Sensitive and In Situ Hemoglobin Detection Based on a Graphene Oxide Functionalized Microfiber
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
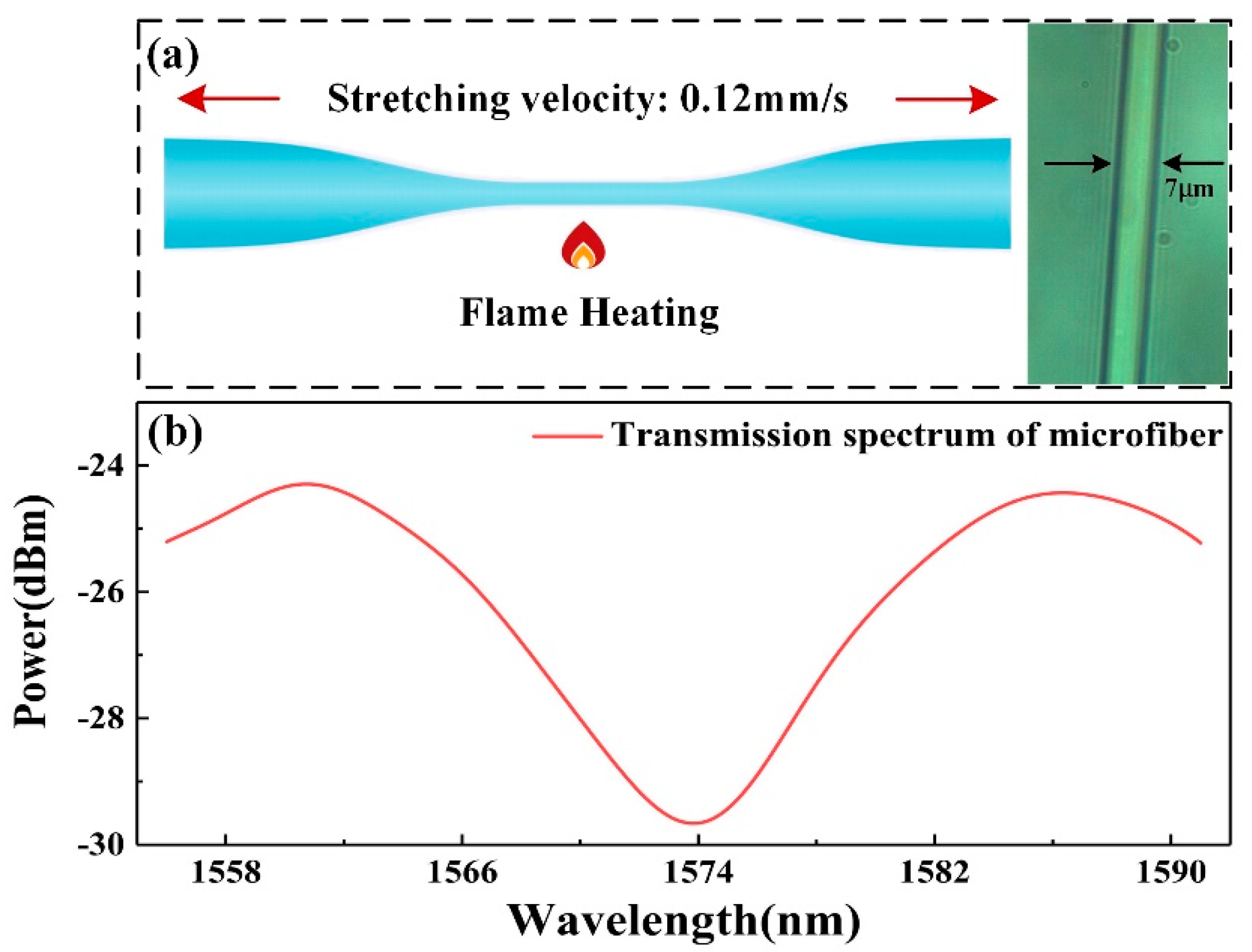
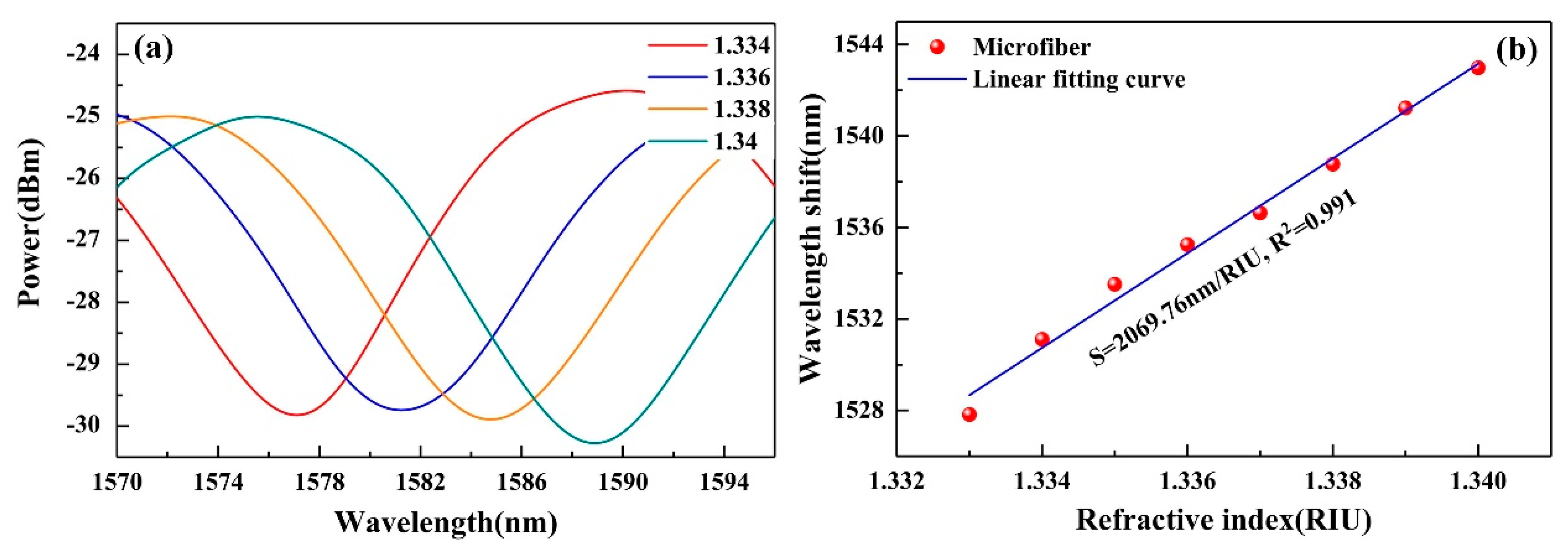
2.2. Fabrication and Refractive Index (RI) Sensitivity of Microfiber
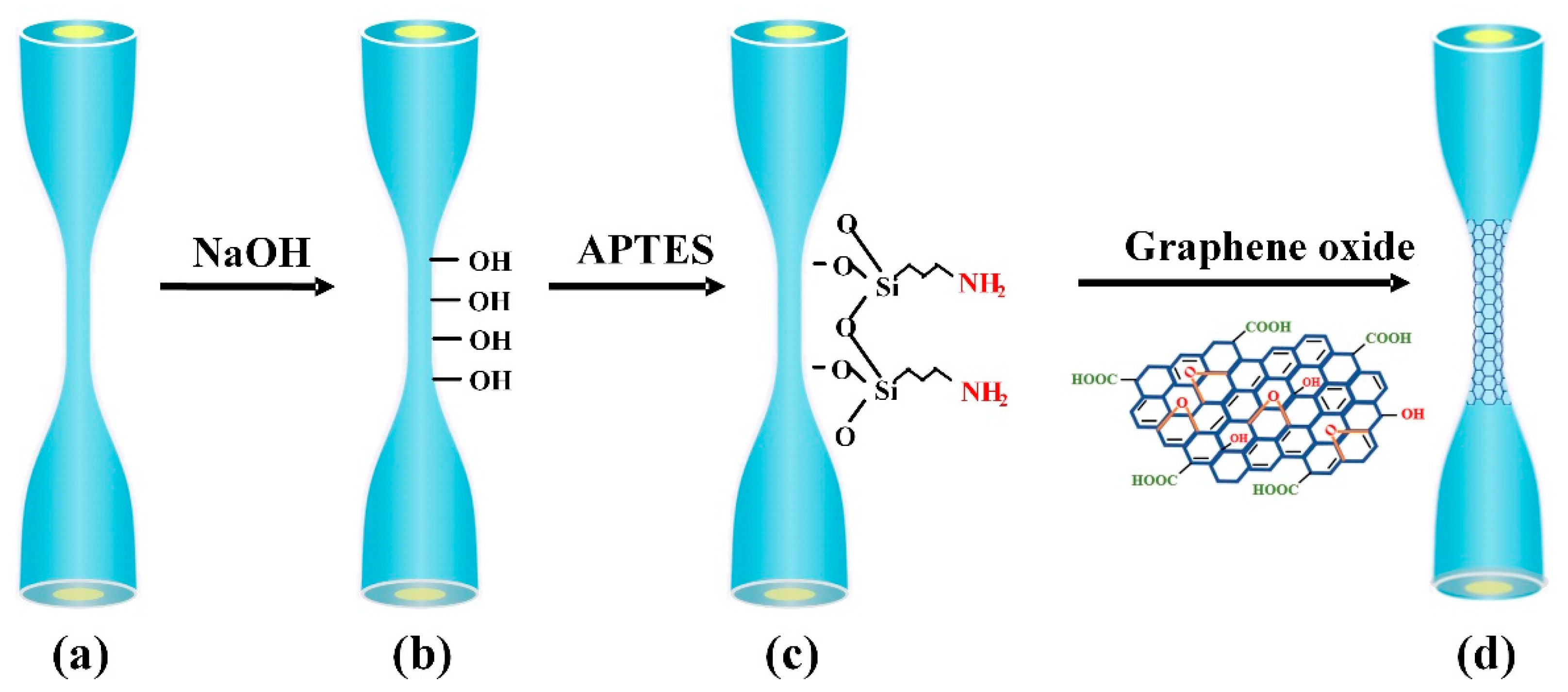
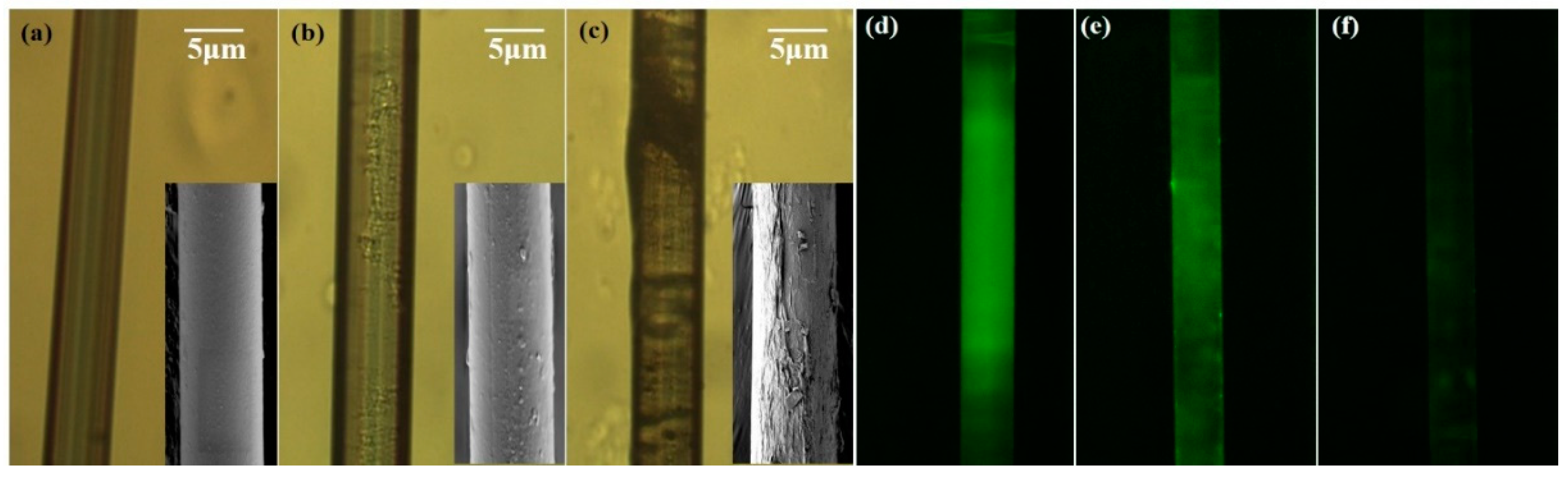
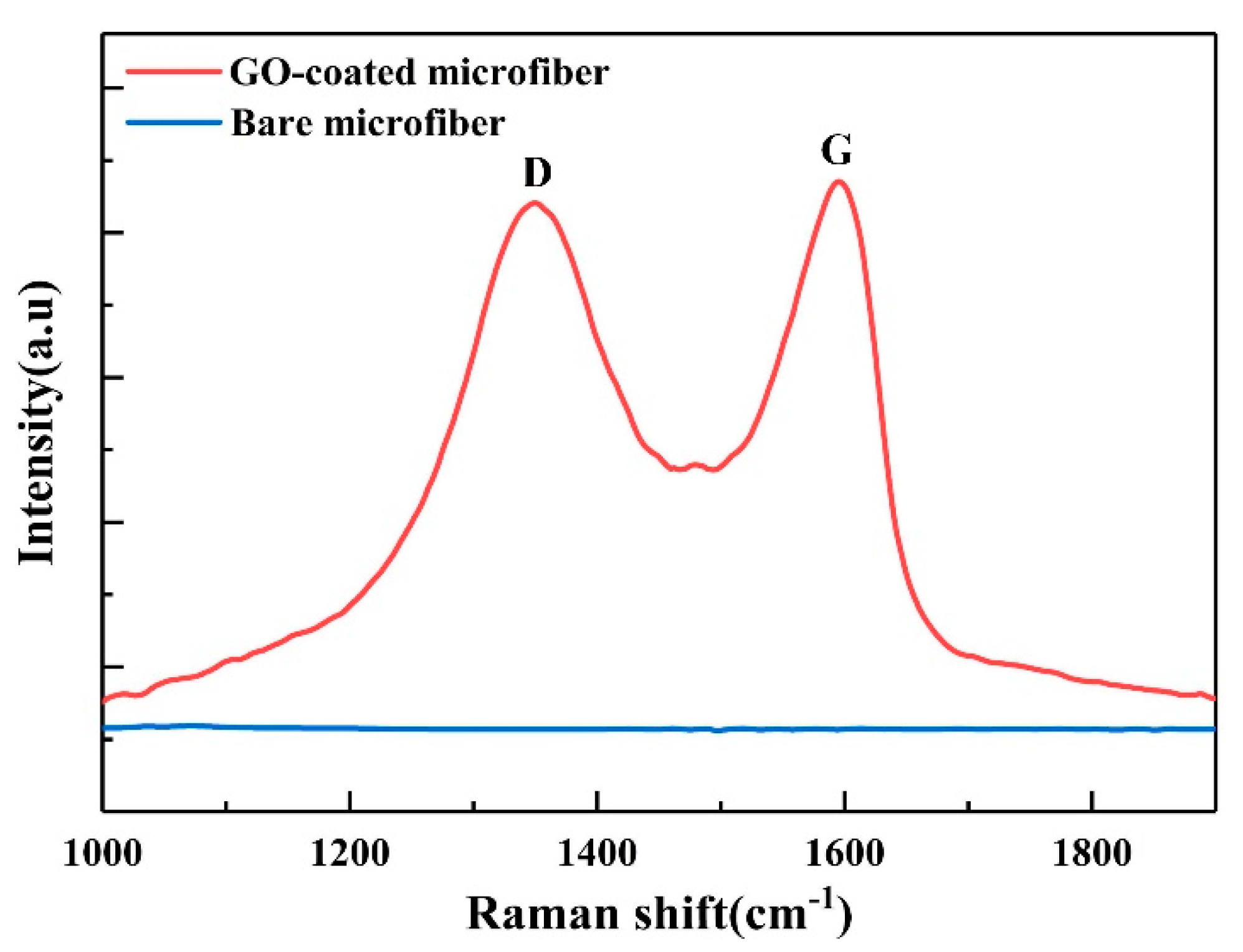
2.3. Surface Functionalization and Characterization of Microfiber
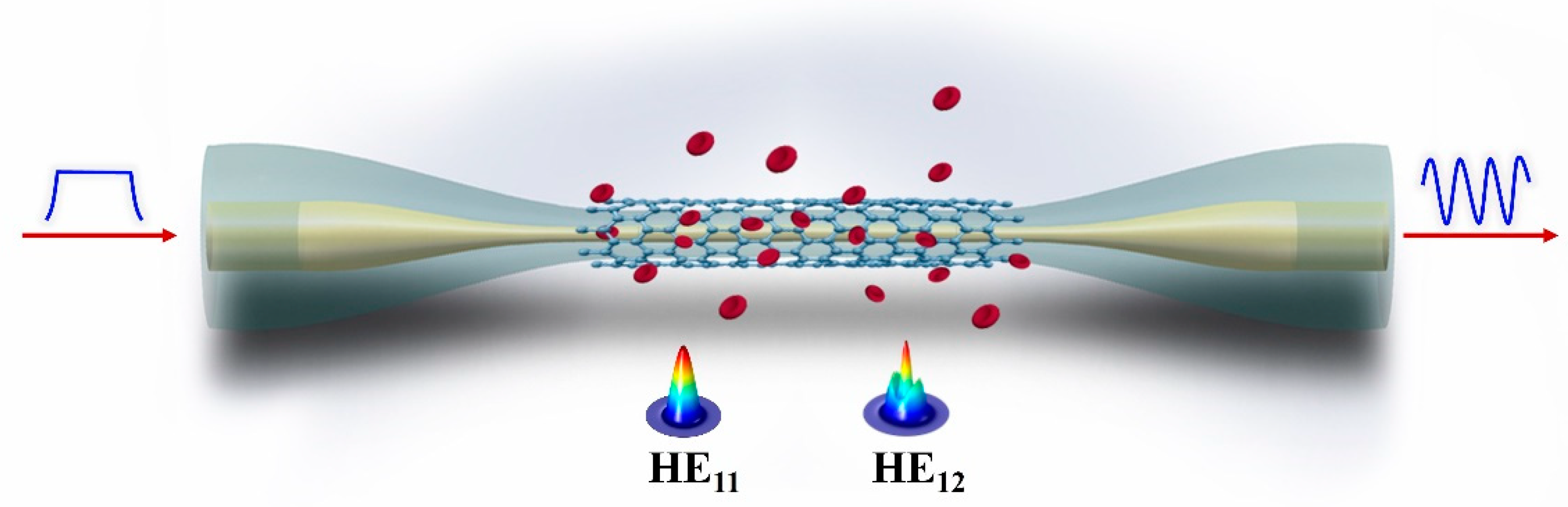
3. Experimental Setup
3.1. Encapsulation of Microfiber
3.2. The Measurement System for Hb Detection
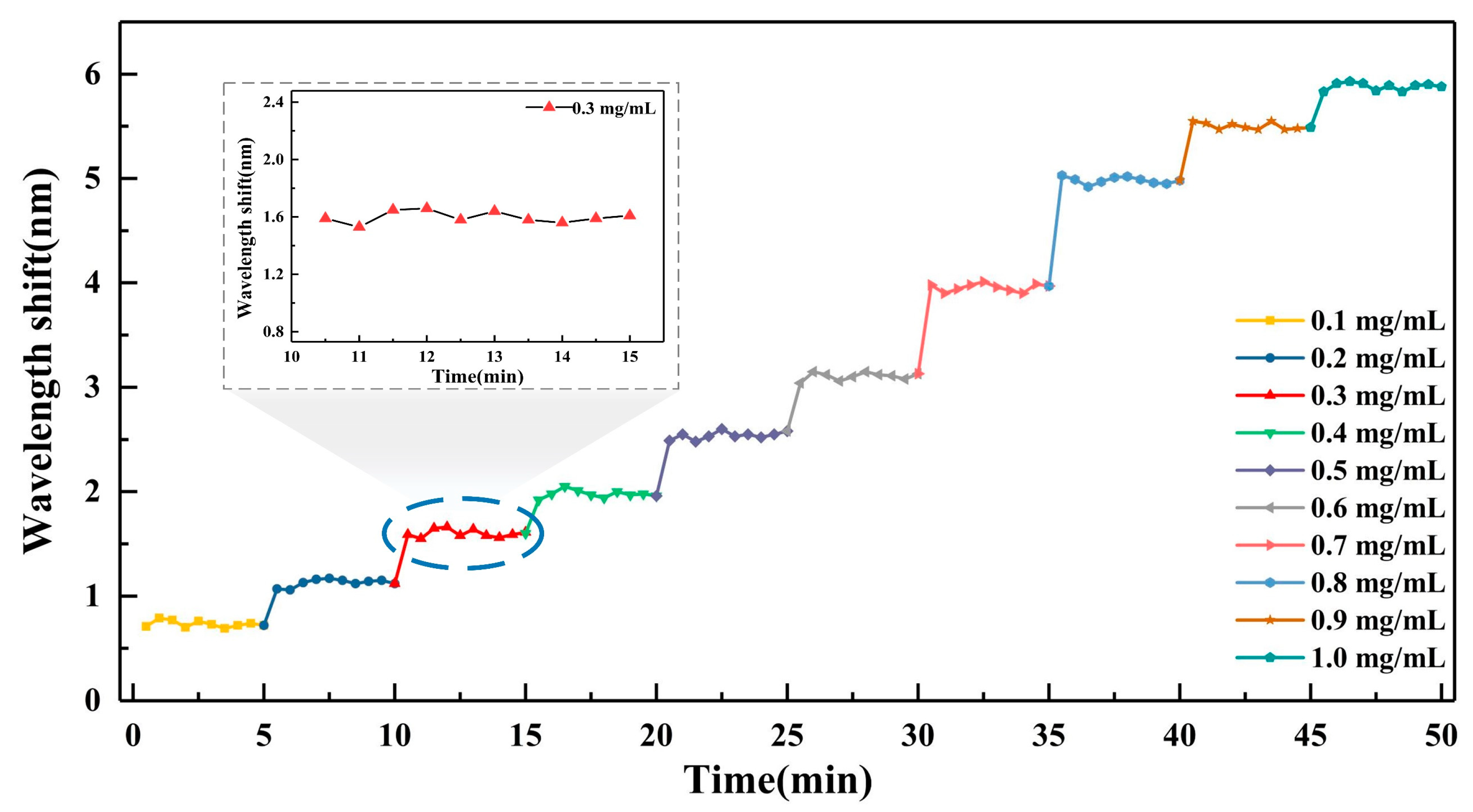
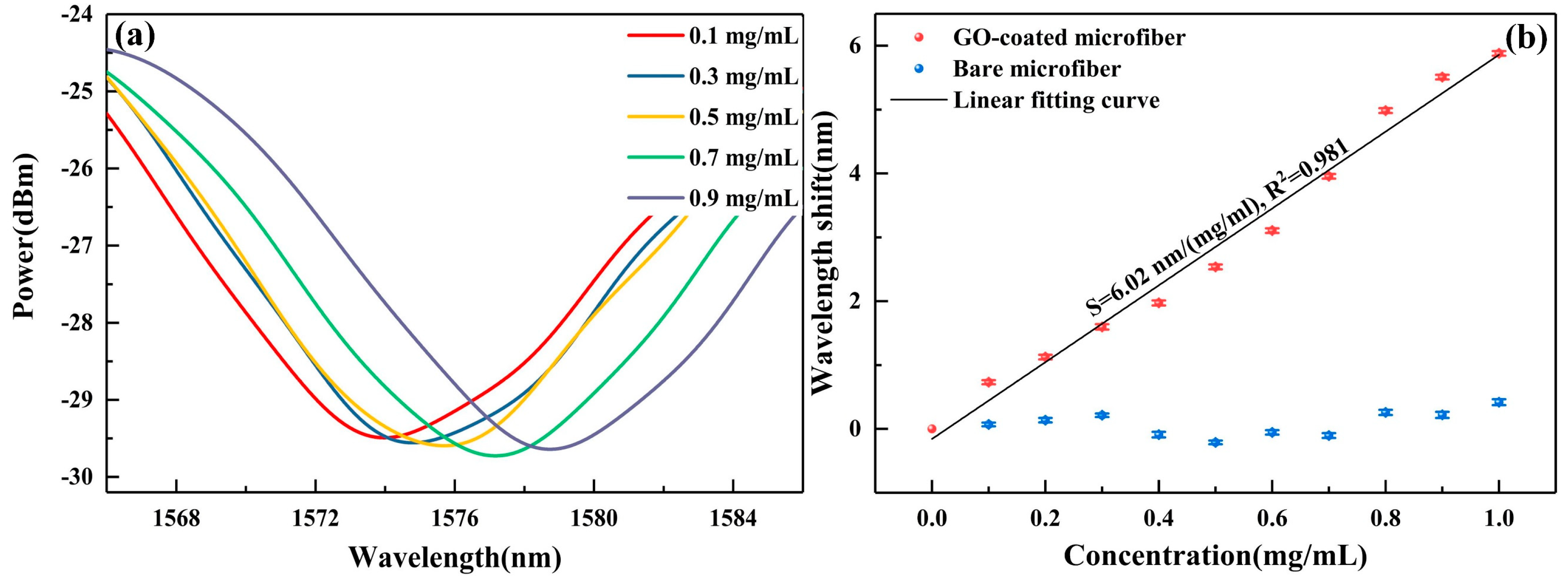
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crispino, J.D.; Weiss, M.J. Erythro-megakaryocytic transcription factors associated with hereditary anemia. Blood 2014, 123, 3080–3088. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.C.; Zhang, W.; Li, Y.; Sehmi, J.; Wass, M.N.; Zabaneh, D.; Hoggart, C.; Bayele, H.; McCarthy, M.I.; Peltonen, L.; et al. Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat. Genet. 2009, 41, 1170–1172. [Google Scholar] [CrossRef]
- Jakovic, L.; Gotic, M.; Gisslinger, H.; Soldatovic, I.; Sefer, D.; Tirnanic, M.; Lekovic, D.; Jovanovic, M.P.; Schalling, M.; Gisslinger, B.; et al. The WHO diagnostic criteria for polycythemia vera—Role of red cell mass versus hemoglobin/hematocrit level and morphology. Ann. Hematol. 2018, 97, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Fairley, K.F.; Birch, D.F. Hematuria: A simple method for identifying glomerular bleeding. Kidney Int. 1982, 21, 105–108. [Google Scholar] [CrossRef]
- Kherad, O.; Restellini, S.; Almadi, M.; Strate, L.L.; Ménard, C.; Martel, M.; Afshar, I.R.; Sadr, M.S.; Barkun, A.N. Systematic review with meta-analysis: Limited benefits from early colonoscopy in acute lower gastrointestinal bleeding. Aliment. Pharmacol. Ther. 2020, 52, 774–788. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.-K.; Capua, E.; Naaman, R. Application of a GaAs-based sensor for detecting hemoglobin in gastrointestinal environments. IEEE Sens. J. 2016, 17, 660–666. [Google Scholar] [CrossRef]
- Chawla, S.; Pundir, C.S. An electrochemical biosensor for fructosyl valine for glycosylated hemoglobin detection based on core–shell magnetic bionanoparticles modified gold electrode. Biosens. Bioelectron. 2011, 26, 3438–3443. [Google Scholar] [CrossRef]
- Akyildiz, B. Noninvasive measurement of hemoglobin using spectrophotometry. J. Pediatr. Hematol. 2018, 40, e19–e22. [Google Scholar] [CrossRef]
- Chen, G.; Chang, T.M.S. Dual effects include antioxidant and pro-oxidation of ascorbic acid on the redox properties of bovine hemoglobin. Artif. Cells Nanomed. Biotechnol. 2018, 46, 983–992. [Google Scholar] [CrossRef]
- Traore, Z.S.; Shah, S.M.; Su, X. Flow-injection chemiluminescence determination of haemoglobin in the blood. Luminescence 2012, 28, 56–62. [Google Scholar] [CrossRef]
- Pourreza, N.; Golmohammadi, H. Hemoglobin detection using curcumin nanoparticles as a colorimetric chemosensor. RSC Adv. 2015, 5, 1712–1717. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, Y.; Hu, X.-G.; Chen, M.-Q. Humidity sensor based on unsymmetrical U-shaped twisted microfiber coupler with wide detection range. Sens. Actuators B Chem. 2019, 290, 406–413. [Google Scholar] [CrossRef]
- Liu, C.; Cai, Q.; Xu, B.; Zhu, W.; Zhang, L.; Zhao, J.; Chen, X. Graphene oxide functionalized long period grating for ultrasensitive label-free immunosensing. Biosens. Bioelectron. 2017, 94, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nan, Y.; Ma, X.; Liu, H.; Liu, W.; Shi, L.; Guo, T. In-situ detection of small biomolecule interactions using a plasmonic tilted fiber grating sensor. J. Light. Technol. 2018, 37, 2792–2799. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, K.; Wang, C.; Sun, Q.; Yin, G.; Tai, Z.; Wilson, K.; Zhao, J.; Zhang, L. Label-free glucose biosensor based on enzymatic graphene oxide-functionalized tilted fiber grating. Sens. Actuators B Chem. 2018, 254, 1033–1039. [Google Scholar] [CrossRef]
- Deep, A.; Tiwari, U.; Kumar, P.; Mishra, V.; Jain, S.C.; Singh, N.; Kapur, P.; Bharadwaj, L.M. Immobilization of enzyme on long period grating fibers for sensitive glucose detection. Biosens. Bioelectron. 2012, 33, 190–195. [Google Scholar] [CrossRef]
- Voisin, V.; Pilate, J.; Damman, P.; Mégret, P.; Caucheteur, C. Highly sensitive detection of molecular interactions with plasmonic optical fiber grating sensors. Biosens. Bioelectron. 2014, 51, 249–254. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Tan, S.; Fang, F.; Yang, L.; Yuan, B.; Sun, Q. Recent advances in microfiber sensors for highly sensitive biochemical detection. J. Phys. D Appl. Phys. 2019, 52, 493002. [Google Scholar] [CrossRef]
- Yap, S.H.K.; Yong, K.-T.; Tan, R.X.; Alauddin, A.R.B.S.; Bin Ji, W.; Tjin, S.C.; Yong, K.-T. An advanced hand-held microfiber-based sensor for ultrasensitive lead ion detection. ACS Sens. 2018, 3, 2506–2512. [Google Scholar] [CrossRef]
- Liu, T.; Liang, L.-L.; Xiao, P.; Sun, L.-P.; Huang, Y.-Y.; Ran, Y.; Jin, L.; Guan, B.-O. A label-free cardiac biomarker immunosensor based on phase-shifted microfiber Bragg grating. Biosens. Bioelectron. 2018, 100, 155–160. [Google Scholar] [CrossRef]
- Sun, D.; Sun, L.-P.; Guo, T.; Guan, B.-O. Label-free thrombin detection using a tapered fiber-optic interferometric aptasensor. J. Light. Technol. 2018, 37, 2756–2761. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Liu, B.; Zhang, H.; Song, B. Square-microfiber-integrated biosensor for label-free DNA hybridization detection. Sens. Actuators B Chem. 2017, 252, 1125–1131. [Google Scholar] [CrossRef]
- Huang, X.; Liu, W.; Zhang, A.-H.; Zhang, Y.; Wang, Z.L. Ballistic transport in single-layer MoS2 piezotronic transistors. Nano Res. 2015, 9, 282–290. [Google Scholar] [CrossRef]
- Lu, N.; Wang, L.; Lv, M.; Tang, Z.; Fan, C. Graphene-based nanomaterials in biosystems. Nano Res. 2018, 12, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Morales-Narváez, E.; Merkoçi, A. Graphene oxide as an optical biosensing platform. Adv. Mater. 2012, 24, 3298–3308. [Google Scholar] [CrossRef]
- Morales-Narvaez, E.; Merkoci, A. Graphene Oxide as an Optical Biosensing Platform: A Progress Report. Adv Mater 2019, 31, e1805043. [Google Scholar] [CrossRef]
- Qin, C.; Guo, W.; Liu, Y.; Liu, Z.; Qiu, J.; Peng, J. A novel electrochemical sensor based on graphene oxide decorated with silver nanoparticles–molecular imprinted polymers for determination of sunset yellow in soft drinks. Food Anal. Methods 2017, 10, 2293–2301. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, B.; Guo, T.; Guan, B.-O. Ultrasensitive and in situ DNA detection in various pH environments based on a microfiber with a graphene oxide linking layer. RSC Adv. 2017, 7, 13177–13183. [Google Scholar] [CrossRef]
- Liu, C.; Xu, B.; Zhou, L.; Sun, Z.; Mao, H.; Zhao, J.; Zhang, L.; Chen, X. Graphene oxide functionalized long period fiber grating for highly sensitive hemoglobin detection. Sens. Actuators B Chem. 2018, 261, 91–96. [Google Scholar] [CrossRef]
- Luo, H.; Sun, Q.; Li, X.; Yan, Z.; Li, Y.; Liu, D.; Zhang, L. Refractive index sensitivity characteristics near the dispersion turning point of the multimode microfiber-based Mach–Zehnder interferometer. Opt. Lett. 2015, 40, 5042–5045. [Google Scholar] [CrossRef]
- Shang, J.; Ma, L.; Li, J.; Ai, W.; Yu, D.Y.W.; Gurzadyan, G.G. The origin of fluorescence from graphene oxide. Sci. Rep. 2012, 2, 792. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Qiu, H.W.; Zhang, C.; Jiang, S.Z.; Li, Z.; Liu, X.Y.; Yue, W.W.; Yang, C.; Huo, Y.; Feng, D.J.; et al. Absorbance response of a graphene oxide coated U-bent optical fiber sensor for aqueous ethanol detection. RSC Adv. 2016, 6, 15808–15815. [Google Scholar] [CrossRef]
- Han, G.-C.; Su, X.; Hou, J.; Ferranco, A.; Feng, X.-Z.; Zeng, R.; Chen, Z.; Kraatz, H. Disposable electrochemical sensors for hemoglobin detection based on ferrocenoyl cysteine conjugates modified electrode. Sens. Actuators B Chem. 2019, 282, 130–136. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, J. Graphene based chalcogenide fiber-optic evanescent wave sensor for detection of hemoglobin in human blood. Opt. Fiber Technol. 2018, 41, 125–130. [Google Scholar] [CrossRef]
- Sampath, U.; Kim, D.-G.; Song, M. Hemoglobin detection using a graphene oxide functionalized side-polished fiber sensor. Opt. Sens. 2019, 11028, 1102822. [Google Scholar] [CrossRef]
- Ayyanar, N.; Khalil, A.E.; Hameed, M.F.O.; Raja, G.T.; Obayya, S.S.A. Enhanced sensitivity of hemoglobin sensor using dual-core photonic crystal fiber. Opt. Quantum Electron. 2018, 50, 453. [Google Scholar] [CrossRef]
- Sun, B.; Ni, X.; Cao, Y.; Cao, G. Electrochemical sensor based on magnetic molecularly imprinted nanoparticles modified magnetic electrode for determination of Hb. Biosens. Bioelectron. 2017, 91, 354–358. [Google Scholar] [CrossRef]


| Sensing Structure | Diameter × Length | Sensitivity | Detection Limit (μg/mL) | Type of Sensor | Ref. |
|---|---|---|---|---|---|
| Long period grating | 125 μm × 15 mm | 1.9 dB/(mg/mL) | 50 | Fiber optic sensor | [29] |
| chalcogenide fiber | – | 6.71 × 10−4/(g/dL) | 1.8 | Fiber optic sensor | [34] |
| Side-polished fiber | – ×17 mm | 0.0025 a.u./(mg/mL) | – | Fiber optic sensor | [35] |
| Dual-core photonic crystal fiber | – ×3.7 cm | ~0.08 nm/(mg/mL) | – | Fiber optic sensor | [36] |
| Nanoparticles modified electrode | 4 mm × 4 mm | 0.17 mA/(mg/mL) | 1 | Electrochemical sensor | [37] |
| Ferrocenoyl cysteine conjugates modified electrode | – | 0.4 mA/(mg/mL) | 0.03 | Electrochemical sensor | [33] |
| GO-coated microfiber | 7 μm × 6.5 mm | 6.02 nm/(mg/mL) | 0.17 | Fiber optic sensor | Our study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, F.; Li, Y.; Yang, L.; Li, L.; Yan, Z.; Sun, Q. Sensitive and In Situ Hemoglobin Detection Based on a Graphene Oxide Functionalized Microfiber. Nanomaterials 2020, 10, 2461. https://doi.org/10.3390/nano10122461
Fang F, Li Y, Yang L, Li L, Yan Z, Sun Q. Sensitive and In Situ Hemoglobin Detection Based on a Graphene Oxide Functionalized Microfiber. Nanomaterials. 2020; 10(12):2461. https://doi.org/10.3390/nano10122461
Chicago/Turabian StyleFang, Fang, Yanpeng Li, Liuyang Yang, Liangye Li, Zhijun Yan, and Qizhen Sun. 2020. "Sensitive and In Situ Hemoglobin Detection Based on a Graphene Oxide Functionalized Microfiber" Nanomaterials 10, no. 12: 2461. https://doi.org/10.3390/nano10122461
APA StyleFang, F., Li, Y., Yang, L., Li, L., Yan, Z., & Sun, Q. (2020). Sensitive and In Situ Hemoglobin Detection Based on a Graphene Oxide Functionalized Microfiber. Nanomaterials, 10(12), 2461. https://doi.org/10.3390/nano10122461




