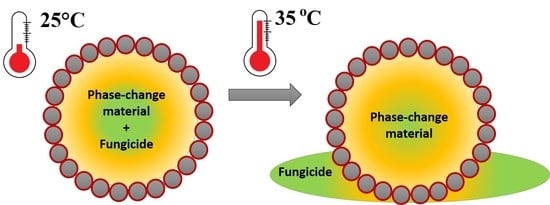
Temperature-Responsive Pyraclostrobin-Loaded Octadecane Submicrocapsules with Lowered Toxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
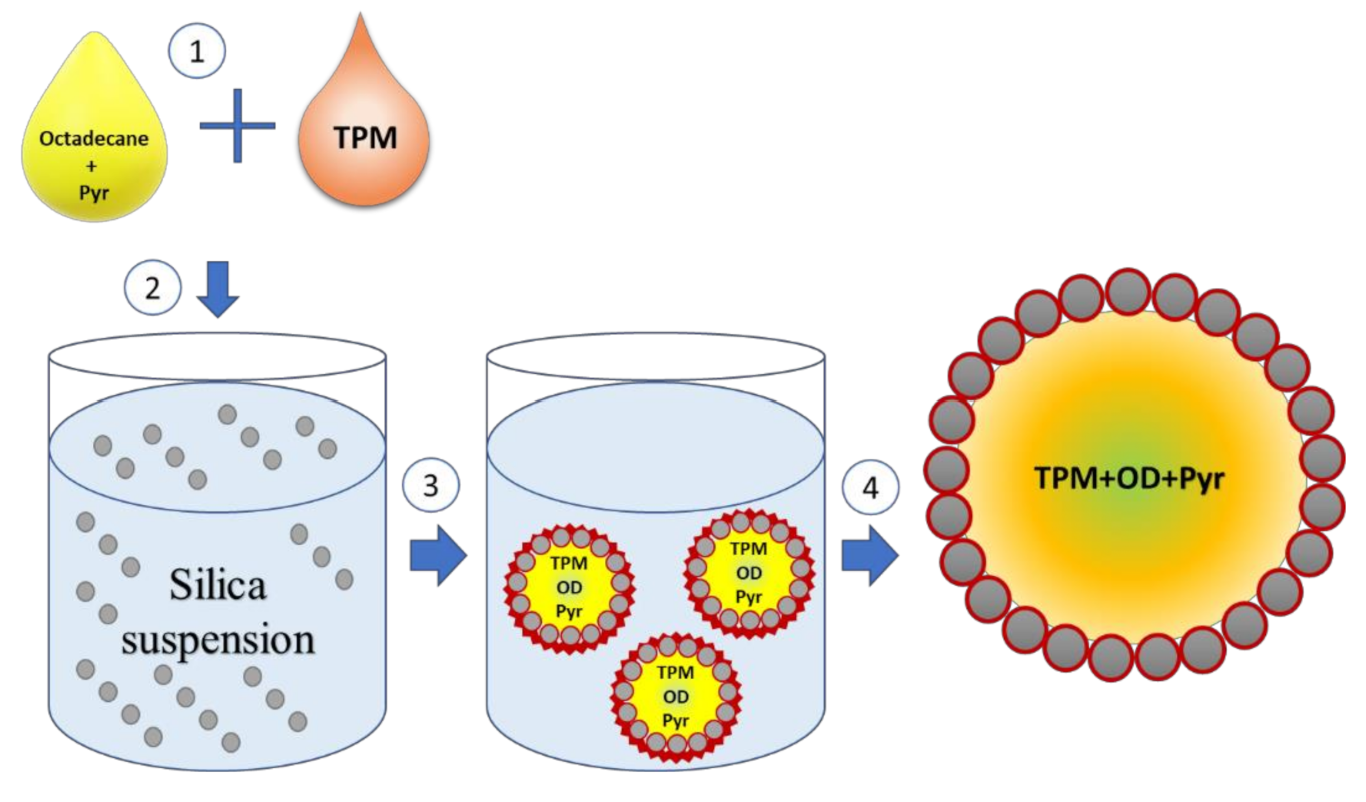
2.2. Preparation of Submicrocapsules
2.3. Characterization of Submicrocapsules
2.4. Temperature-Responsivity of SMCs via Antifungal Activity
2.5. Toxicity of PyrSMCs Using Brine Shrimps (Artemia salina)
2.5.1. Culture and Harvesting of A. Salina
2.5.2. Preparation of Sample
2.5.3. Toxicity Test
3. Results and Discussion
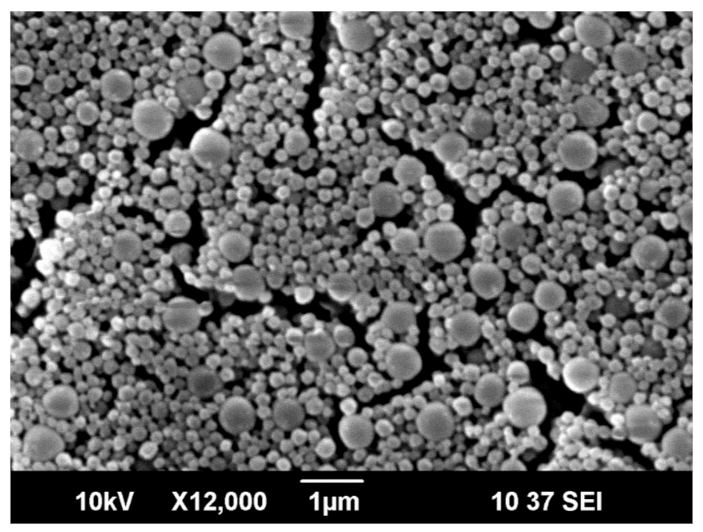
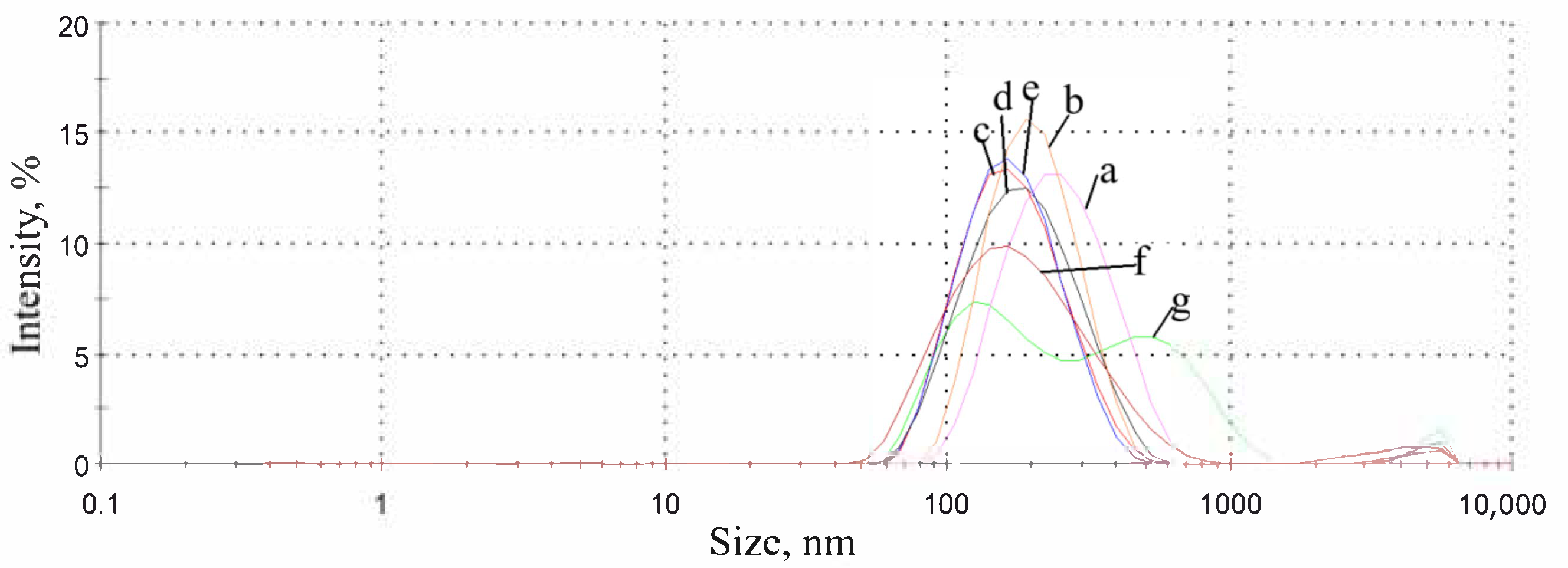
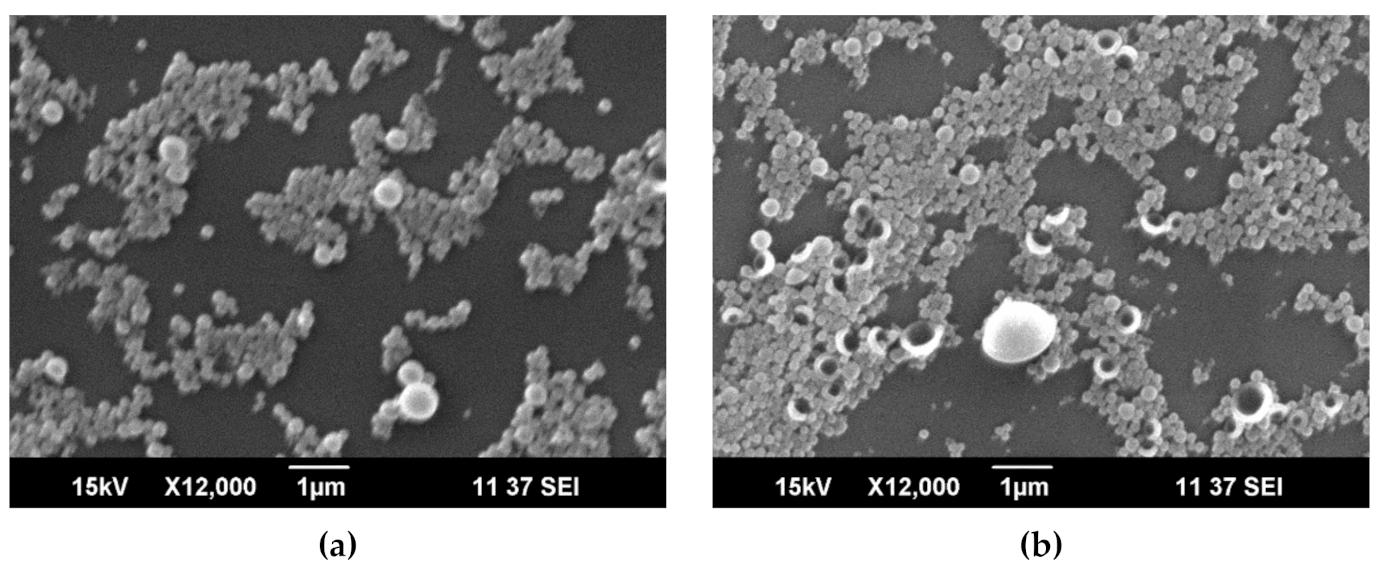
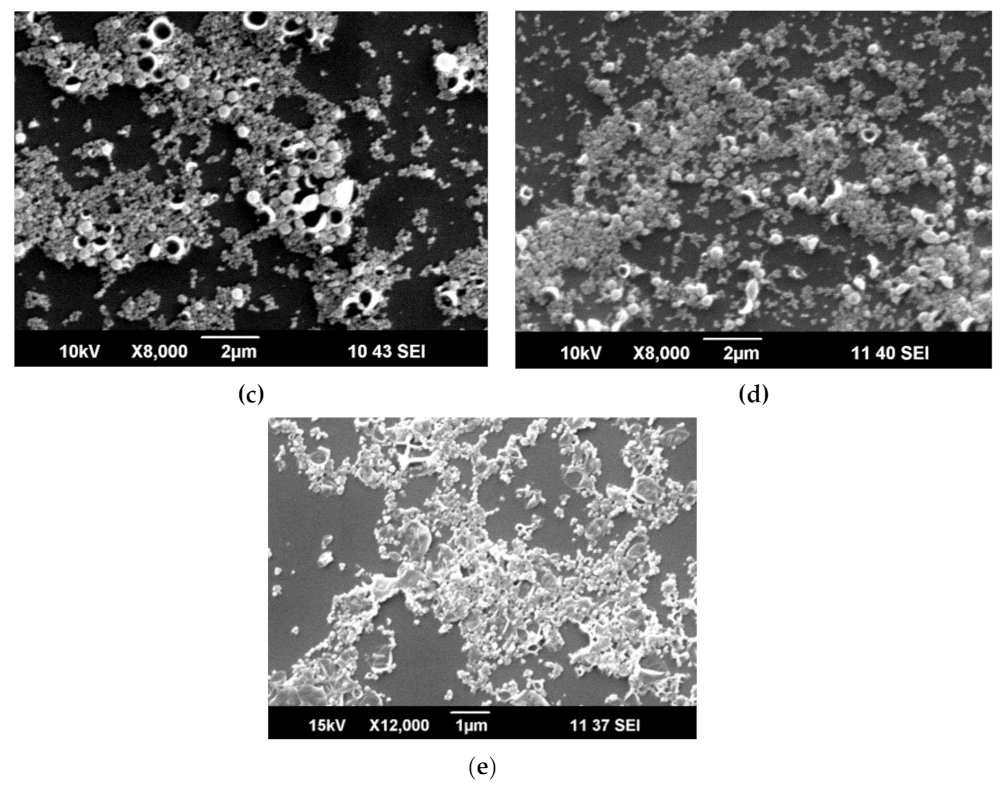
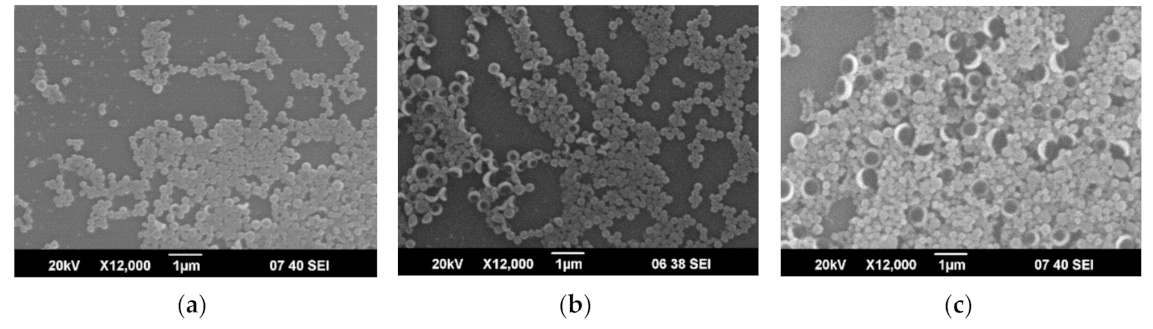
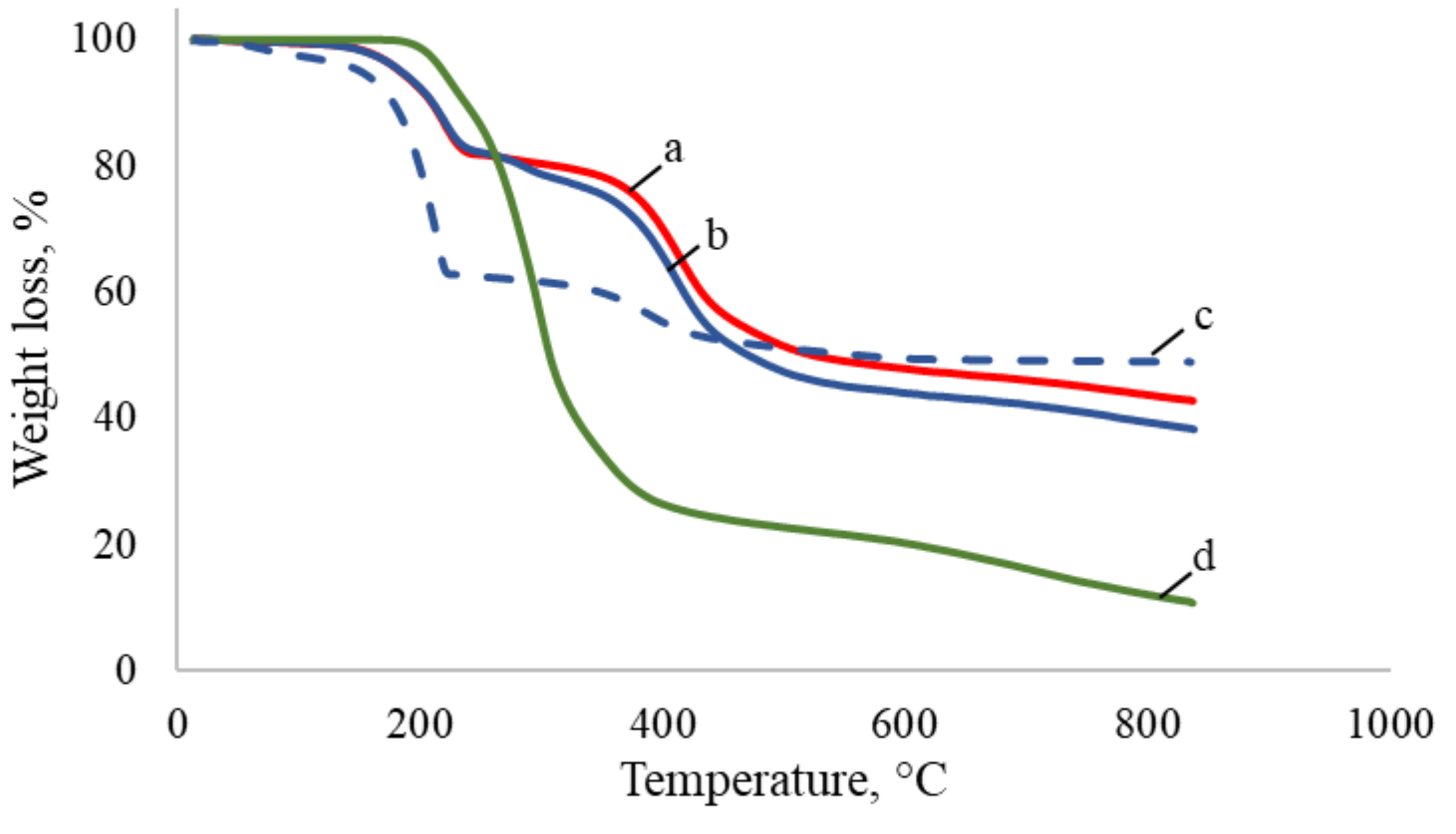
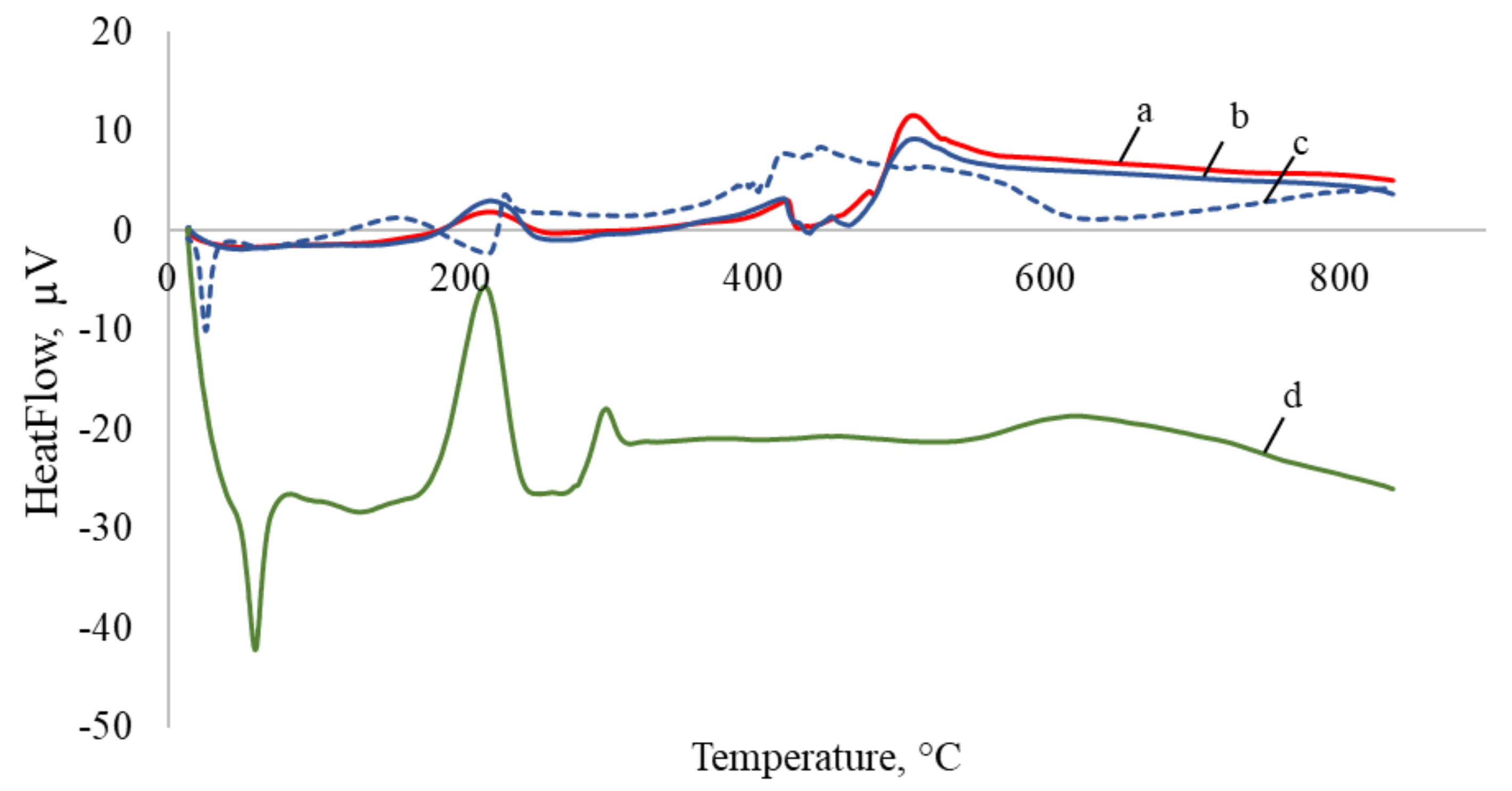
3.1. Capsules Characterization
3.2. Temperature-Responsive Properties through Fungicidal Activity
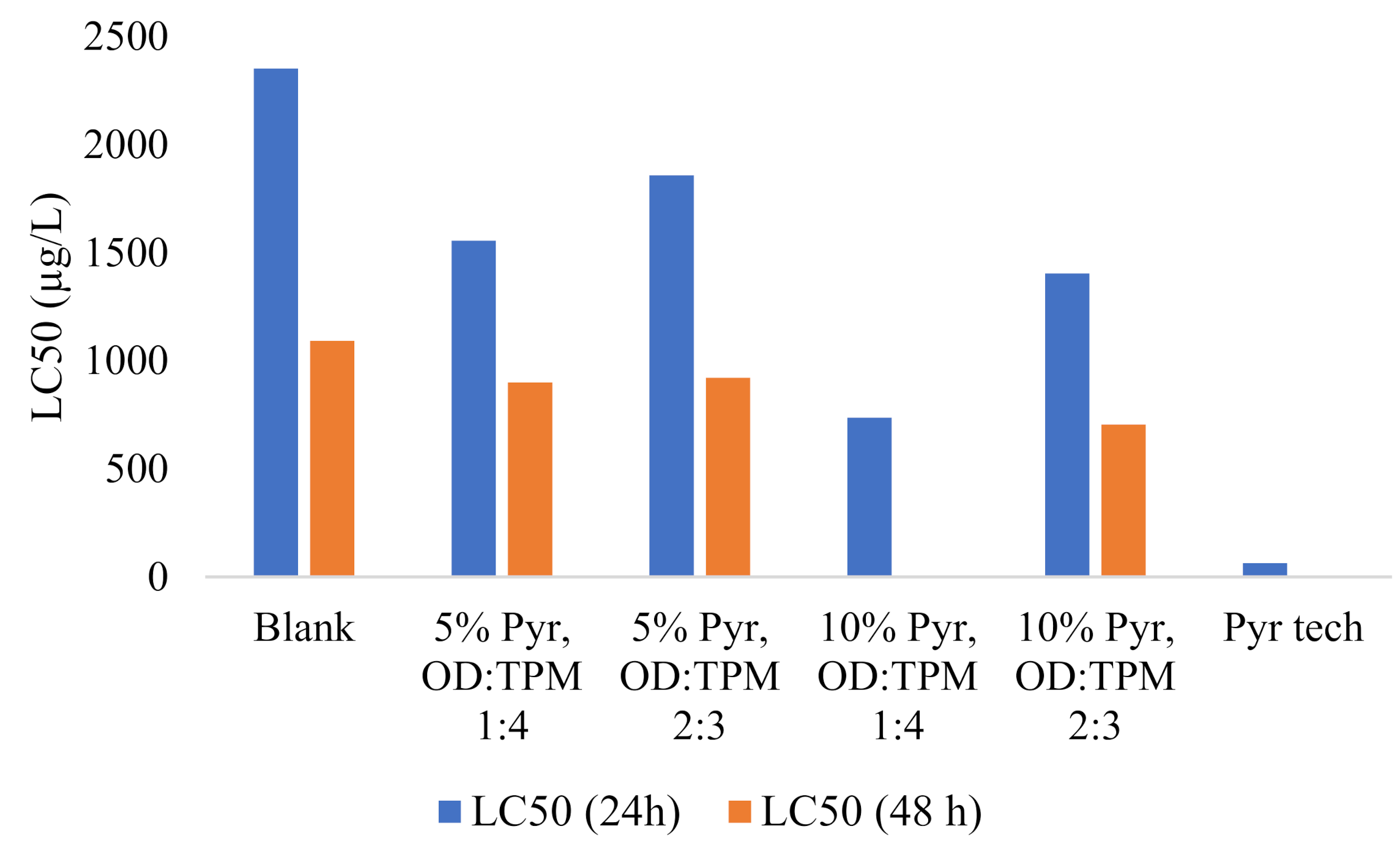
3.3. Toxicity of PyrSMC
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davies, B.; Baulcombe, D.; Crute, I.; Dunwell, J.; Gale, M.; Jones, J. Reaping the Benefits: Science and the Sustainable Intensification of Global Agriculture; The Royal Society: London, UK, 2009; pp. 11–19. [Google Scholar]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.-H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cao, L.; Zhao, P.; Zhou, Z.; Cao, C.; Li, F.; Huang, Q. Emulsion-based synchronous pesticide encapsulation and surface modification of mesoporous silica nanoparticles with carboxymethyl chitosan for controlled azoxystrobin release. Chem. Eng. J. 2018, 348, 244–254. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; He, S.; Xiao, Y.; Qin, X.; Zhang, Y.; Li, D.; Ma, H.; You, H.; Li, J. Fabrication of a hollow mesoporous silica hybrid to improve the targeting of a pesticide. Chem. Eng. J. 2019, 364, 361–369. [Google Scholar] [CrossRef]
- Wibowo, D.; Zhao, C.-X.; Peters, B.C.; Middelberg, A.P.J. Sustained Release of Fipronil Insecticide in Vitro and in Vivo from Biocompatible Silica Nanocapsules. J. Agric. Food Chem. 2014, 62, 12504–12511. [Google Scholar] [CrossRef] [PubMed]
- Tleuova, A.B.; Wielogorska, E.; Talluri, V.P.; Štěpánek, F.; Elliott, C.T.; Grigoriev, D.O. Recent advances and remaining barriers to producing novel formulations of fungicides for safe and sustainable agriculture. J. Control. Release 2020, 326, 468–481. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, H.; Cao, C.; Zhang, J.; Li, F.; Huang, Q. Quaternized Chitosan-Capped Mesoporous Silica Nanoparticles as Nanocarriers for Controlled Pesticide Release. Nanomaterials 2016, 6, 126. [Google Scholar] [CrossRef]
- Li, B.-X.; Wang, W.-C.; Zhang, X.-P.; Zhang, D.-X.; Ren, Y.-P.; Gao, Y.; Mu, W.; Liu, F. Using Coordination Assembly as the Microencapsulation Strategy to Promote the Efficacy and Environmental Safety of Pyraclostrobin. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Li, M.; Xu, W.; Hu, D.; Song, B. Preparation and application of pyraclostrobin microcapsule formulations. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 553, 578–585. [Google Scholar] [CrossRef]
- Yin, M.-M.; Zheng, Y.; Chen, F.-L. Pyraclostrobin-loaded poly (lactic-co-glycolic acid) nanospheres: Preparation and characteristics. J. Integr. Agric. 2018, 17, 1822–1832. [Google Scholar] [CrossRef]
- Ibrahim, M.; Rabah, A.; Liman, B.; Ibrahim, N. Effect of Temperature and Relative Humidity on the Growth of Helminthosporium fulvum. Niger. J. Basic Appl. Sci. 2011, 19. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, K.; Wu, W.; Chen, J.; Zhang, P. Synthesis of temperature-responsive hybrid capsules and their controlled release property. Colloids Surf. A: Physicochem. Eng. Asp. 2011, 385, 126–133. [Google Scholar] [CrossRef]
- Chi, Y.; Zhang, G.; Xiang, Y.; Cai, D.; Wu, Z. Fabrication of reusable temperature-controlled-released fertilizer using a palygorskite-based magnetic nanocomposite. Appl. Clay Sci. 2018, 161, 194–202. [Google Scholar] [CrossRef]
- Hoseini, Z.; Nikje, M.M.A. The Preparation of Novel Microcapsules Based on Palmitic Acid Core and Waterborne Polyurethane/Silane Shell as Phase Change Materials for Thermal Energy Storage. J. Polym. Environ. 2020, 1–8. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Ma, H.; Yang, J. The preparation of a green shape-stabilized composite phase change material of polyethylene glycol/SiO2 with enhanced thermal performance based on oil shale ash via temperature-assisted sol–gel method. Sol. Energy Mater. Sol. Cells 2015, 132, 29–39. [Google Scholar] [CrossRef]
- He, F.; Wang, X.; Wu, D. New approach for sol–gel synthesis of microencapsulated n-octadecane phase change material with silica wall using sodium silicate precursor. Energy 2014, 67, 223–233. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, H.D. Preparation and properties of microencapsulated octadecane with waterborne polyurethane. J. Appl. Polym. Sci. 2005, 96, 1596–1604. [Google Scholar] [CrossRef]
- Devedra, B.N.; Sriniva, N.; Talluri, P.V.; Latha, P.S. Antimicrobial activity of Moringa Olifera Lam Leaf extract against selected bacterial and fungal strains. Int. J. Pharma Biol. Sci. 2011, 2, 13–18. [Google Scholar]
- Carballo, J.L.; Hernández-Inda, Z.L.; Pérez, P.; García-Grávalos, M.D. A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol. 2002, 2, 17. [Google Scholar] [CrossRef]
- Sacanna, S.; Kegel, W.K.; Philipse, A.P. Spontaneous Oil-in-Water Emulsification Induced by Charge-Stabilized Dispersions of Various Inorganic Colloids. Langmuir 2007, 23, 10486–10492. [Google Scholar] [CrossRef]
- Tleuova, A.B.; Aidarova, S.; Sharipova, A.; Bekturganova, N.; Schenderlein, M.; Grigoriev, D. Using profile analysis tensiometry for monitoring auto-oscillations caused by the hydrolysis of 3-(trimethoxysilyl)propyl methacrylate when contacting water. Colloids Surf. A: Physicochem. Eng. Asp. 2016, 505, 18–22. [Google Scholar] [CrossRef]
- Tleuova, A.B.; Schenderlein, M.; Mutaliyeva, B.; Aidarova, S.; Sharipova, A.; Bekturganova, N.; Miller, R.; Grigoriev, D. Selection and study of alkoxysilanes as loading in submicrocapsules for self-lubricating coatings. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 563, 359–369. [Google Scholar] [CrossRef]
- Aidarova, S.B.; Sharipova, A.A.; Issayeva, A.B.; Mutaliyeva, B.Z.; Tleuova, A.B.; Grigoriev, D.O.; Kudasova, D.; Dzhakasheva, M.; Miller, R. Synthesis of Submicrocontainers with “Green” Biocide and Study of Their Antimicrobial Activity. Colloids Interfaces 2018, 2, 67. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Morrison, S.A.; McMurry, S.T.; Smith, L.M.; Belden, J.B. Acute toxicity of pyraclostrobin and trifloxystrobin toHyalella azteca. Environ. Toxicol. Chem. 2013, 32, 1516–1525. [Google Scholar] [CrossRef]
- Hartman, E.A.H.; Belden, J.B.; Smith, L.M.; McMurry, S.T. Acute toxicity of three strobilurin fungicide formulations and their active ingredients to tadpoles. Ecotoxicology 2012, 21, 1458–1464. [Google Scholar] [CrossRef]


| Composition, g | Pyraclostrobin Concentration in the Oil Phase, % | |||
|---|---|---|---|---|
| - | 1% | 5% | 10% | |
| SiNPs | 0.43 | 0.43 | 0.43 | 0.43 |
| OD | 0.43 | 0.4257 | 0.4085 | 0.387 |
| TPM | 1.72 | 1.7028 | 1.634 | 1.548 |
| Pyr | - | 0.0215 | 0.1075 | 0.215 |
| Aqueous phase | 50 mL | 50 mL | 50 mL | 50 mL |
| Composition, % | Pyraclostrobin Concentration in the Oil Phase, % | |||
|---|---|---|---|---|
| - | 1% | 5% | 10% | |
| SiNPs | 16.7 | 16.7 | 16.7 | 16.7 |
| OD | 16.7 | 16.5 | 15.8 | 15 |
| TPM | 66.7 | 66 | 63.3 | 60 |
| Pyr | - | 0.8 | 4.2 | 8.3 |
| OD:TPM wt. Ratio | Pyraclostrobin Theoretical Concentration in SMCs, % | ||
|---|---|---|---|
| 0.8% | 4.2% | 8.3% | |
| 0:1 | 0.96 | 5.065 | 11.088 |
| 1:4 | 0.91 | 4.43 | 9.54 |
| 2:3 | 0.908 | 3.708 | 8.561 |
| OD:TPM 2:3 | 25 °C | 35 °C |
|---|---|---|
| Blank | 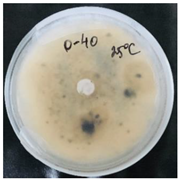 | 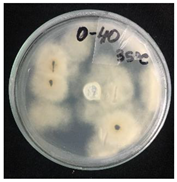 |
| 1% Pyr (41.28 µg) | 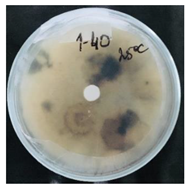 |  |
| 5% Pyr (216.72 µg) |  |  |
| 10% Pyr (428.28 µg) | 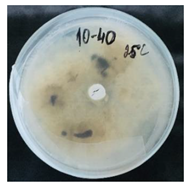 | 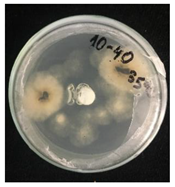 |
| Pyr tech (500 µg) |  | 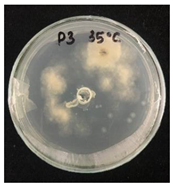 |
| Sample | LC50 (24 h) | Equation | R2 | LC50 (48 h) | Equation | R2 |
|---|---|---|---|---|---|---|
| Blank | 2352 | y = 0.0222x − 2.2222 | 0.89 | 1092 | y = 0.0356x + 11.111 | 0.94 |
| 5% Pyr, OD:TPM 1:4 | 1556 | y = 0.04x − 12.222 | 0.88 | 900 | y = 0.0667x − 10 | 0.97 |
| 5% Pyr, OD:TPM 2:3 | 1858 | y = 0.0311x − 7.7778 | 0.94 | 922 | y = 0.0844x − 27.778 | 0.93 |
| 10% Pyr, OD:TPM 1:4 | 737 | y = 0.0844x − 12.222 | 0.97 | - | - | |
| 10% Pyr, OD:TPM 2:3 | 1405 | y = 0.0356x − 2 × 10−14 | 0.89 | 705 | y = 0.0756x − 3.3333 | 0.94 |
| Pyr tech | 64 | y = 0.2444x + 34.444 | 0.92 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tleuova, A.; Talluri, V.P.; Kumar, R.; Štěpánek, F. Temperature-Responsive Pyraclostrobin-Loaded Octadecane Submicrocapsules with Lowered Toxicity. Nanomaterials 2020, 10, 2374. https://doi.org/10.3390/nano10122374
Tleuova A, Talluri VP, Kumar R, Štěpánek F. Temperature-Responsive Pyraclostrobin-Loaded Octadecane Submicrocapsules with Lowered Toxicity. Nanomaterials. 2020; 10(12):2374. https://doi.org/10.3390/nano10122374
Chicago/Turabian StyleTleuova, Aiym, VSSL Prasad Talluri, Rohitesh Kumar, and František Štěpánek. 2020. "Temperature-Responsive Pyraclostrobin-Loaded Octadecane Submicrocapsules with Lowered Toxicity" Nanomaterials 10, no. 12: 2374. https://doi.org/10.3390/nano10122374
APA StyleTleuova, A., Talluri, V. P., Kumar, R., & Štěpánek, F. (2020). Temperature-Responsive Pyraclostrobin-Loaded Octadecane Submicrocapsules with Lowered Toxicity. Nanomaterials, 10(12), 2374. https://doi.org/10.3390/nano10122374