Enhancing and Tuning the Nonlinear Optical Response and Wavelength-Agile Strong Optical Limiting Action of N-octylamine Modified Fluorographenes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization
2.2. Experimental Setups for the Measurements of the NLO Properties and the OL Performance
3. Results and Discussion
3.1. Measurements of the NLO Properties
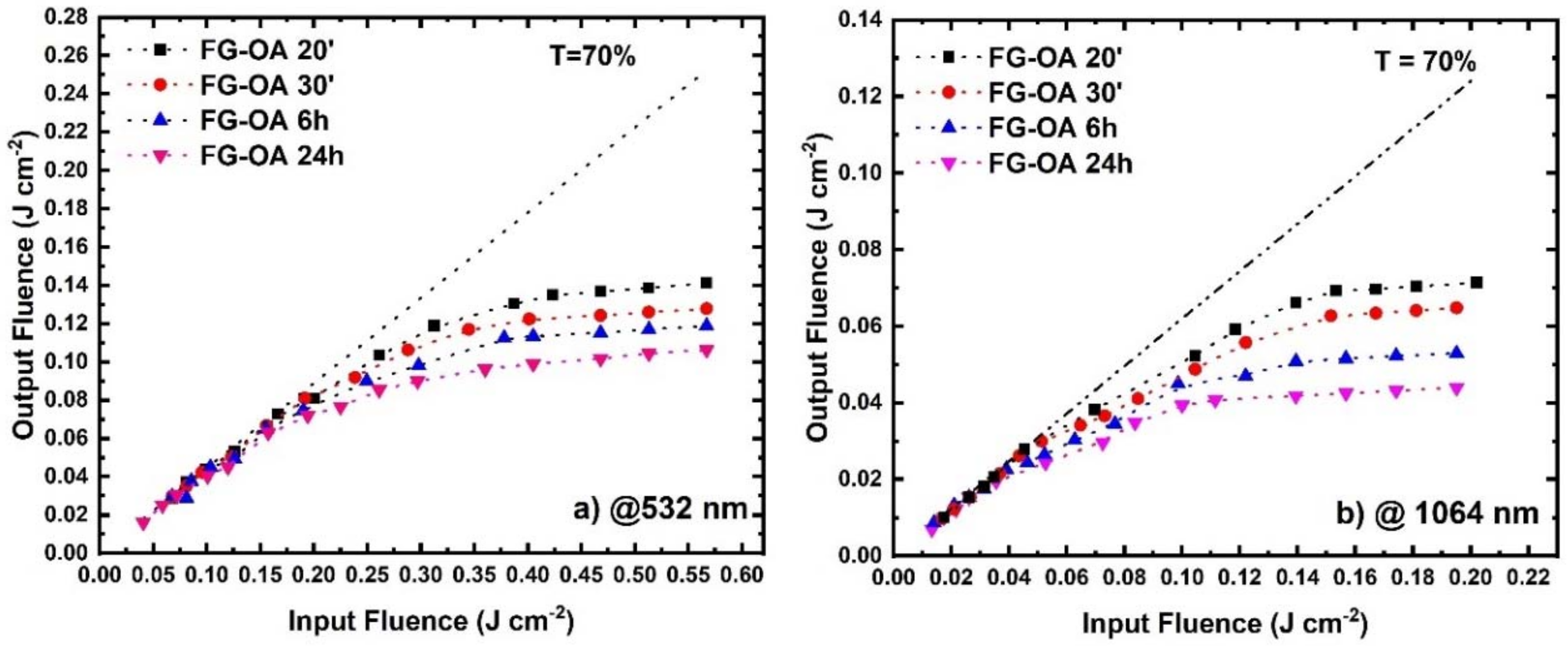
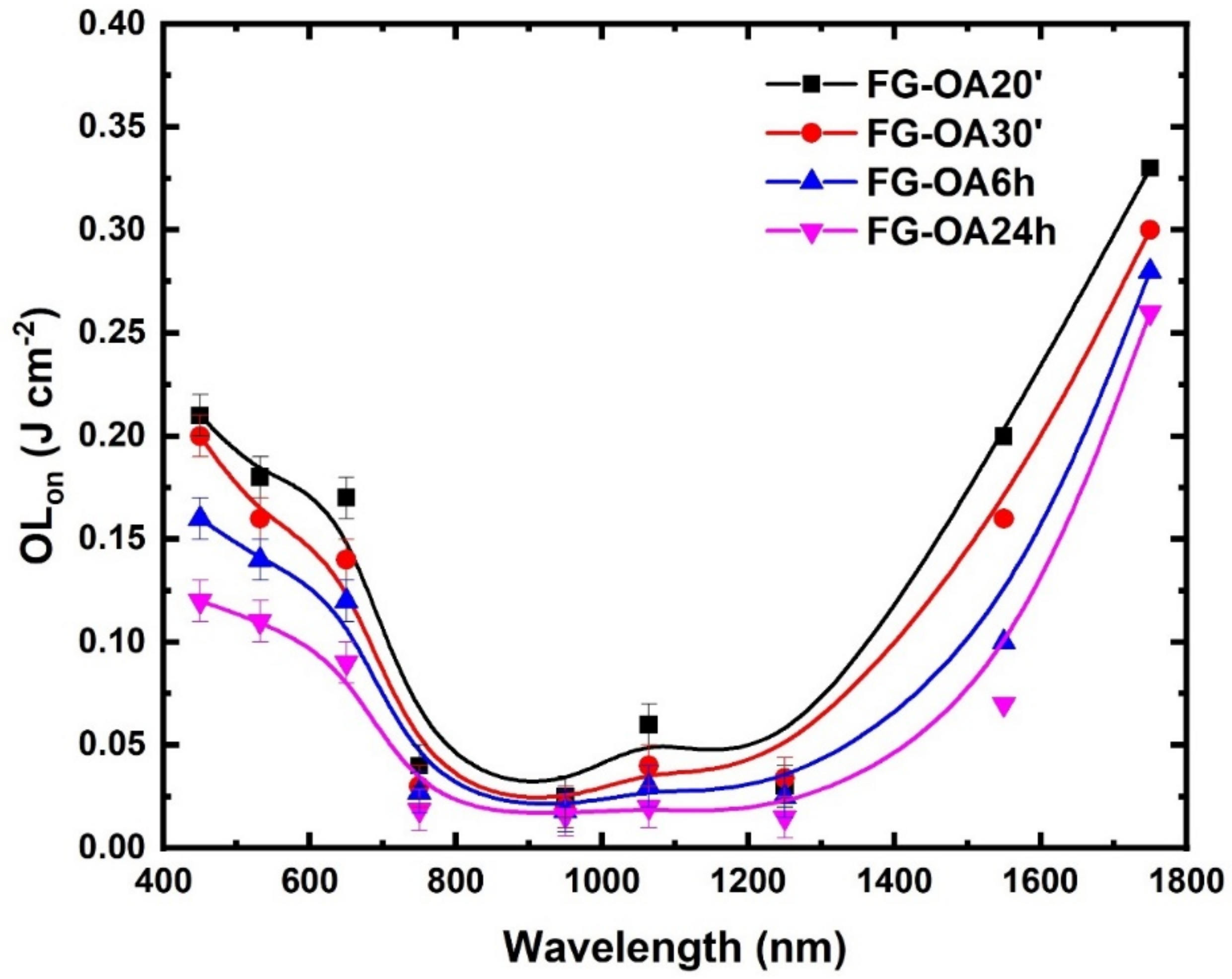
3.2. Optical Limiting Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nair, R.R.; Ren, W.C.; Jalil, R.; Riaz, I.; Kravets, V.G.; Britnell, L.; Blake, P.; Schedin, F.; Mayorov, A.S.; Yuan, S.J.; et al. Fluorographene: A Two-Dimensional Counterpart of Teflon. Small 2010, 6, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Zbořil, R.; Karlický, F.; Bourlinos, A.B.; Steriotis, T.A.; Stubos, A.K.; Georgakilas, V.; Šafářová, K.; Jančík, D.; Trapalis, C.; Otyepka, M. Graphene Fluoride: A Stable Stoichiometric Graphene Derivative and Its Chemical Conversion to Graphene. Small 2010, 6, 2885–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitener, K.E.; Stine, R.; Robinson, J.T.; Sheehan, P.E. Graphene as Electrophile: Reactions of Graphene Fluoride. J. Phys. Chem. C 2015, 119, 10507–10512. [Google Scholar] [CrossRef]
- Bakandritsos, A.; Pykal, M.; Blonski, P.; Jakubec, P.; Chronopoulos, D.D.; Polakova, K.; Georgakilas, V.; Cepe, K.; Tomanec, O.; Ranc, V.; et al. Cyanographene and Graphene Acid: Emerging Derivatives Enabling High-Yield and Selective Functionalization of Graphene. ACS Nano 2017, 11, 2982–2991. [Google Scholar] [CrossRef]
- Urbanova, V.; Hola, K.; Bourlinos, A.B.; Cepe, K.; Ambrosi, A.; Loo, A.H.; Pumera, M.; Karlicky, F.; Otyepka, M.; Zboril, R. Thiofluorographene-Hydrophilic Graphene Derivative with Semiconducting and Genosensing Properties. Adv. Mater. 2015, 27, 2305–2310. [Google Scholar] [CrossRef]
- Urbanova, V.; Karlicky, F.; Matej, A.; Sembera, F.; Janousek, Z.; Perman, J.A.; Ranc, V.; Cepe, K.; Michl, J.; Otyepka, M.; et al. Fluorinated Gaphenes as Advanced Biosensors—Effect of Fluorine Coverage on Electron Transfer Properties and Adsorption of Biomolecules. Nanoscale 2016, 8, 12134–12142. [Google Scholar] [CrossRef]
- Liang, S.-Z.; Chen, G.; Harutyunyan, A.R.; Cole, M.W.; Sofo, J.O. Analysis and Optimization of Carbon Nanotubes and Graphene Sensors Based on Adsorption-Desorption Kinetics. Appl. Phys. Lett. 2013, 103, 233108. [Google Scholar] [CrossRef]
- Das, S.; Sudhagar, P.; Verma, V.; Song, D.; Ito, E.; Lee, S.Y.; Kang, Y.S.; Choi, W. Amplifying Charge-Transfer Characteristics of Graphene for Triiodide Reduction in Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2011, 21, 3729–3736. [Google Scholar] [CrossRef]
- Worsley, K.A.; Ramesh, P.; Mandal, S.K.; Niyogi, S.; Itkis, M.E.; Haddon, R.C. Soluble Graphene Derived from Graphite Fluoride. Chem. Phys. Lett. 2007, 445, 51–56. [Google Scholar] [CrossRef]
- Dubecky, M.; Otyepkova, E.; Lazar, P.; Karlicky, F.; Petr, M.; Cepe, K.; Banas, P.; Zboril, R.; Otyepka, M. Reactivity of Fluorographene: A Facile Way toward Graphene Derivatives. J. Phys. Chem. Lett. 2015, 6, 1430–1434. [Google Scholar] [CrossRef]
- Stathis, A.; Papadakis, I.; Karampitsos, N.; Couris, S.; Potsi, G.; Bourlinos, A.B.; Otyepka, M.; Zboril, R. Thiophenol-Modified Fluorographene Derivatives for Nonlinear Optical Applications. ChemPlusChem 2019, 84, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Zaoralová, D.; Hrubý, V.; Šedajová, V.; Mach, R.; Kupka, V.; Ugolotti, J.; Bakandritsos, A.; Medved’, M.; Otyepka, M. Tunable Synthesis of Nitrogen Doped Graphene from Fluorographene under Mild Conditions. ACS Sustain. Chem. Eng. 2020, 8, 4764–4772. [Google Scholar] [CrossRef]
- Papadakis, I.; Stathis, A.; Bourlinos, A.B.; Couris, S. Diethylamino-Fluorographene: A 2D Material with Broadband and Efficient Optical Limiting Performance (from 500 to 1800 nm) with Very Large Nonlinear Optical Response. Nano Select. 2020, 1, 395–404. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, X.R.; Zhang, L.; Lee, S.W.; Dai, H.J. Chemically Derived, Ultrasmooth Graphene Nanoribbon Semiconductors. Science 2008, 319, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.R.; Li, X.L.; Zhang, L.; Yoon, Y.; Weber, P.K.; Wang, H.L.; Guo, J.; Dai, H.J. N-Doping of Graphene through Electrothermal Reactions with Ammonia. Science 2009, 324, 768–771. [Google Scholar] [CrossRef]
- Tuček, J.; Holá, K.; Bourlinos, A.B.; Błoński, P.; Bakandritsos, A.; Ugolotti, J.; Dubecký, M.; Karlický, F.; Ranc, V.; Čépe, K.; et al. Room Temperature Organic Magnets Derived from sp3 Functionalized Graphene. Nat. Commun. 2017, 8, 14525. [Google Scholar] [CrossRef]
- Matochová, D.; Medved’, M.; Bakandritsos, A.; Steklý, T.; Zbořil, R.; Otyepka, M. 2D Chemistry: Chemical Control of Graphene Derivatization. J. Phys. Chem. Lett. 2018, 9, 3580–3585. [Google Scholar] [CrossRef]
- Stathis, A.; Stavrou, M.; Papadakis, I.; Bakandritsos, A.; Steklý, T.; Otyepka, M.; Couris, S. Octylamine Modified Fluorographenes as a Versatile Platform for the Efficient Engineering of the Nonlinear Optical Properties of Fluorinated Graphenes. Adv. Photonics Res. 2020. accepted. [Google Scholar] [CrossRef]
- Medveď, M.; Zoppellaro, G.; Ugolotti, J.; Matochová, D.; Lazar, P.; Pospíšil, T.; Bakandritsos, A.; Tuček, J.; Zbořil, R.; Otyepka, M. Reactivity of Fluorographene is Triggered by Point Defects: Beyond the Perfect 2D World. Nanoscale 2018, 10, 4696–4707. [Google Scholar] [CrossRef] [Green Version]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.H.; Hagan, D.J.; Van Stryland, E.W. Sensitive Measurement of Optical Nonlinearities Using a Single Beam. IEEE J. Quant. Electr. 1990, 26, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Papagianouli, I.; Iliopoulos, K.; Gindre, D.; Sahraoui, B.; Krupka, O.; Smokal, V.; Kolendo, A.; Couris, S. Third-Order Nonlinear Optical Response of Push–Pull Zzobenzene Polymers. Chem. Phys. Lett. 2012, 554, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable Aqueous Dispersions of Graphene Nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Potsi, G.; Bourlinos, A.B.; Mouselimis, V.; Polakova, K.; Chalmpes, N.; Gournis, D.; Kalytchuk, S.; Tomanec, O.; Błonski, P.; Medved’, M.; et al. Intrinsic Photo-Luminescence of Amine-Functionalized Graphene Derivatives for Bioimaging Applications. Appl. Mater. Today 2019, 17, 112–122. [Google Scholar] [CrossRef]
- Sutherland, R.L. Handbook of Nonlinear Optics, 2nd ed.; Dekker: New York, NY, USA, 2003; pp. 612–615. [Google Scholar]
- Couris, S.; Koudoumas, E.; Ruth, A.A.; Leach, S. Concentration and Wavelength Dependence of the Effective Third-Order Susceptibility and Optical Limiting of C60 in Toluene Solutions. J. Phys. B At. Mol. Opt. Phys. 1995, 28, 4537. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.; Dixit, C.K. Synthesis, Characterization and Potential application of Nitrogen-doped Graphene. J. Sci. Adv. Mater. Devices 2017, 2, 141–149. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.-F.; Polavarapu, L.; Neo, S.T.; Venkatesan, T.; Xu, Q.-H.J. Tunable Broadband Nonlinear Optical Properties of Black Phosphorus Quantum Dots for Femtosecond Laser Pulses. Phys. Chem. Lett. 2012, 3, 785–790. [Google Scholar] [CrossRef]
- Liaros, N.; Bourlinos, A.B.; Zboril, R.; Couris, S. Fluoro-Graphene: Nonlinear Optical Properties. Opt. Express 2013, 21, 21027. [Google Scholar] [CrossRef]
- Papadakis, I.; Bouza, Z.; Couris, S.; Bourlinos, A.B.; Mouselimis, V.; Kouloumpis, A.; Gournis, D.; Bakandritsos, A.; Ugolotti, J.; Zboril, R. Hydrogenated Gluorographene: A 2D Counterpart of Graphene with Enhanced Nonlinear Optical Properties. J. Phys. Chem. C 2017, 121, 22567–22575. [Google Scholar] [CrossRef]
- Liaros, N.; Aloukos, P.; Kolokithas-Ntoukas, A.; Bakandritsos, A.; Szabo, T.; Zboril, R.; Couris, S. Nonlinear Optical Properties and Broadband Optical Power Limiting Action of Graphene Oxide Colloids. J. Phys. Chem. C 2013, 117, 6842–6850. [Google Scholar] [CrossRef]
- Chen, P.; Wu, X.; Sun, X.; Lin, J.; Ji, W.; Tan, K.L. Electronic Structure and Optical Limiting Behavior of Carbon Nanotubes. Phys. Rev. Lett. 1999, 82, 2548–2551. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.; Li, Y.; Feng, Y.; Zhang, S.; Zhang, X.; Chang, C.; Fan, J.; Zhang, L.; Wang, J. Optical Limiting and Theoretical Modelling of Layered Transition Metal Dichalcogenide Nanosheets. Sci. Rep. 2015, 5, 14646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, M.; Zhan, H.; Chen, Y. Nonlinear Optical and Optical Limiting Properties of Graphene Families. Appl. Phys. Lett. 2010, 96, 033107. [Google Scholar] [CrossRef]
- Wang, J.; Hernandez, Y.; Lotya, M.; Coleman, J.N.; Blau, W.J. Broadband Nonlinear Optical Response of Graphene Dispersions. Adv. Mater. 2009, 21, 2430–2435. [Google Scholar] [CrossRef]
- Mansour, K.; Soileau, M.J.; Van Stryland, E.W. Nonlinear Optical Properties of Carbon-Black Suspensions (Ink). J. Opt. Soc. Am. B 1992, 9, 1100–1109. [Google Scholar] [CrossRef]
- Liaros, N.; Orfanos, I.; Papadakis, I.; Couris, S. Nonlinear Optical Response of some Graphene Oxide and Graphene Fluoride Derivatives. Optofluid. Microfluid. Nanofluid. 2016, 3, 53–58. [Google Scholar] [CrossRef]
- Anand, B.; Podila, R.; Ayala, P.; Oliviera, L.; Philip, R.; Siva Sankara Sai, S.; Zakhidov, A.A.; Rao, A.M. Nonlinear Optical Properties of Boron Doped Single-Walled Carbon Nanotubes. Nanoscale 2013, 5, 7271–7276. [Google Scholar] [CrossRef]
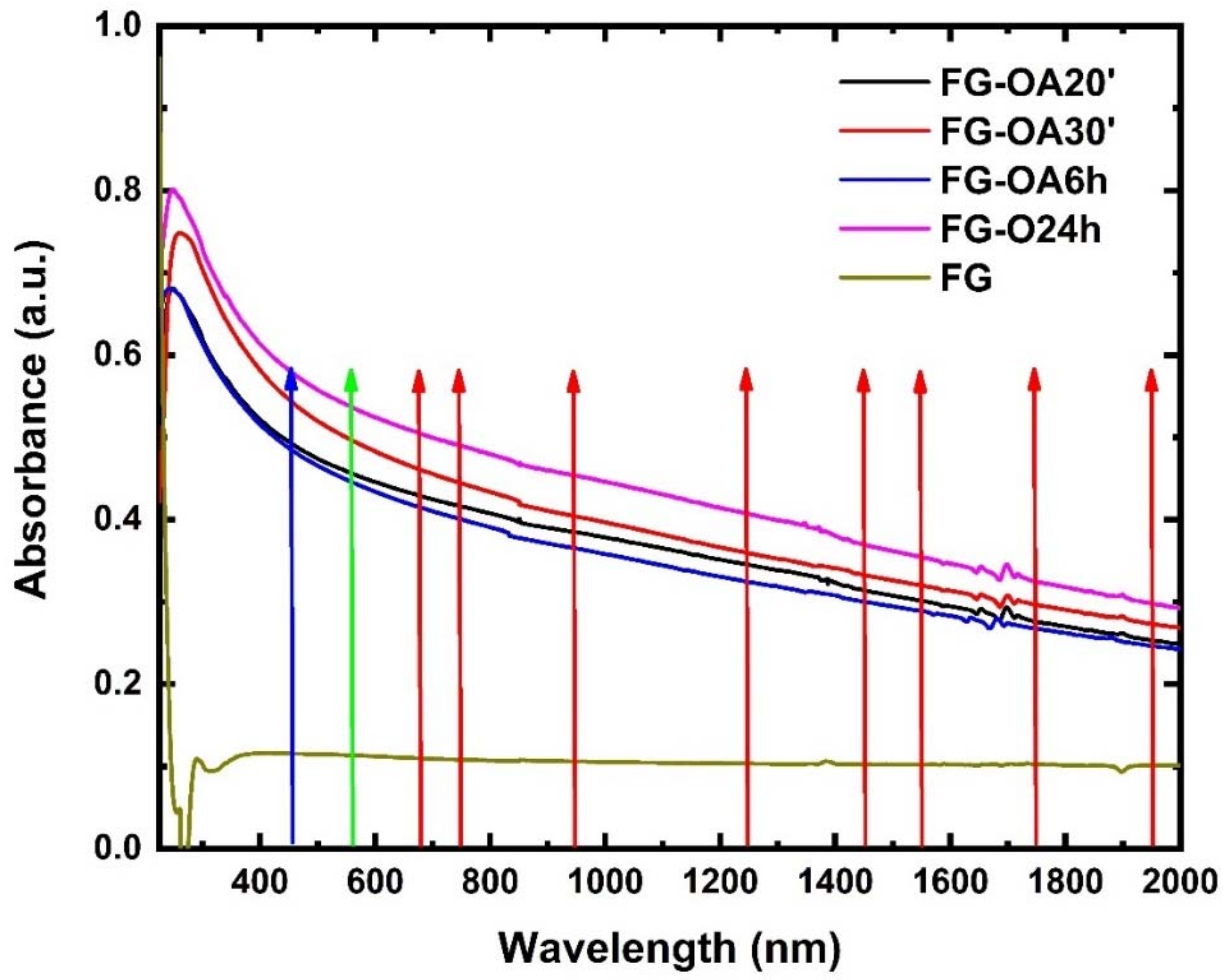
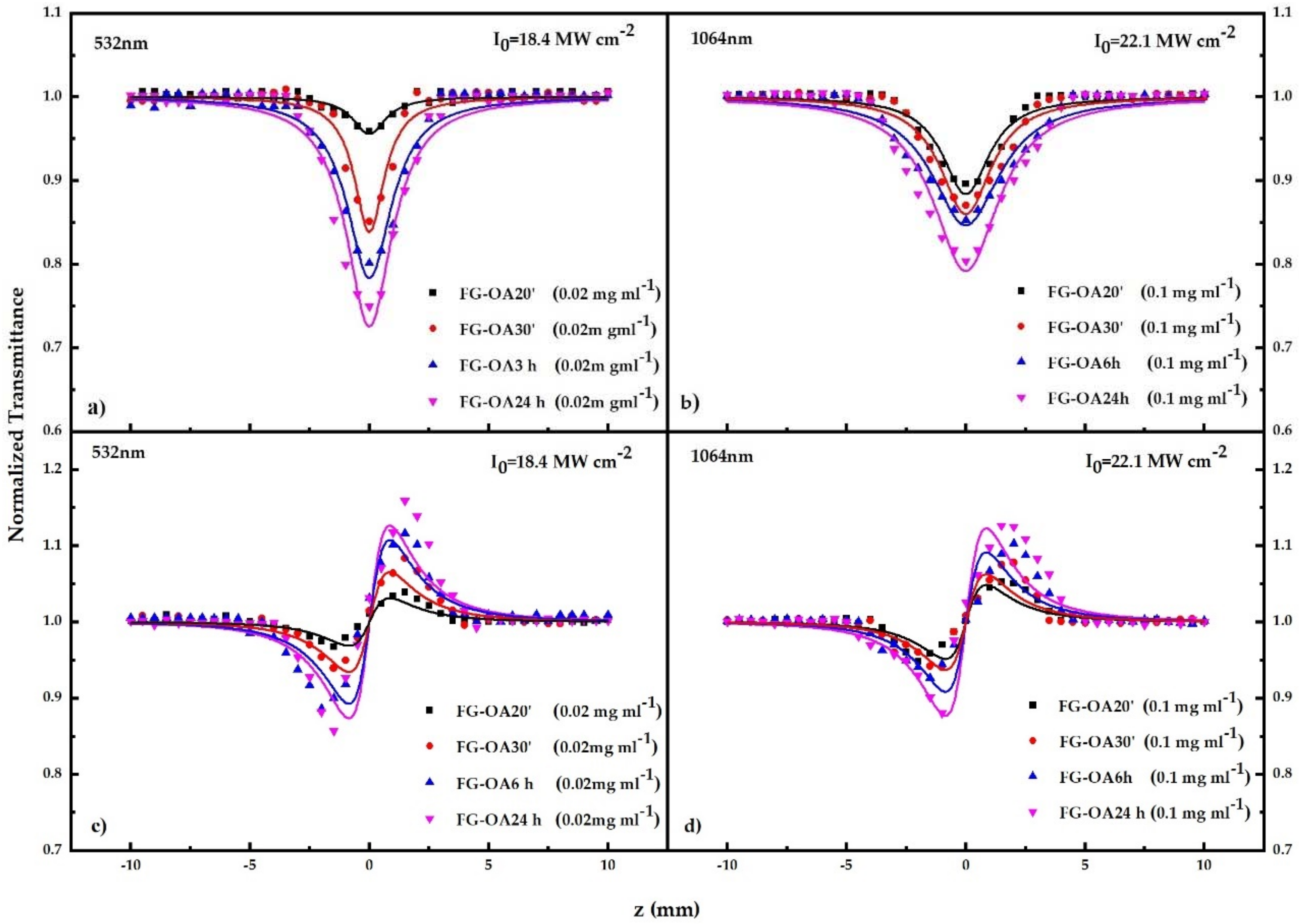
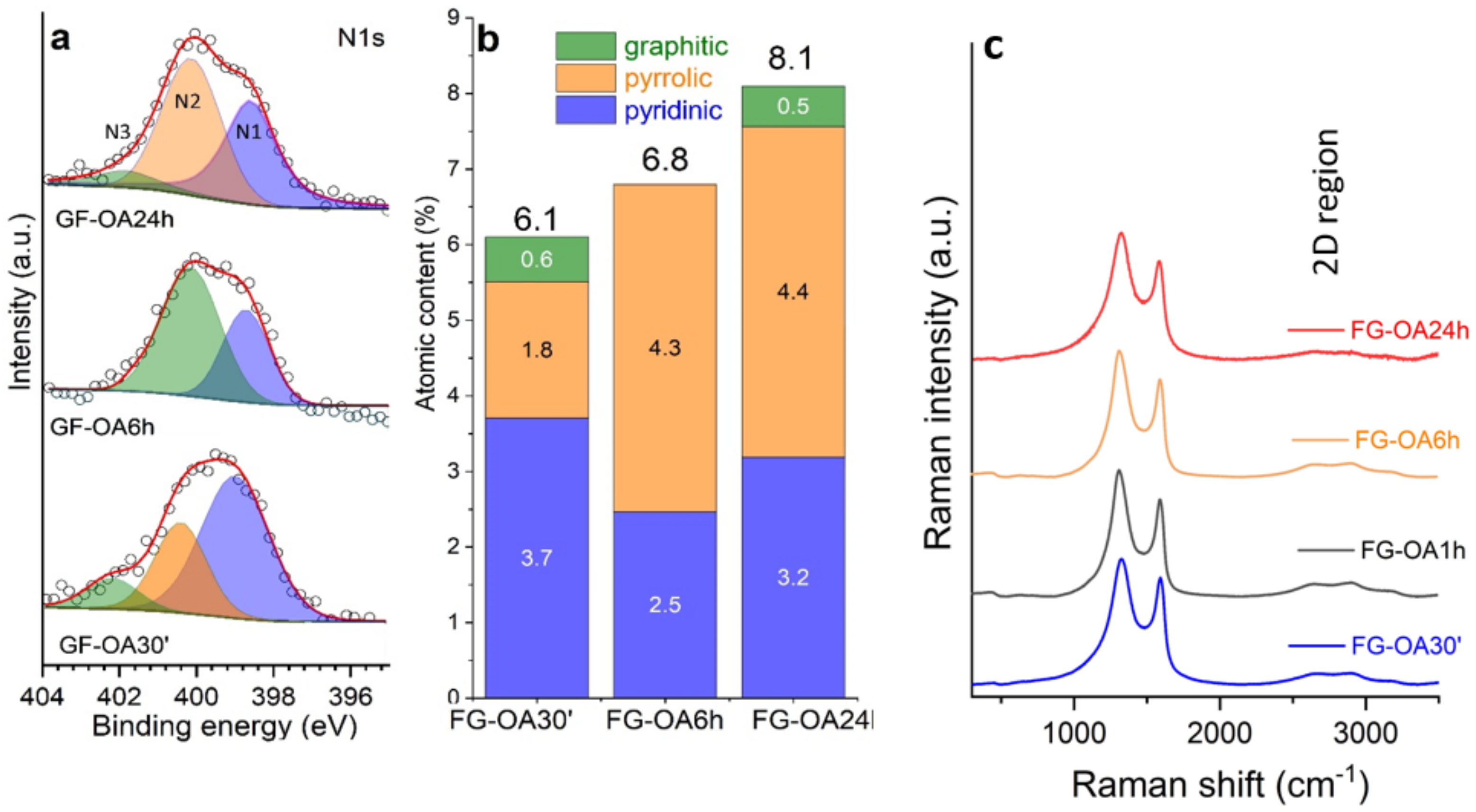



| Wavelength (nm) | Sample | Β (×10−11 m W−1) | γ′ (×10−18 m2 W−1) | Imχ(3) (×10−13 esu) | Reχ(3) (×10−13 esu) | |χ|(3) (×10−13 esu) |
|---|---|---|---|---|---|---|
| 532 nm | FG | 119 ± 19 | −135 ± 31 | 62 ± 12 | −173 ± 38 | 184 ± 40 |
| FG-OA 20′ | 1720 ± 175 | 1325 ± 235 | 900 ± 95 | 1695 ± 300 | 1920 ± 315 | |
| FG-OA 30′ | 5010 ± 1050 | 1680 ± 230 | 2640 ± 550 | 2120 ± 460 | 3380 ± 720 | |
| FG-OA 6 h | 7550 ± 1300 | 4275 ± 425 | 3950 ± 650 | 5475 ± 550 | 6725 ± 770 | |
| FG-OA 24 h | 11,363 ± 663 | 4252 ± 600 | 5950 ± 350 | 5800 ± 775 | 8325 ± 856 | |
| 1064 nm | FG | - | - | - | - | - |
| FG-OA 20′ | 872 ± 55 | 530 ± 100 | 943 ± 59 | 676 ± 128 | 1160 ± 142 | |
| FG-OA 30′ | 2263 ± 345 | 1193 ± 198 | 2448 ± 372 | 1523 ± 350 | 2885 ± 450 | |
| FG-OA 6 h | 3063 ± 200 | 2648 ± 200 | 3313 ± 250 | 3350 ± 250 | 4713 ± 475 | |
| FG-OA 24 h | 6425 ± 388 | 3475 ± 330 | 6950 ± 425 | 4575 ± 725 | 8313 ± 750 |
| Sample | ID cm−1 | FWHM ID cm−1 |
|---|---|---|
| 30 min | 1325 | 103 |
| 1 h | 1316 | 106 |
| 6 h | 1315 | 109 |
| 24 h | 1330 | 118 |
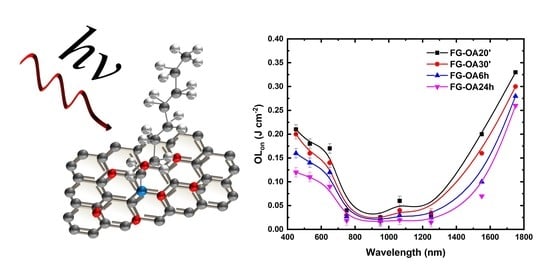
| Wavelength (nm) | Sample | OLon (J cm−2) |
|---|---|---|
| 532 | FG-OA 20′ | 0.18 |
| FG-OA 30′ | 0.16 | |
| FG-OA 6 h | 0.13 | |
| FG-OA 24 h | 0.10 | |
| 1064 | FG-OA 20′ | 0.06 |
| FG-OA 30′ | 0.04 | |
| FG-OA 6 h | 0.03 | |
| FG-OA 24 h | 0.02 |
| Wavelength (nm) | Sample | OLon (J cm−2) |
|---|---|---|
| 450 | FG-OA 20′ | 0.21 |
| FG-OA 30′ | 0.20 | |
| FG-OA 6 h | 0.15 | |
| FG-OA 24 h | 0.12 | |
| 650 | FG-OA 20′ | 0.17 |
| FG-OA 30′ | 0.14 | |
| FG-OA 6 h | 0.12 | |
| FG-OA 24 h | 0.09 | |
| 750 | FG-OA 20′ | 0.04 |
| FG-OA 30′ | 0.03 | |
| FG-OA 6 h | 0.03 | |
| FG-OA 24 h | 0.02 | |
| 950 | FG-OA 20′ | 0.02 |
| FG-OA 30′ | 0.02 | |
| FG-OA 6 h | 0.02 | |
| FG-OA 24 h | 0.01 | |
| 1250 | FG-OA 20′ | 0.03 |
| FG-OA 30′ | 0.03 | |
| FG-OA 6 h | 0.02 | |
| FG-OA 24 h | 0.01 | |
| 1550 | FG-OA 20′ | 0.20 |
| FG-OA 30′ | 0.16 | |
| FG-OA 6 h | 0.10 | |
| FG-OA 24 h | 0.07 | |
| 1750 | FG-OA 20′ | 0.33 |
| FG-OA 30′ | 0.30 | |
| FG-OA 6 h | 0.28 | |
| FG-OA 24 h | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stathis, A.; Stavrou, M.; Papadakis, I.; Obratzov, I.; Couris, S. Enhancing and Tuning the Nonlinear Optical Response and Wavelength-Agile Strong Optical Limiting Action of N-octylamine Modified Fluorographenes. Nanomaterials 2020, 10, 2319. https://doi.org/10.3390/nano10112319
Stathis A, Stavrou M, Papadakis I, Obratzov I, Couris S. Enhancing and Tuning the Nonlinear Optical Response and Wavelength-Agile Strong Optical Limiting Action of N-octylamine Modified Fluorographenes. Nanomaterials. 2020; 10(11):2319. https://doi.org/10.3390/nano10112319
Chicago/Turabian StyleStathis, Aristeidis, Michalis Stavrou, Ioannis Papadakis, Ievgen Obratzov, and Stelios Couris. 2020. "Enhancing and Tuning the Nonlinear Optical Response and Wavelength-Agile Strong Optical Limiting Action of N-octylamine Modified Fluorographenes" Nanomaterials 10, no. 11: 2319. https://doi.org/10.3390/nano10112319
APA StyleStathis, A., Stavrou, M., Papadakis, I., Obratzov, I., & Couris, S. (2020). Enhancing and Tuning the Nonlinear Optical Response and Wavelength-Agile Strong Optical Limiting Action of N-octylamine Modified Fluorographenes. Nanomaterials, 10(11), 2319. https://doi.org/10.3390/nano10112319