Control of Nanostructured Polysulfone Membrane Preparation by Phase Inversion Method
Abstract
1. Introduction
- *
- good solubility in solvents commonly used in the production of membranes (dipolar aprotic solvents such as N-methyl-pyrrolidone, dimethyl-acetamide, dimethyl-formamide, dimethyl-sulfoxide);
- *
- good resistance to acids and bases as well as to other chemical agents over a wide pH range, to oxidation, and mechanical and thermal stresses;
- *
- the possibility to be chemically modified due to the reactive aromatic nuclei;
- *
- relatively high glass transition temperature (Tg = 190 °C); and
- *
- absence of crystallinity.
- -
- Dissolving the polymer in a suitable solvent or mixture of solvents;
- -
- filming the polymer solution on a flat or tubular surface of glass, metal, teflon, or textile; and
- -
- precipitation of the polymer by treatment with a non-solvent (inversion of the actual phase).
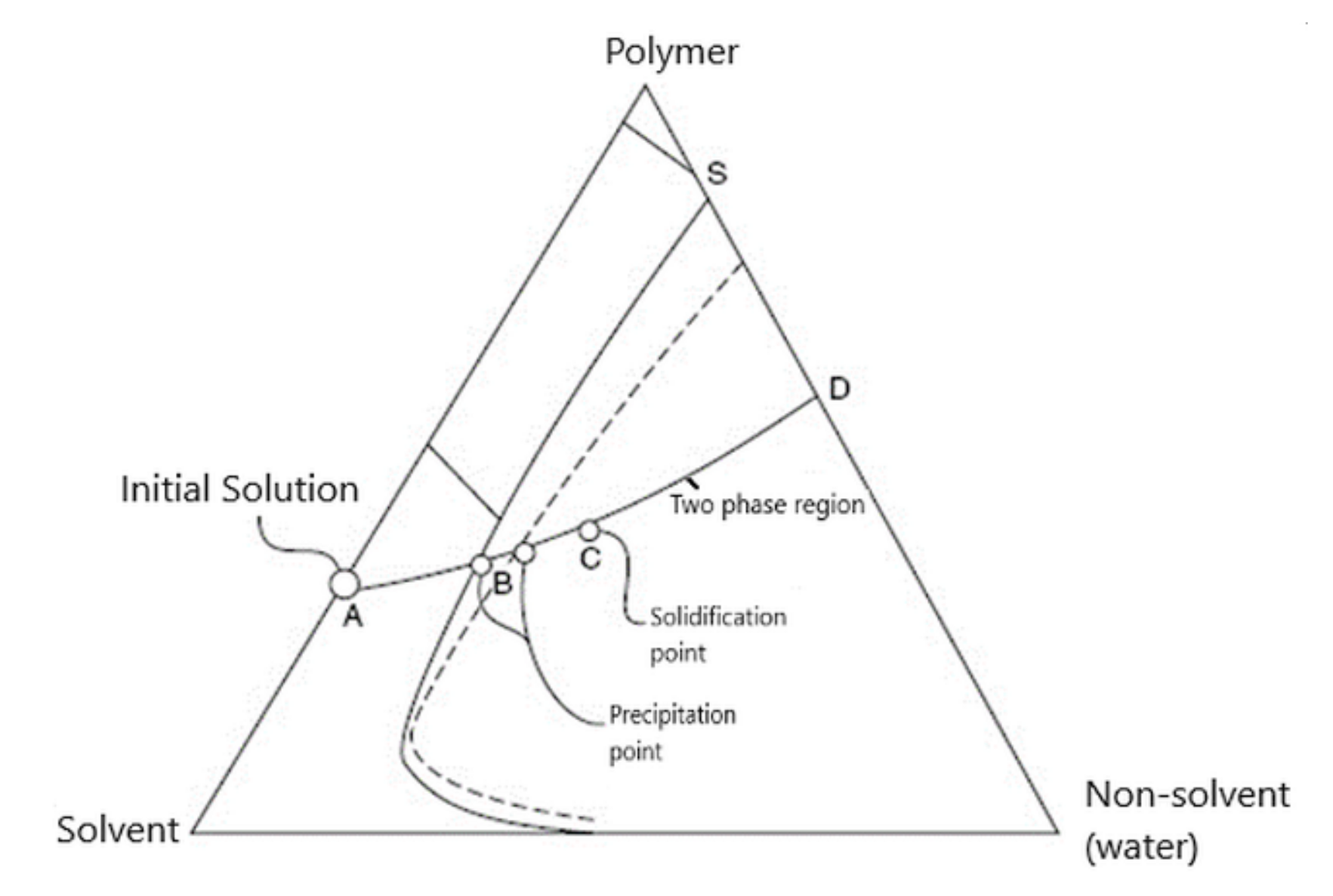
- The ternary system. The process involves at least one polymer phase, one solvent, and one non-solvent. The solvent and the non-solvent must be miscible (Figure 3).
- Mass transfer. The polymer solution is subjected to a mass transfer process to enrich the polymer film in the solvent. Mass transfer begins at the interface between the polymeric film and the coagulation medium (vapor or liquid). The diffusion phenomenon governs the changes in the composition of the polymeric film.
- The precipitation. An increase in the non-solvent concentration leads to a polymer solution thermodynamically unstable, and phase separation will occur. An essential aspect of the phase inversion is represented by a separation phenomenon in the ternary polymeric system. This phenomenon includes the equilibrium and kinetics of phase separation as the formation of membranes is a dynamic process [27,28,29].
- -
- Choice of polymer–solvent–nonsolvent system.
- -
- Diffusion rate of the solvent outside the polymer and the non-solvent inside the polymer.
2. Materials and Methods
2.1. Materials
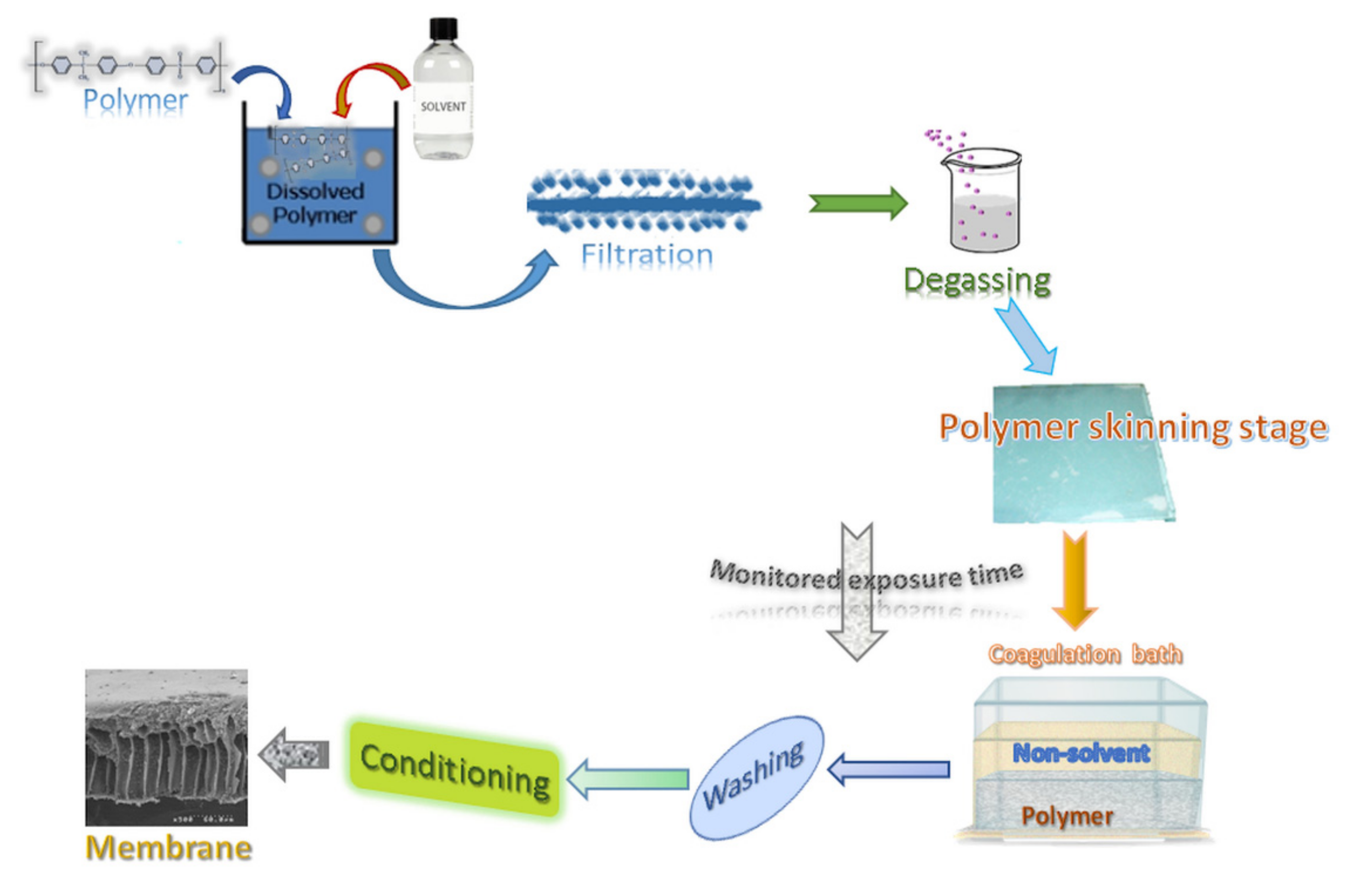
2.2. Preparation of the Polymer Solution
2.3. Characterization of Porous Membranes
- V = apparent membrane volume (cm3), and
- V′ = actual membrane volume (cm3).
- J = hydrodynamic flow;
- r = average pore radius;
- Δp = pressure difference when measuring the flow J, and
- η = viscosity of the reference liquid (water).
- J = flow (L/m2h);
- V = the volume of fluid passed through the membrane (L);
- S = membrane surface (m2), and
- t = time (h).
- J = flow (L/m2h bar);
- V = distilled water volume (L);
- S = membrane surface (m2);
- t = time (h), and
- Δp = pressure difference (bar).
- cf = solute concentration in the supply fluid (% mass, g/L, mols/L);
- cp = concentration of solute in the permeate (% mass, g/L, mols/L).
2.4. Preparation of Membrane Samples
2.5. Structural, Thermal, and Electrochemical Investigations
3. Results
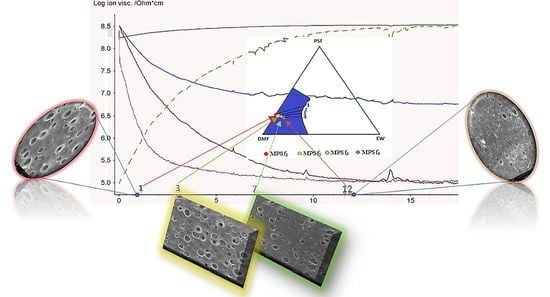
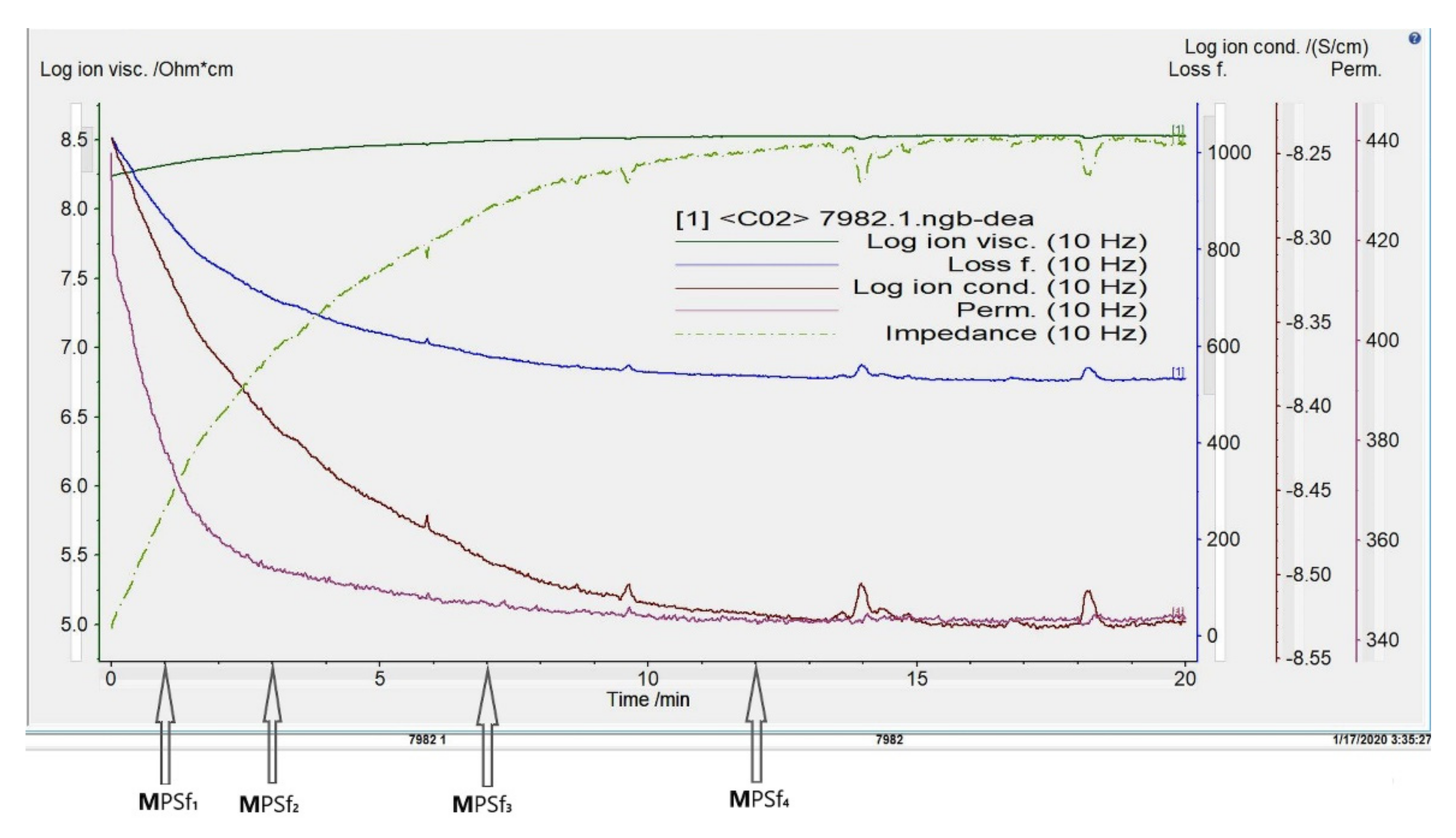
3.1. Evolution of Electrochemical Parameters
3.2. Scanning Electron Microscopy
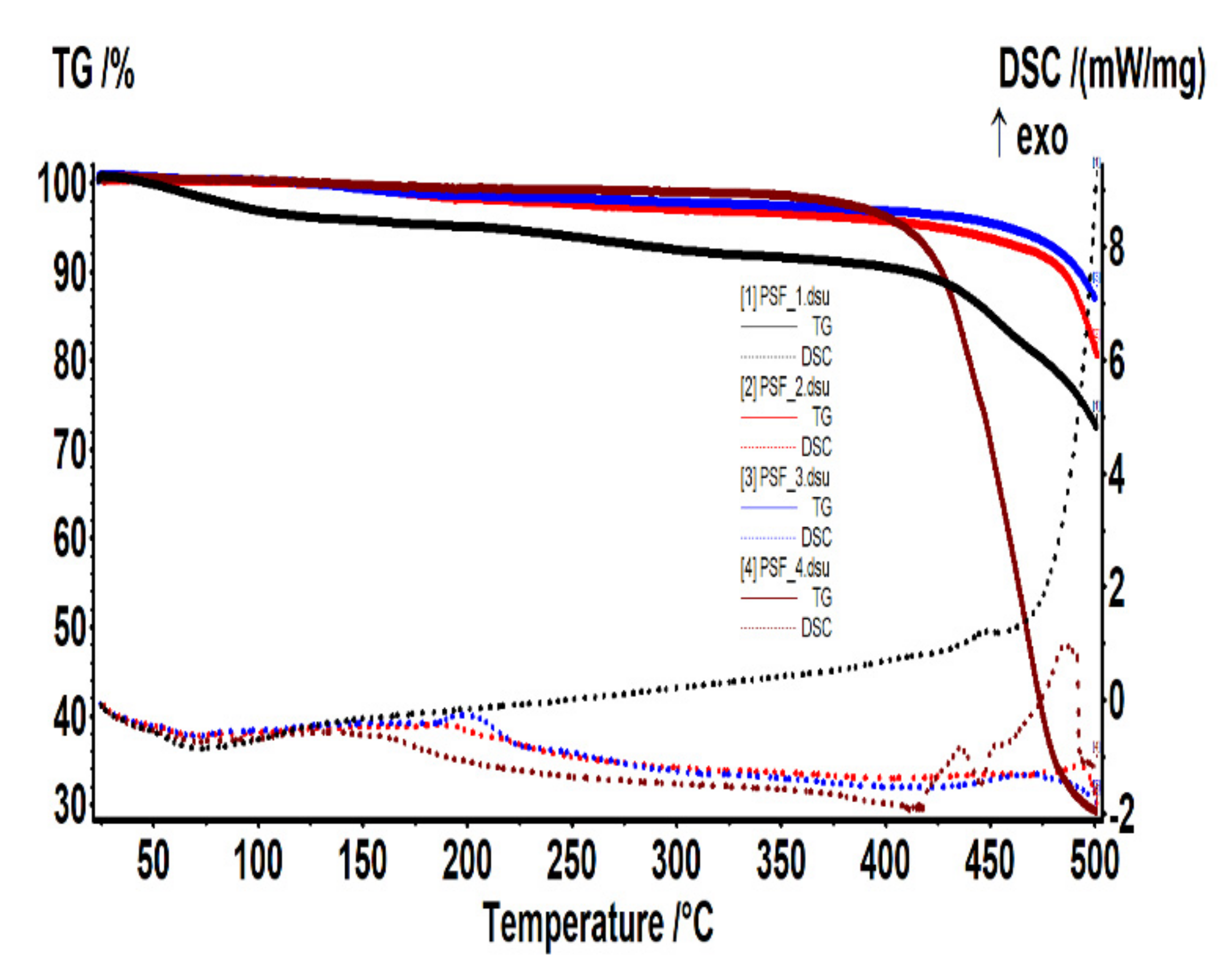
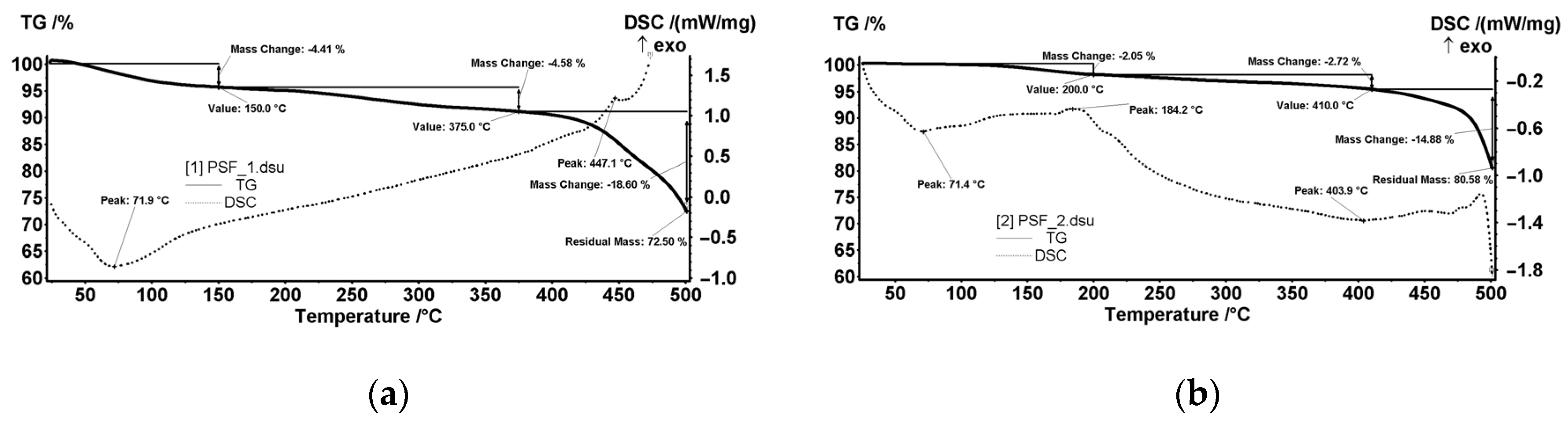
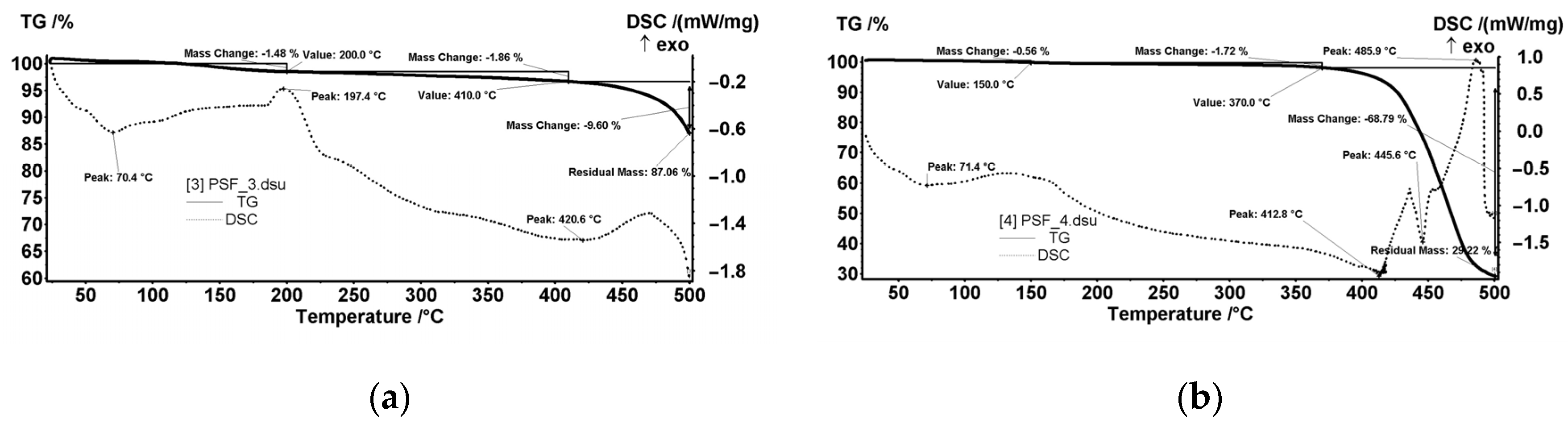
3.3. Thermal Analysis
3.4. Determination of Water Flows and Retention of Bovine Serum Albumin (BSA)
4. Discussion
- The thickness of the membranes may indirectly influence this process. For analysis, the crucible usually contains at least a 4–5 mg sample. It means to use several round cut membranes (disks), one on top of the other. The membrane disks’ thickness conditions the number of disks used: 2–3 or 4–6 disks if it is too thin. The oxygen access (easier or difficult) towards the sample depends on its thickness. Consequently, depending on how many membrane disks are in the crucible, the decomposition, or oxidation processes at high temperatures may predominate.
- The size of the polymeric nanoparticles that form the membranes and the fact that they superficially retain the liquid components of the thermal system influence the objectivity of results.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loeb, S. The Loeb-Sourirajan Membrane. In Hyper and Ultrafiltration Uses; ACS Symposium Series; Volume 2 of Synthetic Membranes: Based on the 20th Anniversary Symposium Honoring Drs. Loeb and Sourirajan; American Chemical Society: Washington, DC, USA, 1981; ISBN 1947-5918. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; ISBN 9780470743720. [Google Scholar]
- Kesting, R. Synthetic polymeric membranes. J. Colloid Interface Sci. 1988. [Google Scholar] [CrossRef]
- Broens, L.; Altena, F.W.; Smolders, C.A.; Koenhen, D.M. Asymmetric membrane structures as a result of phase separation phenomena. Desalination 1980. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Kluwer Academic Publishers: Dordrecht, Netherlands, 1996; ISBN 0792309782. [Google Scholar]
- Baker, R.W. Membrane Transport Theory. In Membrane Technology and Applications; John Wiley&Sons Ltd.: Hoboken, NJ, USA, 2012; ISBN 0-470-85445-6. [Google Scholar]
- Nechifor, G.; Popescu, G. Asymmetric membranes prepared by immersion–precipitation technique. Rev. Roum. Chim. 1990, 35, 899. [Google Scholar]
- Stannett, V.T.; Koros, W.J.; Paul, D.R.; Lonsdale, H.K.; Baker, R.W. Recent advances in membrane science and technology. Adv. Polym. Sci. 1979. [Google Scholar] [CrossRef]
- Kammermeyer, K. Technical Gas Permeation Processes. Chemie Ing. Tech. 1976, 48, 672. [Google Scholar] [CrossRef]
- Loeb, S.; Mehta, G.D. A two-coefficient water transport equation for pressure-retarded osmosis. J. Memb. Sci. 1978. [Google Scholar] [CrossRef]
- Cuciureanu, A.; Nechifor, G. Protein Separation Using Polysulfone-Polyaniline Composite Membranes; University “Politehnica” of Bucharest: Bucharest, Romania, 2012. [Google Scholar]
- He, X.; Chen, C.; Jiang, Z.; Su, Y. Computer simulation of formation of polymeric ultrafiltration membrane via immersion precipitation. J. Memb. Sci. 2011. [Google Scholar] [CrossRef]
- Kesting, R.E. Phase Inversion Membranes. In Materials Science of Synthetic Membranes—Chapter 7; ACS Symposium Series; 1985; Volume 269, pp. 131–164. [Google Scholar]
- Kesting, R.E. Structural Aspects of Asymmetric (Skinned) Synthetic Polymeric Membranes. In Chemistry for the Welfare of Mankind—The 26th International Congress of Pure and Applied Chemistry, Tokyo, Japan, 4–10 September 1977; Elsevier Ltd: Pergamon, Turkey, 1979. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A. Novel porous membranes from chemically modified poly(vinylidene fluoride). J. Membr. Sci. 2006, 273, 20–24. [Google Scholar] [CrossRef]
- Wojciechowski, C.; Chwojnowski, A.; Dudzinski, K.; Lukowska, E. Evaluation of separation characteristic of polysulfone membranes modified by means of weak solvent of polymers. Proc. Conf. Slovacia 2004, 35, 263–270. [Google Scholar]
- Nystrom, M.; Jarvinen, P. Modification of polysulfone ultrafiltration membranes with UV irradiation and hydrophilicity increasing agents. J. Membr. Sci. 1987, 60, 275–296. [Google Scholar] [CrossRef]
- Idris, A.; Man, Z.; Maulud, A.S.; Khan, M.S. Effects of phase separation behavior on morphology and performance of polycarbonate membranes. Membranes 2017. [Google Scholar] [CrossRef]
- Sivakumar, M.; Susithra, L.; Mohan, D.R.; Rangarajan, R. Preparation and performance of polysulfone-cellulose acetate blend ultrafiltration membrane. J. Macromol. Sci. Part A Pure Appl. Chem. 2006. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Anatharaman, N.; Raajenthiren, M. Characteristics, Performance of Blend CA/SPEEK Ultrafiltration Membranes Prepared by Phase Inversion Method using PEG 600 as an Additive. J. Appl. Membr. Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Mohan, D.; Raajenthiren, M. Preparation, characterization and performance studies of ultrafiltration membranes with polymeric additive. J. Memb. Sci. 2010. [Google Scholar] [CrossRef]
- Ochoa, N.; Prádanos, P.; Palacio, L.; Pagliero, C.; Marchese, J.; Hernández, A. Pore size distributions based on AFM imaging and retention of multidisperse polymer solutes. J. Memb. Sci. 2001. [Google Scholar] [CrossRef]
- Qin, J.J.; Chen, S.; Oo, M.H.; Kekre, K.A.; Cornelissen, E.R.; Ruiken, C.J. Experimental studies and modeling on concentration polarization in forward osmosis. Water Sci. Technol. 2010. [Google Scholar] [CrossRef]
- Barth, C.; Gonçalves, M.C.; Pires, A.T.N.; Roeder, J.; Wolf, B.A. Asymmetric polysulfone and polyethersulfone membranes: Effects of thermodynamic conditions during formation on their performance. J. Memb. Sci. 2000. [Google Scholar] [CrossRef]
- Totu, E.; Segal, E.; Covington, A.K. On the thermal behaviour of some polyimide membranes. J. Therm. Anal. Calorim. 1998, 52. [Google Scholar] [CrossRef]
- Cheng, L.P.; Lin, D.J.; Yang, K.C. Formation of mica-intercalated-Nylon 6 nanocomposite membranes by phase inversion method. J. Memb. Sci. 2000. [Google Scholar] [CrossRef]
- Boom, R.M.; Wienk, I.M.; van den Boomgaard, T.; Smolders, C.A. Microstructures in phase inversion membranes. Part 2. The role of a polymeric additive. J. Memb. Sci. 1992. [Google Scholar] [CrossRef]
- Han, B.; Zhang, D.; Shao, Z.; Kong, L.; Lv, S. Preparation and characterization of cellulose acetate/carboxymethyl cellulose acetate blend ultrafiltration membranes. Desalination 2013. [Google Scholar] [CrossRef]
- Han, M.J.; Bhattacharyya, D. Characterization of reverse osmosis cellulose acetate membranes by gas adsorption method: Effect of casting variables and chlorine damage. J. Memb. Sci. 1991. [Google Scholar] [CrossRef]
- Guizard, C.; Rios, G. Chapter 12 Transport and fouling phenomena in liquid phase separation with inorganic and hybrid membranes. Membr. Sci. Technol. 1996, 4, 569–618. [Google Scholar] [CrossRef]
- Duncan, B.; Urquhart, J.; Roberts, S. Review of Measurement and Modelling of Permeation and Diffusion in Polymers. In U.K. National Physical Laboratory Report; National Physical Laboratory: Teddington-Middlesex, UK, 2005; pp. 1–73. ISSN 1744-0270. [Google Scholar]
- Qin, J.J.; Oo, M.H.; Li, Y. Development of high flux polyethersulfone hollow fiber ultrafiltration membranes from a low critical solution temperature dope via hypochlorite treatment. J. Memb. Sci. 2005. [Google Scholar] [CrossRef]
- Zheng, Q.Z.; Wang, P.; Yang, Y.N.; Cui, D.J. The relationship between porosity and kinetics parameter of membrane formation in PSF ultrafiltration membrane. J. Memb. Sci. 2006. [Google Scholar] [CrossRef]
- Jiang, B.T.; Chan, P.K. Effect of concentration gradient on the morphology development in polymer solutions undergoing thermally induced phase separation. Macromol. Theory Simul. 2007. [Google Scholar] [CrossRef]
- Blanco, J.F.; Sublet, J.; Nguyen, Q.T.; Schaetzel, P. Formation and morphology studies of different polysulfones-based membranes made by wet phase inversion process. J. Memb. Sci. 2006. [Google Scholar] [CrossRef]
- Wang, B.; Ji, J.; Chen, C.; Li, K. Porous membranes prepared by a combined crystallisation and diffusion (CCD) method: Study on formation mechanisms. J. Memb. Sci. 2018. [Google Scholar] [CrossRef]
- Feng, C.; Wang, R.; Shi, B.; Li, G.; Wu, Y. Factors affecting pore structure and performance of poly(vinylidene fluoride-co-hexafluoro propylene) asymmetric porous membrane. J. Memb. Sci. 2006. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010. [Google Scholar] [CrossRef]
- Peinemann, K.V.; Abetz, V.; Simon, P.F.W. Asymmetric superstructure formed in a block copolymer via phase separation. Nat. Mater. 2007. [Google Scholar] [CrossRef] [PubMed]
- Leisi, R.; Bieri, J.; Roth, N.J.; Ros, C. Determination of parvovirus retention profiles in virus filter membranes using laser scanning microscopy. J. Memb. Sci. 2020. [Google Scholar] [CrossRef]
- Yang, S.Y.; Park, J.; Yoon, J.; Ree, M.; Jang, S.K.; Kim, J.K. Virus filtration membranes prepared from nanoporous block copolymers with good dimensional stability under high pressures and excellent solvent resistance. Adv. Funct. Mater. 2008. [Google Scholar] [CrossRef]
- Akthakul, A.; McDonald, W.F.; Mayes, A.M. Noncircular pores on the surface of asymmetric polymer membranes: Evidence of pore formation via spinodal demixing. J. Memb. Sci. 2002. [Google Scholar] [CrossRef]
- Radovanovic, P.; Thiel, S.W.; Hwang, S.T. Formation of asymmetric polysulfone membranes by immersion precipitation. Part I. Modelling mass transport during gelation. J. Memb. Sci. 1992. [Google Scholar] [CrossRef]
- Radjabian, M.; Abetz, V. Advanced porous polymer membranes from self-assembling block copolymers. Prog. Polym. Sci. 2020, 102, 101219. [Google Scholar] [CrossRef]
- Tompa, H. Polymer Solutions; Butterworths Scientific Publications: London, UK, 1956; ISBN 57003566. [Google Scholar]
- Altena, F.W.; Smolders, C.A. Calculation of Liquid-Liquid Phase Separation in a Ternary System of a Polymer in a Mixture of a Solvent and a Nonsolvent. Macromolecules 1982. [Google Scholar] [CrossRef]
- Cohen, C.; Tanny, G.B.; Prager, S. Diffusion-controlled formation of porous structures in ternary polymer systems. J. Polym. Sci. Polym. Phys. Ed. 1979. [Google Scholar] [CrossRef]
- Reuvers, A.J.; Smolders, C.A. Formation of membranes by means of immersion precipitation. Part II. The mechanism of formation of membranes prepared from the system cellulose-acetate-acetone-water. J. Memb. Sci. 1987, 34, 67–86. [Google Scholar] [CrossRef]
- Reuvers, A.J.; van den Berg, J.W.A.; Smolders, C.A. Formation of membranes by means of immersion precipitation. Part I. A model to describe mass transfer during immersion precipitation. J. Memb. Sci. 1987, 34, 45–65. [Google Scholar] [CrossRef]
- Bulte, A.M.W.; Mulder, M.H.V.; Smolders, C.A.; Strathmann, H. Diffusion induced phase separtion with crystallizable nylons. I. J. Memb. Sci. 1996, 121, 37–49. [Google Scholar] [CrossRef]
- Kim, Y.D.; Kim, J.Y.; Lee, H.K.; Kim, S.C. A new modeling of asymmetric membrane formation in rapid mass transfer system. J. Memb. Sci. 2001. [Google Scholar] [CrossRef]
- Akthakul, A.; Scott, C.E.; Mayes, A.M.; Wagner, A.J. Lattice Boltzmann simulation of asymmetric membrane formation by immersion precipitation. J. Memb. Sci. 2005. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Raoufi, N.; Rezakazemi, M.; Shirazian, S. Simulation of Nonporous Polymeric Membranes Using CFD for Bioethanol Purification. Macromol. Theory Simulations 2018. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Sriyamuna Devi, T.K.; Mohan, D. Development, characterization and separation performance of organic-inorganic membranes. Part II. Effect of additives. Sep. Purif. Technol. 2009. [Google Scholar] [CrossRef]
- Nechifor, G.; Voicu, S.I.; Nechifor, A.C.; Garea, S. Nanostructured hybrid membrane polysulfone-carbon nanotubes for hemodialysis. Desalination 2009, 241, 342–348. [Google Scholar] [CrossRef]
- Totu, E.E.; Ruse, E.; Gârdea, R.; Grigorescu, A. FT-IR analysis on polyimide selective membranes. Optoelectron. Adv. Mater. Rapid Commun. 2008, 2, 442–445. [Google Scholar]
- Nechifor, G.; Totu, E.E.; Nechifor, A.C.; Isildak, I.; Oprea, O.; Cristache, C.M. Non-resorbable nanocomposite membranes for guided bone regeneration based on polysulfone-quartz fiber grafted with nano-TiO2. Nanomaterials 2019, 9, 985. [Google Scholar] [CrossRef]
- Totu, E.E.; Ruse, E.; Diaconu, I.G. Characterisation of polyimide membranes using thermochemical data. Optoelectron. Adv. Mater. Rapid Commun. 2009, 3, 808–811. [Google Scholar]
- Miricioiu, M.G.; Iacob, C.; Nechifor, G.; Niculescu, V.C. High selective mixed membranes based on mesoporous MCM-41 and MCM-41-NH2 particles in a polysulfone matrix. Front. Chem. 2019, 7, 332. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Mohan, D.; Raajenthiren, M. Preparation and performance of polysulfone-sulfonated poly(ether ether ketone) blend ultrafiltration membranes. Part I. Appl. Surf. Sci. 2007. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P.; Raajenthiren, M. Sulfonated poly(ether ether ketone)-induced porous poly(ether sulfone) blend membranes for the separation of proteins and metal ions. J. Appl. Polym. Sci. 2010. [Google Scholar] [CrossRef]
- Scutariu, R.E.; Batrinescu, G.; Nechifor, G.; Popescu, M.; Tenea, A.G. Separation of the collagen protein by ultrafiltration: Effects of concentration on the membrane’s characteristics. Polym. Eng. Sci. 2020, 60, 2487–2495. [Google Scholar] [CrossRef]
- Eftimie Totu, E.; Ruse, E.; Diaconu, I.; Gîrdea, R. On the behaviour of polyimide ion-selective membranes in electrolyte solutions. Optoelectron. Adv. Mater. Rapid Commun. 2009, 3, 1327–1330. [Google Scholar]
- Trombley, C.I.; Ekiel-Jeżewska, M.L. Basic Concepts of Stokes Flows. In Flowing Matter Soft and Biological Matter; Toschi, F.M.S., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]



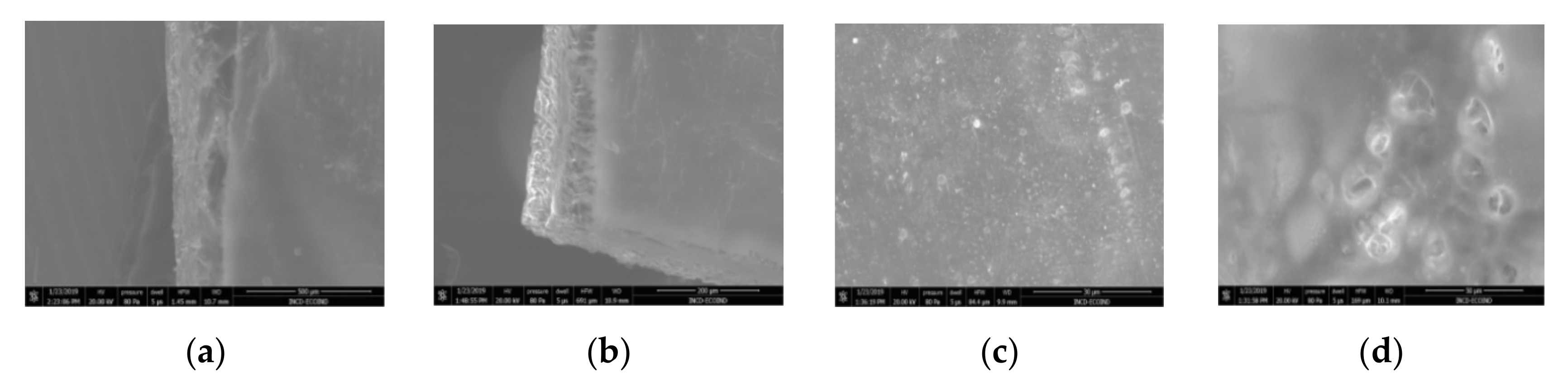



| Membrane * | Surface | Bottom | L (µm) | (%) |
|---|---|---|---|---|
| MPSf1 |  | 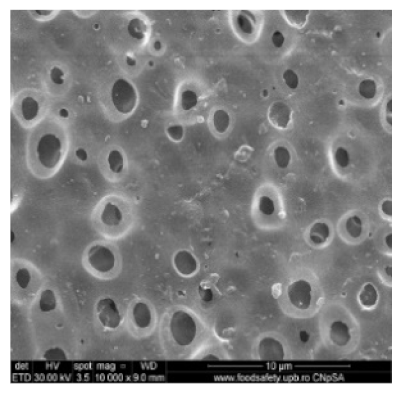 | 126 ± 2 | 72 ± 3 |
| MPSf2 | 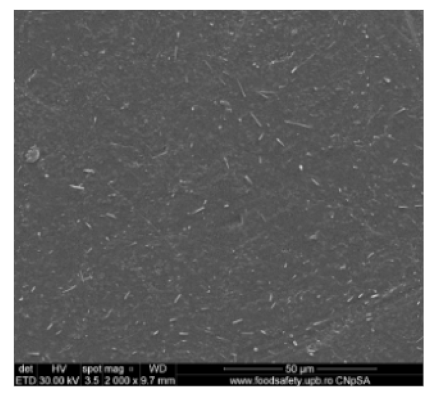 | 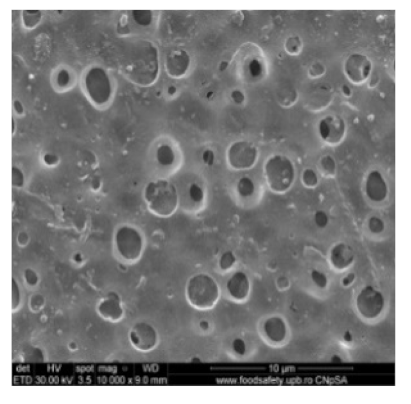 | 124 ± 2 | 69 ± 3 |
| MPSf3 |  |  | 123 ± 2 | 67 ± 3 |
| MPSf4 |  | 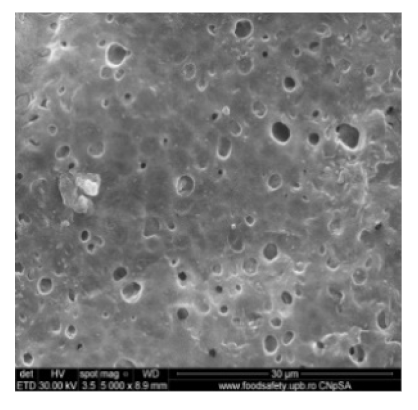 | 117 ± 6 | 63 ± 5 |
| Membrane Type | Normalized Flow, J (L/m2·h·bar) | Retention, R (%) |
|---|---|---|
| MPSf1 | 73 ± 2 | 75 ± 3 |
| MPSf2 | 61 ± 2 | 82 ± 3 |
| MPSf3 | 54 ± 2 | 93 ± 3 |
| MPSf4 | 51 ± 2 | 84 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bărdacă Urducea, C.; Nechifor, A.C.; Dimulescu, I.A.; Oprea, O.; Nechifor, G.; Totu, E.E.; Isildak, I.; Albu, P.C.; Bungău, S.G. Control of Nanostructured Polysulfone Membrane Preparation by Phase Inversion Method. Nanomaterials 2020, 10, 2349. https://doi.org/10.3390/nano10122349
Bărdacă Urducea C, Nechifor AC, Dimulescu IA, Oprea O, Nechifor G, Totu EE, Isildak I, Albu PC, Bungău SG. Control of Nanostructured Polysulfone Membrane Preparation by Phase Inversion Method. Nanomaterials. 2020; 10(12):2349. https://doi.org/10.3390/nano10122349
Chicago/Turabian StyleBărdacă Urducea, Cristina, Aurelia Cristina Nechifor, Ioana Alina Dimulescu, Ovidiu Oprea, Gheorghe Nechifor, Eugenia Eftimie Totu, Ibrahim Isildak, Paul Constantin Albu, and Simona Gabriela Bungău. 2020. "Control of Nanostructured Polysulfone Membrane Preparation by Phase Inversion Method" Nanomaterials 10, no. 12: 2349. https://doi.org/10.3390/nano10122349
APA StyleBărdacă Urducea, C., Nechifor, A. C., Dimulescu, I. A., Oprea, O., Nechifor, G., Totu, E. E., Isildak, I., Albu, P. C., & Bungău, S. G. (2020). Control of Nanostructured Polysulfone Membrane Preparation by Phase Inversion Method. Nanomaterials, 10(12), 2349. https://doi.org/10.3390/nano10122349


.jpg)