Self-Assembled Nano-Fe3C Embedded in Reduced Graphene Oxide Aerogel with Efficient Fenton-Like Catalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Self-Assembled Nano-Fe3C@RGO Aerogel
2.3. Characterization
2.4. Catalytic Degradation Experimental of Methyl Orange by Self-Assembled Nano-Fe3C@RGO Aerogel
2.5. Analytical Methods
3. Results and Discussion
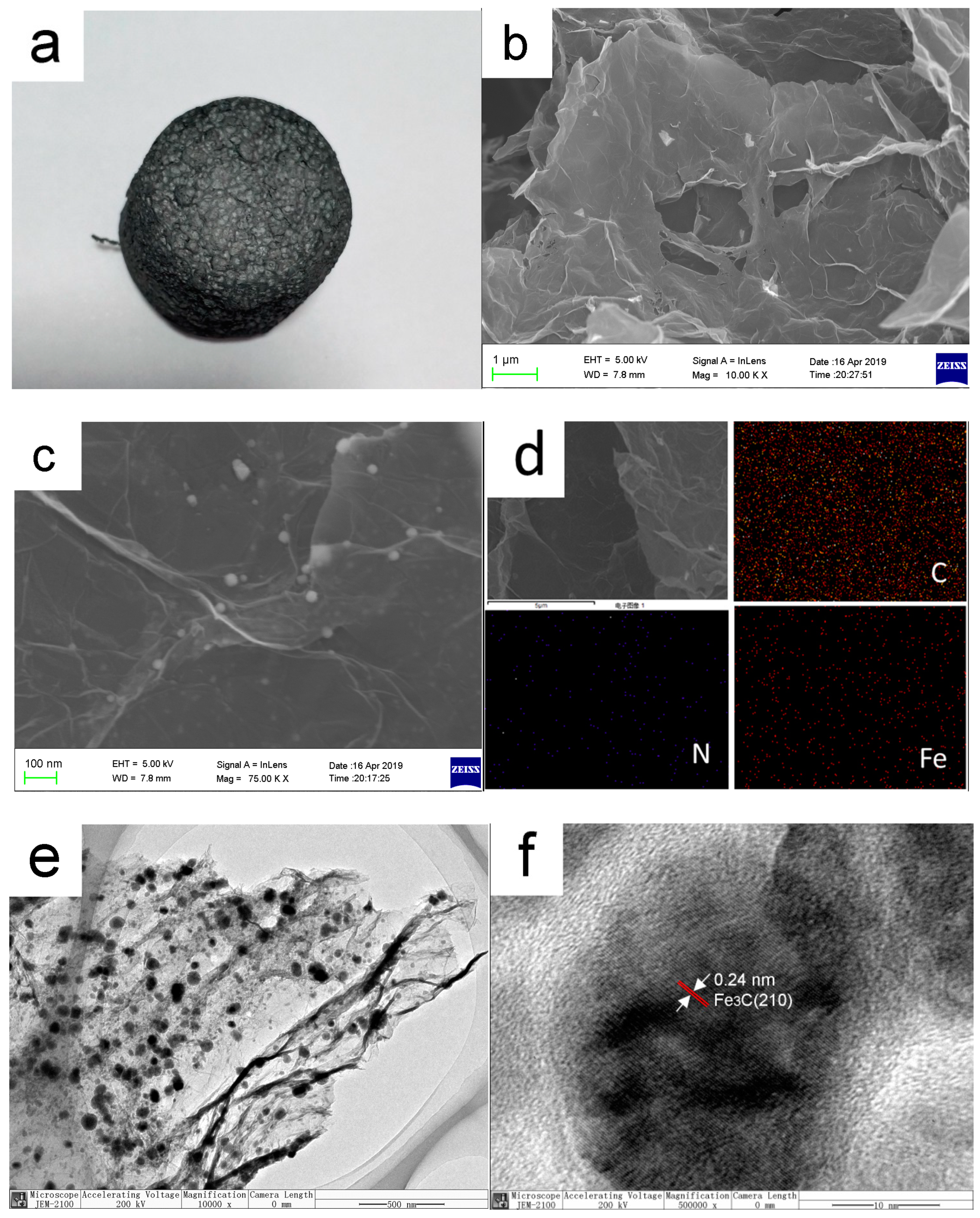
3.1. The Morphology of Self-Assembled Nano-Fe3C@RGO Aerogel
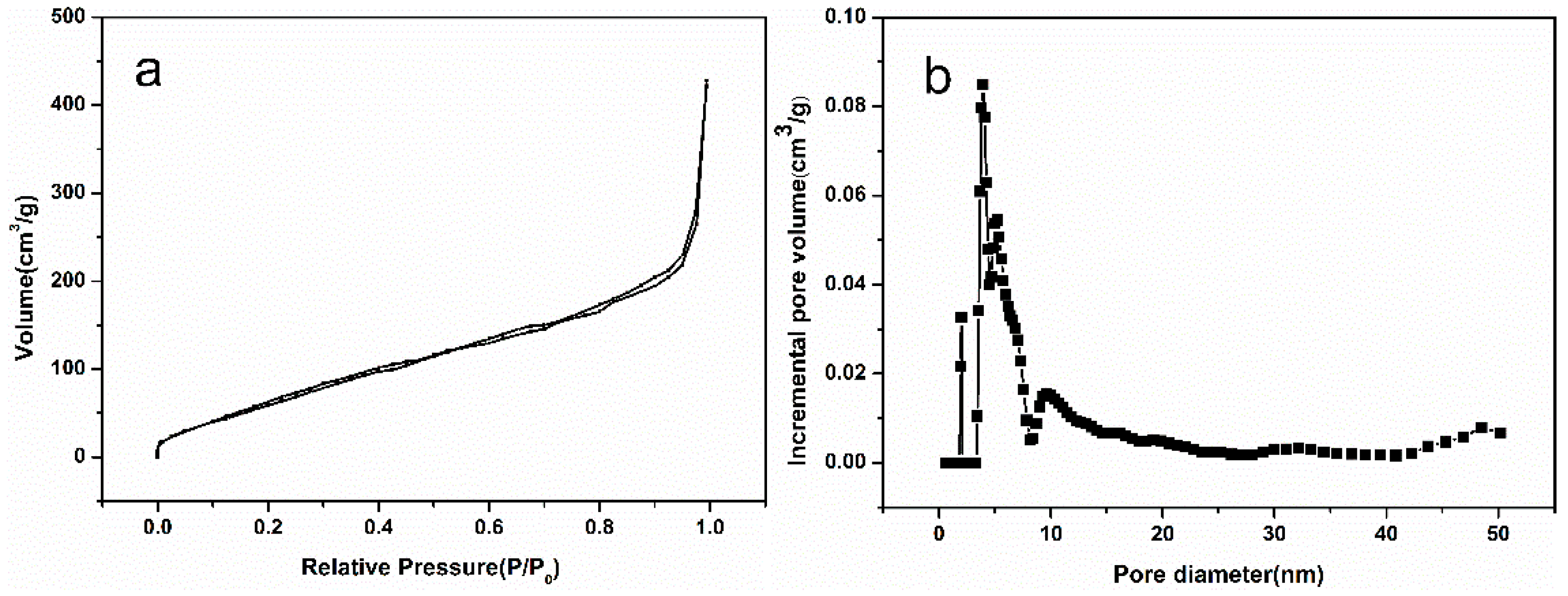
3.2. Pore Size Distribution
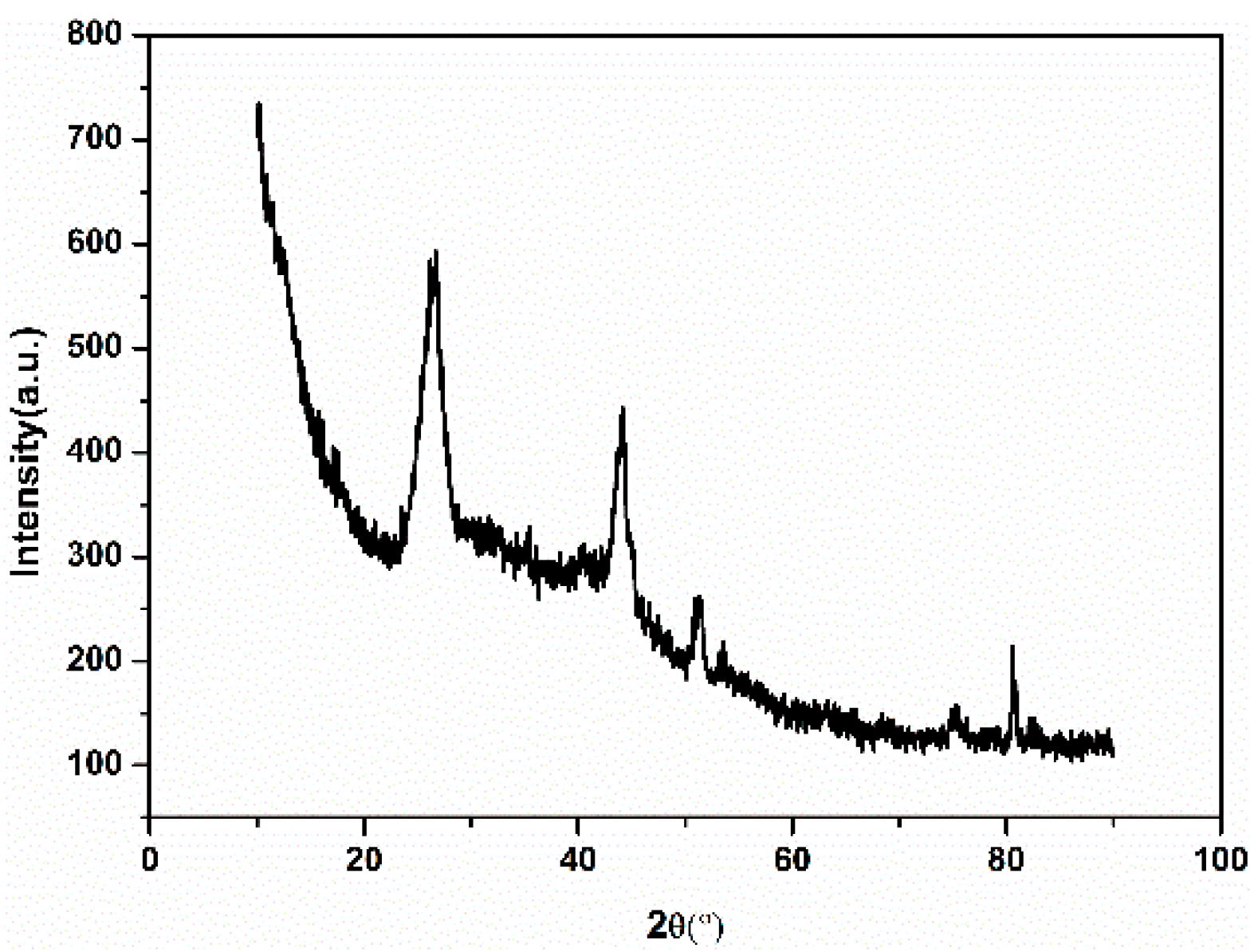
3.3. XRD
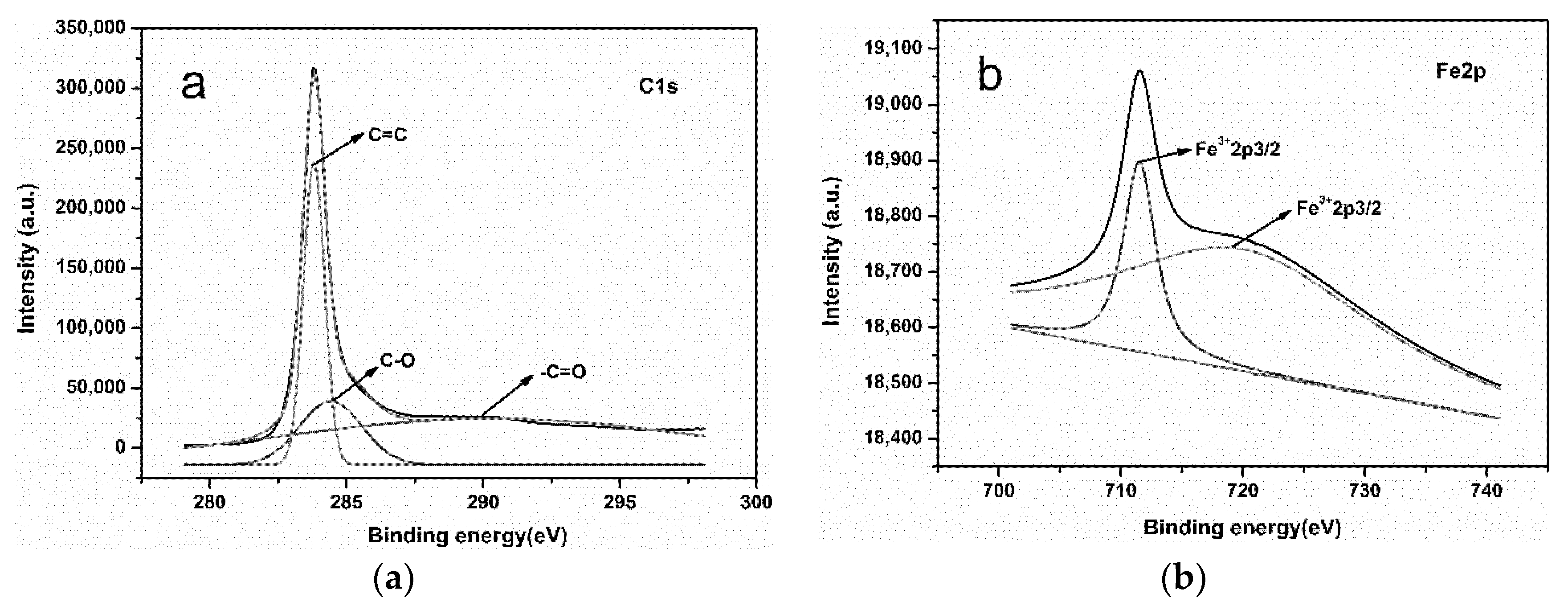
3.4. XPS
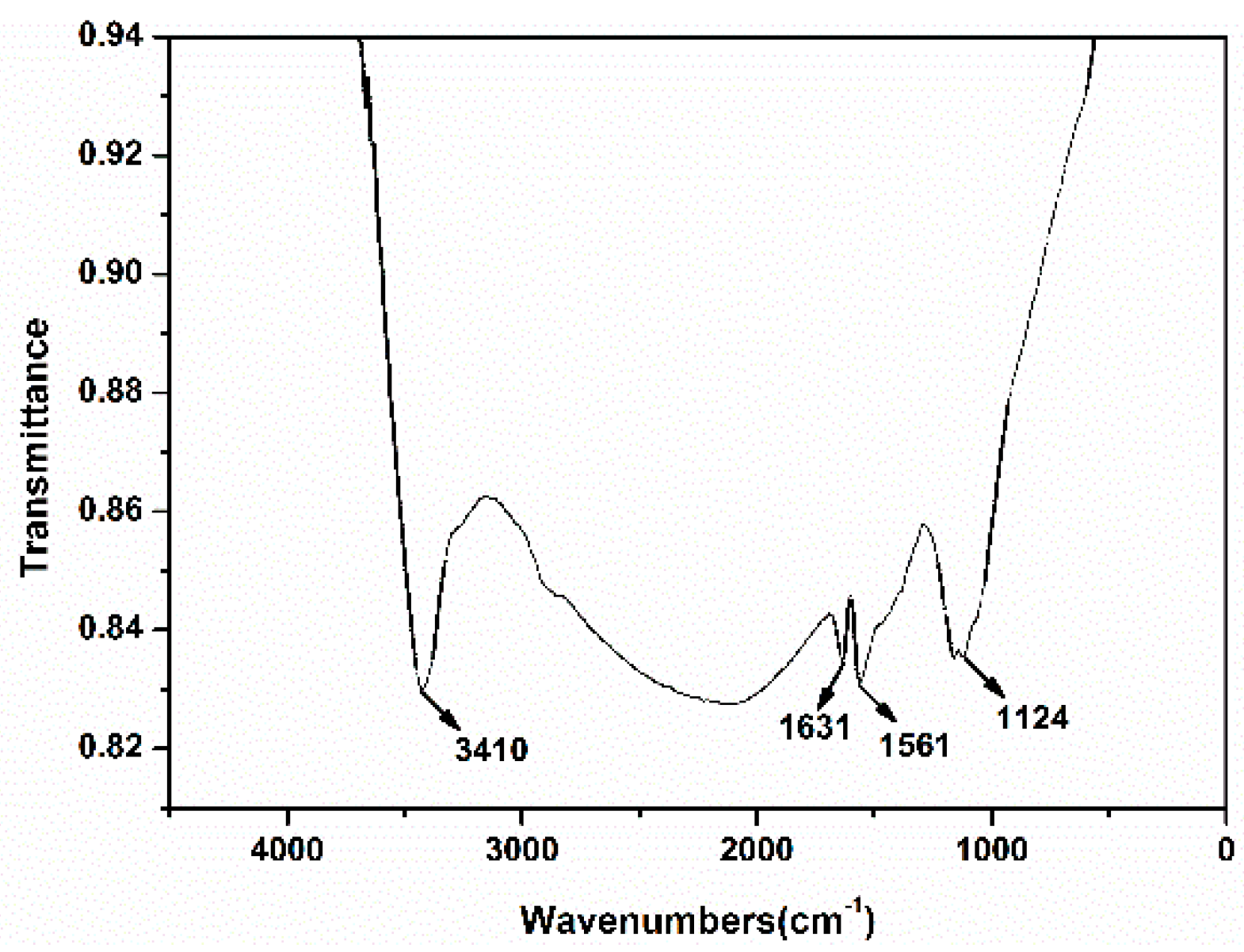
3.5. FTIR
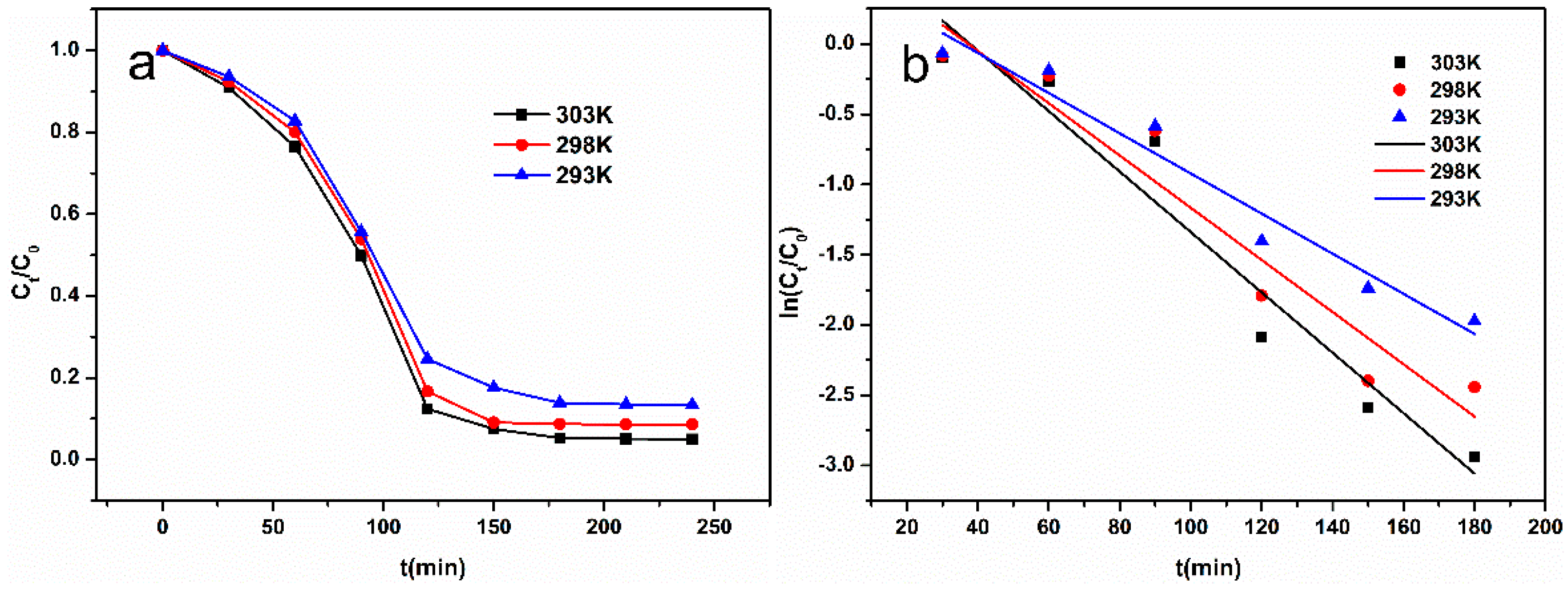
3.6. Catalytic Degradation Property of Self-Assembled Nano-Fe3C@RGO Aerogel for Methyl Orange
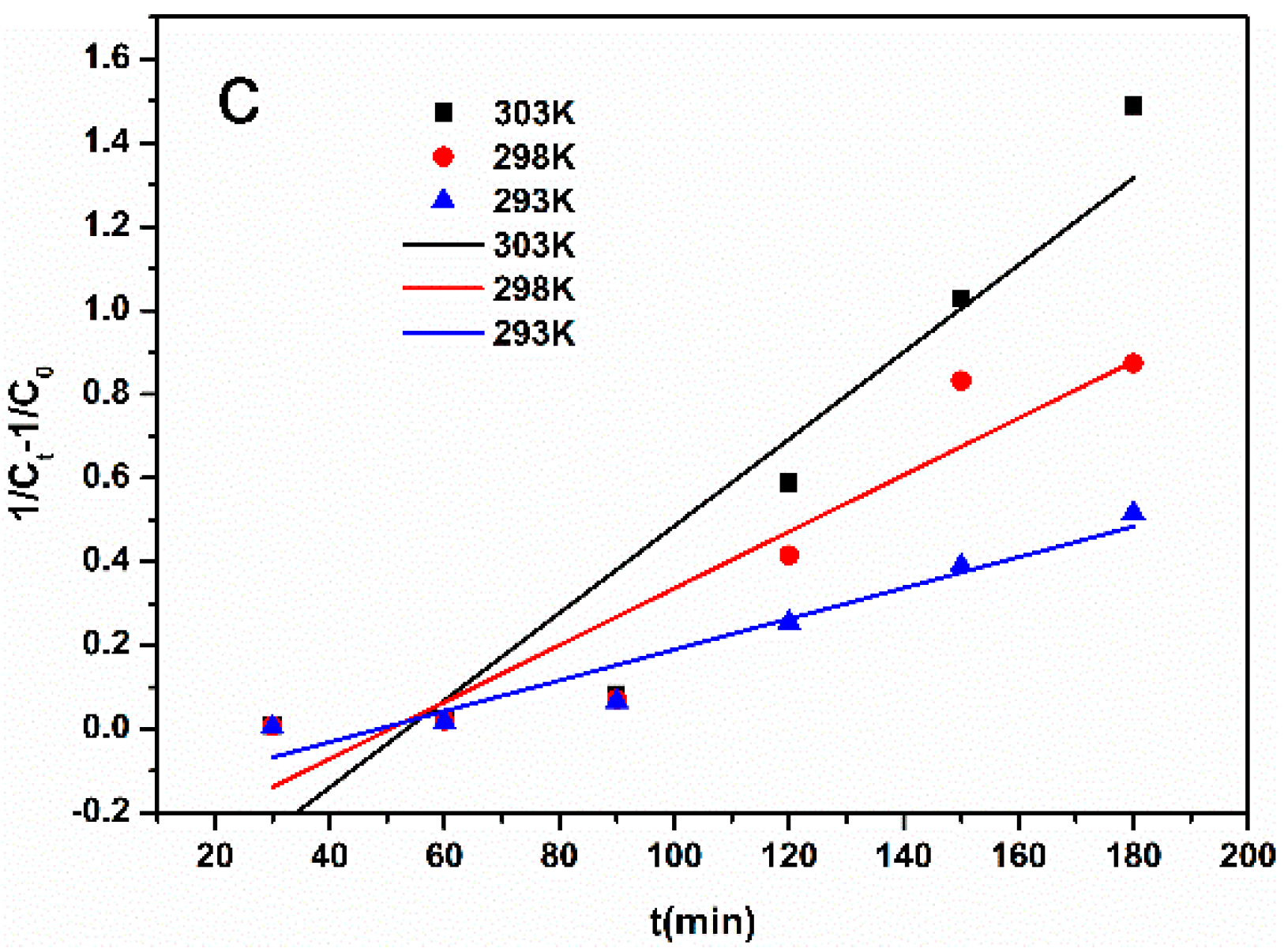
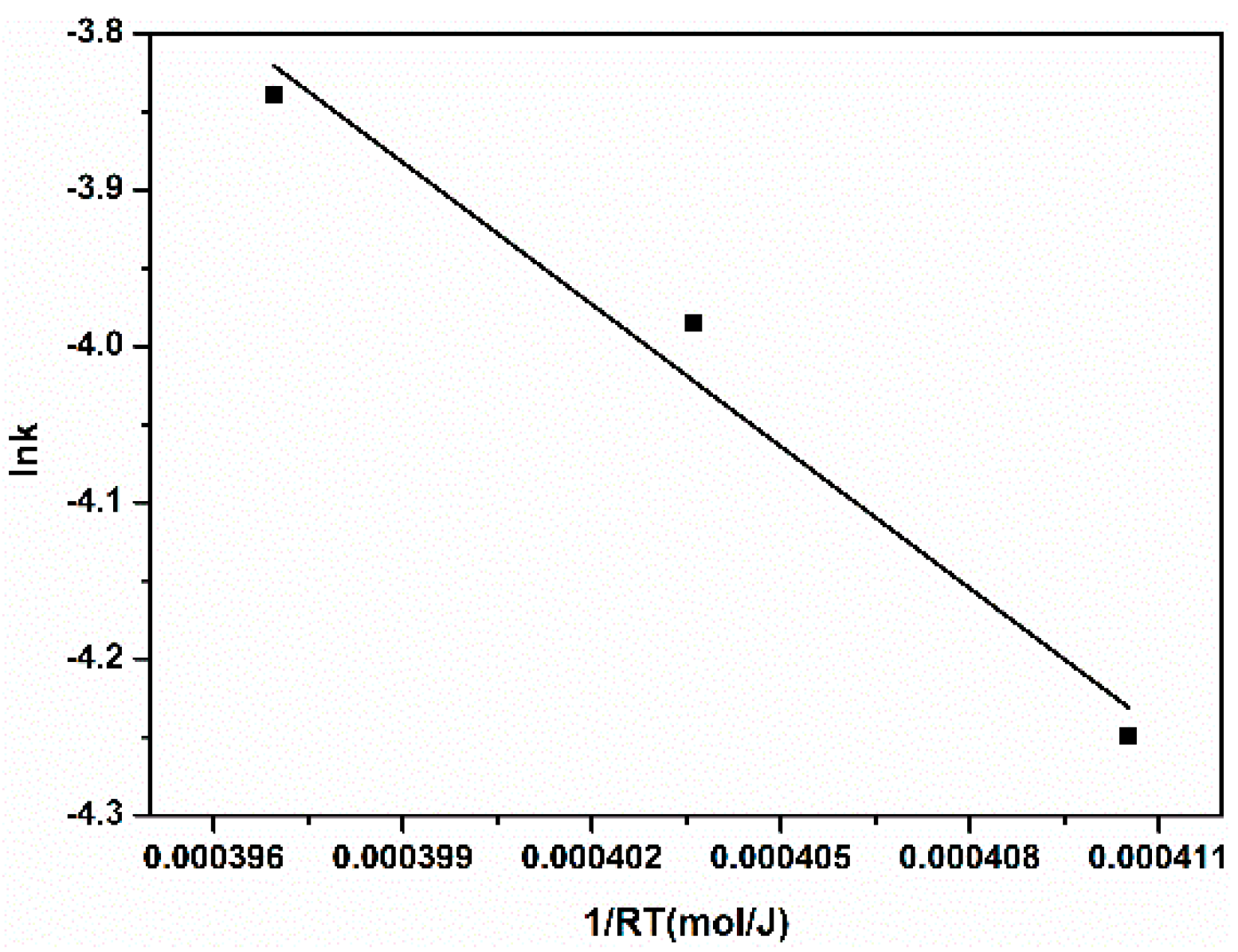
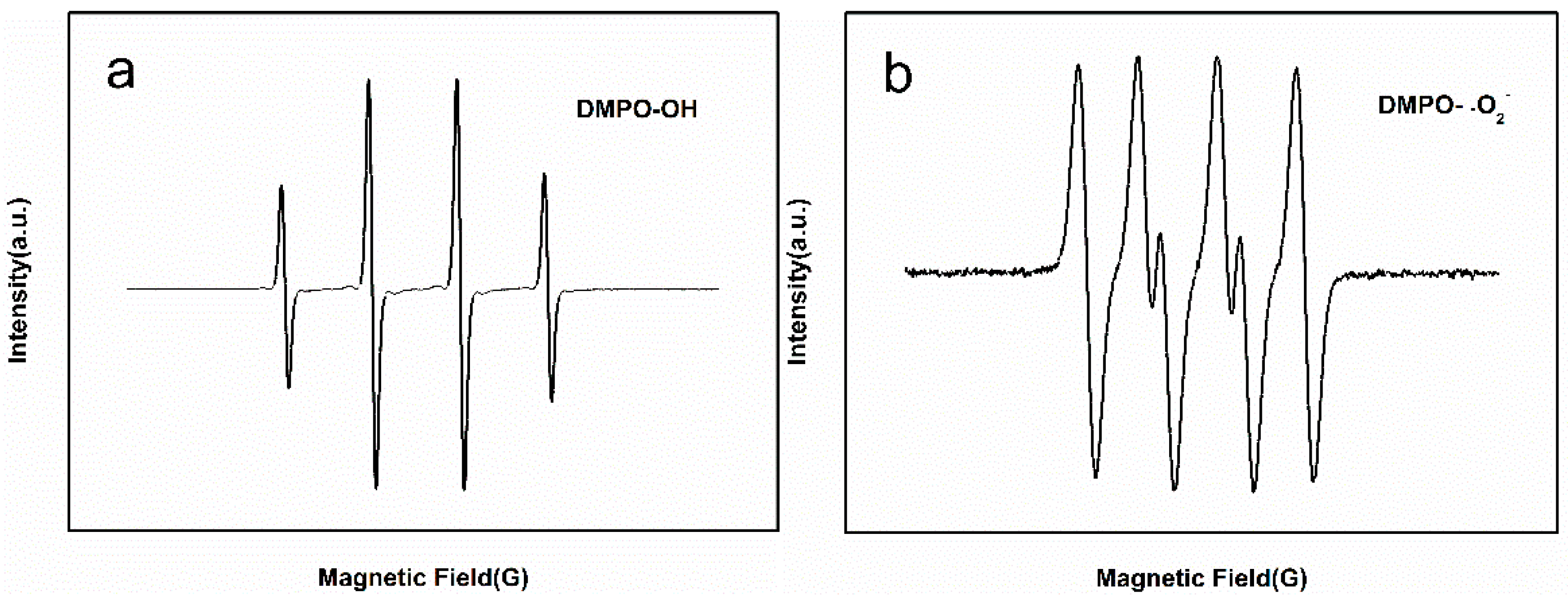
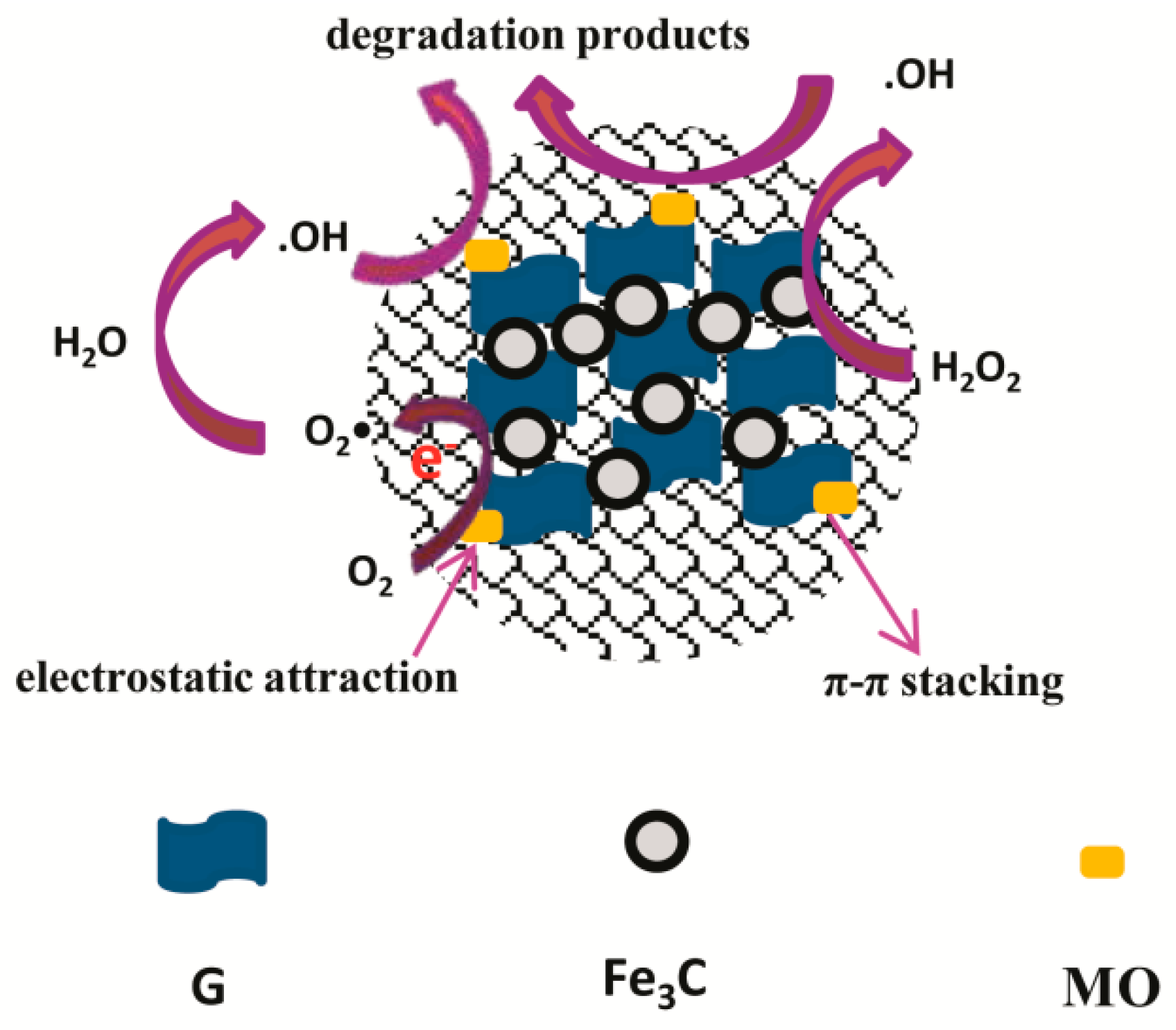
3.7. Catalytic Degradation Mechanism of Self-Assembled Nano-Fe3C@RGO Aerogel for Methyl Orange
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, J.-H.; Wang, J.J.; Xiao, R.; Tafti, N.; DeLaune, R.D.; Seo, D.-C. Degradation of Orange G by Fenton-like reaction with Fe-impregnated biochar catalyst. Bioresour. Technol. 2018, 249, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tian, A.; You, J.; Yang, H.; Wang, Y.; Xue, X. Degradation of organic dyes by a new heterogeneous Fenton reagent-Fe2GeS4 nanoparticle. J. Hazard. Mater. 2018, 353, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Cheng, N.; Feng, L.; Gu, B.-H.; Liu, Y. Multivalent Supramolecular Self-Assembly between β-Cyclodextrin Derivatives and Polyoxometalate for Photodegradation of Dyes and Antibiotics. ACS Appl. Bio Mater. 2019, 2, 5898–5904. [Google Scholar] [CrossRef]
- Mei, Y.; Zeng, J.; Sun, M.; Ma, J.; Komarneni, S. A novel Fenton-like system of Fe2O3 and NaHSO3 for Orange II degradation. Sep. Purif. Technol. 2020, 230, 115866. [Google Scholar] [CrossRef]
- Shi, B.; Zhao, C.; Ji, Y.; Shi, J.; Yang, H. Promotion effect of PANI on Fe-PANI/Zeolite as an active and recyclable Fenton-like catalyst under near-neutral condition. Appl. Surf. Sci. 2020, 508, 145298. [Google Scholar] [CrossRef]
- Weng, X.; Owens, G.; Chen, Z. Synergetic adsorption and Fenton-like oxidation for simultaneous removal of ofloxacin and enrofloxacin using green synthesized Fe NPs. Chem. Eng. J. 2020, 382, 122871. [Google Scholar] [CrossRef]
- Wu, J.; Wang, B.; Cagnetta, G.; Huang, J.; Wang, Y.; Deng, S.; Yu, G. Nanoscale zero valent iron-activated persulfate coupled with Fenton oxidation process for typical pharmaceuticals and personal care products degradation. Sep. Purif. Technol. 2020, 239, 116534. [Google Scholar] [CrossRef]
- Yi, Y.; Tu, G.; Eric Tsang, P.; Fang, Z. Insight into the influence of pyrolysis temperature on Fenton-like catalytic performance of magnetic biochar. Chem. Eng. J. 2020, 380, 122518. [Google Scholar] [CrossRef]
- Banerjee, S.; Benjwal, P.; Singh, M.; Kar, K.K. Graphene oxide (rGO)-metal oxide (TiO2/Fe3O4) based nanocomposites for the removal of methylene blue. Appl. Surf. Sci. 2018, 439, 560–568. [Google Scholar] [CrossRef]
- Wang, W.; Cao, Y.; Hu, X.; Zhou, S.; Zhu, D.; Qi, D.; Deng, S. Granular reduced graphene oxide/Fe3O4 hydrogel for efficient adsorption and catalytic oxidation of p-perfluorous nonenoxybenzene sulfonate. J. Hazard. Mater. 2020, 386, 121662. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, X.; Liu, Q.; Shi, B. N-doped FeOOH/RGO hydrogels with a dual-reaction-center for enhanced catalytic removal of organic pollutants. Chem. Eng. J. 2020, 379, 122310. [Google Scholar] [CrossRef]
- Bao, T.; Jin, J.; Damtie, M.M.; Wu, K.; Yu, Z.M.; Wang, L.; Chen, J.; Zhang, Y.; Frost, R.L. Green synthesis and application of nanoscale zero-valent iron/rectorite composite material for P-chlorophenol degradation via heterogeneous Fenton reaction. J. Saudi Chem. Soc. 2019, 23, 864–878. [Google Scholar] [CrossRef]
- Deng, J.; Dong, H.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zhang, L.; Fan, C. Nanoscale zero-valent iron/biochar composite as an activator for Fenton-like removal of sulfamethazine. Sep. Purif. Technol. 2018, 202, 130–137. [Google Scholar] [CrossRef]
- Lin, J.; Sun, M.; Liu, X.; Chen, Z. Functional kaolin supported nanoscale zero-valent iron as a Fenton-like catalyst for the degradation of Direct Black, G. Chemosphere 2017, 184, 664–672. [Google Scholar] [CrossRef]
- Morshed, M.N.; Bouazizi, N.; Behary, N.; Guan, J.; Nierstrasz, V. Stabilization of zero valent iron (Fe0) on plasma/dendrimer functionalized polyester fabrics for Fenton-like removal of hazardous water pollutants. Chem. Eng. J. 2019, 374, 658–673. [Google Scholar] [CrossRef]
- Gao, P.; Chen, X.; Hao, M.; Xiao, F.; Yang, S. Oxygen vacancy enhancing the Fe2O3-CeO2 catalysts in Fenton-like reaction for the sulfamerazine degradation under O2 atmosphere. Chemosphere 2019, 228, 521–527. [Google Scholar] [CrossRef]
- Ren, B.; Miao, J.; Xu, Y.; Zhai, Z.; Dong, X.; Wang, S.; Zhang, L.; Liu, Z. A grape-like N-doped carbon/CuO-Fe2O3 nanocomposite as a highly active heterogeneous Fenton-like catalyst in methylene blue degradation. J. Clean. Prod. 2019, 240, 118143. [Google Scholar] [CrossRef]
- Vu, A.-T.; Xuan, T.N.; Lee, C.-H. Preparation of mesoporous Fe2O3·SiO2 composite from rice husk as an efficient heterogeneous Fenton-like catalyst for degradation of organic dyes. J. Water Process Eng. 2019, 28, 169–180. [Google Scholar] [CrossRef]
- Gong, Q.; Liu, Y.; Dang, Z. Core-shell structured Fe3O4@GO@MIL-100(Fe) magnetic nanoparticles as heterogeneous photo-Fenton catalyst for 2,4-dichlorophenol degradation under visible light. J. Hazard. Mater. 2019, 371, 677–686. [Google Scholar] [CrossRef]
- Pan, X.; Cheng, S.; Su, T.; Zuo, G.; Zhao, W.; Qi, X.; Wei, W.; Dong, W. Fenton-like catalyst Fe3O4@polydopamine-MnO2 for enhancing removal of methylene blue in wastewater. Colloids Surf. B Biointerfaces 2019, 181, 226–233. [Google Scholar] [CrossRef]
- Roy, K.; Agarkoti, C.; Malani, R.S.; Thokchom, B.; Moholkar, V.S. Mechanistic study of sulfadiazine degradation by ultrasound-assisted Fenton-persulfate system using yolk-shell Fe3O4@hollow@mSiO2 nanoparticles. Chem. Eng. Sci. 2020, 217, 115522. [Google Scholar] [CrossRef]
- Sun, C.; Yang, S.T.; Gao, Z.; Yang, S.; Yilihamu, A.; Ma, Q.; Zhao, R.S.; Xue, F. Fe3O4/TiO2/reduced graphene oxide composites as highly efficient Fenton-like catalyst for the decoloration of methylene blue. Mater. Chem. Phys. 2019, 223, 751–757. [Google Scholar] [CrossRef]
- Tong, M.; Liu, F.; Dong, Q.; Ma, Z.; Liu, W. Magnetic Fe3O4-deposited flower-like MoS2 nanocomposites for the Fenton-like Escherichia coli disinfection and diclofenac degradation. J. Hazard. Mater. 2020, 385, 121604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, P.; Chen, Z.; Zhou, L.; Zhao, Y.; Lai, Y.; Duan, Y.; Wang, F.; Shuai, L. Synergistic effect in heterogeneous Fenton degradation of tetrabromobisphenol A by MWCNT and β-CD co-modified Fe3O4. Mater. Res. Bull. 2019, 113, 14–24. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zhao, Y.; Dionysiou, D.D. Aligned α-FeOOH nanorods anchored on a graphene oxide-carbon nanotubes aerogel can serve as an effective Fenton-like oxidation catalyst. Appl. Catal. B Environ. 2017, 213, 74–86. [Google Scholar] [CrossRef]
- Qian, X.; Wu, Y.; Kan, M.; Fang, M.; Yue, D.; Zeng, J.; Zhao, Y. FeOOH quantum dots coupled g-C3N4 for visible light driving photo- Fenton degradation of organic pollutants. Appl. Catal. B Environ. 2018, 237, 513–520. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Crittenden, J.C.; Shen, C. Novel RGO/α-FeOOH supported catalyst for Fenton oxidation of phenol at a wide pH range using solar-light-driven irradiation. J. Hazard. Mater. 2017, 329, 321–329. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, H.; Yu, Y.; Hu, C. Enhanced catalytic activity of α-FeOOH-rGO supported on active carbon fiber (ACF) for degradation of phenol and quinolone in the solar-Fenton system. Chemosphere 2018, 208, 931–941. [Google Scholar] [CrossRef]
- Xiao, F.; Li, W.; Fang, L.; Wang, D. Synthesis of akageneite (beta-FeOOH)/reduced graphene oxide nanocomposites for oxidative decomposition of 2-chlorophenol by Fenton-like reaction. J. Hazard. Mater. 2016, 308, 11–20. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.; Chen, B. Self-Assembled Nano-FeO(OH)/Reduced Graphene Oxide Aerogel as a Reusable Catalyst for Photo-Fenton Degradation of Phenolic Organics. Environ. Sci. Technol. 2018, 52, 7043–7053. [Google Scholar] [CrossRef]
- Feng, L.; Xie, R.; Wang, C.; Gai, S.; He, F.; Yang, D.; Yang, P.; Lin, J. Magnetic Targeting, Tumor Microenvironment-Responsive Intelligent Nanocatalysts for Enhanced Tumor Ablation. ACS Nano 2018, 12, 11000–11012. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhao, F.; Gao, W.; Yang, X.; Ju, Y.; Zhao, L.; Guo, W.; Xie, J.; Liang, X.J.; Tao, X.; et al. Magnetic Reactive Oxygen Species Nanoreactor for Switchable Magnetic Resonance Imaging Guided Cancer Therapy Based on pH-Sensitive Fe5C2@Fe3O4 Nanoparticles. ACS Nano 2019, 13, 10002–10014. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Li, Y.; Kim, J.; Takei, T.; Wang, Z.; Xu, X.; Wang, J.; Bando, Y.; Kang, Y.M.; Tang, J.; et al. Sub-50 nm Iron–Nitrogen-Doped Hollow Carbon Sphere-Encapsulated Iron Carbide Nanoparticles as Efficient Oxygen Reduction Catalysts. Adv. Sci. 2018, 5, 1800120. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, M.; Weng, X.; Owens, G.; Chen, Z. Removal mechanism of mitoxantrone by a green synthesized hybrid reduced graphene oxide @ iron nanoparticles. Chemosphere 2020, 246, 125700. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.J.; Teel, A.L. Hydroxyl radical and non-hydroxyl radical pathways for trichloroethylene and perchloroethylene degradation in catalyzed H2O2 propagation systems. Water Res. 2019, 159, 46–54. [Google Scholar] [CrossRef]
- Zhao, H.; Joseph, J.; Zhang, H.; Karoui, H.; Kalyanaraman, B. Synthesis and biochemical applications of a solid cyclic nitrone spin trap: A relatively superior trap for detecting superoxide anions and glutathiyl radicals. Free Radic. Biol. Med. 2001, 31, 599–606. [Google Scholar] [CrossRef]
- Zhu, M.; Muhammad, Y.; Hu, P.; Wang, B.; Wu, Y.; Sun, X.; Tong, Z.; Zhao, Z. Enhanced interfacial contact of dopamine bridged melamine-graphene/TiO2 nano-capsules for efficient photocatalytic degradation of gaseous formaldehyde. Appl. Catal. B Environ. 2018, 232, 182–193. [Google Scholar] [CrossRef]

| T | 303 K | 298 K | 293 K |
|---|---|---|---|
| k1 (min−1) | 0.0215 | 0.01858 | 0.01428 |
| r | 0.9634 | 0.9544 | 0.9734 |
| T | 303 K | 298 K | 293 K |
|---|---|---|---|
| k2 (min−1) | 0.00925 | 0.00528 | 0.00309 |
| r | 0.9148 | 0.8472 | 0.9190 |
| Ea (kJ·mol−1) | lnA | r |
|---|---|---|
| 30.25 | 8.1865 | 0.9761 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhang, M.; Xie, J. Self-Assembled Nano-Fe3C Embedded in Reduced Graphene Oxide Aerogel with Efficient Fenton-Like Catalysis. Nanomaterials 2020, 10, 2348. https://doi.org/10.3390/nano10122348
Wang L, Zhang M, Xie J. Self-Assembled Nano-Fe3C Embedded in Reduced Graphene Oxide Aerogel with Efficient Fenton-Like Catalysis. Nanomaterials. 2020; 10(12):2348. https://doi.org/10.3390/nano10122348
Chicago/Turabian StyleWang, Liping, Mingyu Zhang, and Jiawei Xie. 2020. "Self-Assembled Nano-Fe3C Embedded in Reduced Graphene Oxide Aerogel with Efficient Fenton-Like Catalysis" Nanomaterials 10, no. 12: 2348. https://doi.org/10.3390/nano10122348
APA StyleWang, L., Zhang, M., & Xie, J. (2020). Self-Assembled Nano-Fe3C Embedded in Reduced Graphene Oxide Aerogel with Efficient Fenton-Like Catalysis. Nanomaterials, 10(12), 2348. https://doi.org/10.3390/nano10122348




