Post-Synthesis Modification of Photoluminescent and Electrochemiluminescent Au Nanoclusters with Dopamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Post-Synthesis Modification of NCs
2.3. Characterization
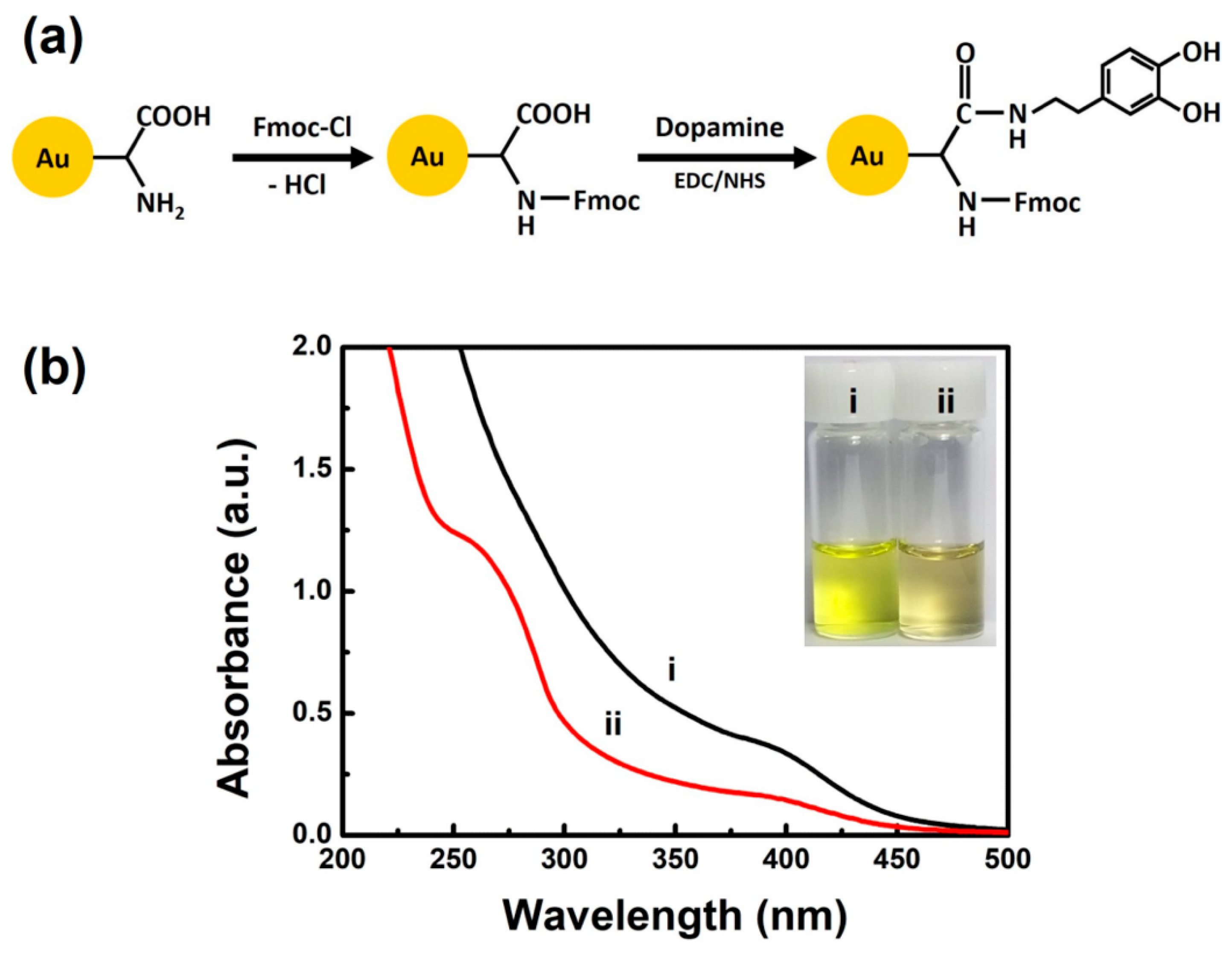
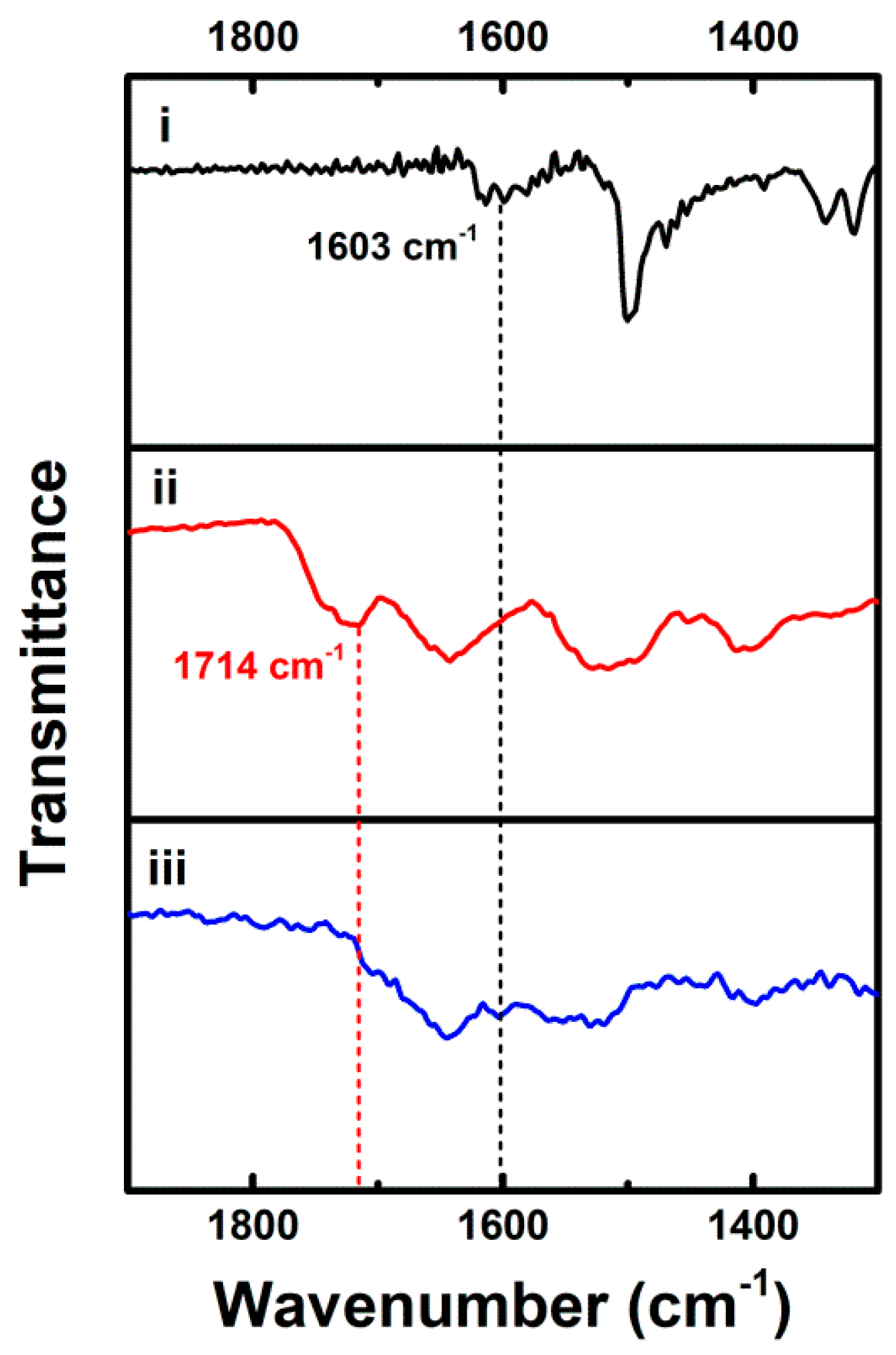
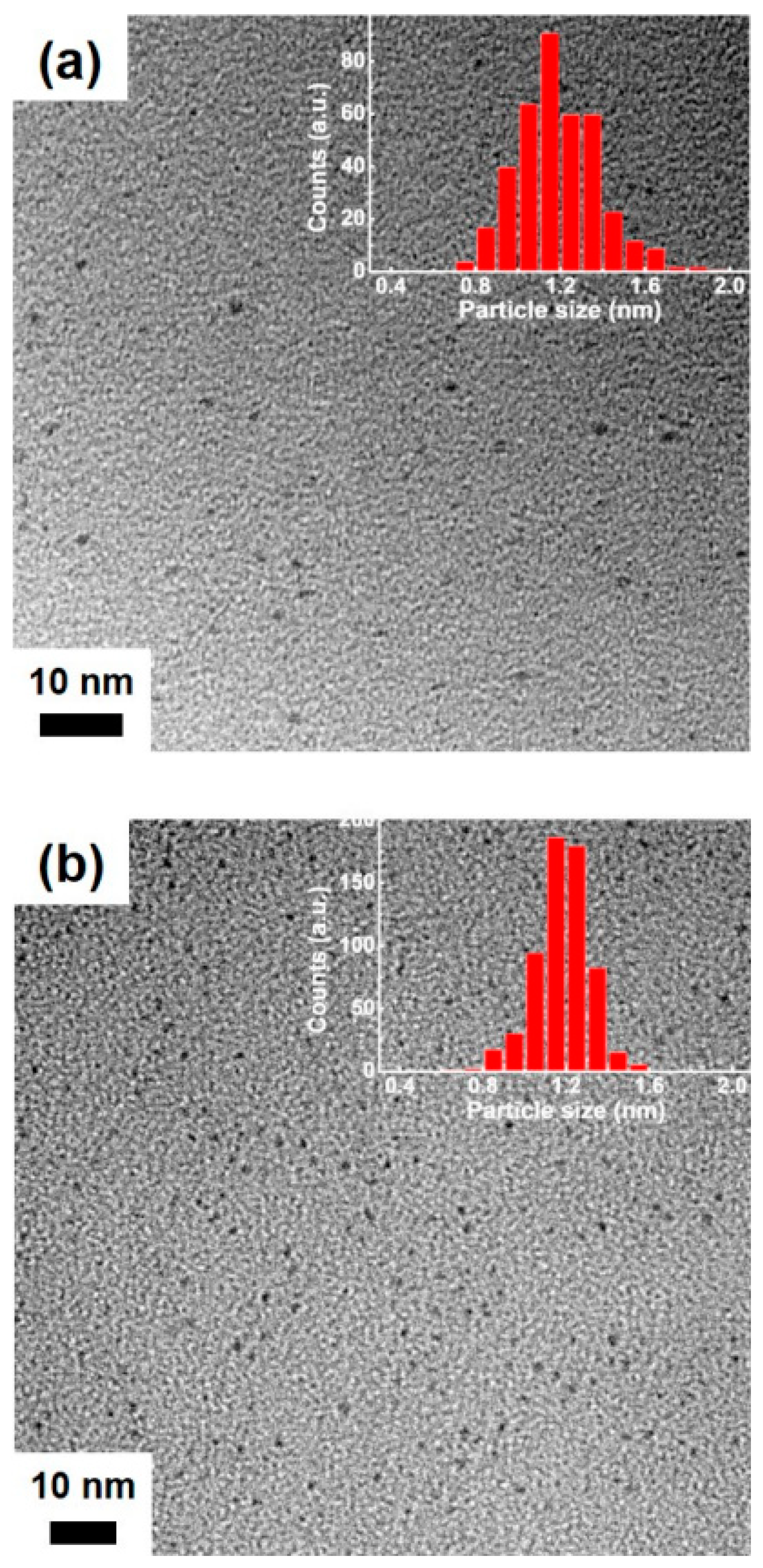
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zhao, Y.; Song, Q. Synthesis, optical properties and applications of ultra-small luminescent gold nanoclusters. Trends Anal. Chem. 2014, 57, 73–82. [Google Scholar] [CrossRef]
- Kaur, N.; Aditya, R.N.; Singh, A.; Kuo, T.-R. Biomedical Applications for Gold Nanoclusters: Recent Developments and Future Perspectives. Nanoscale Res. Lett. 2018, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, D.; Jiang, Y.; Ni, P.; Zhang, C.; Wang, B.; Yang, F.; Lu, Y.; Sun, J. Logically Regulating Peroxidase-Like Activity of Gold Nanoclusters for Sensing Phosphate-Containing Metabolites and Alkaline Phosphatase Activity. Anal. Chem. 2019, 91, 15017–15024. [Google Scholar] [CrossRef]
- Latorre, A.; Latorre, A.; Castellanos, M.; Rodriguez Diaz, C.; Lazaro-Carrillo, A.; Aguado, T.; Lecea, M.; Romero-Pérez, S.; Calero, M.; Sanchez-Puelles, J.M.; et al. Multifunctional Albumin-Stabilized Gold Nanoclusters for the Reduction of Cancer Stem Cells. Cancers 2019, 11, 969. [Google Scholar] [CrossRef]
- Lim, D.C.; Seo, B.Y.; Nho, S.; Kim, D.H.; Hong, E.M.; Lee, J.Y.; Park, S.-Y.; Lee, C.-L.; Kim, Y.D.; Cho, S. Emissive Nanoclusters Based on Subnanometer-Sized Au38 Cores for Boosting the Performance of Inverted Organic Photovoltaic Cells. Adv. Energy Mater. 2015, 5, 1500393. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, M.; Zhang, Y.; Hou, Y.; Wu, Y.; Yao, M.; Li, L.; Shi, L.; Liu, T.; Hu, B.; et al. Monoclonal-Antibody-Templated Gold Nanoclusters for HER2 Receptors Targeted Fluorescence Imaging. ACS Appl. Bio. Mater. 2020, 3, 7061–7066. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, H.; Zhang, L.; Wang, L.; Zeng, Y.; Xia, X.; Liu, F.; Chen, Y. Strategy to Fabricate Naked-Eye Readout Ultrasensitive Plasmonic Nanosensor Based on Enzyme Mimetic Gold Nanoclusters. Anal. Chem. 2016, 88, 1412–1418. [Google Scholar] [CrossRef]
- Wang, G.; Huang, T.; Murray, R.W.; Menard, L.; Nuzzo, R.G. Near-IR Luminescence of Monolayer-Protected Metal Clusters. J. Am. Chem. Soc. 2005, 127, 812–813. [Google Scholar] [CrossRef]
- Miles, D.T.; Murray, R.W. Temperature-Dependent Quantized Double Layer Charging of Monolayer-Protected Gold Clusters. Anal. Chem. 2003, 75, 1251–1257. [Google Scholar] [CrossRef]
- Fang, Y.-M.; Song, J.; Li, J.; Wang, Y.-W.; Yang, H.-H.; Sun, J.-J.; Chen, G.-N. Electrogenerated chemiluminescence from Au nanoclusters. Chem. Commun. 2011, 47, 2369–2371. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Zhu, M.; Wu, Z.; Jin, R. Quantum Sized Gold Nanoclusters with Atomic Precision. Acc. Chem. Res. 2012, 45, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Plascencia-Villa, G.; Demeler, B.; Whetten, R.L.; Griffith, W.P.; Alvarez, M.; Black, D.M.; José-Yacamán, M. Analytical Characterization of Size-Dependent Properties of Larger Aqueous Gold Nanoclusters. J. Phys. Chem. C 2016, 120, 8950–8958. [Google Scholar] [CrossRef]
- Li, G.; Hu, W.; Sun, Y.; Xu, J.; Cai, X.; Cheng, X.; Zhang, Y.; Tang, A.; Liu, X.; Chen, M.; et al. Reactivity and Lability Modulated by a Valence Electron Moving in and out of 25-Atom Gold Nanoclusters. Angew. Chem. Int. Ed. 2020, 59, 21135–21142. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Takasugi, Y.; Sato, S.; Yao, H.; Kimura, K.; Tsukuda, T. Magic-Numbered Aun Clusters Protected by Glutathione Monolayers (n = 18, 21, 25, 28, 32, 39): Isolation and Spectroscopic Characterization. J. Am. Chem. Soc. 2004, 126, 6518–6519. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R. On the Ligand’s Role in the Fluorescence of Gold Nanoclusters. Nano Lett. 2010, 10, 2568–2573. [Google Scholar] [CrossRef]
- Schaaff, T.G.; Knight, G.; Shafigullin, M.N.; Borkman, R.F.; Whetten, R.L. Isolation and Selected Properties of a 10.4 kDa Gold:Glutathione Cluster Compound. J. Phys. Chem. B 1998, 102, 10643–10646. [Google Scholar] [CrossRef]
- Luo, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From Aggregation-Induced Emission of Au(I)–Thiolate Complexes to Ultrabright Au(0)@Au(I)–Thiolate Core–Shell Nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar] [CrossRef]
- Negishi, Y.; Nobusada, K.; Tsukuda, T. Glutathione-Protected Gold Clusters Revisited: Bridging the Gap between Gold(I)−Thiolate Complexes and Thiolate-Protected Gold Nanocrystals. J. Am. Chem. Soc. 2005, 127, 5261–5270. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, Z.; Chevrier, D.M.; Leong, D.T.; Zhang, P.; Jiang, D.-E.; Xie, J. Identification of a Highly Luminescent Au22(SG)18 Nanocluster. J. Am. Chem. Soc. 2014, 136, 1246–1249. [Google Scholar] [CrossRef]
- Pyo, K.; Thanthirige, V.D.; Kwak, K.; Pandurangan, P.; Ramakrishna, G.; Lee, D. Ultrabright Luminescence from Gold Nanoclusters: Rigidifying the Au(I)–Thiolate Shell. J. Am. Chem. Soc. 2015, 137, 8244–8250. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, J.; Wang, C.; Zhao, Y.; Lin, Q. Tunable near-infrared fluorescent gold nanoclusters: Temperature sensor and targeted bioimaging. New J. Chem. 2017, 41, 5412–5419. [Google Scholar] [CrossRef]
- Peng, H.; Huang, Z.; Sheng, Y.; Zhang, X.; Deng, H.; Chen, W.; Liu, J. Pre-oxidation of Gold Nanoclusters Results in a 66 % Anodic Electrochemiluminescence Yield and Drives Mechanistic Insights. Angew. Chem. Int. Ed. 2019, 58, 11691–11694. [Google Scholar] [CrossRef] [PubMed]
- Cantelli, A.; Guidetti, G.; Manzi, J.; Caponetti, V.; Montalti, M. Towards Ultra-Bright Gold Nanoclusters. Eur. J. Inorg. Chem. 2017, 2017, 5068–5084. [Google Scholar] [CrossRef]
- Genji Srinivasulu, Y.; Yao, Q.; Goswami, N.; Xie, J. Interfacial engineering of gold nanoclusters for biomedical applications. Mater. Horiz. 2020, 7, 2596–2618. [Google Scholar] [CrossRef]
- Pyo, K.; Thanthirige, V.D.; Yoon, S.Y.; Ramakrishna, G.; Lee, D. Enhanced luminescence of Au22(SG)18 nanoclusters via rational surface engineering. Nanoscale 2016, 8, 20008–20016. [Google Scholar] [CrossRef]
- Tang, Z.; Ahuja, T.; Wang, S.; Wang, G. Near infrared luminescence of gold nanoclusters affected by the bonding of 1,4-dithiolate durene and monothiolate phenylethanethiolate. Nanoscale 2012, 4, 4119–4124. [Google Scholar] [CrossRef]
- Montalti, M.; Zaccheroni, N.; Prodi, L.; O’Reilly, N.; James, S.L. Enhanced Sensitized NIR Luminescence from Gold Nanoparticles via Energy Transfer from Surface-Bound Fluorophores. J. Am. Chem. Soc. 2007, 129, 2418–2419. [Google Scholar] [CrossRef]
- Yahia-Ammar, A.; Sierra, D.; Mérola, F.; Hildebrandt, N.; Le Guével, X. Self-Assembled Gold Nanoclusters for Bright Fluorescence Imaging and Enhanced Drug Delivery. ACS Nano 2016, 10, 2591–2599. [Google Scholar] [CrossRef]
- Kim, J.M.; Jeong, S.; Song, J.K.; Kim, J. Near-infrared electrochemiluminescence from orange fluorescent Au nanoclusters in water. Chem. Commun. 2018, 54, 2838–2841. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, J. Electrochemiluminescence of Glutathione-Stabilized Au Nanoclusters Fractionated by Gel Electrophoresis in Water. ChemElectroChem 2020, 7, 1092–1096. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, J.; Yu, Y.; Lee, J.Y. Monodispersity control in the synthesis of monometallic and bimetallic quasi-spherical gold and silver nanoparticles. Nanoscale 2010, 2, 1962–1975. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Panigrahi, S.; Praharaj, S.; Kumar Ghosh, S.; Pande, S.; Jana, S.; Pal, T. Dipole–dipole plasmon interactions in self-assembly of gold organosol induced by glutathione. New J. Chem. 2006, 30, 1333–1339. [Google Scholar] [CrossRef]
- Wu, Z.; Gayathri, C.; Gil, R.R.; Jin, R. Probing the Structure and Charge State of Glutathione-Capped Au25(SG)18 Clusters by NMR and Mass Spectrometry. J. Am. Chem. Soc. 2009, 131, 6535–6542. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Jin, R. Stability of the Two Au−S Binding Modes in Au25(SG)18 Nanoclusters Probed by NMR and Optical Spectroscopy. ACS Nano 2009, 3, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, X.; Yao, Q.; Yu, Y.; Yan, N.; Xie, J. Scalable and Precise Synthesis of Thiolated Au10–12, Au15, Au18, and Au25 Nanoclusters via pH Controlled CO Reduction. Chem. Mater. 2013, 25, 946–952. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef]
- Lyu, Q.; Song, H.; Yakovlev, N.L.; Tan, W.S.; Chai, C.L.L. In situ insights into the nanoscale deposition of 5,6-dihydroxyindole-based coatings and the implications on the underwater adhesion mechanism of polydopamine coatings. RSC Adv. 2018, 8, 27695–27702. [Google Scholar] [CrossRef]
- Goswami, N.; Lin, F.; Liu, Y.; Leong, D.T.; Xie, J. Highly Luminescent Thiolated Gold Nanoclusters Impregnated in Nanogel. Chem. Mater. 2016, 28, 4009–4016. [Google Scholar] [CrossRef]
- Gao, F.; Qu, H.; Duan, Y.; Wang, J.; Song, X.; Ji, T.; Cao, L.; Nie, G.; Sun, S. Dopamine coating as a general and facile route to biofunctionalization of superparamagnetic Fe3O4 nanoparticles for magnetic separation of proteins. RSC Adv. 2014, 4, 6657–6663. [Google Scholar] [CrossRef]
- Bachman, R.E.; Bodolosky-Bettis, S.A.; Glennon, S.C.; Sirchio, S.A. Formation of a Novel Luminescent Form of Gold(I) Phenylthiolate via Self-Assembly and Decomposition of Isonitrilegold(I) Phenylthiolate Complexes. J. Am. Chem. Soc. 2000, 122, 7146–7147. [Google Scholar] [CrossRef]
- Pyo, K.; Ly, N.H.; Han, S.M.; Hatshan, M.b.; Abuhagr, A.; Wiederrecht, G.; Joo, S.-W.; Ramakrishna, G.; Lee, D. Unique Energy Transfer in Fluorescein-Conjugated Au22 Nanoclusters Leading to 160-Fold pH-Contrasting Photoluminescence. J. Phys. Chem. Lett. 2018, 9, 5303–5310. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, M.; Guo, Z.; Wang, H.; Dong, H.; Yang, W. Aggregation-Enhanced Emission of Gold Nanoclusters Induced by Serum Albumin and Its Application to Protein Detection and Fabrication of Molecular Logic Gates. ACS Omega 2018, 3, 12763–12769. [Google Scholar] [CrossRef] [PubMed]
- You, J.G.; Tseng, W.L. Peptide-induced aggregation of glutathione-capped gold nanoclusters: A new strategy for designing aggregation-induced enhanced emission probes. Anal. Chim. Acta 2019, 1078, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.-H.; Xiao, K.-P.; Wang, Y.-S.; Liu, L.; Li, J.-Q.; Li, M.; Qu, Y.-N.; Xiao, X.-L. Aggregation-induced photoluminescence enhancement of protamine-templated gold nanoclusters for 1-hydroxypyrene detection using 9-hydroxyphenanthrene as a sensitizer. Colloids Surf. B 2020, 189, 110873. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Kim, J. Post-Synthesis Modification of Photoluminescent and Electrochemiluminescent Au Nanoclusters with Dopamine. Nanomaterials 2021, 11, 46. https://doi.org/10.3390/nano11010046
Kim JH, Kim J. Post-Synthesis Modification of Photoluminescent and Electrochemiluminescent Au Nanoclusters with Dopamine. Nanomaterials. 2021; 11(1):46. https://doi.org/10.3390/nano11010046
Chicago/Turabian StyleKim, Jae Hyun, and Joohoon Kim. 2021. "Post-Synthesis Modification of Photoluminescent and Electrochemiluminescent Au Nanoclusters with Dopamine" Nanomaterials 11, no. 1: 46. https://doi.org/10.3390/nano11010046
APA StyleKim, J. H., & Kim, J. (2021). Post-Synthesis Modification of Photoluminescent and Electrochemiluminescent Au Nanoclusters with Dopamine. Nanomaterials, 11(1), 46. https://doi.org/10.3390/nano11010046




