.S.; Babalar, M. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Sci. Hortic. 2010, 124, 40–45. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, F.; Lu, Y.; Deng, J. Combination of chitosan and salicylic acid to control postharvest green mold caused by Penicillium digitatum in grapefruit fruit. Sci. Hortic. 2018, 233, 54–60. [Google Scholar] [CrossRef]
- da Rocha Neto, A.C.; Luiz, C.; Maraschin, M.; Di Piero, R.M. Efficacy of salicylic acid to reduce Penicillium expansum inoculum and preserve apple fruits. Int. J. Food Microbiol. 2016, 221, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yang, H.; Jiao, J.; Wang, F.; Lu, Y.; Deng, J. Effects of graft copolymer of chitosan and salicylic acid on reducing rot of postharvest fruit and retarding cell wall degradation in grapefruit during storage. Food Chem. 2019, 283, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Bugatti, V.; Vertuccio, L.; Vittoria, V.; Pace, B.; Cefola, M.; Quintieri, L.; Bernardo, P.; Clarizia, G. Active packaging for table grapes: Evaluation of antimicrobial performances of packaging for shelf life of the grapes under thermal stress. Food Packag. Shelf Life 2020, 25, 100545. [Google Scholar] [CrossRef]
- da Rocha Neto, A.C.; Maraschin, M.; Di Piero, R.M. Antifungal activity of salicylic acid against Penicillium expansum and its possible mechanisms of action. Int. J. Food Microbiol. 2015, 215, 64–70. [Google Scholar] [CrossRef]
- Quansah, J.K.; Gazula, H.; Holland, R.; Scherm, H.; Li, C.; Takeda, F.; Chen, J. Microbial quality of blueberries for the fresh market. Food Control 2019, 100, 92–96. [Google Scholar] [CrossRef]
- Callahan, S.; Perry, J.J. Survival of Listeria innocua and Native Microflora in Sanitizer-Treated Wild Blueberries (Vaccinium Angustifolium). Int. J. Fruit Sci. 2019, 1–16. [Google Scholar] [CrossRef]
- Crowe, K.M.; Bushway, A.A.; Bushway, R.J.; Davis-Dentici, K. Microbial degradation of phosmet on blueberry fruit and in aqueous systems by indigenous bacterial flora on lowbush blueberries (Vaccinium angustifolium). J. Food Sci. 2007, 72, 293–299. [Google Scholar] [CrossRef]
- Gazula, H.; Quansah, J.; Allen, R.; Scherm, H.; Li, C.; Takeda, F.; Chen, J. Microbial loads on selected fresh blueberry packing lines. Food Control 2019, 100, 315–320. [Google Scholar] [CrossRef]
- Palumbo, M.; Capotorto, I.; Cefola, M.; Burbaci, S.; Pace, B. Modified atmosphere packaging to improve the shelf-life of Goji berries during cold storage. Adv. Hortic. Sci. 2020, 34, 21–26. [Google Scholar] [CrossRef]
- Ramos, B.; Miller, F.A.; Brandão, T.R.; Teixeira, P.; Silva, C.L. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. Technol. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Madrid, M.; Beaudry, R. Small fruits: Raspberries, blackberries, blueberries. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Gil, M.I., Beaudry, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 335–346. [Google Scholar] [CrossRef]
- Liu, B.; Wang, K.; Shu, X.; Liang, J.; Fan, X.; Sun, L. Changes in fruit firmness, quality traits and cell wall constituents of two highbush blueberries (Vaccinium corymbosum L.) during postharvest cold storage. Sci. Hortic. 2019, 246, 557–562. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Pertuzatti, P.B.; Barcia, M.T.; Rodrigues, D.; da Cruz, P.N.; Hermosín-Gutiérrez, I.; Smith, R.; Godoy, H.T. Antioxidant activity of hydrophilic and lipophilic extracts of Brazilian blueberries. Food Chem. 2014, 164, 81–88. [Google Scholar] [CrossRef] [PubMed]

| Simulant | Global Migration Average in the Simulant (mg/dm2) | |
|---|---|---|
| A | Ethanol at 10% (v/v) | 2.1 |
| B | Acetic acid at 3% (v/v) | 4.2 |
| C | Ethanol at 20% (v/v) | 2.5 |
| D1 | Ethanol at 50% (v/v) | 3.7 |
| D2 | Oil | <2 |
| Limit | 10 | |
| Quality Parameter | A | B | A × B |
|---|---|---|---|
| Respiration rate (mL CO2/kg h) | **** | **** | **** |
| Weight loss % | **** | **** | **** |
| Off-odor (5-1) | ns | ns | ns |
| Visual quality (5-1) | **** | **** | **** |
| Color (hue angle) | ns | ns | ns |
| Firmness (N/g) | ns | ns | * |
| Total soluble solids (°Brix) | ns | ns | ns |
| Titratable acidity (citric acid %) | ns | ns | ns |
| pH | ns | ns | ns |
| Total phenols (mg gallic acid/100 g fw) | ns | ns | ns |
| Antioxidant activity (mg Trolox/100 g fw) | ns | ns | ns |
| Carotenoids (mg/100 g fw) | ns | ns | ns |
| Quality Parameter | |||
|---|---|---|---|
| Total soluble solids (°Brix) | 10.0 | ± | 0.4 |
| pH | 4.5 | ± | 0.3 |
| Hue angle (h°) | 106.6 | ± | 6.3 |
| Total phenols (mg gallic acid/100 g fw) | 222.4 | ± | 29.3 |
| Antioxidant activity (mg Trolox/100 g fw) | 216.1 | ± | 27.5 |
| Titratable acidity (citric acid %) | 1.19 | ± | 0.10 |
| Carotenoids (mg/100 g fw) | 0.52 | ± | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugatti, V.; Cefola, M.; Montemurro, N.; Palumbo, M.; Quintieri, L.; Pace, B.; Gorrasi, G. Combined Effect of Active Packaging of Polyethylene Filled with a Nano-Carrier of Salicylate and Modified Atmosphere to Improve the Shelf Life of Fresh Blueberries. Nanomaterials 2020, 10, 2513. https://doi.org/10.3390/nano10122513
Bugatti V, Cefola M, Montemurro N, Palumbo M, Quintieri L, Pace B, Gorrasi G. Combined Effect of Active Packaging of Polyethylene Filled with a Nano-Carrier of Salicylate and Modified Atmosphere to Improve the Shelf Life of Fresh Blueberries. Nanomaterials. 2020; 10(12):2513. https://doi.org/10.3390/nano10122513
Chicago/Turabian StyleBugatti, Valeria, Maria Cefola, Nicola Montemurro, Michela Palumbo, Laura Quintieri, Bernardo Pace, and Giuliana Gorrasi. 2020. "Combined Effect of Active Packaging of Polyethylene Filled with a Nano-Carrier of Salicylate and Modified Atmosphere to Improve the Shelf Life of Fresh Blueberries" Nanomaterials 10, no. 12: 2513. https://doi.org/10.3390/nano10122513
APA StyleBugatti, V., Cefola, M., Montemurro, N., Palumbo, M., Quintieri, L., Pace, B., & Gorrasi, G. (2020). Combined Effect of Active Packaging of Polyethylene Filled with a Nano-Carrier of Salicylate and Modified Atmosphere to Improve the Shelf Life of Fresh Blueberries. Nanomaterials, 10(12), 2513. https://doi.org/10.3390/nano10122513








