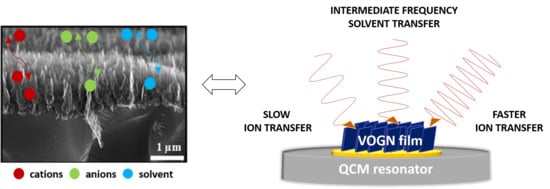
Deciphering the Influence of Electrolytes on the Energy Storage Mechanism of Vertically-Oriented Graphene Nanosheet Electrodes by Using Advanced Electrogravimetric Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Growth of VOGNs
2.3. Morphological Characterization
2.4. Electrochemical Characterization
2.5. Electrogravimetric Analysis
3. Results
3.1. VOGNs
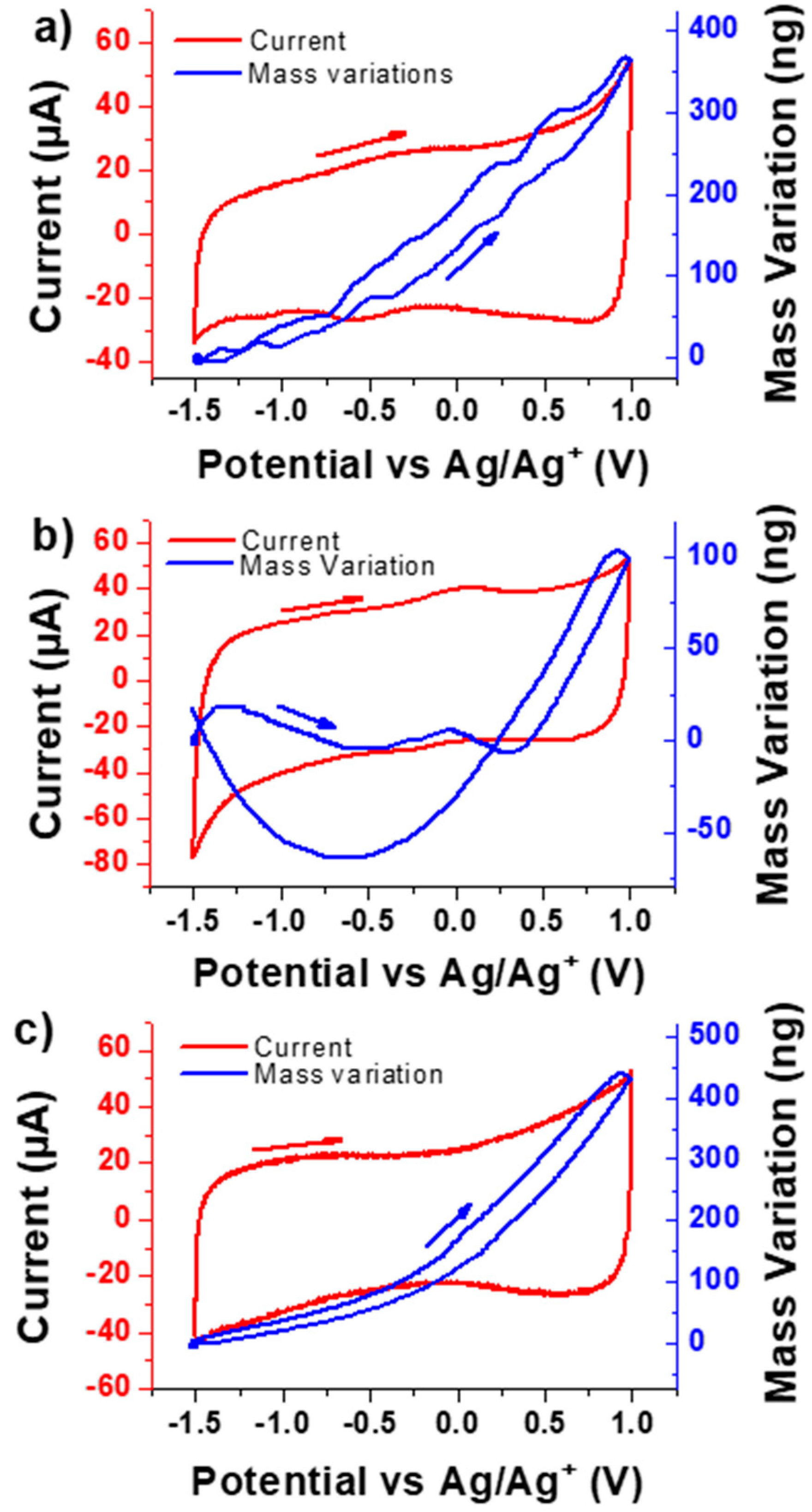
3.2. Electrochemical Performance and EQCM Results
3.3. Ac-Electrogravimetry
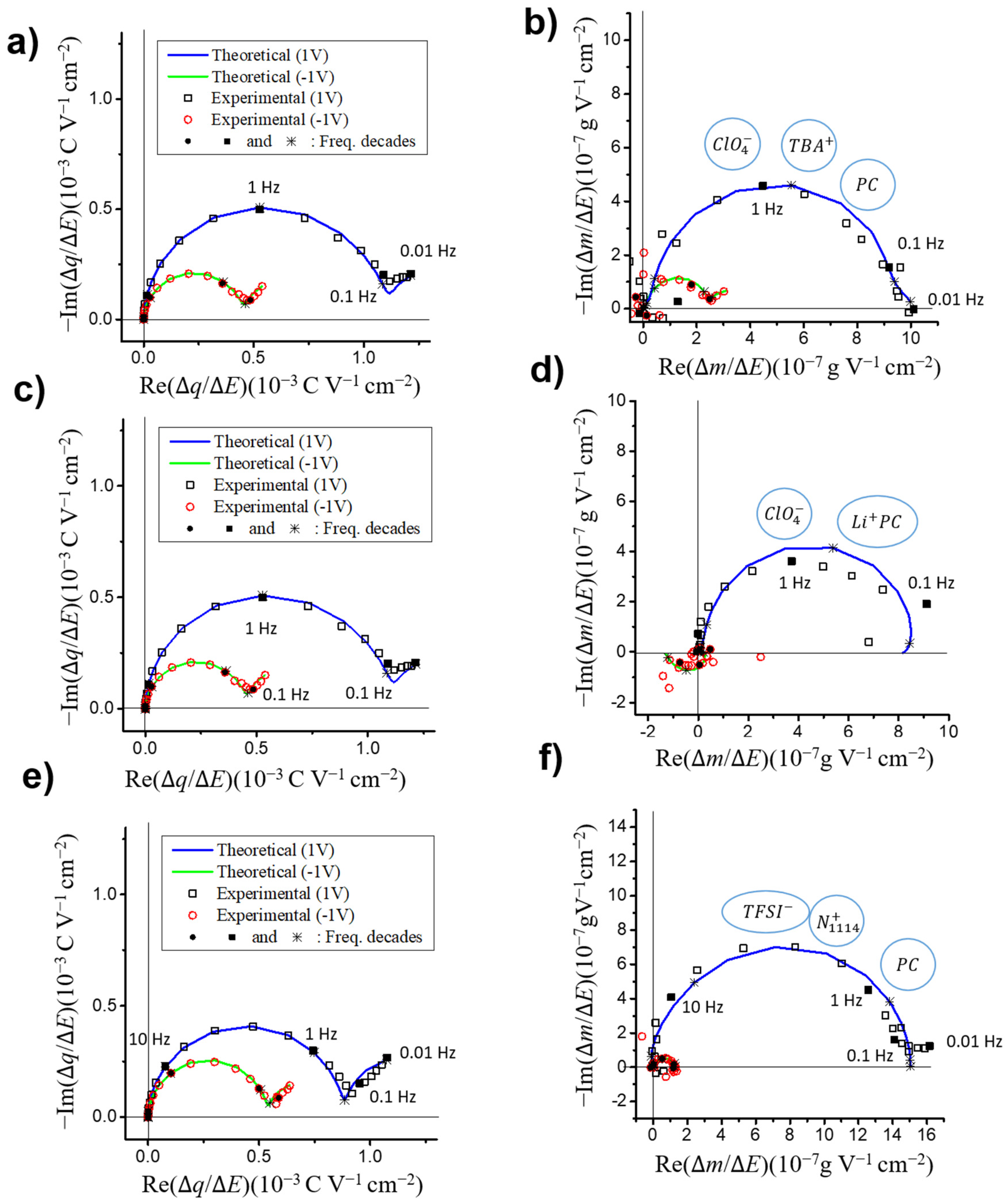
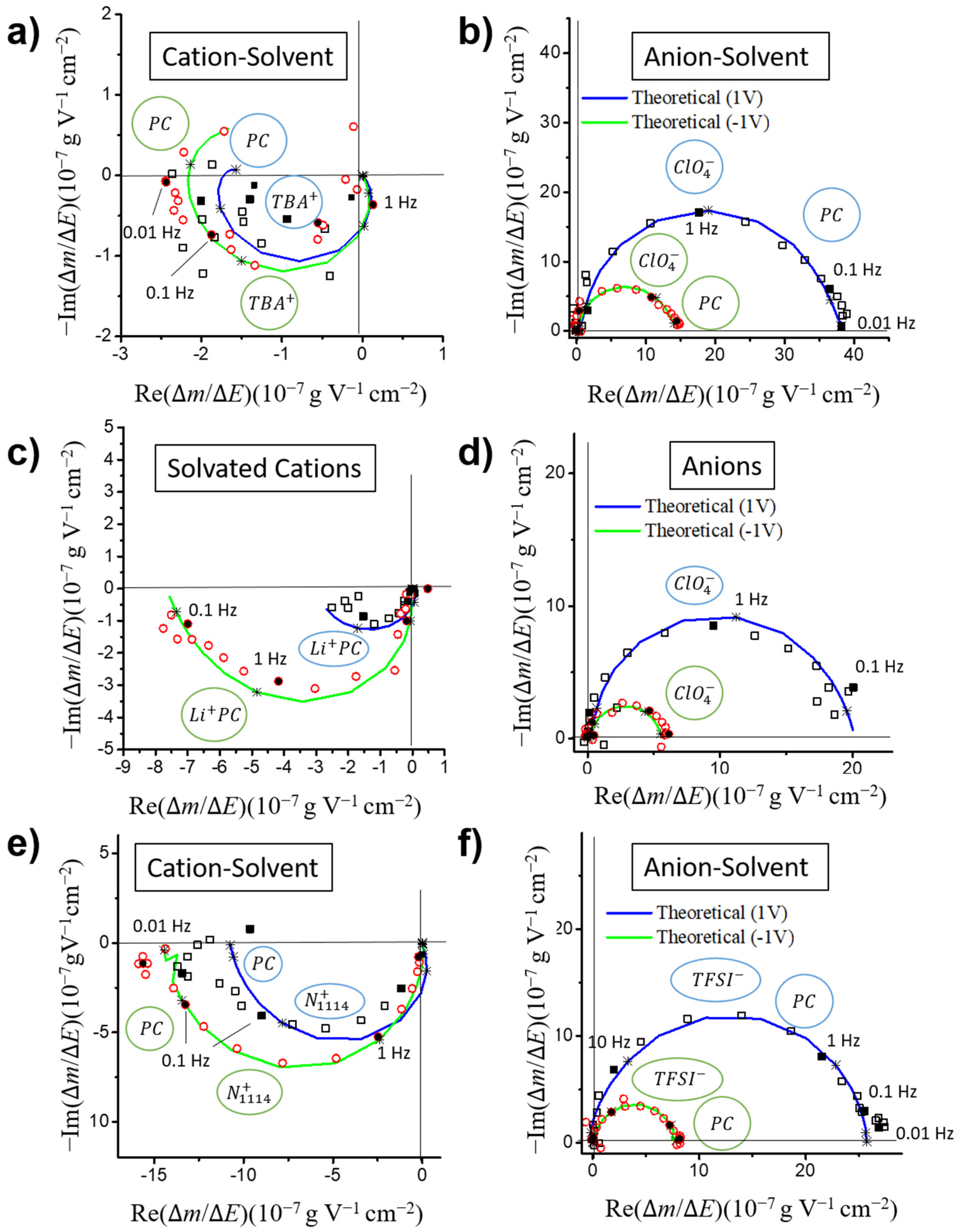
3.3.1. Partial Mass/Potential TFs-Separating Each Species Contribution
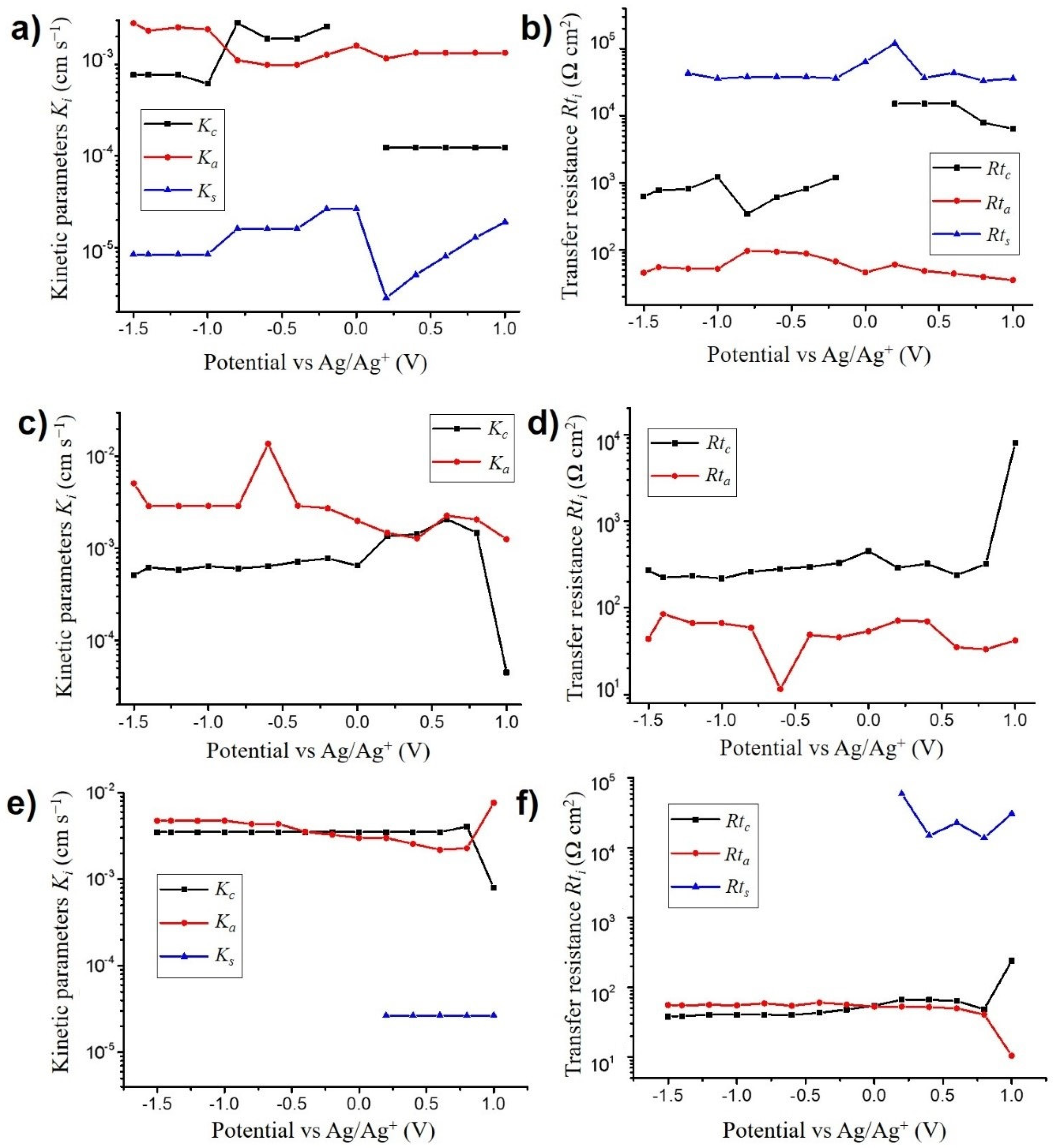
3.3.2. Kinetic Parameters and Transfer Resistance
3.3.3. Concentration and Mass Variations for Each Species
4. Discussion
- (a)
- VOGNs-PC/TBAClO4: A major contribution of ClO4− anions was found that they are faster than their corresponding cation (TBA+) counterparts at most of the potentials, as well as at higher concentration variations along the whole electrochemical window (−1.5 to 1 V). Consequently, an anion electro-adsorption process was identified to be the most predominant energy storage mechanism, while cations and free solvent molecules are given non-negligible supporting roles. This tendency was found to be similar to PC/TBABF4 already examined through similar electrogravimetric methods [45].
- (b)
- VOGNs-PC/LiClO4: The nature of the cation greatly affects the electrochemical behavior as evidenced in the particular case of PC/LiClO4. Monosolvated Li+ cations (PC-Li+) and ClO4− anions were found to play key roles in the energy storage mechanism of VOGNs. Thus, solvated Li+ cations predominates at negative potentials (−1.5 to −0.5 V) as shown by its higher concentration, whereas ClO4− is more predominant at positive potentials. A V-shape gravimetric response was obtained in this case.
- (c)
- VOGNs-PC/N1114TFSI: The high deviation of MPE values compared to the theoretical molar mass of N1114+ and TFSI− showed that anions are not the only species exchanged on the energy storage mechanism of VOGN. The ac-electrogravimetry allowed unveiling that N1114+ cations and TFSI− anions, with an addition of PC molecules, play a key role. More specifically, both cations and anions exhibited similar kinetic parameters and transfer resistance values, as well as the same magnitude of concentration variations in the studied potential window. Consequently, both species contributed equally to the capacitive properties of VOGNs.
- (d)
- The areal capacitance values obtained by varying the nature of the electrolyte have shown no significant difference in spite of the change in size and the molar mass of the anions and cations. However, the VOGNs-PC/TBAClO4 electrode/electrolyte configuration shows a good permselectivity behavior in the larger potential window and for this reason can be recommended, among the others investigated in this work.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lethien, C.; Le Bideau, J.; Brousse, T. Challenges and prospects of 3D micro-supercapacitors for powering the internet of things. Energy Environ. Sci. 2019, 12, 96–115. [Google Scholar] [CrossRef]
- Anuradha, J.; Tripathy, B.K. (Eds.) Internet of Things (IoT): Technologies, Applications, Challenges and Solutions; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon-on-Thames, UK, 2018. [Google Scholar]
- Xiao, P. Designing Embedded Systems and the Internet of Things (IoT) with the ARM MbedTM; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Kyeremateng, N.A.; Brousse, T.; Pech, D. Micro-supercapacitors as miniaturized energy-storage components for on-chip electronics. Nat. Nanotechnol. 2017, 12, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, Y.; Chee, M.O.L.; Dong, P.; Ye, M.; Shen, J. Recent advances in micro-supercapacitors. Nanoscale 2019, 11, 5807–5821. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zao, H.; Lei, Y. Advances on three-dimensional electrodes for micro-supercapacitors: A mini-review. InfoMat 2019, 1, 74–84. [Google Scholar] [CrossRef]
- Beidaghi, M.; Gogotsi, Y. Capacitive energy storage in micro-scale devices: Recent advances in design and fabrication of micro-supercapacitors. Energy Environ. Sci. 2014, 7, 867–884. [Google Scholar] [CrossRef]
- Lin, F.; Simon, P. MXene for supercapacitor applications. In 2D Metal Carbides and Nitrides (MXenes), 1st ed.; Anasori, B., Gogotsi, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 7, pp. 349–365. [Google Scholar]
- Aradilla, D.; Sadki, S.; Bidan, G. Beyond conventional supercapacitors: Hierarchically conducting polymer-coated 3D nanostructures for integrated on-chip micro-supercapacitors employing ionic liquid electrolytes. Synth. Met. 2019, 247, 131–143. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Karuppasamy, K.; Durai, G.; Rana, A.; Arunachalam, P.; Sangeetha, K.; Kuppusami, P.; Kim, H.S. Recent advances in metal chalcogenides (MX; X=S, Se) nanostructures for electrochemical supercapacitor applications: A brief review. Nanomaterials 2018, 8, 256. [Google Scholar] [CrossRef]
- Abdah, M.A.A.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Des. 2020, 186, 108199. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Chen, Y. An overview of the applications of graphene-based materials in supercapacitors. Small 2012, 8, 1805–1834. [Google Scholar] [CrossRef]
- Thomas, P.; Rumjit, N.P.; Lai, C.W.; Johan, M.R.B. Future prospects and challenges of graphene-based supercapacitors. Mater. Res. Found. 2020, 64, 257–275. [Google Scholar]
- Urade, A.; Kaur, G.; Lahiri, I. Chapter 5—Graphene based materials for micro-supercapacitor. In Graphene as Energy Storage Material for Supercapacitors, 1st ed.; Boddula, R., Faraz, M., Asiri, A.M., Eds.; Material Research Forum LLC: Millersville, PA, USA, 2020; Volume 64, pp. 129–166. [Google Scholar]
- Bo, Z.; Mao, S.; Han, Z.J.; Cen, K.; Chen, J.; Ostrikov, K. Emerging energy and environmental applications of vertically-oriented graphenes. Chem. Soc. Rev. 2015, 44, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Feng, X.; Cheng, H.-M. Recent advances in graphene-based planar micro-supercapacitors for on-chip energy storage. Natl. Sci. Rev. 2014, 1, 277–292. [Google Scholar] [CrossRef]
- Chang, Z.; Lee, C.-S.; Zhang, W. Vertically aligned graphene nanosheet array: Synthesis, properties and applications in electrochemical energy conversion and storage. Adv. Energy Mater. 2017, 7, 1700678. [Google Scholar]
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetic, M. Synthesis of vertically oriented graphene sheets or carbon nanowalls: Review and challenges. Materials 2019, 12, 2968. [Google Scholar] [CrossRef]
- Chen, J.; Bo, Z.; Lu, G. Vertically-oriented graphene for supercapacitors. In Vertically-Oriented Graphene: PECVD Synthesis and Applications; Springer: Berlin/Heidelberg, Germany, 2016; Chapter 7; pp. 79–95. [Google Scholar]
- Rajendran, S.; Naushad, M.; Balakumar, S. Nanostructured emerging vertical nanostructures for high-performance supercapacitor applications. In Nanostructured Materials for Energy Related Applications; Springer: Berlin/Heidelberg, Germany, 2019; Chapter 7; pp. 163–187. [Google Scholar]
- Forse, A.C.; Merlet, C.; Griffin, J.M.; Grey, C.P. New perspectives on the charging mechanisms of supercapacitors. J. Am. Chem. Soc. 2016, 138, 5731–5744. [Google Scholar] [CrossRef]
- Lin, Z.; Taberna, P.-L.; Simon, P. Electrochemical double layer capacitors: What is next beyond the corner? Curr. Opin. Electrochem. 2017, 6, 115–119. [Google Scholar] [CrossRef]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.-L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient storage mechanisms for building better supercapacitors. Nat. Energy 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Salanne, M. Theoretical studies of supercapacitors. Chem. Modell. 2016, 12, 119–150. [Google Scholar]
- Marrocchelli, D.; Merlet, C.; Salanne, M. Molecular dynamics simulations of electrochemical energy storage devices. In Physical Multiscale Modeling and Numerical Simulation of Electrochemical devices for Energy Conversion and Storage; Green Energy and Technology; Franco, A., Doublet, M., Bessler, W., Eds.; Springer: London, UK, 2016. [Google Scholar]
- Pean, C.; Rotenberg, B.; Simon, P.; Salanne, M. Understanding the different (dis)charging steps of supercapacitors: Influence of potential and solvation. Electrochim. Acta 2016, 206, 504–512. [Google Scholar] [CrossRef]
- Lin, Z.; Taberna, P.-L.; Simon, P. Advanced analytical techniques to characterize materials for electrochemical capacitors. Curr. Opin. Electrochem. 2018, 9, 18–25. [Google Scholar] [CrossRef]
- Hui, J.; Gossage, Z.T.; Sarbapalli, D.; Hernandez-Burgos, K.; Rodriguez-Lopez, J. Advanced electrochemical analysis for energy storage interfaces. Anal Chem. 2019, 91, 60–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Forse, A.C.; Griffin, J.M.; Trease, N.M.; Trongko, L.; Taberna, P.-L.; Simon, P.; Grey, C.P. In situ NMR spectroscopy of supercapacitors: Insight into the charge storage mechanism. J. Am. Chem. Soc. 2013, 135, 18968–18980. [Google Scholar] [CrossRef] [PubMed]
- Forse, A.; Griffin, J.; Merlet, C.; Gonzalez, J.C.; Raji, A.; Trease, N.; Grey, C. Direct observation of ion dynamics in supercapacitor electrodes using in situ diffusion NMR spectroscopy. Nat. Energy 2017, 2, 16216. [Google Scholar] [CrossRef]
- Oukali, G.; Salager, E.; Ammar, M.R.; Dutoit, C.-E.; Sarou-Kanian, V.; Simon, P.; Raymundo Pinero, E.; Deschamps, M. In situ magnetic resonance imaging of a complete supercapacitor giving additional insight on the role of nanopores. ACS Nano 2019, 13, 12810–12815. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Malveau, C.; Rochefort, D. Solid-state NMR and electrochemical dilatometry study of charge storage in supercapacitor with redox ionic liquid electrolyte. Energy Storage Mater. 2019, 20, 80–88. [Google Scholar] [CrossRef]
- Griffin, J.M.; Forse, A.C.; Tsai, W.-Y.; Taberna, P.-L.; Simon, P.; Grey, C.P. In situ NMR and electrochemical quartz crystal microbalance techniques reveal the structure of the electrical double layer in supercapacitors. Nat. Mater. 2015, 14, 812–819. [Google Scholar] [CrossRef]
- Wang, B.; Fielding, A.J.; Dryfe, R.A.W. In situ electrochemical electron paramagnetic resonance spectroscopy as a tool to probe electrical double layer capacitance. Chem. Commun. 2018, 54, 3827–3830. [Google Scholar] [CrossRef]
- Tsai, W.-Y.; Taberna, P.-L.; Simon, P. Eelctrochemical quartz crystal microbalance (EQCM) study of ion dynamics in nanoporous carbons. J. Am. Chem. Soc. 2014, 136, 8722–8728. [Google Scholar] [CrossRef]
- Levi, M.D.; Daikhin, L.; Aurbarch, D.; Presser, V. Quartz crystal microbalance with dissipation monitoring (EQCM-D) for in-situ studies of electrodes for supercapacitors and batteries: A mini-review. Electrochem. Commun. 2016, 67, 16–21. [Google Scholar] [CrossRef]
- Shpigel, N.; Levi, M.D.; Aurbach, D. EQCM-D technique for complex mechanical characterization of energy storage electrodes: Background and practical guide. Energy Stor. Mater. 2019, 21, 399–413. [Google Scholar] [CrossRef]
- Levi, M.D.; Shpigel, N.; Sigalov, S.; Dargel, V.; Daikhin, L.; Aubarch, D. In situ porous structure characterization of electrodes for energy storage and conversion by EQCM-D: A review. Electrochim. Acta 2017, 232, 271–284. [Google Scholar] [CrossRef]
- Shpigel, N.; Levi, M.D.; Sigalov, S.; Daikhin, L.; Aubarch, D. In situ real-time mechanical and morphological characterization of electrodes for electrochemical energy storage and conversion by electrochemical quartz crystal microbalance with dissipation monitoring. Acc. Chem. Res. 2018, 51, 69–79. [Google Scholar] [CrossRef]
- Shao, H.; Wu, Y.-C.; Lin, Z.; Taberna, P.-L.; Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 2020, 49, 3005–3039. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Teran, F.; Perrot, H.; Sel, O. Ion dynamics at the single wall carbon nanotube based composite electrode/electrolyte interface: Influence of the cation size and electrolyte pH. J. Phys. Chem. C 2019, 123, 4263–4273. [Google Scholar] [CrossRef]
- Yi, F.; Huang, Y.; Gao, A.; Zhang, F.; Shu, D.; Chen, W.; Cheng, H.; Zhou, X.; Zeng, R. Investigation on the pseudocapacitive charge storage mechanism of MnO2 in various electrolytes by electrochemical quartz crystal microbalance (EQCM). Ionics 2019, 25, 2393–2399. [Google Scholar] [CrossRef]
- Arias, C.R.; Debiemme-Chouvy, C.; Gabrielli, C.; Laberty-Robert, C.; Pailleret, A.; Perrot, H.; Sel, O. New insights into pseudocapacitive charge-storage mechanisms in Li-birnessite type MnO2 monitored by fast quartz crystal microbalance methods. J. Phys. Chem. C 2014, 118, 26551–26559. [Google Scholar] [CrossRef]
- Djire, A.; Pande, P.; Deb, A.; Siegel, J.B.; Ajenifujah, O.T.; He, L.; Sleightholme, A.E.; Rasmussen, P.G.; Thompson, L.T. Unveiling the pseudocapacitive charge storage mechanisms of nanostructured vanadium nitrides using in-situ analyses. Nano Energy 2019, 60, 72–81. [Google Scholar] [CrossRef]
- Lé, T.; Aradilla, D.; Bidan, G.; Billon, F.; Delaunay, M.; Gérard, J.-M.; Perrot, H.; Sel, O. Unveiling the ionic exchange mechanisms in vertically-oriented graphene nanosheet supercapacitor electrodes with electrochemical quartz crystal microbalance and ac-electrogravimetry. Electrochem. Commun. 2018, 93, 5–9. [Google Scholar] [CrossRef]
- Lé, T.; Bidan, G.; Gentile, P.; Billon, F.; Debiemme-Chouvy, C.; Perrot, H.; Sel, O.; Aradilla, D. Understanding the energy storage mechanisms of poly(3,4-ethylenedioxythiophene)-coated silicon nanowires by electrochemical quartz crystal microbalance. Mater. Lett. 2019, 5, 31–34. [Google Scholar] [CrossRef]
- Lé, T.; Aradilla, D.; Bidan, G.; Billon, F.; Debiemme-Chouvy, F.; Perrot, H.; Sel, O. Charge storage properties of nanostructured poly(3,4-ethylenedioxythiophene) electrodes revealed by advanced electrogravimetry. Nanomaterials 2019, 9, 962. [Google Scholar] [CrossRef]
- Bourkane, S.; Gabrielli, C.; Keddam, M. Kinetic study of electrode processes by ac quartz electrogravimetry. J. Electroanal. Chem. Interfacial Electrochem. 1988, 256, 471–475. [Google Scholar] [CrossRef]
- Delaunay, M. Permanent magnet linear microwave plasma source. U.S. Patent Application No. 6319372 B1, 26 January 1987. [Google Scholar]
- Aradilla, D.; Delaunay, M.; Sadki, S.; Gérard, J.-M.; Bidan, G. Vertically aligned graphene nanosheets on silicon using an ionic liquid electrolyte: Towards high performance on-chip micro-supercapacitors. J. Mater. Chem. A 2015, 3, 19254–19262. [Google Scholar] [CrossRef]
- Lé, T.; Gentile, P.; Bidan, G.; Aradilla, D. New electrolyte mixture of propylene carbonate and butyltrimethylammonium bis (trifluoromethylsulfonyl) imide (N1114 TFSI) for high performance silicon nanowire (SiNW)-based supercapacitor applications. Electrochim. Acta 2017, 254, 368–374. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Für Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Jakab, S.; Picart, S.; Tribollet, B.; Rousseau, P.; Perrot, H.; Gabrielli, C. Study of the dissolution of thin films of cerium oxide by using a GaPO4 crystal microbalance. Anal. Chem. 2009, 81, 5139–5145. [Google Scholar] [CrossRef]
- Escobar-Teran, F.; Arnau, A.; Garcia, J.; Jiménez, Y.; Perrot, H.; Sel, O. Gravimetric and dynamic deconvolution of global EQCM response of carbon nanotube based electrodes by ac-electrogravimetry. Electrochem. Commun. 2016, 70, 73–77. [Google Scholar] [CrossRef]
- Gao, W.; Debiemme-Chouvy, C.; Lahcini, M.; Perrot, H.; Sel, O. Tuning charge storage properties of supercapacitive electrodes evidenced by in situ gravimetric and viscoelastic explorations. Anal. Chem. 2019, 91, 2885–2893. [Google Scholar] [CrossRef]
- Gabrielli, C.; Jareno, J.; Keddam, M.; Perrot, H.; Vicente, F. Ac-electrogravimetry study of electroactive thin films I. Application to prussian blue. J. Phys. Chem. B 2002, 106, 3182–3191. [Google Scholar] [CrossRef]
- Gabrielli, C.; Jareno, J.; Keddam, M.; Perrot, H.; Vicente, F. Ac-electrogravimetry study of electroactive thin films II. Application to polypyrrole. J. Phys. Chem. B 2002, 106, 3192–3201. [Google Scholar] [CrossRef]
- Daikhin, L.; Gileadi, E.; Katz, G.; Tsionsky, V.; Urbakh, M.; Zagidulin, D. Influence of roughness on the admittance of the quartz crystal microbalance immersed in liquids. Anal. Chem. 2002, 74, 554–561. [Google Scholar] [CrossRef]
- Ghosh, S.; Mathews, T.; Gupta, B.; Das, A.; Krishna, N.G.; Kamruddin, M. Supercapacitive vertical graphene nanosheets in aqueous electrolytes. Nano Struct. Nano Objects 2017, 10, 42–50. [Google Scholar] [CrossRef]
- Shi, P.; Lin, M.; Zheng, H.; He, X.; Xue, Z.; Xiang, H.; Chen, C. Effect of propylene carbonate-Li solvation structures on graphite exfoliation and its application in Li-ion batteries. Electrochim. Acta 2017, 247, 12–18. [Google Scholar] [CrossRef]
- Lemaire, P.; Sel, O.; Corte, D.A.; Iadecola, A.; Perrot, H.; Tarascon, J.-M. Elucidating the origin of the electrochemical capacity in a proton-based battery HxIrO4 via advanced electrogravimetry. ACS Appl. Mater. Interfaces 2020, 12, 4510–4519. [Google Scholar] [CrossRef] [PubMed]
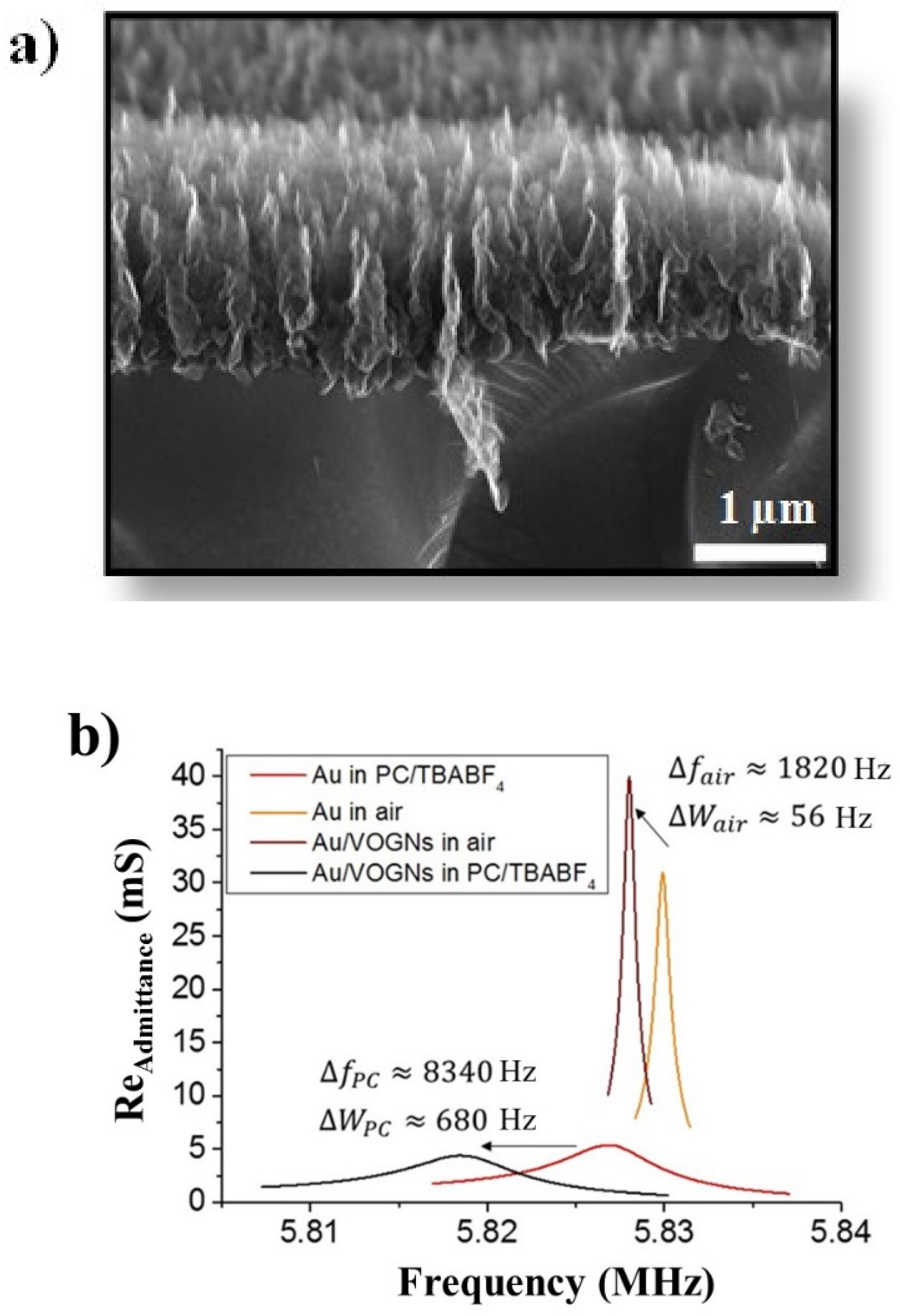

| System | Fluid Viscosity η (mPa s) | Fluid Density ρ (kg m−3) | Resonant Frequency f (Hz) | Peak Width W (Hz) | Motional Resistance, Rm (Ω) | Quality Factor, Q |
|---|---|---|---|---|---|---|
| Au in air | 0.0184 | 1.17 | 5,830,000 (±15) | 700 (±27) | 33 (±5) | 8300 (±135) |
| Au in PC | 2.5 | 1200 | 5,827,000 (±25) | 5046 (±64) | 188 (±8) | 1155 (±85) |
| VOGNs in air | 0.0184 | 1.17 | 5,828,000 (±17) | 756 (±25) | 24 (±6) | 7700 (±120) |
| VOGNs in PC | 2.5 | 1200 | 5,819,000 (±27) | 5726 (±72) | 229 (±12) | 1016 (±70) |
| Electrolyte | MPE(experimental) (g mol−1) | Anion Mass (g mol−1) | Cation Mass (g mol−1) |
|---|---|---|---|
| TBAClO4 | 70 (above −1 V) 30 (below −1 V) | 99.4 | 242 |
| LiClO4 | 34 (above −0.75 V) −31 (below −0.75 V) | 99.4 | 6.9 |
| PC/N1114TFSI | 90 (above 0 V) 25 (below −0.3 V) | 280.1 | 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lé, T.; Bidan, G.; Billon, F.; Delaunay, M.; Gérard, J.-M.; Perrot, H.; Sel, O.; Aradilla, D. Deciphering the Influence of Electrolytes on the Energy Storage Mechanism of Vertically-Oriented Graphene Nanosheet Electrodes by Using Advanced Electrogravimetric Methods. Nanomaterials 2020, 10, 2451. https://doi.org/10.3390/nano10122451
Lé T, Bidan G, Billon F, Delaunay M, Gérard J-M, Perrot H, Sel O, Aradilla D. Deciphering the Influence of Electrolytes on the Energy Storage Mechanism of Vertically-Oriented Graphene Nanosheet Electrodes by Using Advanced Electrogravimetric Methods. Nanomaterials. 2020; 10(12):2451. https://doi.org/10.3390/nano10122451
Chicago/Turabian StyleLé, Tao, Gérard Bidan, Florence Billon, Marc Delaunay, Jean-Michel Gérard, Hubert Perrot, Ozlem Sel, and David Aradilla. 2020. "Deciphering the Influence of Electrolytes on the Energy Storage Mechanism of Vertically-Oriented Graphene Nanosheet Electrodes by Using Advanced Electrogravimetric Methods" Nanomaterials 10, no. 12: 2451. https://doi.org/10.3390/nano10122451
APA StyleLé, T., Bidan, G., Billon, F., Delaunay, M., Gérard, J.-M., Perrot, H., Sel, O., & Aradilla, D. (2020). Deciphering the Influence of Electrolytes on the Energy Storage Mechanism of Vertically-Oriented Graphene Nanosheet Electrodes by Using Advanced Electrogravimetric Methods. Nanomaterials, 10(12), 2451. https://doi.org/10.3390/nano10122451