Inflammatory Bowel Disease: The Emergence of New Trends in Lifestyle and Nanomedicine as the Modern Tool for Pharmacotherapy
Abstract
1. Introduction
2. Epidemiology of IBD
3. Genetic Factors
4. Gut Microbiome and Immunity
4.1. Mucosal Barriers and Intestinal Epithelial Cells (IECs)
4.2. Innate Immune Cells and Myeloid Cells
4.3. T Cells and B Cells
5. Nutrition
6. Conventional Therapies for IBD
6.1. Probiotics
6.2. Fecal Microbiota Transplantation
7. The General Considerations of the Physiology of GIT during IBD for Oral Drug Delivery
8. Nano Drug Delivery Systems as an Alternative
8.1. Surface Charge-Dependent Drug Delivery Systems
8.2. ROS-Responsive Delivery System
8.3. pH-Dependent Drug Delivery System
8.4. Biodegradable Drug Delivery Systems
8.5. Active Targeting-Dependent Nano Delivery Systems
9. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Osada, T.; Ohkusa, T.; Okayasu, I.; Yoshida, T.; Hirai, S.; Beppu, K.; Shibuya, T.; Sakamoto, N.; Kobayashi, O.; Nagahara, A.; et al. Correlations among total colonoscopic findings, clinical symptoms, and laboratory markers in ulcerative colitis. J. Gastroenterol. Hepatol. 2008, 23, S262–S267. [Google Scholar] [CrossRef] [PubMed]
- Head, K.A.; Jurenka, J.S. Inflammatory bowel disease part I: Ulcerative colitis—Pathophysiology and conventional and alternative treatment options. Altern. Med. Rev. 2003, 8, 247–283. [Google Scholar] [PubMed]
- Sartor, R.B. Genetics and Environmental Interactions Shape the Intestinal Microbiome to Promote Inflammatory Bowel Disease Versus Mucosal Homeostasis. Gastroenterology 2010, 139, 1816–1819. [Google Scholar] [CrossRef]
- López-Serrano, P.; Pérez-Calle, J.L.; Pérez-Fernández, M.T.; Fernández-Font, J.M.; de Miguel, D.B.; Fernández-Rodríguez, C.M. Environmental risk factors in inflammatory bowel diseases. Investigating the hygiene hypothesis: A Spanish case–control study. Scand. J. Gastroenterol. 2010, 45, 1464–1471. [Google Scholar] [CrossRef]
- Hansen, R.; Thomson, J.M.; El-Omar, E.M.; Hold, G.L. The role of infection in the aetiology of inflammatory bowel disease. J. Gastroenterol. 2010, 45, 266–276. [Google Scholar] [CrossRef]
- Keshteli, A.H.; Madsen, K.L.; Dieleman, L.A. Diet in the Pathogenesis and Management of Ulcerative Colitis; A Review of Randomized Controlled Dietary Interventions. Nutrients 2019, 11, 1498. [Google Scholar] [CrossRef]
- Zaltman, C.; Braulio, V.B.; Outeiral, R.; Nunes, T.; de Castro, C.L.N. Lower extremity mobility limitation and impaired muscle function in women with ulcerative colitis. J. Crohn’s Colitis 2014, 8, 529–535. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Inoue, N.; Yajima, T.; Izumiya, M.; Ichikawa, H.; Hibi, T. Psychological aspects of inflammatory bowel disease. J. Gastroenterol. 2007, 42, 34–40. [Google Scholar] [CrossRef]
- Kostić, M.; Djakovic, L.; Šujić, R.; Godman, B.; Janković, S.M. Inflammatory Bowel Diseases (Crohn’s Disease and Ulcerative Colitis): Cost of Treatment in Serbia and the Implications. Appl. Health Econ. Health Policy 2017, 15, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Klampfer, L. Cytokines, Inflammation and Colon Cancer. Curr. Cancer Drug Targets 2011, 11, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J. Crohn’s disease and ulcerative colitis: Occurrence, course and prognosis during the first year of disease in a European population-based inception cohort. Dan. Med. J. 2014, 61, B4778. [Google Scholar] [PubMed]
- Itzkowitz, S.H.; Yio, X. Inflammation and Cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am. J. Physiol. Liver Physiol. 2004, 287, G7–G17. [Google Scholar] [CrossRef]
- Annese, V.; Duricova, D.; Gower-Rousseau, C.; Jess, T.; Langholz, E. Impact of New Treatments on Hospitalisation, Surgery, Infection, and Mortality in IBD: A Focus Paper by the Epidemiology Committee of ECCO. J. Crohn’s Colitis 2016, 10, 216–225. [Google Scholar] [CrossRef]
- Lyakhovich, A.; Gasche, C. Systematic review: Molecular chemoprevention of colorectal malignancy by mesalazine. Aliment. Pharmacol. Ther. 2009, 31, 202–209. [Google Scholar] [CrossRef]
- Dayharsh, G.A.; Loftus, E.V., Jr.; Sandborn, W.J.; Tremaine, W.J.; Zinsmeister, A.R.; Witzig, T.E.; Macon, W.R.; Burgart, L.J. Epstein-Barr virus–positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology 2002, 122, 72–77. [Google Scholar] [CrossRef]
- Talley, N.J.; Abreu, M.T.; Achkar, J.; Bernstein, C.N.; Dubinsky, M.C.; Hanauer, S.B.; Kane, S.V.; Sandborn, W.J.; Ullman, T.A.; Moayyedi, P.; et al. An Evidence-Based Systematic Review on Medical Therapies for Inflammatory Bowel Disease. Am. J. Gastroenterol. 2011, 106, S2–S25. [Google Scholar] [CrossRef]
- Coco, R.; Plapied, L.; Pourcelle, V.; Jerome, C.; Brayden, D.J.; Schneider, Y.; Preat, V. Drug delivery to inflamed colon by nanoparticles: Comparison of different strategies. Int. J. Pharm. 2013, 440, 3–12. [Google Scholar] [CrossRef]
- Hua, S. Orally administered liposomal formulations for colon targeted drug delivery. Front. Pharmacol. 2014, 5, 138. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Munkholm, P. The epidemiology of inflammatory bowel disease. Scand. J. Gastroenterol. 2015, 50, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.L. The burden of inflammatory bowel disease in Europe. J. Crohn’s Colitis 2013, 7, 322–337. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Bernstein, C.N.; Coward, S.; Bitton, A.; Murthy, S.K.; Nguyen, G.C.; Lee, K.; Cooke-Lauder, J.; Benchimol, E.I. The Impact of Inflammatory Bowel Disease in Canada 2018: Epidemiology. J. Can. Assoc. Gastroenterol. 2019, 2, S6–S16. [Google Scholar] [CrossRef] [PubMed]
- M’koma, A.E. Inflammatory Bowel Disease: An Expanding Global Health Problem. Clin. Med. Insights Gastroenterol. 2013, 6, CGast.S12731. [Google Scholar]
- Shah, S.C.; Khalili, H.; Gower-Rousseau, C.; Olen, O.; Benchimol, E.I.; Lynge, E.; Nielsen, K.R.; Brassard, P.; Vutcovici, M.; Bitton, A.; et al. Sex-Based Differences in Incidence of Inflammatory Bowel Diseases—Pooled Analysis of Population-Based Studies From Western Countries. Gastroenterology 2018, 155, 1079–1089. [Google Scholar] [CrossRef]
- Greuter, T.; Manser, C.; Pittet, V.; Vavricka, S.R.; Biedermann, L. Gender Differences in Inflammatory Bowel Disease. Digestion 2020, 101, 98–104. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- van der Heide, F.; Dijkstra, A.; Weersma, R.K.; Albersnagel, F.A.; van der Logt, E.M.J.; Faber, K.N.; Sluiter, W.J.; Kleibeuker, J.; Dijkstra, G. Effects of active and passive smoking on disease course of Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2009, 15, 1199–1207. [Google Scholar] [CrossRef]
- Berkowitz, L.; Schultz, B.M.; Salazar, G.A.; Pardo-Roa, C.; Sebastian, V.P.; Alvarez-Lobos, M.M.; Bueno, S.M. Impact of Cigarette Smoking on the Gastrointestinal Tract Inflammation: Opposing Effects in Crohn’s Disease and Ulcerative Colitis. Front. Immunol. 2018, 9, 74. [Google Scholar] [CrossRef]
- Monstad, I.; Hovde, O.; Solberg, I.C.; Moum, B.A. Clinical course and prognosis in ulcerative colitis: Results from population-based and observational studies. Ann. Gastroenterol. 2014, 27, 95–104. [Google Scholar] [PubMed]
- Limketkai, B.N.; Parian, A.M.; Shah, N.D.; Colombel, J.-F. Short Bowel Syndrome and Intestinal Failure in Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Boucher, G.; Lees, C.; Franke, A.; D’Amato, M.; Taylor, K.D.; Lee, J.C.; Goyette, P.; Imielinski, M.; Latiano, A.; et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 2011, 43, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.H.; Kamada, N.; Warner, N.; Kim, Y.G.; Nuñez, G. The ever-expanding function of NOD2: Autophagy, viral recognition, and T cell activation. Trends Immunol. 2011, 32, 73–79. [Google Scholar] [CrossRef]
- Philpott, D.J.; Sorbara, M.T.; Robertson, S.J.; Croitoru, K.; Girardin, S.E. NOD proteins: Regulators of inflammation in health and disease. Nat. Rev. Immunol. 2014, 14, 9–23. [Google Scholar] [CrossRef]
- Barnich, N.; Aguirre, J.E.; Reinecker, H.-C.; Xavier, R.; Podolsky, D.K. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor–κB activation in muramyl dipeptide recognition. J. Cell Biol. 2005, 170, 21–26. [Google Scholar] [CrossRef]
- Rubino, S.J.; Selvanantham, T.; Girardin, S.E.; Philpott, D.J. Nod-like receptors in the control of intestinal inflammation. Curr. Opin. Immunol. 2012, 24, 398–404. [Google Scholar] [CrossRef]
- Chassaing, B.; Rolhion, N.; de Vallée, A.; Sa’ad, Y.S.; Prorok-Hamon, M.; Neut, C.; Campbell, B.J.; Söderholm, J.D.; Hugot, J.P.; Colombel, J.F.; et al. Crohn disease–associated adherent-invasive E. coli bacteria target mouse and human Peyer’s patches via long polar fimbriae. J. Clin. Investig. 2011, 121, 966–975. [Google Scholar] [CrossRef]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-κB and Rel Proteins: Evolutionarily Conserved Mediators of Immune Responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef]
- Richmond, A. NF-κB, chemokine gene transcription and tumour growth. Nat. Rev. Immunol. 2002, 2, 664–674. [Google Scholar] [CrossRef]
- Seril, D.N. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in humans and animal models. Carcinogenesis 2003, 24, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Arkan, M.C.; Bollrath, J.; Hsu, L.C.; Goode, J.; Miething, C.; Göktuna, S.I.; Neuenhahn, M.; Fierer, J.; Paxian, S.; et al. NF-κB Is a Negative Regulator of IL-1β Secretion as Revealed by Genetic and Pharmacological Inhibition of IKKβ. Cell 2007, 130, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Huttenhower, C. Meta’omic Analytic Techniques for Studying the Intestinal Microbiome. Gastroenterology 2014, 146, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Azcárate-Peril, M.A.; Sikes, M.; Bruno-Bárcena, J.M. The intestinal microbiota, gastrointestinal environment and colorectal cancer: A putative role for probiotics in prevention of colorectal cancer? Am. J. Physiol. Liver Physiol. 2011, 301, G401–G424. [Google Scholar] [CrossRef]
- Schippa, S.; Conte, M. Dysbiotic Events in Gut Microbiota: Impact on Human Health. Nutrients 2014, 6, 5786–5805. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baez, Y.; Treuren, W.V.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.; Blugeon, S.; Bridonneau, C.; Furet, J.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Thiennimitr, P.; Winter, S.E.; Winter, M.G.; Xavier, M.N.; Tolstikov, V.; Huseby, D.L.; Sterzenbach, T.; Tsolis, R.M.; Roth, J.R.; Baumler, A.J. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 17480–17485. [Google Scholar] [CrossRef]
- Sassone-Corsi, M.; Nuccio, S.; Liu, H.; Hernandez, D.; Vu, C.T.; Takahashi, A.A.; Edwards, R.A.; Raffatellu, M. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature 2016, 540, 280–283. [Google Scholar] [CrossRef]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A.; et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe 2017, 22, 25–37. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- van der Sluis, M.; De Koning, B.A.E.; de Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; van Goudoever, J.B.; Buller, H.A.; Dekker, J.; van Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Jakobsson, H.E.; Holmen-Larsson, J.; Schutte, A.; Ermund, A.; Rodriguez-Pineiro, A.M.; Arike, L.; Wising, C.; Svensson, F.; Backhed, F.; et al. Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization. Cell Host Microbe 2015, 18, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Frantz, A.L.; Rogier, E.W.; Weber, C.R.; Shen, L.; Cohen, D.A.; Fenton, L.A.; Bruno, M.E.C.; Kaetzel, C.S. Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2 and antibacterial peptides. Mucosal Immunol. 2012, 5, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Cliffe, L.J. Accelerated Intestinal Epithelial Cell Turnover: A New Mechanism of Parasite Expulsion. Science 2005, 308, 1463–1465. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Larsson, J.M.H.; Hansson, G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 4659–4665. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Vaishnava, S.; Behrendt, C.L.; Ismail, A.S.; Eckmann, L.; Hooper, L.V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA 2008, 105, 20858–20863. [Google Scholar] [CrossRef]
- Matamouros, S.; Miller, S.I. S. Typhimurium strategies to resist killing by cationic antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2015, 1848, 3021–3025. [Google Scholar] [CrossRef]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The Antibacterial Lectin RegIII Promotes the Spatial Segregation of Microbiota and Host in the Intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef]
- Kobayashi, K.S. Nod2-Dependent Regulation of Innate and Adaptive Immunity in the Intestinal Tract. Science 2005, 307, 731–734. [Google Scholar] [CrossRef]
- Bostick, J.W.; Zhou, L. Innate lymphoid cells in intestinal immunity and inflammation. Cell. Mol. Life Sci. 2016, 73, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Vermi, W.; Lee, J.S.; Lonardi, S.; Gilfillan, S.; Newberry, R.D.; Cella, M.; Colonna, M. Intraepithelial Type 1 Innate Lymphoid Cells Are a Unique Subset of IL-12- and IL-15-Responsive IFN-γ-Producing Cells. Immunity 2013, 38, 769–781. [Google Scholar] [CrossRef]
- Kernbauer, E.; Ding, Y.; Cadwell, K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 2014, 516, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Abt, M.C.; Lewis, B.B.; Caballero, S.; Xiong, H.; Carter, R.A.; Suvsac, B.; Ling, L.; Leiner, I.; Pamer, E.G. Innate Immune Defenses Mediated by Two ILC Subsets Are Critical for Protection against Acute Clostridium difficile Infection. Cell Host Microbe 2015, 18, 27–37. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Artis, D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med. 2015, 21, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; de Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 2013, 39, 72–385. [Google Scholar] [CrossRef]
- Sabat, R.; Ouyang, W.; Wolk, K. Therapeutic opportunities of the IL-22–IL-22R1 system. Nat. Rev. Drug Discov. 2014, 13, 21–38. [Google Scholar] [CrossRef]
- Pham, T.A.N.; Clare, S.; Goulding, D.; Arasteh, J.M.; Stares, M.D.; Browne, H.P.; Keane, J.A.; Page, A.J.; Kumasaka, N.; Kane, L.; et al. Epithelial IL-22RA1-Mediated Fucosylation Promotes Intestinal Colonization Resistance to an Opportunistic Pathogen. Cell Host Microbe 2014, 16, 504–516. [Google Scholar] [CrossRef]
- Rescigno, M. Intestinal Dendritic Cells; Academic Press: Cambridge, MA, USA, 2010; pp. 109–138. [Google Scholar]
- Khosravi, A.; Yanez, A.; Price, J.G.; Chow, A.; Merad, M.; Goodridge, H.S.; Mazmanian, S.K. Gut Microbiota Promote Hematopoiesis to Control Bacterial Infection. Cell Host Microbe 2014, 15, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Kamada, N.; Jiao, Y.; Liu, M.Z.; Núñez, G.; Inohara, N. Protective Role of Commensals against Clostridium difficile Infection via an IL-1β–Mediated Positive-Feedback Loop. J. Immunol. 2012, 189, 3085–3091. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Inflammatory bowel disease as a disorder of an imbalance between pro- and anti-inflammatory molecules and deficiency of resolution bioactive lipids. Lipids Health Dis. 2016, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of Splenic Reservoir Monocytes and Their Deployment to Inflammatory Sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef]
- Ubeda, C.; Djukovic, A.; Isaac, S. Roles of the intestinal microbiota in pathogen protection. Clin. Transl. Immunol. 2017, 6, e128. [Google Scholar] [CrossRef]
- Zhou, L.; Chong, M.M.W.; Littman, D.R. Plasticity of CD4+ T Cell Lineage Differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef]
- Murphy, K.M.; Reiner, S.L. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2002, 2, 933–944. [Google Scholar] [CrossRef]
- Brand, S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: New immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut 2009, 58, 1152–1167. [Google Scholar] [CrossRef]
- Siakavellas, S.I.; Bamias, G. Tumor Necrosis Factor–like Cytokine TL1A and Its Receptors DR3 and DcR3. Inflamm. Bowel Dis. 2015, 21, 2441–2452. [Google Scholar] [CrossRef]
- Geddes, K.; Rubino, S.J.; Magalhaes, J.G.; Streutker, C.; le Bourhis, L.; Cho, J.H.; Robertson, S.J.; Kim, C.J.; Kaul, R.; Philpott, D.J.; et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat. Med. 2011, 17, 837–844. [Google Scholar] [CrossRef]
- Brain, O.; Owens, B.M.J.; Pichulik, T.; Allan, P.; Khatamzas, E.; Leslie, A.; Steevels, T.; Sharma, S.; Mayer, A.; Catuneanu, A.M.; et al. The Intracellular Sensor NOD2 Induces MicroRNA-29 Expression in Human Dendritic Cells to Limit IL-23 Release. Immunity 2013, 39, 521–536. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kole, A.; Maloy, K.J. Control of Intestinal Inflammation by Interleukin-10; Springer: Berlin/Heidelberg, Germany, 2014; pp. 19–38. [Google Scholar]
- Kim, S.C.; Tonkonogy, S.L.; Albright, C.A.; Tsang, J.; Balish, E.J.; Braun, J.; Huycke, M.M.; Sartor, R.B. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology 2005, 128, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.N.; Peng, Y.; Rengarajan, S.; Solomon, B.D.; Ai, T.L.; Shen, Z.; Perry, J.S.A.; Knoop, K.A.; Tanoue, T.; Narushima, S.; et al. Helicobacter species are potent drivers of colonic T cell responses in homeostasis and inflammation. Sci. Immunol. 2017, 2, eaal5068. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.V.; Xiang, W.V.; Kwak, C.; Yang, Y.; Lin, X.W.; Ota, M.; Sarpel, U.; Rifkin, D.B.; Xu, R.; Littman, D.R. GPR15-Mediated Homing Controls Immune Homeostasis in the Large Intestine Mucosa. Science 2013, 340, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Lathrop, S.K.; Bloom, S.M.; Rao, S.M.; Nutsch, K.; Lio, C.; Santacruz, N.; Peterson, D.A.; Stappenbeck, T.S.; Hsieh, C. Peripheral education of the immune system by colonic commensal microbiota. Nature 2011, 478, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Lo, B.C.; Núñez, G. Host–microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020, 3, 332–344. [Google Scholar] [CrossRef]
- Bunker, J.J.; Bendelac, A. IgA Responses to Microbiota. Immunity 2018, 49, 211–224. [Google Scholar] [CrossRef]
- Grootjans, J.; Krupka, N.; Hosomi, S.; Matute, J.D.; Hanley, T.; Saveljeva, S.; Gensollen, T.; Heijmans, J.; Li, H.; Limenitakis, J.P.; et al. Epithelial endoplasmic reticulum stress orchestrates a protective IgA response. Science 2019, 363, 993–998. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Cisalpino, D.; Varadarajan, S.; Hellman, J.; Warren, H.S.; Cascalho, M.; Inohara, N.; Nunez, G. Gut Microbiota-Induced Immunoglobulin G Controls Systemic Infection by Symbiotic Bacteria and Pathogens. Immunity 2016, 44, 647–658. [Google Scholar] [CrossRef]
- Han, P.D.; Burke, A.; Baldassano, R.N.; Rombeau, J.L.; Lichtenstein, G.R. Nutrition and Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 1999, 28, 423–443. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Duncan, S.H.; Louis, P.; Flint, H.J. Nutritional influences on the gut microbiota and the consequences for gastrointestinal health. Biochem. Soc. Trans. 2011, 39, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Mcrorie, J.W.; Fahey, G.C. A review of gastrointestinal physiology and the mechanisms underlying the health benefits of dietary fiber: Matching an effective fiber with specific patient needs. Clin. Nurs. Stud. 2013, 1, 82–92. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- de Filippo, C.; Cavalieri, D.; di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Carbonnel, F. Animal Protein Intake and Risk of Inflammatory Bowel Disease: The E3N Prospective Study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2007, 104, 305–344. [Google Scholar] [CrossRef]
- Martens, E.C.; Lowe, E.C.; Chiang, H.; Pudlo, N.A.; Wu, M.; McNulty, N.P.; Abbott, D.W.; Henrissat, B.; Gilbert, H.J.; Bolam, D.N.; et al. Recognition and Degradation of Plant Cell Wall Polysaccharides by Two Human Gut Symbionts. PLoS Biol. 2011, 9, e1001221. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D.; Milner, J.A. Gastrointestinal microflora, food components and colon cancer prevention. J. Nutr. Biochem. 2009, 20, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; van de Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef]
- Jan, G.; Belzacq, A.S.; Haouzi, D.; Rouault, A.; Metivier, D.; Kroemer, G.; Brenner, C. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 2002, 9, 179–188. [Google Scholar] [CrossRef]
- Zapolska-Downar, M.N.D. Propionate Reduces the Cytokine-Induced VCAM-1 and ICAM-1 Expression by Inhibiting Nuclear Factor-Kappa B (NF-kappaB) Activation. J. Physiol. Pharmacol. 2009, 60, 123–131. [Google Scholar]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef]
- Khan, A.K.A.; Piris, J.; Truelove, S.C. An Experiment to Determine the Active Therapeutic Moiety of Sulphasalazine. Lancet 1977, 310, 892–895. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Fleisher, D.; Pao, L.H.; Li, C.; Winward, B. Intestinal Metabolism and Transport of 5-aminosalicylate. Drug Metab. Dispos. 1999, 27, 479–485. [Google Scholar]
- Feagan, B.G.; MacDonald, J.K. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. In Cochrane Database of Systematic Reviews; Feagan, B.G., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Gionchetti, P.; Rizzello, F.; Annese, V.; Armuzzi, A.; Biancone, L.; Castiglione, F.; Comberlato, M.; Cottone, M.; Danese, S.; Daperno, M.; et al. Use of corticosteroids and immunosuppressive drugs in inflammatory bowel disease: Clinical practice guidelines of the Italian Group for the Study of Inflammatory Bowel Disease. Dig. Liver Dis. 2017, 49, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.; Lindsay, J.O.; Sturm, A.; Windsor, A.; Colombel, J.; Allez, M.; D’Haens, G.; D’Hoore, A.; Mantzaris, G.; Novacek, G.; et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 2: Current management. J. Crohn’s Colitis 2012, 6, 991–1030. [Google Scholar] [CrossRef] [PubMed]
- Toruner, M.; Loftus, E.V., Jr.; Harmsen, W.S.; Zinsmeister, A.R.; Orenstein, R.; Sandborn, W.J.; Colombel, J.F.; Egan, L.J. Risk Factors for Opportunistic Infections in Patients With Inflammatory Bowel Disease. Gastroenterology 2008, 134, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; Boutati, E.; Krania, M.; Raptis, S. Hypertension and other morbidities with Cushing’s amprsquos syndrome associated with corticosteroids: A review. Integr. Blood Press. Control 2011, 4, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ford, A.C.; Bernstein, C.N.; Khan, K.J.; Abreu, M.T.; Marshall, J.K.; Talley, N.J.; Moayyedi, P. Glucocorticosteroid Therapy in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2011, 106, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Fasci Spurio, F.; Aratari, A.; Margagnoni, G.; Clemente, V.; Moretti, A.; Papi, C. Low bioavailability and traditional systemic steroids in IBD: Can the former take over the latter? J. Gastrointest. Liver Dis. 2013, 22. [Google Scholar]
- Rutgeerts, P.; Lofberg, R.; Malchow, H.; Lamers, C.; Olaison, G.; Jewell, D.; Danielsson, A.; Goebell, H.; Thomsen, O.O.; Lorenz-Meyer, H.; et al. A Comparison of Budesonide with Prednisolone for Active Crohn’s Disease. N. Engl. J. Med. 1994, 331, 842–845. [Google Scholar] [CrossRef]
- Papi, C.; Luchetti, R.; Gili, L.; Montanti, S.; Koch, M.; Capurso, L. Budesonide in the treatment of Crohn’s disease: A meta-analysis. Aliment. Pharmacol. Ther. 2000, 14, 1419–1428. [Google Scholar] [CrossRef]
- Schoon, E.J.; Bollani, S.; Mills, P.R.; Israeli, E.; Felsenberg, D.; Ljunghall, S.; Persson, T.; Hapten-White, L.; Graffner, H.; Porro, G.B.; et al. Bone mineral density in relation to efficacy and side effects of budesonide and prednisolone in Crohn’s disease. Clin. Gastroenterol. Hepatol. 2005, 3, 113–121. [Google Scholar] [CrossRef]
- Al-Sukhni, W.; McLeod, R.S.; MacRae, H.; O’Connor, B.; Huang, H.; and Cohen, Z. Oncologic Outcome in Patients With Ulcerative Colitis Associated With Dyplasia or Cancer Who Underwent Stapled or Handsewn Ileal Pouch-Anal Anastomosis. Dis. Colon Rectum. 2010, 53, 1495–1500. [Google Scholar] [CrossRef]
- Gionchetti, P.; Dignass, A.; Danese, S.; Magro Dias, F.J.; Rogler, G.; Lakatos, P.L.; Adamina, M.; Ardizzone, S.; Buskens, C.J.; Sebastian, S.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J. Crohn’s Colitis 2017, 11, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Tomé, A.M.; Filipe, A. Quinolones. Drug Saf. 2011, 34, 465–488. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, A.; Jackson, J.L.; Doi, A.; Kamiya, T. Metronidazole-Induced Central Nervous System Toxicity. Clin. Neuropharmacol. 2011, 34, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Scarpignato, C.; Pelosini, I. Rifaximin, a Poorly Absorbed Antibiotic: Pharmacology and Clinical Potential. Chemotherapy 2005, 51, 36–66. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.; Ordas, I.; Cabre, E.; Garcia-Sanchez, V.; Bastida, G.; Penalva, M.; Gomollon, F.; Garcia-Planella, E.; Merino, O.; Gutierrez, A.; et al. Safety of Thiopurine Therapy in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 1404–1410. [Google Scholar] [CrossRef]
- Saibeni, S.; Virgilio, T.; D’Inca, R.; Spina, L.; Bortoli, A.; Paccagnella, M.; Peli, M.; Sablich, R.; Meucci, G.; Colombo, E.; et al. The use of thiopurines for the treatment of inflammatory bowel diseases in clinical practice. Dig. Liver Dis. 2008, 40, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Vos, A.C.W.; Bakkal, N.; Minnee, R.C.; Casparie, M.K.; de Jong, D.J.; Dijkstra, G.; Stokkers, P.; van Bodegraven, A.A.; Pierik, M.; van der Woude, C.J.; et al. Risk of malignant lymphoma in patients with inflammatory bowel diseases: A Dutch nationwide study. Inflamm. Bowel Dis. 2011, 17, 1837–1845. [Google Scholar] [CrossRef]
- Engel, M.A.; Neurath, M.F. New pathophysiological insights and modern treatment of IBD. J. Gastroenterol. 2010, 45, 571–583. [Google Scholar] [CrossRef]
- Feagan, B.G.; Fedorak, R.N.; Irvine, E.J.; Wild, G.; Sutherland, L.; Steinhart, A.H.; Greenberg, G.R.; Koval, J.; Wong, C.J.; Hopkins, M.; et al. A Comparison of Methotrexate with Placebo for the Maintenance of Remission in Crohn’s Disease. N. Engl. J. Med. 2000, 342, 1627–1632. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Abreu, M.T.; Cohen, R.; Tremaine, W. American Gastroenterological Association Institute Technical Review on Corticosteroids, Immunomodulators, and Infliximab in Inflammatory Bowel Disease. Gastroenterology 2006, 130, 940–987. [Google Scholar] [CrossRef]
- Naganuma, M.; Fujii, T.; Watanabe, M. The use of traditional and newer calcineurin inhibitors in inflammatory bowel disease. J. Gastroenterol. 2011, 46, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Dretzke, J.; Edlin, R.; Round, J.; Connock, M.; Hulme, C.; Czeczot, J.; Fry-Smith, A.; McCabe, C.; Meads, C. A systematic review and economic evaluation of the use of tumour necrosis factor-alpha (TNF-α) inhibitors, adalimumab and infliximab, for Crohn’s disease. Health Technol. Assess. (Rockville) 2011, 15. [Google Scholar] [CrossRef] [PubMed]
- Danese, S. Mechanisms of action of infliximab in inflammatory bowel disease: An anti-inflammatory multitasker. Dig. Liver Dis. 2008, 40, S225–S228. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, W.; et al. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Diamond, R.H.; Wagner, C.L.; Fasanmade, A.A.; Olson, A.D.; Marano, C.W.; Johanns, J.; Lang, Y.; Sandborn, W.J. Clinical trial: Benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment. Pharmacol. Ther. 2009, 30, 210–226. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.; Reinisch, W.; et al. Subcutaneous Golimumab Induces Clinical Response and Remission in Patients With Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2014, 146, 85–95. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Rosh, J.; Faubion, W.A., Jr.; Kierkus, J.; Ruemmele, F.; Hyams, J.S.; Eichner, S.; Li, Y.; Huang, B.; Mostafa, N.M.; et al. Efficacy and Safety of Escalation of Adalimumab Therapy to Weekly Dosing in Pediatric Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 886–893. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Hanauer, S.B.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.G.; Panaccione, R.; Wolf, D.; Kent, J.D.; Bittle, B.; et al. Adalimumab for maintenance treatment of Crohn’s disease: Results of the CLASSIC II trial. Gut 2007, 56, 1232–1239. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Marín, A.C.; McNicholl, A.G.; Chaparro, M. Systematic review with meta-analysis: The efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment. Pharmacol. Ther. 2015, 41, 613–623. [Google Scholar] [CrossRef]
- Sebastian, S.; Ashton, K.; Houston, Y.; Diggory, T.M.; Dore, P. Anti-TNF therapy induced immune neutropenia in Crohns disease- report of 2 cases and review of literature. J. Crohn’s Colitis 2012, 6, 713–716. [Google Scholar] [CrossRef]
- Biancone, L.; Armuzzi, A.; Scribano, M.L.; D’Inca, R.; Castiglione, F.; Papi, C.; Angelucci, E.; Daperno, M.; Mocciaro, F.; Riegler, G.; et al. Inflammatory Bowel Disease Phenotype as Risk Factor for Cancer in a Prospective Multicentre Nested Case-Control IG-IBD Study. J. Crohn’s Colitis 2016, 10, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Peyrin-Biroulet, L. Opportunistic Infections With Anti-Tumor Necrosis Factor-α Therapy in Inflammatory Bowel Disease: Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2013, 108, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Grimaud, J.; Peyrin-Biroulet, L.; Filippi, J.; Pariente, B.; Roblin, X.; Buisson, A.; Stefanescu, C.; Trang-Poisson, C.; Altwegg, R.; et al. Effectiveness and Safety of Vedolizumab Induction Therapy for Patients With Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 1593–1601. [Google Scholar] [CrossRef]
- Schrezenmeir, J.; de Vrese, M. Probiotics, prebiotics, and synbiotics—Approaching a definition. Am. J. Clin. Nutr. 2001, 73, 361s–364s. [Google Scholar] [CrossRef]
- Gill, H.; Prasad, J. Probiotics, Immunomodulation, and Health Benefits. In Bioactive Components of Milk; Springer: New York, NY, USA, 2008; pp. 423–454. [Google Scholar]
- Kruis, W. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef]
- Sood, A.; Midha, V.; Makharia, G.K.; Ahuja, V.; Singal, D.; Goswami, P.; Tandon, R.K. The Probiotic Preparation, VSL#3 Induces Remission in Patients With Mild-to-Moderately Active Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2009, 7, 1202–1209. [Google Scholar] [PubMed]
- Braat, H.; Rottiers, P.; Hommes, D.W.; Huyghebaert, N.; Remaut, E.; Remon, J.P.; van Deventer, S.J.H.; Neirynck, S.; Peppelenbosch, M.P.; Steidler, L.; et al. A Phase I Trial With Transgenic Bacteria Expressing Interleukin-10 in Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2006, 4, 754–759. [Google Scholar] [CrossRef]
- Worthley, D.L.; Le Leu, R.K.; Whitehall, V.L.; Conlon, M.; Christophersen, C.; Belobrajdic, D.; Mallitt, K.; Hu, Y.; Irahara, N.; Ogino, S.; et al. A human, double-blind, placebo-controlled, crossover trial of prebiotic, probiotic, and synbiotic supplementation: Effects on luminal, inflammatory, epigenetic, and epithelial biomarkers of colorectal cancer. Am. J. Clin. Nutr. 2009, 90, 578–586. [Google Scholar] [CrossRef]
- Jia, K.; Tong, X.; Wang, R.; Song, X. The clinical effects of probiotics for inflammatory bowel disease. Medicine 2018, 97, e13792. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Kelly, C.R.; Kahn, S.; Kashyap, P.; Laine, L.; Rubin, D.; Atreja, A.; Moore, T.; Wu, G. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms and Outlook. Gastroenterology 2015, 149, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Paramsothy, R.; Rubin, D.T.; Kamm, M.A.; Kaakoush, N.O.; Mitchell, H.M.; Castano-Rodriguez, N. Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Crohn’s Colitis 2017, 11, 1180–1199. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, B.P.; Vatanen, T.; Allegretti, J.R.; Bai, A.; Xavier, R.J.; Korzenik, J.; Gevers, D.; Ting, A.; Robson, S.C.; Moss, A.C. Increased Intestinal Microbial Diversity Following Fecal Microbiota Transplant for Active Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Collnot, E.-M.; Ali, H.; Lehr, C.-M. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J. Control. Release 2012, 161, 235–246. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Fadda, H.M.; Basit, A.W. Gut instincts: Explorations in intestinal physiology and drug delivery. Int. J. Pharm. 2008, 364, 213–226. [Google Scholar] [CrossRef]
- Sasaki, A.M.Y.; Hada, R.; Nakajima, H.; Fukuda, S. Improved Localizing Method of Radiopill in Measurement of Entire Gastrointestinal pH Profiles: Colonic Luminal pH in Normal Subjects and Patients with Crohn’s Disease. Am. J. Gastroenterol. 1997, 92, 114–118. [Google Scholar]
- Philip, A.; Philip, B. Colon Targeted Drug Delivery Systems: A Review on Primary and Novel Approaches. Oman Med. J. 2010, 25, 70–78. [Google Scholar] [CrossRef]
- Van Citters, G.W.; Lin, H.C. Ileal brake: Neuropeptidergic control of intestinal transit. Curr. Gastroenterol. Rep. 2006, 8, 367–373. [Google Scholar] [CrossRef]
- Yang, L. Biorelevant dissolution testing of colon-specific delivery systems activated by colonic microflora. J. Control. Release 2008, 125, 77–86. [Google Scholar] [CrossRef]
- Musch, M.W.; Wang, Y.; Claud, E.C.; Chang, E.B. Lubiprostone Decreases Mouse Colonic Inner Mucus Layer Thickness and Alters Intestinal Microbiota. Dig. Dis. Sci. 2013, 58, 668–677. [Google Scholar] [CrossRef]
- Goggins, B.J.; Chaney, C.; Radford-Smith, G.L.; Horvat, J.C.; Keely, S. Hypoxia and Integrin-Mediated Epithelial Restitution during Mucosal Inflammation. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Gavhane, Y.N.; Yadav, A.V. Loss of orally administered drugs in GI tract. Saudi Pharm. J. 2012, 20, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Creed, T.J.; Probert, C.S.J. Review article: Steroid resistance in inflammatory bowel disease-mechanisms and therapeutic strategies. Aliment. Pharmacol. Ther. 2007, 25, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Mattingly, C.A.; Tseng, M.T.; Cho, M.J.; Liu, Y.; Adams, V.R.; Mumper, R.J. Doxorubicin and Paclitaxel-Loaded Lipid-Based Nanoparticles Overcome Multidrug Resistance by Inhibiting P-Glycoprotein and Depleting ATP. Cancer Res. 2009, 69, 3918–3926. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Merlin, D. Oral colon-specific therapeutic approaches toward treatment of inflammatory bowel disease. Expert Opin. Drug Deliv. 2012, 9, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, C.A.; Quatacker, J.; Mielants, H.; Vos, M.D.; Veys, E.; Roels, H.J. M cells are damaged and increased in number in inflamed human ileal mucosa. Eur. J. Morphol. 1993, 31, 87–91. [Google Scholar] [CrossRef]
- Pichai, M.V. Potential prospects of nanomedicine for targeted therapeutics in inflammatory bowel diseases. World J. Gastroenterol. 2012, 18, 2895. [Google Scholar] [CrossRef]
- Antoni, L. Intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 1165. [Google Scholar] [CrossRef]
- Han, H.-K.; Shin, H.-J.; Ha, D.H. Improved oral bioavailability of alendronate via the mucoadhesive liposomal delivery system. Eur. J. Pharm. Sci. 2012, 46, 500–507. [Google Scholar] [CrossRef]
- Niebel, W.; Walkenbach, K.; Béduneau, A.; Pellequer, Y.; Lamprecht, A. Nanoparticle-based clodronate delivery mitigates murine experimental colitis. J. Control. Release 2012, 160, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Lautenschläger, C.; Schmidt, C.; Lehr, C.-M.; Fischer, D.; Stallmach, A. PEG-functionalized microparticles selectively target inflamed mucosa in inflammatory bowel disease. Eur. J. Pharm. Biopharm. 2013, 85, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, B.; Khatib, N.; Barenholz, Y.; Nissan, A.; Rubinstein, A. Transferrin as a Luminal Target for Negatively Charged Liposomes in the Inflamed Colonic Mucosa. Mol. Pharm. 2009, 6, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Liu, Z.; Kerdsakundee, N.; Zhang, M.; Zhang, F.; Liu, X.; Bauleth-Ramos, T.; Lian, W.; Makila, E.; et al. Hierarchical structured and programmed vehicles deliver drugs locally to inflamed sites of intestine. Biomaterials 2018, 185, 322–332. [Google Scholar] [CrossRef]
- Jubeh, T.T.; Barenholz, Y.; Rubinstein, A. Differential Adhesion of Normal and Inflamed Rat Colonic Mucosa by Charged Liposomes. Pharm. Res. 2004, 21, 447–453. [Google Scholar] [CrossRef]
- Talaei, F.; Atyabi, F.; Azhdarzadeh, M.; Dinarvand, R.; Saadatzadeh, A. Overcoming therapeutic obstacles in inflammatory bowel diseases: A comprehensive review on novel drug delivery strategies. Eur. J. Pharm. Sci. 2013, 49, 712–722. [Google Scholar] [CrossRef]
- Wilson, D.S.; Dalmasso, G.; Wang, L.; Sitaraman, S.V.; Merlin, D.; Murthy, N. Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines. Nat. Mater. 2010, 9, 923–928. [Google Scholar] [CrossRef]
- Vong, L.B.; Tomita, T.; Yoshitomi, T.; Matsui, H.; Nagasaki, Y. An Orally Administered Redox Nanoparticle That Accumulates in the Colonic Mucosa and Reduces Colitis in Mice. Gastroenterology 2012, 143, 1027–1036. [Google Scholar] [CrossRef]
- Vong, L.B.; Yoshitomi, T.; Morikawa, K.; Saito, S.; Matsui, H.; Nagasaki, Y. Oral nanotherapeutics: Effect of redox nanoparticle on microflora in mice with dextran sodium sulfate-induced colitis. J. Gastroenterol. 2014, 49, 806–813. [Google Scholar] [CrossRef]
- Liu, L.; Yao, W.; Rao, Y.; Lu, X.; Gao, J. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Agoram, B.; Woltosz, W.S.; Bolger, M.B. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv. Drug Deliv. Rev. 2001, 50, S41–S67. [Google Scholar] [CrossRef]
- Beloqui, A.; Coco, R.; Memvanga, P.B.; Ucakar, B.; Rieux, A.D.; Préat, V. pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. Int. J. Pharm. 2014, 473, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Barea, M.J.; Jenkins, M.J.; Gaber, M.H.; Bridson, R.H. Evaluation of liposomes coated with a pH responsive polymer. Int. J. Pharm. 2010, 402, 89–94. [Google Scholar] [CrossRef]
- Lamprecht, A.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. Design of pH-sensitive microspheres for the colonic delivery of the immunosuppressive drug tacrolimus. Eur. J. Pharm. Biopharm. 2004, 58, 37–43. [Google Scholar] [CrossRef]
- Lamprecht, A.; Yamamoto, H.; Ubrich, N.; Takeuchi, H.; Maincent, P.; Kawashima, Y. FK506 Microparticles Mitigate Experimental Colitis with Minor Renal Calcineurin Suppression. Pharm. Res. 2005, 22, 193–199. [Google Scholar] [CrossRef]
- Krishnamachari, Y.; Madan, P.; Lin, S. Development of pH- and time-dependent oral microparticles to optimize budesonide delivery to ileum and colon. Int. J. Pharm. 2007, 338, 238–247. [Google Scholar] [CrossRef]
- Makhlof, A.; Tozuka, Y.; Takeuchi, H. pH-Sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur. J. Pharm. Biopharm. 2009, 72, 1–8. [Google Scholar] [CrossRef]
- Ribeiro, L.N.M.; Alcântara, A.C.S.; Darder, M.; Aranda, P.; Araújo-Moreira, F.M.; Ruiz-Hitzky, E. Pectin-coated chitosan–LDH bionanocomposite beads as potential systems for colon-targeted drug delivery. Int. J. Pharm. 2014, 463, 1–9. [Google Scholar] [CrossRef]
- Naeem, M.; Bae, J.; Oshi, M.A.; Kim, M.; Moon, H.R.; Lee, B.L.; Im, E.; Jung, Y.; Yoo, J. Colon-targeted delivery of cyclosporine A using dual-functional Eudragit® FS30D/PLGA nanoparticles ameliorates murine experimental colitis. Int. J. Nanomed. 2018, 13, 1225–1240. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, N.; Qin, W.; Liu, J.; Jia, Z.; Liu, H. Preparation, In Vitro and In Vivo Evaluation of Budesonide Loaded Core/Shell Nanofibers as Oral Colonic Drug Delivery System. J. Nanosci. Nanotechnol. 2013, 13, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Mongia, P.; Khatik, R.; Raj, R.; Jain, N.; Pathak, A.K. pH-Sensitive Eudragit S-100 Coated Chitosan Nanoparticles of 5-Amino Salicylic Acid for Colon Delivery. J. Biomater. Tissue Eng. 2014, 4, 738–743. [Google Scholar] [CrossRef]
- Oshi, M.A.; Naeem, M.; Bae, J.; Kim, J.; Lee, J.; Hasan, N.; Kim, W.; Im, E.; Jung, Y.; Yoo, J. Colon-targeted dexamethasone microcrystals with pH-sensitive chitosan/alginate/Eudragit S multilayers for the treatment of inflammatory bowel disease. Carbohydr. Polym. 2018, 198, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Asghar, L.F.A.; Chandran, S. Multiparticulate Formulation Approach to Colon Specific Drug Delivery: Current Perspectives. J. Pharm. Pharm. Sci. 2006, 9, 327–338. [Google Scholar] [PubMed]
- Sharpe, L.A.; Daily, A.M.; Horava, S.D.; Peppas, N.A. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin. Drug Deliv. 2014, 11, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-S.; Wang, G.-F.; Zhou, J.; Gao, L.-N.; Cui, Y.-L. Colon targeted oral drug delivery system based on alginate-chitosan microspheres loaded with icariin in the treatment of ulcerative colitis. Int. J. Pharm. 2016, 515, 176–185. [Google Scholar] [CrossRef]
- Oliva, N.; Conde, J.; Wang, K.; Artzi, N. Designing Hydrogels for On-Demand Therapy. Acc. Chem. Res. 2017, 50, 669–679. [Google Scholar] [CrossRef]
- Laroui, H.; Dalmasso, G.; Nguyen, H.T.T.; Yan, Y.; Sitaraman, S.V.; Merlin, D. Drug-Loaded Nanoparticles Targeted to the Colon With Polysaccharide Hydrogel Reduce Colitis in a Mouse Model. Gastroenterology 2010, 138, 843–853. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Chen, L.; Xie, F. Starch film-coated microparticles for oral colon-specific drug delivery. Carbohydr. Polym. 2018, 191, 242–254. [Google Scholar] [CrossRef]
- Bhavsar, M.D.; Amiji, M.M. Gastrointestinal distribution and in vivo gene transfection studies with nanoparticles-in-microsphere oral system (NiMOS). J. Control. Release 2007, 119, 339–348. [Google Scholar] [CrossRef]
- Dongowski, G.; Lorenz, A.; Anger, H. Degradation of Pectins with Different Degrees of Esterification by Bacteroides thetaiotaomicron Isolated from Human Gut Flora. Appl. Environ. Microbiol. 2000, 66, 1321–1327. [Google Scholar] [CrossRef]
- Lee, C.-M.; Kim, D.-W.; Lee, H.-C.; Lee, K.-Y. Pectin microspheres for oral colon delivery: Preparation using spray drying method andin vitro release of indomethacin. Biotechnol. Bioprocess. Eng. 2004, 9, 191–195. [Google Scholar] [CrossRef]
- Günter, E.A.; Markov, P.A.; Melekhin, A.K.; Belozerov, V.S.; Martinson, E.A.; Litvinets, S.G.; Popov, S.V. Preparation and release characteristics of mesalazine loaded calcium pectin-silica gel beads based on callus cultures pectins for colon-targeted drug delivery. Int. J. Biol. Macromol. 2018, 120, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Patole, V.C.; Pandit, A.P. Mesalamine-loaded alginate microspheres filled in enteric coated HPMC capsules for local treatment of ulcerative colitis: In vitro and in vivo characterization. J. Pharm. Investig. 2018, 48, 257–267. [Google Scholar] [CrossRef]
- Iglesias, N.; Galbis, E.; Díaz-Blanco, M.; Lucas, R.; Benito, E.; De-Paz, M.-V. Nanostructured Chitosan-Based Biomaterials for Sustained and Colon-Specific Resveratrol Release. Int. J. Mol. Sci. 2019, 20, 398. [Google Scholar] [CrossRef] [PubMed]
- Helmy, A.M.; Elsabahy, M.; Abd-Elkareem, M.; Ibrahim, E.A.; Soliman, G.M. High-Payload chitosan microparticles for the colonic delivery of quercetin: Development and in-vivo evaluation in a rabbit colitis model. J. Drug Deliv. Sci. Technol. 2020, 58, 101832. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, W. Ligand-mediated active targeting for enhanced oral absorption. Drug Discov. Today 2014, 19, 898–904. [Google Scholar] [CrossRef]
- Laroui, H.; Geem, D.; Xiao, B.; Viennois, E.; Rakhya, P.; Denning, T.; Merlin, D. Targeting Intestinal Inflammation With CD98 siRNA/PEI–loaded Nanoparticles. Mol. Ther. 2014, 22, 69–80. [Google Scholar] [CrossRef]
- Sans, M.; Panes, J.; Ardite, E.; Elizalde, J.I.; Arce, Y.; Elena, M.; Palacin, A.; Fernandez-Checa, J.C.; Anderson, D.C.; Lobb, R.; et al. VCAM-1 and ICAM-1 mediate leukocyte-endothelial cell adhesion in rat experimental colitis. Gastroenterology 1999, 116, 874–883. [Google Scholar] [CrossRef]
- Muro, S.; Mane, V. Biodistribution and endocytosis of ICAM-1-targeting antibodies versus nanocarriers in the gastrointestinal tract in mice. Int. J. Nanomed. 2012, 7, 4223. [Google Scholar] [CrossRef]
- Xiao, B.; Laroui, H.; Ayyadurai, S.; Viennois, E.; Charania, M.A.; Zhang, Y.; Merlin, D. Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-α RNA interference for IBD therapy. Biomaterials 2013, 34, 7471–7482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, C.; Yin, C. Galactosylated trimethyl chitosan–cysteine nanoparticles loaded with Map4k4 siRNA for targeting activated macrophages. Biomaterials 2013, 34, 3667–3677. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, Z.; Viennois, E.; Kang, Y.; Zhang, M.; Han, M.K.; Chen, J.; Merlin, D. Combination Therapy for Ulcerative Colitis: Orally Targeted Nanoparticles Prevent Mucosal Damage and Relieve Inflammation. Theranostics 2016, 6, 2250–2266. [Google Scholar] [CrossRef]
- Vafaei, S.Y.; Esmaeili, M.; Amini, M.; Atyabi, F.; Ostad, S.N.; Dinarvand, R. Self assembled hyaluronic acid nanoparticles as a potential carrier for targeting the inflamed intestinal mucosa. Carbohydr. Polym. 2016, 144, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Moulari, B.; Béduneau, A.; Pellequer, Y.; Lamprecht, A. Lectin-decorated nanoparticles enhance binding to the inflamed tissue in experimental colitis. J. Control. Release 2014, 188, 9–17. [Google Scholar] [CrossRef]
- Naserifar, M.; Hosseinzadeh, H.; Abnous, K.; Mohammadi, M.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Oral delivery of folate-targeted resveratrol-loaded nanoparticles for inflammatory bowel disease therapy in rats. Life Sci. 2020, 262, 118555. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Harel, E.; Rubinstein, A.; Nissan, A.; Khazanov, E.; Milbauer, M.N.; Barenholz, Y.; Tirosh, B. Enhanced Transferrin Receptor Expression by Proinflammatory Cytokines in Enterocytes as a Means for Local Delivery of Drugs to Inflamed Gut Mucosa. PLoS ONE 2011, 6, e24202. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T.; Dalmasso, G.; Yan, Y.; Laroui, H.; Dahan, S.; Mayer, L.; Sitaraman, S.V.; Merlin, D. MicroRNA-7 Modulates CD98 Expression during Intestinal Epithelial Cell Differentiation. J. Biol. Chem. 2010, 285, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, T.; Kraal, G. A function for the macrophage F4/80 molecule in tolerance induction. Trends Immunol. 2005, 26, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Laroui, H.; Viennois, E.; Xiao, B.; Canup, B.S.B.; Geem, D.; Denning, T.L.; Merlin, D. Fab’-bearing siRNA TNFα-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis. J. Control. Release 2014, 186, 41–53. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Peterson, D.A.; Frank, D.N.; Pace, N.R.; Gordon, J.I. Metagenomic Approaches for Defining the Pathogenesis of Inflammatory Bowel Diseases. Cell Host Microbe 2008, 3, 417–427. [Google Scholar] [CrossRef]
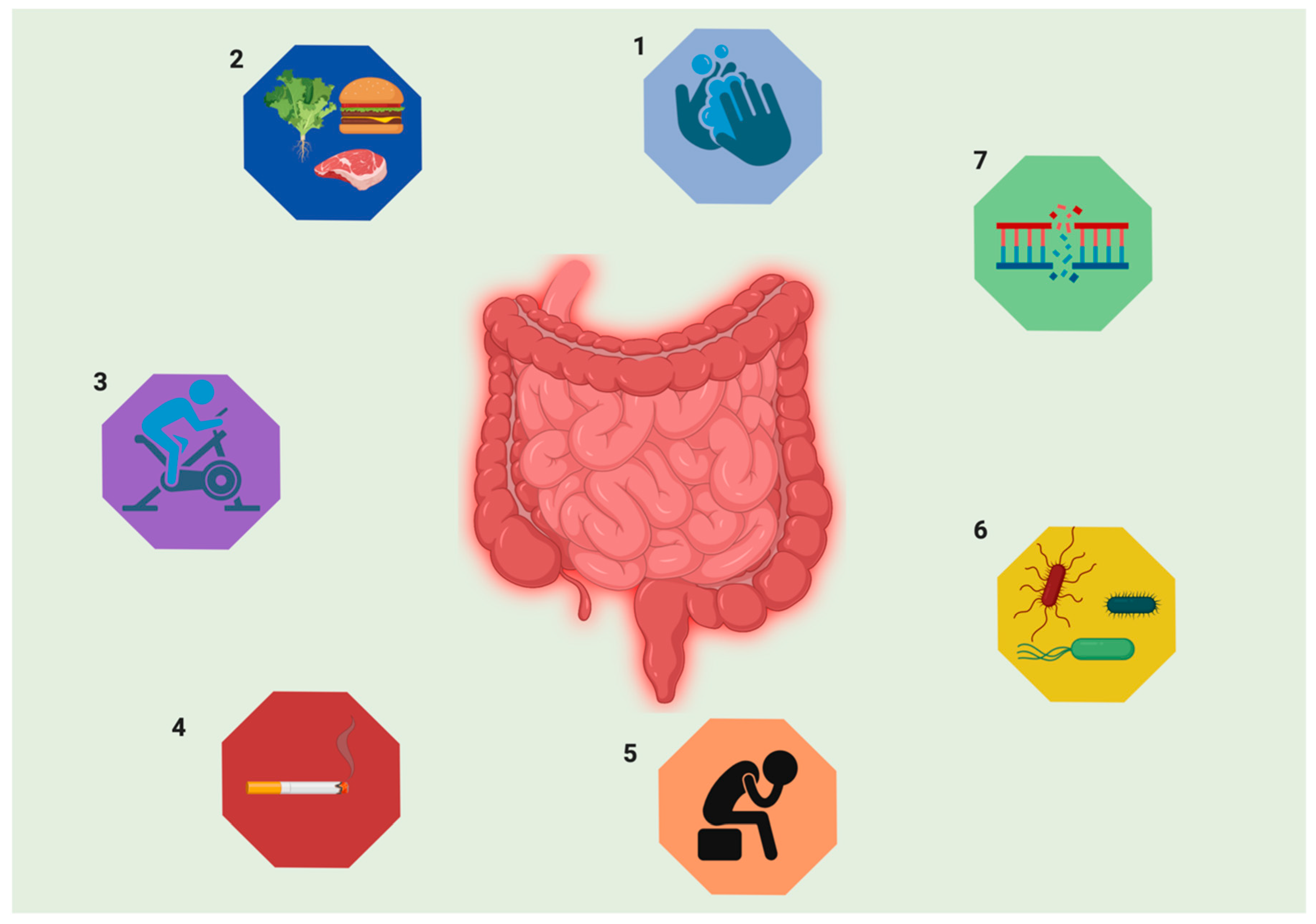
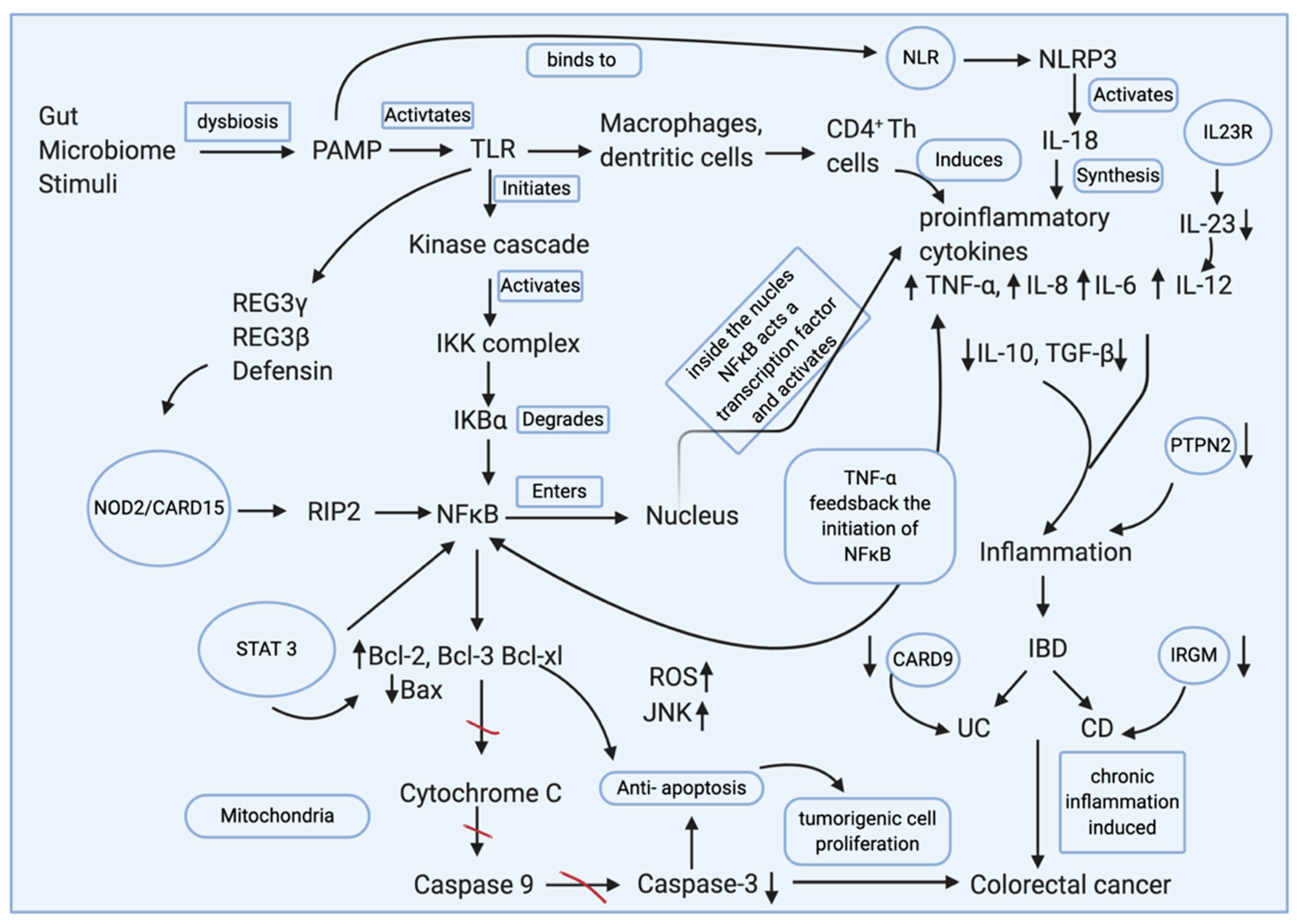
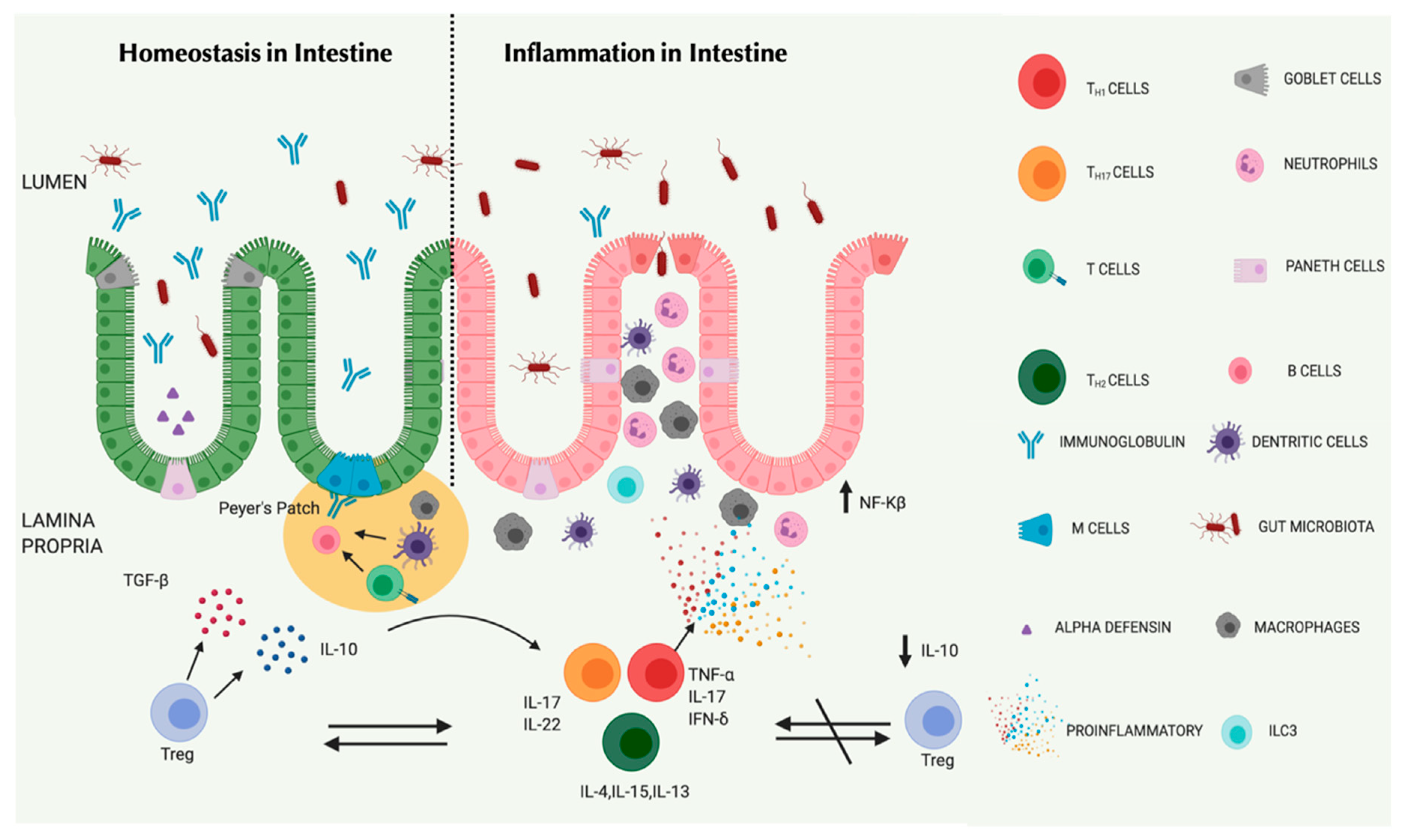
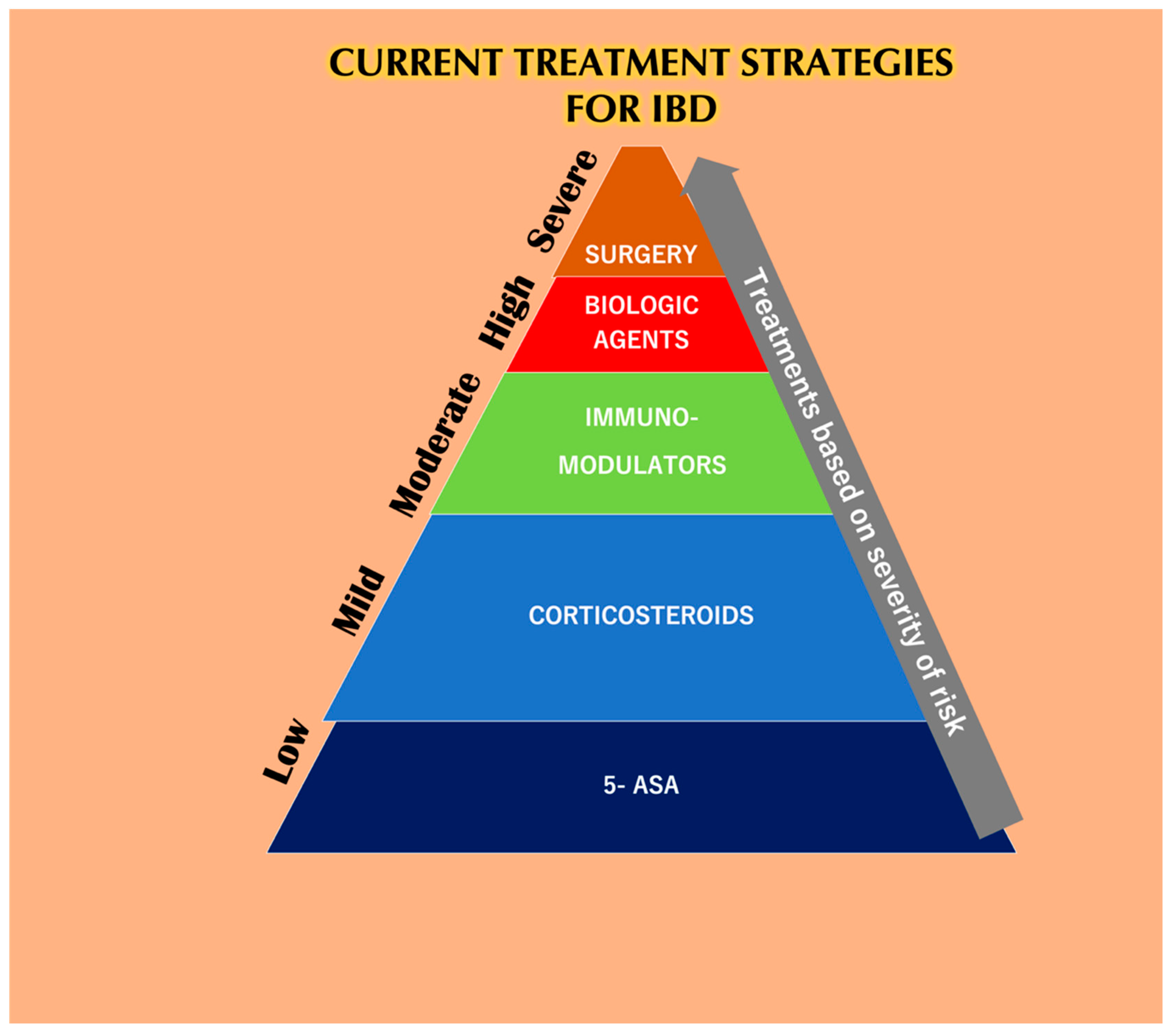
| Parameters | UC | CD |
|---|---|---|
| Gender [26,27] | Males | Females |
| Onset [28] | 30–40 years of age | 20–30 years of age |
| Site | Distal colon [3] | Any part of the gastrointestinal tract (GIT) [2] |
| Complications | Cryptitis, fistulas, severe bleeding [3], and colon cancer | Strictures, abscesses, fistulas [2], colon cancer |
| Smoking | Protective role-reduced colectomy and sclerosing cholangitis [29] | Threatening—leading to gut irritation, an impaired immune response, and respiratory disease [30] |
| Post-Surgery | Surgically curable but higher risk for adults above 50 years due to postoperative complications like infections and abscesses [31] | Relapse even after the surgical removal of the affected portion and postoperative complications like short bowel syndrome [32] |
| Nanoparticles | Therapeutic Agent | Characteristics |
|---|---|---|
| Cationic Polymethacrylate (Eudragit RL) NPs | Clodronate | Decreased MPO activity in TNBS- and OXA-induced-colitis compared to free clodronate [175] |
| Polymeric (poly 1,4-phenyl acetone dimethylene-thioketal) NPs complexed with DOTAP | TNF- small interfering ribonucleic acid (siRNA) | Thioketal NPs degrade in response to ROS, and DOTAP enhances mucosal transport. Protects UC by decreasing TNF- level [182] |
| Nitroxide-radical NPs comprising of block copolymer methoxy-poly(ethylene glycol)-b-poly(4-(2,26,6-tetramethyl-piperidine-1-oxyl)oxymethylstyrene (MeO-PEG-b-PMOT) | TEMPOL | Reduction in colitis with successful scavenging of ROS [183] |
| Poly(lactic-co-glycolic acid) (PLGA) NPs encapsulated inside Eudragit P4135F microspheres | Tacrolimus | Provided protection to the drug at acidic pH [189] |
| PLGA/Eudragit NPs | Budesonide (BSD) | Sustained release of BSD at colonic pH and improved therapeutic effects of BSD against TNBS-induced colitis [192] |
| PLGA/Eudragit S100 NPs | Curcumin | Reduced TNF- secretion in LPS-activated macrophages. Reduced neutrophil infiltration in a DSS-colitis model [187] |
| Pectin coated-chitosan-hydroxide biohybrid beads | 5-ASA | Protection to NPs to transport through gastric juices in upper GIT and sustained release of the drugs at 7.4 [193] |
| PLGA-coated Eudragit S100 nanospheres | Cyclosporine | Sustained release at 7.4 and used in UC therapy [194] |
| Eudragit S100/ethyl cellulose nanofibers | Budesonide (BSD) | Sustained release of drug at a pH of 7.4 [195] |
| Chitosan and alginate coated with Eudragit S100 microcrystals | Dexamethasone | Release of drug in colonic pH and alleviate inflammation in DSS-colitis model [197] |
| Chitosan and alginate hydrogel/PLA NPs | Anti-inflammatory tripeptide Lys–Pro–Val (KPV) | Reduced MPO activity and colitis symptoms [202] |
| Cross-linked pectin microspheres | Indomethacin | Increased colon-specific delivery at a pH of 7.4 [206] |
| Calcium pectin–silica gel beads | Mesalazine | Controlled release of mesalazine at the simulated upper GIT condition and increased mesalazine release in the simulated colonic fluid [207] |
| Grapefruit-derived nanovesicles | Methotrexate | Downregulation of IL-1 and TNF- by upregulating release of heme-oxygenase I and induced anti-inflammatory properties against DSS-IBD [211] |
| Ginger-derived NPs | Ginger | Elevated anti-inflammatory cytokines and downregulated concertation’s of pro-inflammatory cytokines TNF-, IL-1, and IL-6 [212] |
| PEI NPs | Anti-cluster of differentiation (CD)98 siRNA | Reduction of CD98 expression in DSS-induced colitis [215] |
| Mannosylated bio-reducible cationic polymeric NPs | TNF- siRNA | Reduction in TNF- expression in DSS-induced colitis [218] |
| Galactosylated trimethyl chitosan cysteine NPs | Mitogen-Activated Protein Kinase Kinase Kinase Kinase (Map4k4) siRNA | Blocks the TNF- production in DSS-induced UC [219] |
| Peanut and wheat germ-lecithin decorated NPs | Betamethasone | Specificity towards inflamed tissues having high expression of lecithin [222] |
| PLA-PEG (poly (lactic acid) poly (ethylene glycol) block copolymer) grafted with the Fab’ portion of F4/F80 Ab | TNF- | Enhanced macrophage-targeting and endocytosis of NPs. Oral administration led to attention in DSS-induced colitis [229] |
| Folic acid-conjugated PLGA NPs | Resveratrol | Oral delivery reduced colitis in TNBS-induced rat model [223] |
| Chitosan microparticle | Quercetin | Oral administration reduced colitis in an acetic acid-induced rabbit model compared to free quercetin [210] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, E.M.; Borah, A.; Pillai, S.C.; Kumar, D.S. Inflammatory Bowel Disease: The Emergence of New Trends in Lifestyle and Nanomedicine as the Modern Tool for Pharmacotherapy. Nanomaterials 2020, 10, 2460. https://doi.org/10.3390/nano10122460
Jacob EM, Borah A, Pillai SC, Kumar DS. Inflammatory Bowel Disease: The Emergence of New Trends in Lifestyle and Nanomedicine as the Modern Tool for Pharmacotherapy. Nanomaterials. 2020; 10(12):2460. https://doi.org/10.3390/nano10122460
Chicago/Turabian StyleJacob, Eden Mariam, Ankita Borah, Sindhu C Pillai, and D. Sakthi Kumar. 2020. "Inflammatory Bowel Disease: The Emergence of New Trends in Lifestyle and Nanomedicine as the Modern Tool for Pharmacotherapy" Nanomaterials 10, no. 12: 2460. https://doi.org/10.3390/nano10122460
APA StyleJacob, E. M., Borah, A., Pillai, S. C., & Kumar, D. S. (2020). Inflammatory Bowel Disease: The Emergence of New Trends in Lifestyle and Nanomedicine as the Modern Tool for Pharmacotherapy. Nanomaterials, 10(12), 2460. https://doi.org/10.3390/nano10122460




