Tuning Redox State and Ionic Transfers of Mg/Fe-Layered Double Hydroxide Nanosheets by Electrochemical and Electrogravimetric Methods
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
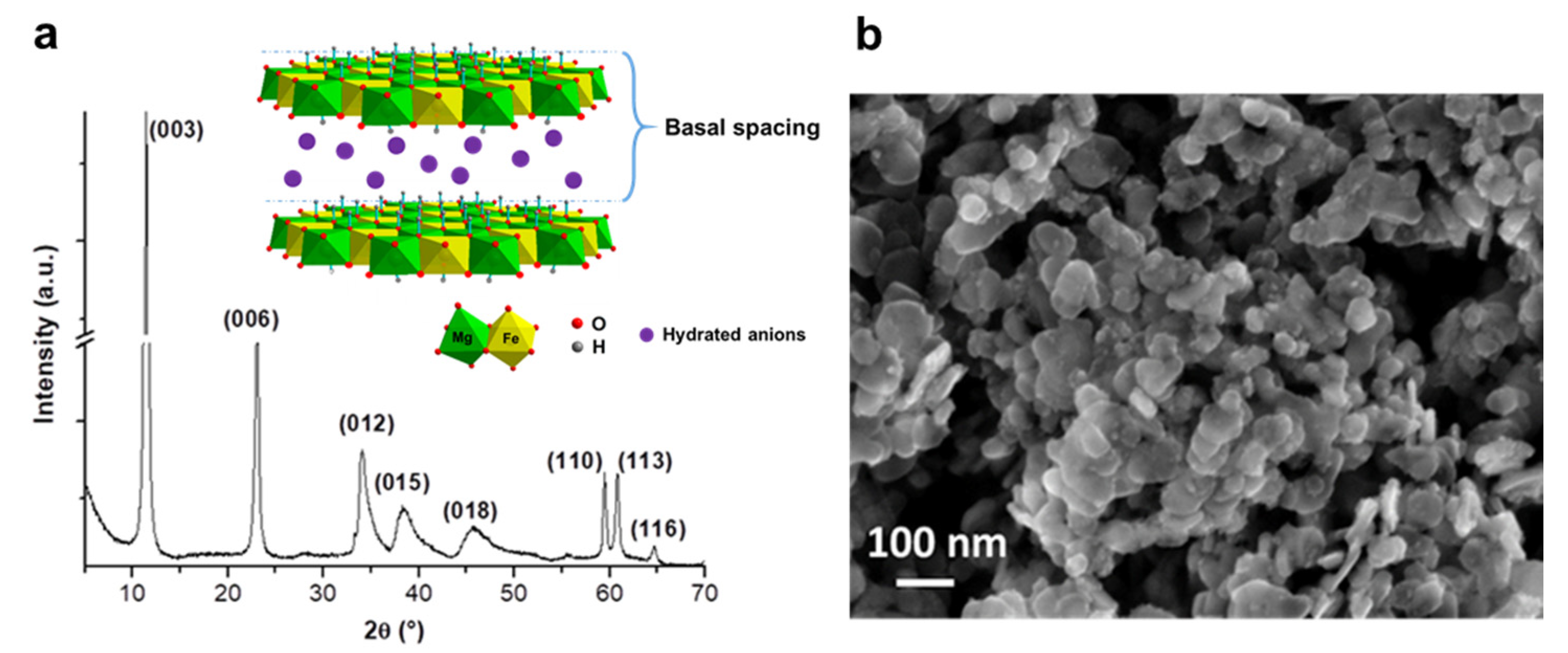
3.1. Structural and Morphological Characterization of the LDH
3.2. Polarization Induced Ionic Transfers
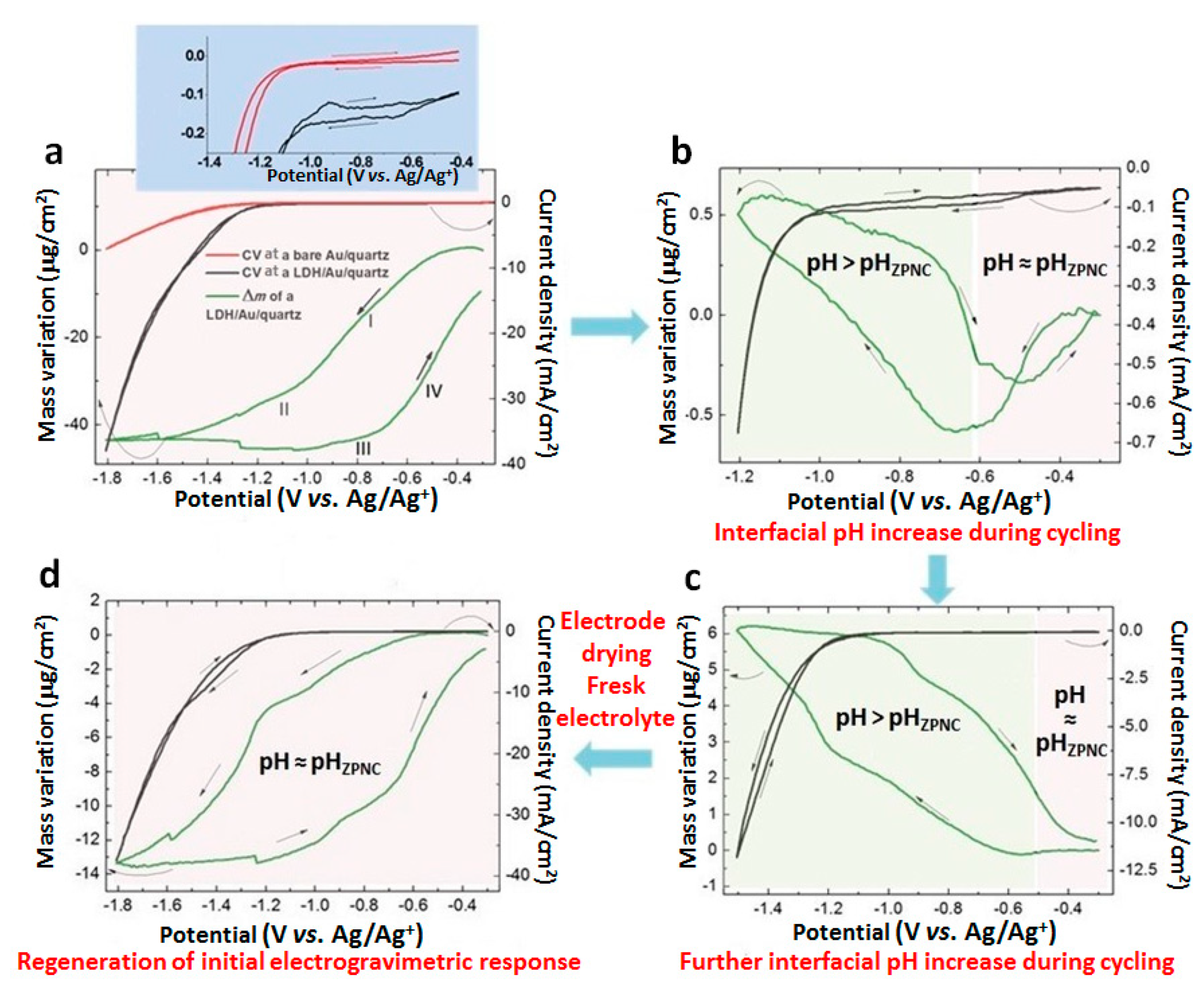
3.2.1. EQCM in the [−1.8; −0.3] V Range
[Mg4IIFe2−xIIIFexII(OH)16](2−x)+[(CO32−)1–0.5x](2−x)−,(m−y)H2O + 0.5x CO32− + 0.5y H2 + y OH−
3.2.2. EQCM in the [−1.2; −0.3] V Range
3.2.3. EQCM in the [−1.5; −0.3] V Range
[Mg4IIFe2−xIIIFexII(OH)16−z (ONa)z](2−x)+[(CO32−)1−0.5x](2−x)−,(m−y) H2O + (y−z) OH− + z H2O
3.2.4. EQCM in the [−1.8; −0.3] V Range–Regeneration of the Initial Mg/Fe-LDH
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bish, D.L. Anion-exchangein takovite—Applications to other hydroxide minerals. Bull. Mineral. 1980, 103, 170–175. [Google Scholar]
- Wang, Y.F.; Gao, H.Z. Compositional and structural control on anion sorption capability of layered double hydroxides (LDHs). J. Colloid Interface Sci. 2006, 301, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Nomura, K.; Yagi, I. Adsorption and Electroreduction of Oxygen on Gold in Acidic Media: In Situ Spectroscopic Identification of Adsorbed Molecular Oxygen and Hydrogen Superoxide. J. Phys. Chem. C 2012, 116, 14390–14400. [Google Scholar] [CrossRef]
- Szabados, M.; Varga, G.; Konya, Z.; Kukovecz, A.; Carlson, S.; Sipos, P.; Palinko, I. Ultrasonically-enhanced preparation, characterization of CaFe-layered double hydroxides with various interlayer halide, azide and oxo anions (CO32-, NO3-, ClO4-). Ultrason. Sonochem. 2018, 40, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Taviot-Gueho, C.; Vialat, P.; Leroux, F.; Razzaghi, F.; Perrot, H.; Sel, O.; Jensen, N.D.; Nielsen, U.G.; Peulon, S.; Elkaim, E.; et al. Dynamic Characterization of Inter- and Intralamellar Domains of Cobalt-Based Layered Double Hydroxides upon Electrochemical Oxidation. Chem. Mater. 2016, 28, 7793–7806. [Google Scholar] [CrossRef]
- Bhave, C.; Shejwalkar, S. A review on the synthesis and applications of green rust for environmental pollutant remediation. Int. J. Environ. Sci. Technol. 2018, 15, 1243–1248. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, P.; Kumar, P.; Wang, W.; Liu, B.; Li, J. Controlled Growth of LDH Films with Enhanced Photocatalytic Activity in a Mixed Wastewater Treatment. Nanomaterials 2019, 9, 807. [Google Scholar] [CrossRef]
- Chubar, N.; Gilmour, R.; Gerda, V.; Micusik, M.; Omastova, M.; Heister, K.; Man, P.; Fraissard, J.; Zaitsev, V. Layered double hydroxides as the next generation inorganic anion exchangers: Synthetic methods versus applicability. Adv. Colloid Interface Sci. 2017, 245, 62–80. [Google Scholar] [CrossRef]
- Goh, K.H.; Lim, T.T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’azu, N.D.; Qureshi, A. Magnetic Mg-Fe/LDH Intercalated Activated Carbon Composites for Nitrate and Phosphate Removal from Wastewater: Insight into Behavior and Mechanisms. Nanomaterials 2020, 10, 1361. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Jin, W. Recent Advances in the Synthesis of Layered, Double-Hydroxide-Based Materials and Their Applications in Hydrogen and Oxygen Evolution. Energy Technol. 2016, 4, 354–368. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, D.; El Hankari, S.; Zou, Y.; Wang, S. Recent Progress on Layered Double Hydroxides and Their Derivatives for Electrocatalytic Water Splitting. Adv. Sci. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Arrabito, G.; Bonasera, A.; Prestopino, G.; Orsini, A.; Mattoccia, A.; Martinelli, E.; Pignataro, B.; Medaglia, P.G. Layered Double Hydroxides: A Toolbox for Chemistry and Biology. Crystals 2019, 9, 361. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, K.Y.; Kim, S.W.; Kang, K. Aqueous Rechargeable Li and Na Ion Batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, M.; Shakir, I. Recent advances in layered double hydroxides as electrode materials for high-performance electrochemical energy storage devices. J. Energy Storage 2017, 13, 103–122. [Google Scholar] [CrossRef]
- Antony, H.; Legrand, L.; Chausse, A. Carbonate and sulphate green rusts—Mechanisms of oxidation and reduction. Electrochim. Acta 2008, 53, 7146–7156. [Google Scholar] [CrossRef]
- Duquesne, E.; Betelu, S.; Bazin, C.; Seron, A.; Ignatiadis, I.; Perrot, H.; Sel, O.; Debiemme-Chouvy, C. Insights into Redox Reactions and Ionic Transfers in Nickel/Iron Layered Double Hydroxide in Potassium Hydroxide. J. Phys. Chem. C 2020, 124, 3037–3049. [Google Scholar] [CrossRef]
- Yao, K.; Taniguchi, M.; Nakata, M.; Shimazu, K.; Takahashi, M.; Yamagishi, A. Mass transport on an anionic clay-modified electrode as studied by a quartz crystal microbalance. J. Electroanal. Chem. 1998, 457, 119–128. [Google Scholar] [CrossRef]
- Roto, R.; Yamagishi, A.; Villemure, G. Electrochemical quartz crystal microbalance study of mass transport in thin film of a redox active Ni-Al-Cl layered double hydroxide. J. Electroanal. Chem. 2004, 572, 101–108. [Google Scholar] [CrossRef]
- Roto, R.; Villemure, G. Mass transport in thin films of [Fe(CN)6]4− exchanged Ni-Al layered double hydroxide monitored with an electrochemical quartz crystal microbalance. J. Electroanal. Chem. 2006, 588, 140–146. [Google Scholar] [CrossRef]
- Mignani, A.; Ballarin, B.; Giorgetti, M.; Scavetta, E.; Tonelli, D.; Boanini, E.; Prevot, V.; Mousty, C.; Iadecola, A. Heterostructure of Au Nanoparticles-NiAl Layered Double Hydroxide: Electrosynthesis, Characterization, and Electrocatalytic Properties. J. Phys. Chem. C 2013, 117, 16221–16230. [Google Scholar] [CrossRef]
- Hu, W.G.; Su, Y.L.; Sun, D.J.; Zhang, C.G. Studies on zero point of charge and permanent charge density of Mg-Fe hydrotalcite-like compounds. Langmuir 2001, 17, 1885–1888. [Google Scholar] [CrossRef]
- Seron, A.; Delorme, F. Synthesis of layered double hydroxides (LDHs) with varying pH: A valuable contribution to the study of Mg/Al LDH formation mechanism. J. Phys. Chem. Solids 2008, 69, 1088–1090. [Google Scholar] [CrossRef]
- Delorme, F.; Seron, A.; Gautier, A.; Crouzet, C. Comparison of the fluoride, arsenate and nitrate anions water depollution potential of a calcined quintinite, a layered double hydroxide compound. Asian J. Mater. Sci. 2007, 42, 5799–5804. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von schwingquarzen zur wagung dunner schichten und zur mikrowagung. Z. Angew. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Bizet, K.; Gabrielli, C.; Perrot, H. Immunodetection by quartz crystal microbalance—A new approach for direct detection of rabbit IgG and peroxidase. Appl. Biochem. Biotechnol. 2000, 89, 139–149. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Miyata, S. Anion exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duquesne, E.; Betelu, S.; Seron, A.; Ignatiadis, I.; Perrot, H.; Debiemme-Chouvy, C. Tuning Redox State and Ionic Transfers of Mg/Fe-Layered Double Hydroxide Nanosheets by Electrochemical and Electrogravimetric Methods. Nanomaterials 2020, 10, 1832. https://doi.org/10.3390/nano10091832
Duquesne E, Betelu S, Seron A, Ignatiadis I, Perrot H, Debiemme-Chouvy C. Tuning Redox State and Ionic Transfers of Mg/Fe-Layered Double Hydroxide Nanosheets by Electrochemical and Electrogravimetric Methods. Nanomaterials. 2020; 10(9):1832. https://doi.org/10.3390/nano10091832
Chicago/Turabian StyleDuquesne, Elise, Stéphanie Betelu, Alain Seron, Ioannis Ignatiadis, Hubert Perrot, and Catherine Debiemme-Chouvy. 2020. "Tuning Redox State and Ionic Transfers of Mg/Fe-Layered Double Hydroxide Nanosheets by Electrochemical and Electrogravimetric Methods" Nanomaterials 10, no. 9: 1832. https://doi.org/10.3390/nano10091832
APA StyleDuquesne, E., Betelu, S., Seron, A., Ignatiadis, I., Perrot, H., & Debiemme-Chouvy, C. (2020). Tuning Redox State and Ionic Transfers of Mg/Fe-Layered Double Hydroxide Nanosheets by Electrochemical and Electrogravimetric Methods. Nanomaterials, 10(9), 1832. https://doi.org/10.3390/nano10091832




