Novel Pt-Ag3PO4/CdS/Chitosan Nanocomposite with Enhanced Photocatalytic and Biological Activities
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization Methods
2.3. Synthesis Steps
2.3.1. Synthesis of CdS
2.3.2. Synthesis of CdS/Ag3PO4 Nanocomposite with a Mass Ratio of 3:7
2.3.3. Synthesis of Pt-Ag3PO4/CdS Nanocomposite
2.3.4. Synthesis of Pt-Ag3PO4/CdS/Chitosan Nanocomposite with a Mass Ratio of 3:2
2.4. Photocatalytic Degradation of MB
2.5. Antibacterial Test under Visible Light and Dark Conditions
2.6. MTT Assay
3. Results and Discussion
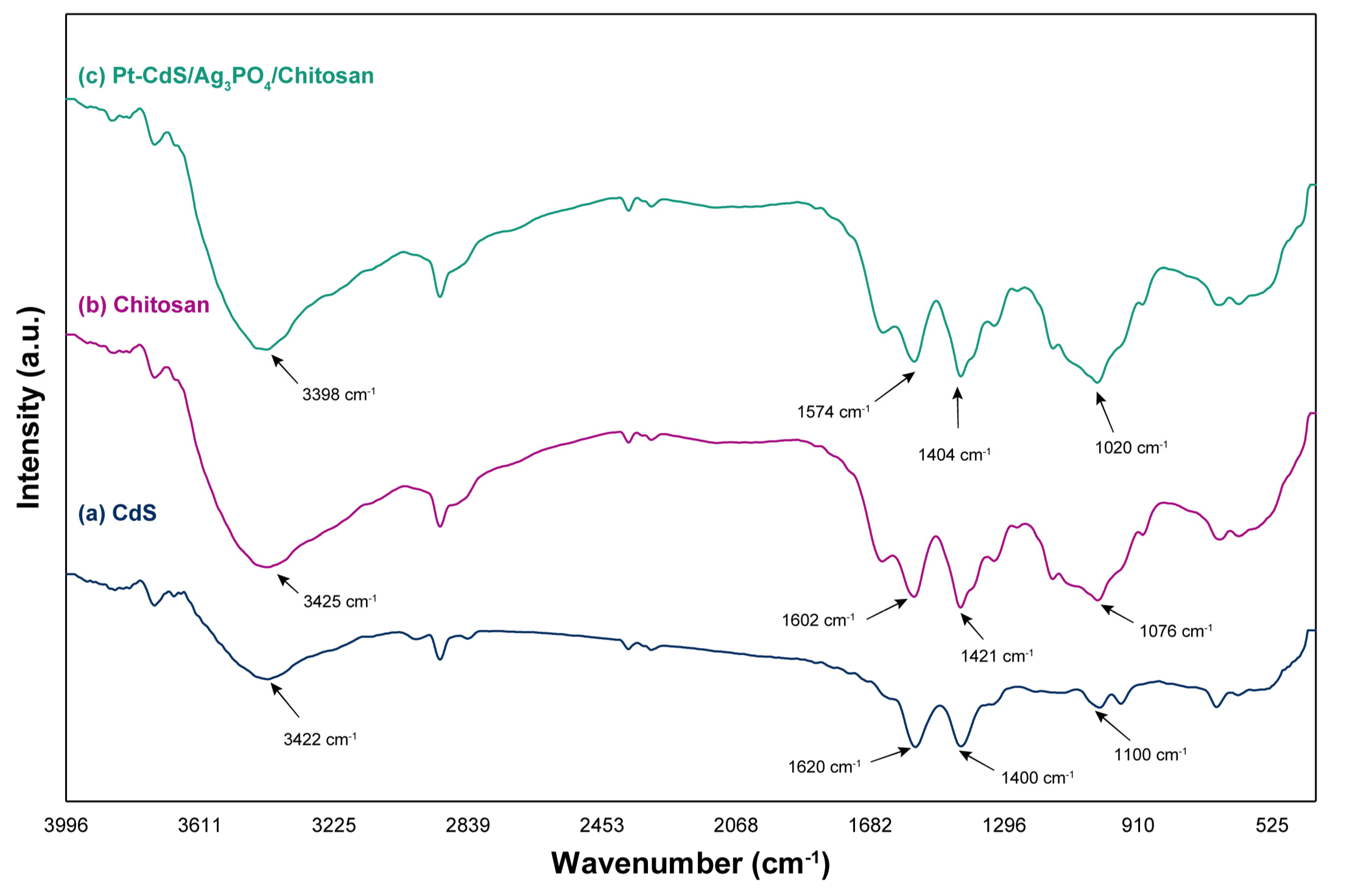
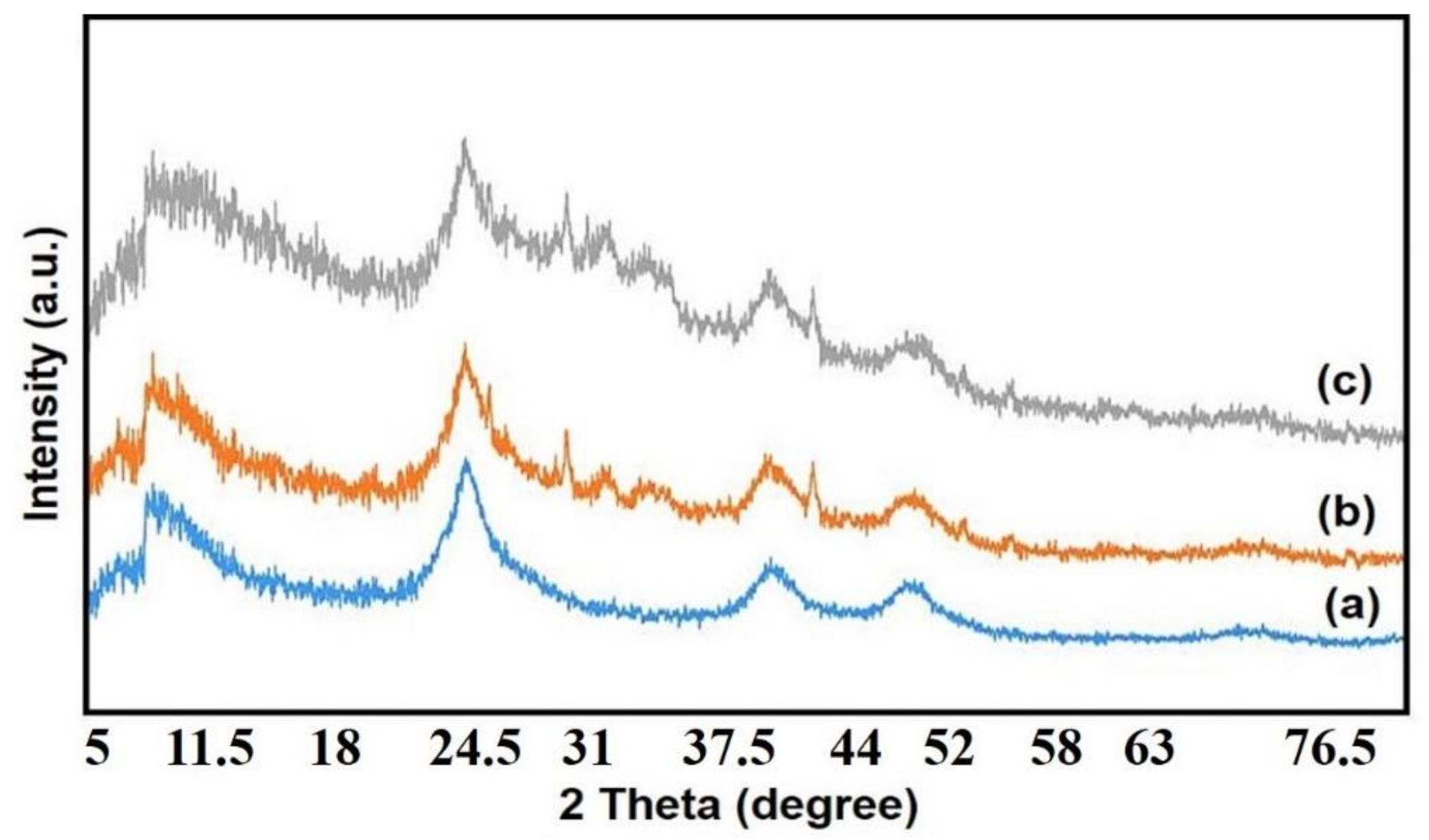
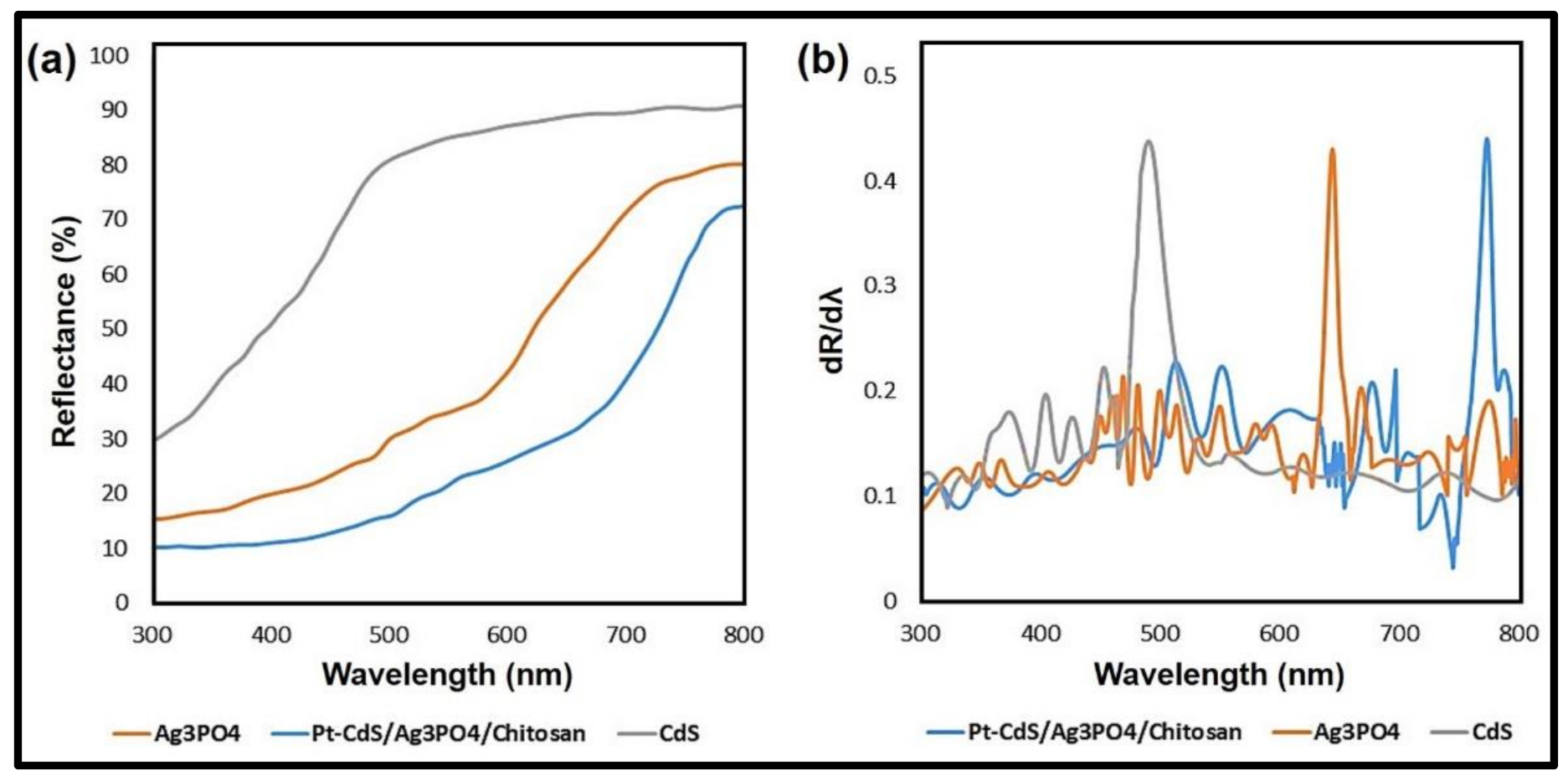
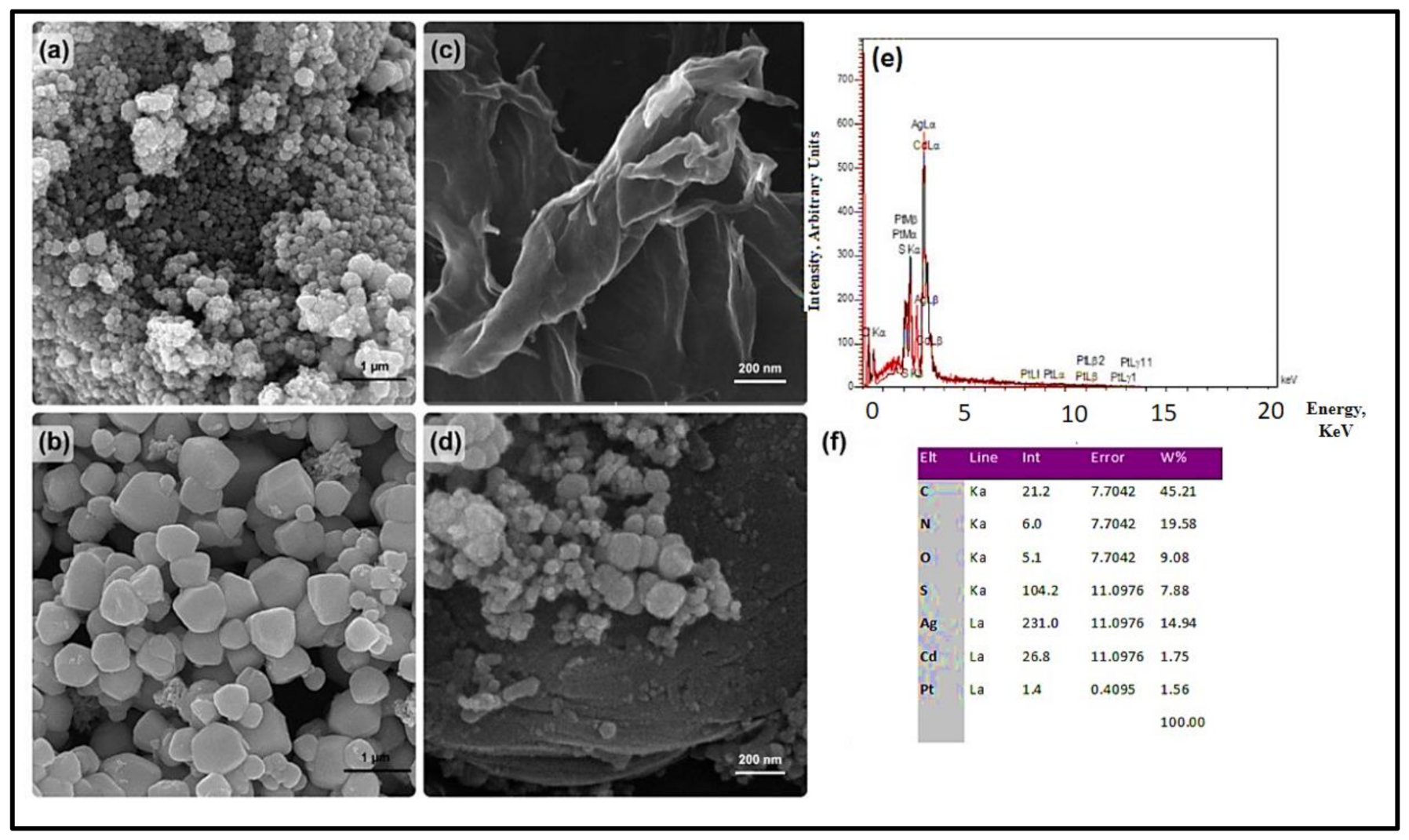
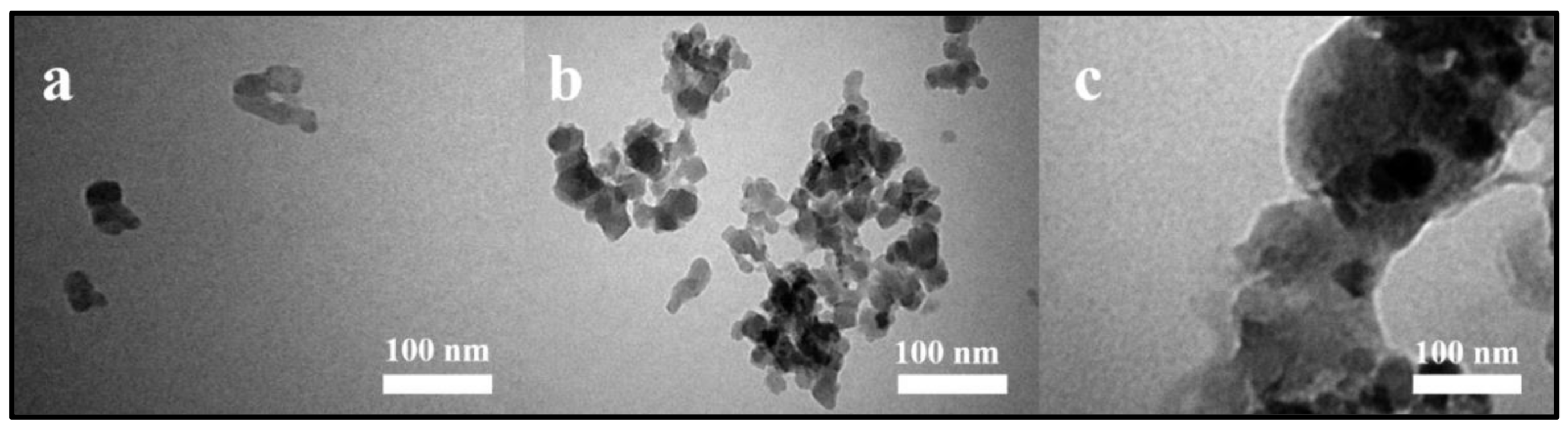
3.1. Characterizations
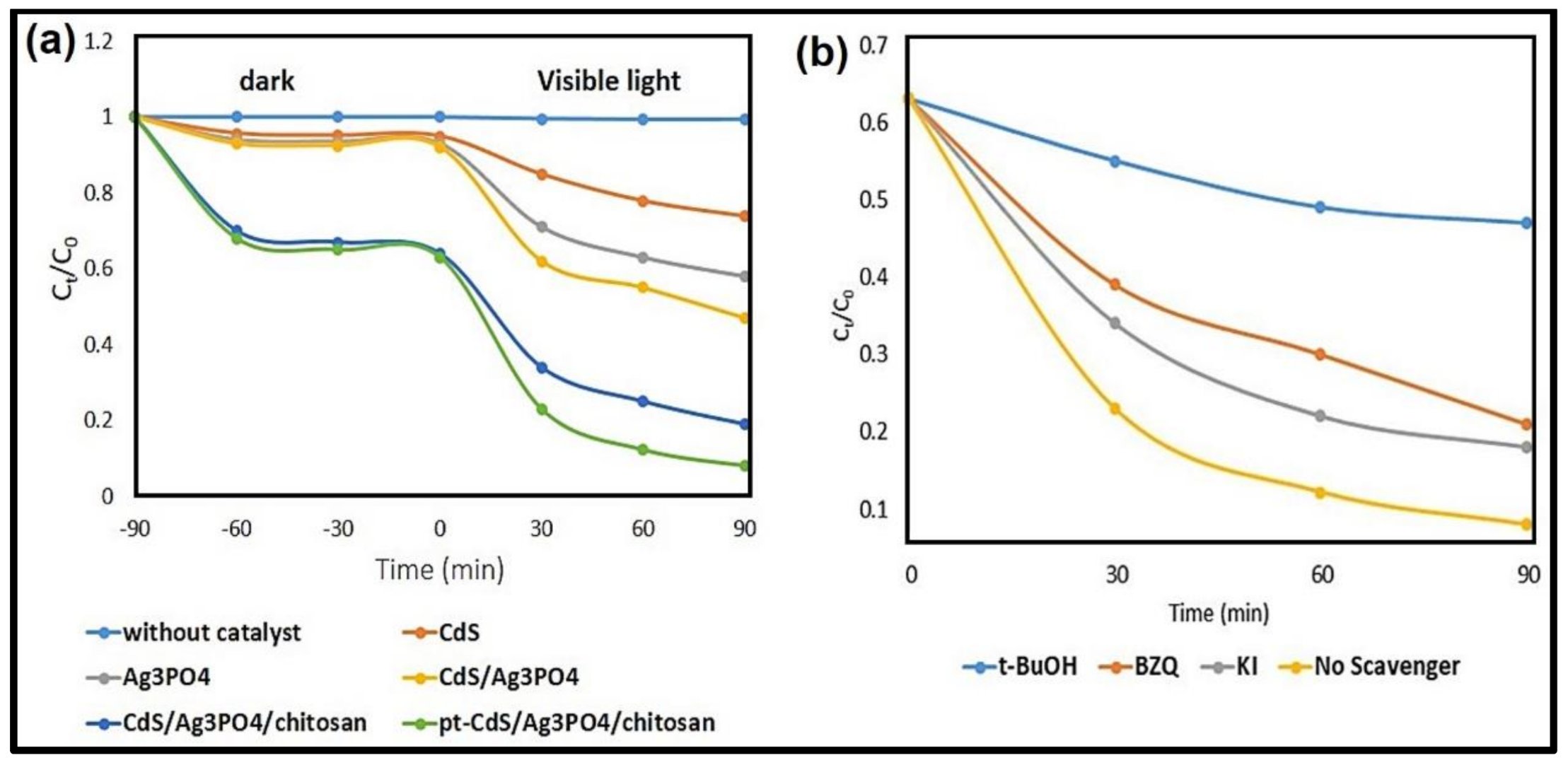
3.2. Photocatalytic Performance for MB Degradation
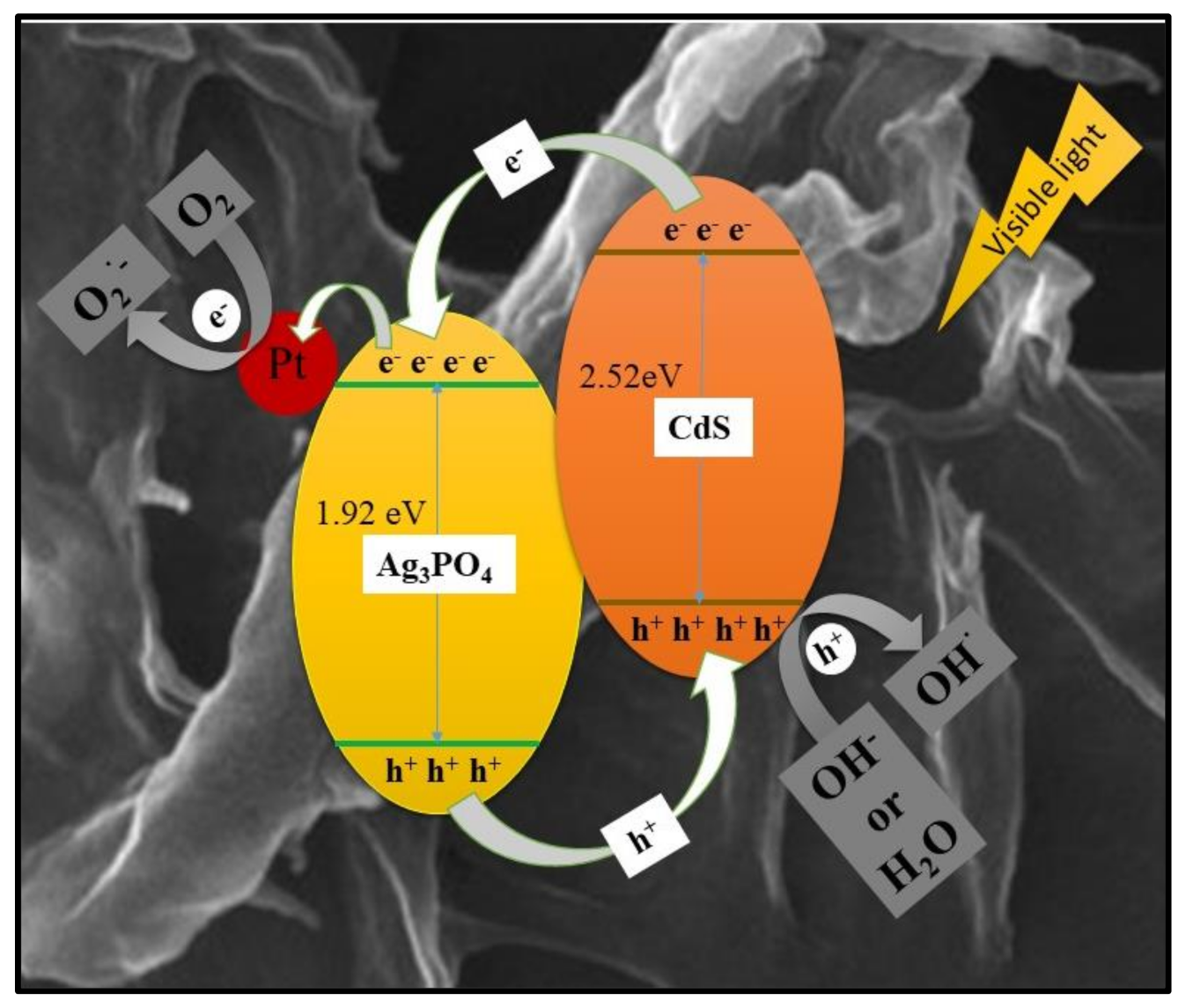
3.3. Possible Photocatalytic Mechanism
3.4. Effect of Reactive Species on Photodegradation of MB
3.5. Recyclability of Pt-Ag3PO4/CdS/Chitosan Nanocomposites
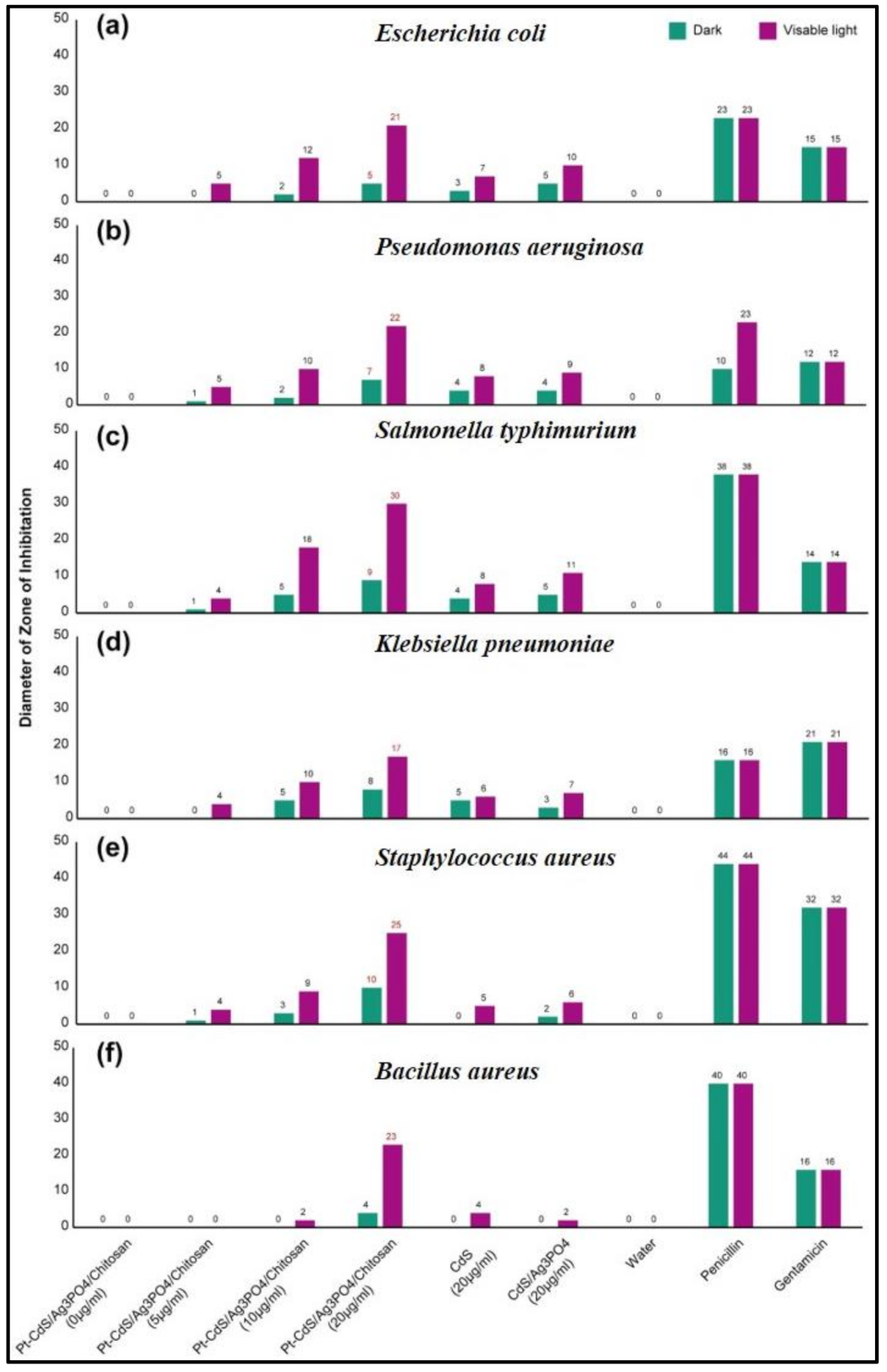
3.6. Antibacterial Tests
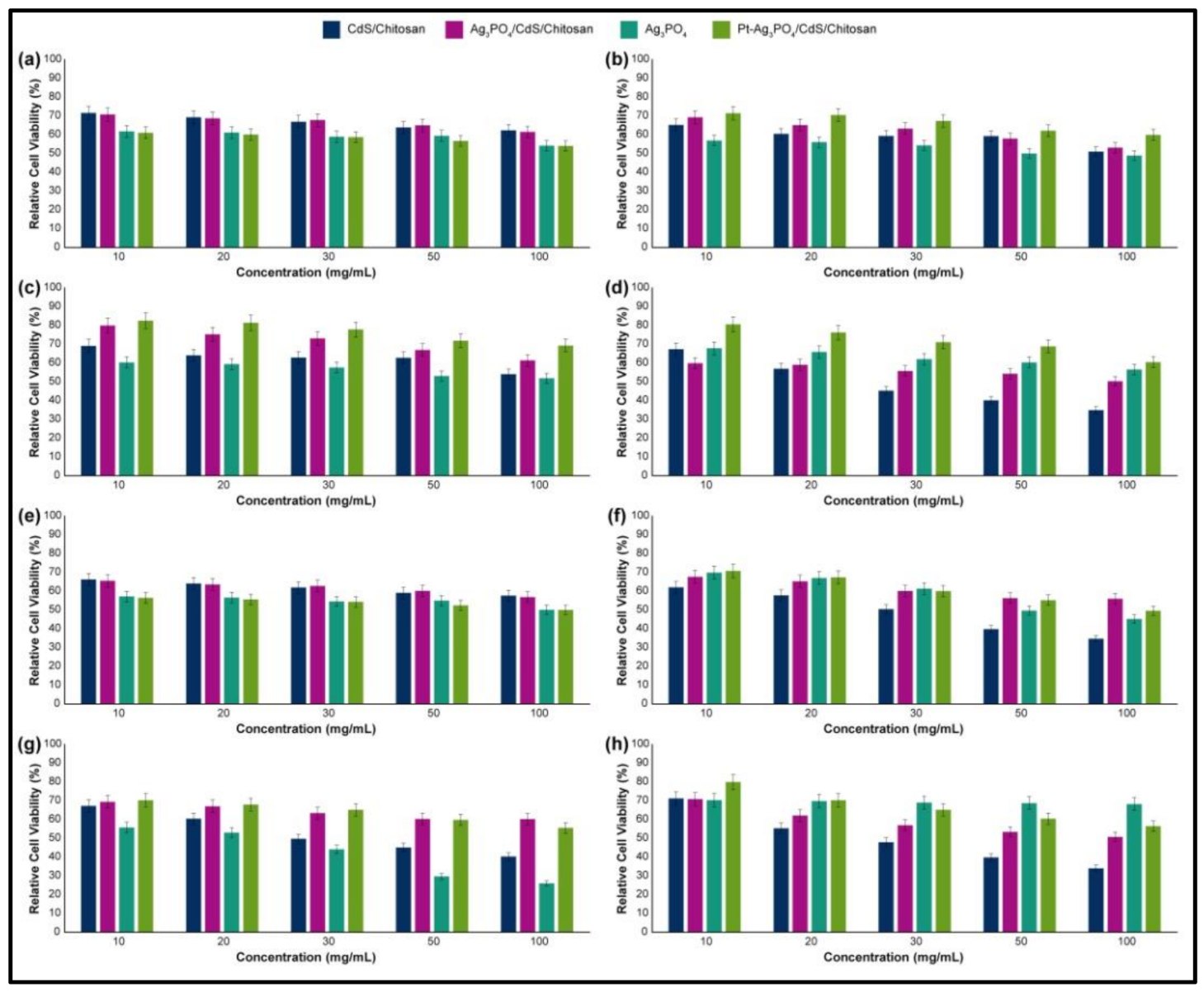
3.7. Cytotoxicity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zakerzadeh, E.; Alizadeh, E.; Samadi Kafil, H.; Mohammad Hassanzadeh, A.; Salehi, R.; Mahkam, M. Novel antibacterial polymeric nanocomposite for smart co-delivery of anticancer drugs. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, G.; Xu, B.; Feng, X.; Bai, Q.; Yang, G.; Li, H. Thermosensitive antibacterial Ag nanocomposite hydrogels made by a one-step green synthesis strategy. New J. Chem. 2016, 40, 6650–6657. [Google Scholar] [CrossRef]
- Yadollahi, M.; Farhoudian, S.; Namazi, H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int. J. Biol. Macromol. 2015, 79, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Kumar, A.; Sharma, N. Photocatalytic and antibacterial activity studies of ZnO nanoparticles synthesized by thermal decomposition of mechanochemically processed oxalate precursor. Chem. Sel. 2016, 1, 6925–6932. [Google Scholar] [CrossRef]
- Amornpitoksuk, P.; Suwanboon, S.; Sangkanu, S.; Sukhoom, A.; Wudtipan, J.; Srijan, K.; Kaewtaro, S. Synthesis, photocatalytic and antibacterial activities of ZnO particles modified by diblock copolymer. Powder Technol. 2011, 212, 432–438. [Google Scholar] [CrossRef]
- Safdar, M.; Ozaslan, M.; Khailany, R.A.; Latif, S.; Junejo, Y.; Saeed, M.; Al-Attar, M.S.; Kanabe, B.O. Synthesis, Characterization and Applications of a Novel Platinum-Based Nanoparticles: Catalytic, Antibacterial and Cytotoxic Studies. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2430–2439. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B. Green Synthesis of Biogenic Silver Nanoparticles for Efficient Catalytic Removal of Harmful Organic Dyes. Nanomaterials 2020, 10, 202. [Google Scholar] [CrossRef]
- Liu, X.; Qi, S.; Li, Y.; Yang, L.; Cao, B.; Tang, C.Y. Synthesis and characterization of novel antibacterial silver nanocomposite nanofiltration and forward osmosis membranes based on layer-by-layer assembly. Water Res. 2013, 47, 3081–3092. [Google Scholar] [CrossRef]
- Mahiuddin, M.; Saha, P.; Ochiai, B. Green Synthesis and Catalytic Activity of Silver Nanoparticles Based on Piper chaba Stem Extracts. Nanomaterials 2020, 10, 1777. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections—United States. Morb. Mortal. Wkly. Rep. 2019, 68, 214. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Brahmachari, S.; Das, P.K. In Situ Synthesised Silver Nanoparticle-Infused l-Lysine-Based Injectable Hydrogel: Development of a Biocompatible, Antibacterial, Soft Nanocomposite. ChemPlusChem 2014, 79, 1733–1746. [Google Scholar]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Ogugbue, C.J.; Sawidis, T. Bioremediation and detoxification of synthetic wastewater containing triarylmethane dyes by Aeromonas hydrophila isolated from industrial effluent. Biotechnol. Res. Int. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- de Campos Ventura-Camargo, B.; Marin-Morales, M.A. Azo dyes: Characterization and toxicity-a review. Text. Light Ind. Sci. Technol. 2013, 2, 85–103. [Google Scholar]
- Saif, S.; Tahir, A.; Chen, Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, L.; Ma, J.; Alam, N.; Zhou, X.; Ni, Y. Nano-cellulose/MOF derived carbon doped CuO/Fe3O4 nanocomposite as high efficient catalyst for organic pollutant remedy. Nanomaterials 2019, 9, 277. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, Q.; Li, H.; Zhang, B.; Yan, R.; Chen, J.; Sun, M. Photocatalytic activity of silver oxide capped Ag nanoparticles constructed by air plasma irradiation. Appl. Phys. Lett. 2018, 112, 163101. [Google Scholar] [CrossRef]
- Ouyang, W.; Kuna, E.; Yepez, A.; Balu, A.M.; Romero, A.A.; Colmenares, J.C.; Luque, R. Mechanochemical synthesis of TiO2 nanocomposites as photocatalysts for benzyl alcohol photo-oxidation. Nanomaterials 2016, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Dasineh Khiavi, N.; Katal, R.; Kholghi Eshkalak, S.; Masudy-Panah, S.; Ramakrishna, S.; Jiangyong, H. Visible light driven heterojunction photocatalyst of CuO–Cu2O thin films for photocatalytic degradation of organic pollutants. Nanomaterials 2019, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Tryk, D.; Fujishima, A.; Honda, K. Recent topics in photoelectrochemistry: Achievements and future prospects. Electrochim. Acta 2000, 45, 2363–2376. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Mohan, R.; Kim, S.-J. Graphene oxide as a photocatalytic material. Appl. Phys. Lett. 2011, 98, 244101. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef]
- Gatto, F.; Moglianetti, M.; Pompa, P.P.; Bardi, G. Platinum nanoparticles decrease reactive oxygen species and modulate gene expression without alteration of immune responses in THP-1 monocytes. Nanomaterials 2018, 8, 392. [Google Scholar] [CrossRef]
- Baeg, E.; Sooklert, K.; Sereemaspun, A. Copper oxide nanoparticles cause a dose-dependent toxicity via inducing reactive oxygen species in drosophila. Nanomaterials 2018, 8, 824. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef]
- McEvoy, J.G.; Zhang, Z. Antimicrobial and photocatalytic disinfection mechanisms in silver-modified photocatalysts under dark and light conditions. J. Photochem. Photobiol. C Photochem. Rev. 2014, 19, 62–75. [Google Scholar] [CrossRef]
- Amornpitoksuk, P.; Suwanboon, S.; Sangkanu, S.; Sukhoom, A.; Muensit, N.; Baltrusaitis, J. Synthesis, characterization, photocatalytic and antibacterial activities of Ag-doped ZnO powders modified with a diblock copolymer. Powder Technol. 2012, 219, 158–164. [Google Scholar] [CrossRef]
- Xu, Y.-S.; Zhang, W.-D. Monodispersed Ag3PO4 nanocrystals loaded on the surface of spherical Bi2MoO6 with enhanced photocatalytic performance. Dalton Trans. 2013, 42, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh, M.; Kaveh, R. New Magnetically Recyclable Reduced Graphene Oxide rGO/MFe2O4 (M= Ca, Mg)/Ag3PO4 Nanocomposites with Remarkably Enhanced Visible-light Photocatalytic Activity and Stability. Photochem. Photobiol. 2018, 94, 1210–1224. [Google Scholar] [CrossRef]
- Cao, Q.; Xiao, L.; Zeng, L.; Cao, C.; Wang, J. Ag3PO4/chitosan/CdS nanocomposites exhibiting high photocatalytic activities under visible-light illumination. Powder Technol. 2017, 321, 1–8. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, B.; Huang, C.; Ma, C.; Song, X.; Xu, Q. Synthesis and characterization of high efficiency and stable Ag3PO4/TiO2 visible light photocatalyst for the degradation of methylene blue and rhodamine B solutions. J. Mater. Chem. 2012, 22, 4050–4055. [Google Scholar] [CrossRef]
- Zhao, F.-M.; Pan, L.; Wang, S.; Deng, Q.; Zou, J.-J.; Wang, L.; Zhang, X. Ag3PO4/TiO2 composite for efficient photodegradation of organic pollutants under visible light. Appl. Surf. Sci. 2014, 317, 833–838. [Google Scholar] [CrossRef]
- Li, Y.; Yu, L.; Li, N.; Yan, W.; Li, X. Heterostructures of Ag3PO4/TiO2 mesoporous spheres with highly efficient visible light photocatalytic activity. J. Colloid Interface Sci. 2015, 450, 246–253. [Google Scholar] [CrossRef]
- Samal, A.; Baral, A.; Das, D.P. Silver Phosphate Based Photocatalysis: A Brief Review from Fundamentals to Applications. Photocatalytic Nanomater. Environ. Appl. 2018, 27, 276–315. [Google Scholar]
- Miyasato, R.; Fujiwara, M.; Sato, H.; Yano, T.; Hashimoto, H. Particle size effects of tetrahedron-shaped Ag3PO4 photocatalyst on water-oxidation activity and carrier recombination dynamics. Chem. Phys. Lett. X 2019, 2, 100023. [Google Scholar] [CrossRef]
- Guo, J.; Ouyang, S.; Zhou, H.; Kako, T.; Ye, J. Ag3PO4/In(OH)3 composite photocatalysts with adjustable surface-electric property for efficient photodegradation of organic dyes under simulated solar-light irradiation. J. Phys. Chem. C 2013, 117, 17716–17724. [Google Scholar] [CrossRef]
- Tang, J.; Gong, W.; Cai, T.; Xie, T.; Deng, C.; Peng, Z.; Deng, Q. Novel visible light responsive Ag@(Ag2S/Ag3PO4) photocatalysts: Synergistic effect between Ag and Ag2S for their enhanced photocatalytic activity. RSC Adv. 2013, 3, 2543–2547. [Google Scholar] [CrossRef]
- Taddesse, D.; Anjejo, G.; Kebede, D. Zeolite Supported CdS/ZnO/Ag3PO4 Nano-Composite: Synthesis, Characterization and Photocatalytic Activity for the Degradation of Methylene Blue. Ph.D. Thesis, Haramaya University, Haramaya, Ethiopia, 2017. [Google Scholar]
- Berr, M.; Vaneski, A.; Susha, A.S.; Rodríguez-Fernández, J.; Döblinger, M.; Jäckel, F.; Rogach, A.L.; Feldmann, J. Colloidal CdS nanorods decorated with subnanometer sized Pt clusters for photocatalytic hydrogen generation. Appl. Phys. Lett. 2010, 97, 093108. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Kaveh, R.; Ozkar, S.; Akbayrak, S. Preparation and characterization of a new CdS-NiFe2O4/reduced graphene oxide photocatalyst and its use for degradation of methylene blue under visible light irradiation. Res. Chem. Intermed. 2018, 44, 5953–5979. [Google Scholar] [CrossRef]
- Lin, R.; Ding, Y. A review on the synthesis and applications of mesostructured transition metal phosphates. Materials 2013, 6, 217–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, X.; Chen, S.; Zhu, Y. Electronic structure and optical properties of Ag3PO4 photocatalyst calculated by hybrid density functional method. Appl. Phys. Lett. 2011, 99, 191903. [Google Scholar] [CrossRef]
- Zhu, G.; Cheng, Z.; Lv, T.; Pan, L.; Zhao, Q.; Sun, Z. Zn-doped nanocrystalline TiO2 films for CdS quantum dot sensitized solar cells. Nanoscale 2010, 2, 1229–1232. [Google Scholar] [CrossRef]
- Zhai, T.; Fang, X.; Li, L.; Bando, Y.; Golberg, D. One-dimensional CdS nanostructures: Synthesis, properties, and applications. Nanoscale 2010, 2, 168–187. [Google Scholar] [CrossRef]
- Yu, J.; Yang, B.; Cheng, B. Noble-metal-free carbon nanotube-Cd0.1Zn0.9S composites for high visible-light photocatalytic H2-production performance. Nanoscale 2012, 4, 2670–2677. [Google Scholar] [CrossRef]
- Cao, J.; Sun, J.Z.; Hong, J.; Li, H.Y.; Chen, H.Z.; Wang, M. Carbon Nanotube/CdS Core–Shell Nanowires Prepared by a Simple Room-Temperature Chemical Reduction Method. Adv. Mater. 2004, 16, 84–87. [Google Scholar] [CrossRef]
- Bahnemann, D.; Kholuiskaya, S.; Dillert, R.; Kulak, A.; Kokorin, A. Photodestruction of dichloroacetic acid catalyzed by nano-sized TiO2 particles. Appl. Catal. B Environ. 2002, 36, 161–169. [Google Scholar] [CrossRef]
- Jo, Y.K.; Kim, I.Y.; Lee, J.M.; Nahm, S.; Choi, J.-W.; Hwang, S.-J. Surface-anchored CdS@ Ag3PO4 nanocomposite with efficient visible light photocatalytic activity. Mater. Lett. 2014, 114, 152–155. [Google Scholar] [CrossRef]
- Chava, R.K.; Do, J.Y.; Kang, M. Fabrication of CdS-Ag3PO4 heteronanostructures for improved visible photocatalytic hydrogen evolution. J. Alloy. Compd. 2017, 727, 86–93. [Google Scholar] [CrossRef]
- Mirsalari, S.A.; Nezamzadeh-Ejhieh, A. CdS–Ag3PO4 nano-catalyst: A brief characterization and kinetic study towards methylene blue photodegradation. Mater. Sci. Semicond. Process. 2021, 122, 105455. [Google Scholar] [CrossRef]
- Jing, Z.; Wang, C.; Wang, G.; Li, W.; Lu, D. Preparation and antibacterial activities of undoped and palladium doped titania nanoparticles. J. Sol Gel Sci. Technol. 2010, 56, 121–127. [Google Scholar] [CrossRef]
- Lin, J.; Qu, W.; Zhang, S. Disposable biosensor based on enzyme immobilized on Au–chitosan-modified indium tin oxide electrode with flow injection amperometric analysis. Anal. Biochem. 2007, 360, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Bandara, S.; Du, H.; Carson, L.; Bradford, D.; Kommalapati, R. Agricultural and Biomedical Applications of Chitosan-Based Nanomaterials. Nanomaterials 2020, 10, 1903. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczesna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, R.; Xiao, L.; Chang, Y.; Guan, Y.; Li, X.; Zeng, G. Photocatalytic decolorization and degradation of Congo Red on innovative crosslinked chitosan/nano-CdS composite catalyst under visible light irradiation. J. Hazard. Mater. 2009, 169, 933–940. [Google Scholar] [CrossRef]
- Liu, S.; Guo, Z.; Qian, X.; Zhang, J.; Liu, J.; Lin, J. Sonochemical deposition of ultrafine metallic Pt nanoparticles on CdS for efficient photocatalytic hydrogen evolution. Sustain. Energy Fuels 2019, 3, 1048–1054. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.; Li, J.; Li, X.; Wang, H.; Huo, P.; Yan, Y. CeO2/3D g-C3N4 heterojunction deposited with Pt cocatalyst for enhanced photocatalytic CO2 reduction. Appl. Surf. Sci. 2021, 537, 147891. [Google Scholar] [CrossRef]
- Wang, D.; Song, Y.; Cai, J.; Wu, L.; Li, Z. Effective photo-reduction to deposit Pt nanoparticles on MIL-100 (Fe) for visible-light-induced hydrogen evolution. New J. Chem. 2016, 40, 9170–9175. [Google Scholar] [CrossRef]
- Boomi, P.; Prabu, H.G.; Mathiyarasu, J. Synthesis, characterization and antibacterial activity of polyaniline/Pt–Pd nanocomposite. Eur. J. Med. Chem. 2014, 72, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, N.; Doroodmand, M.M.; Ghahremani, A. Fabrication of a novel casein phosphopeptides/multi-walled carbon nanotubes/micro hybrid resin as mixed matrix membrane-junction reference electrode. J. Electroanal. Chem. 2015, 745, 98–105. [Google Scholar] [CrossRef]
- Bharathi, D.; Vasantharaj, S.; Bhuvaneshwari, V. Green synthesis of silver nanoparticles using Cordia dichotoma fruit extract and its enhanced antibacterial, anti-biofilm and photo catalytic activity. Mater. Res. Express 2018, 5, 055404. [Google Scholar] [CrossRef]
- Mutalik, C.; Wang, D.-Y.; Krisnawati, D.I.; Jazidie, A.; Yougbare, S.; Kuo, T.-R. Light-activated heterostructured nanomaterials for antibacterial applications. Nanomaterials 2020, 10, 643. [Google Scholar] [CrossRef]
- Zhao, C.; Feng, B.; Li, Y.; Tan, J.; Lu, X.; Weng, J. Preparation and antibacterial activity of titanium nanotubes loaded with Ag nanoparticles in the dark and under the UV light. Appl. Surf. Sci. 2013, 280, 8–14. [Google Scholar] [CrossRef]
- Plachá, D.; Muñoz-Bonilla, A.; Škrlová, K.; Echeverria, C.; Chiloeches, A.; Petr, M.; Lafdi, K.; Fernández-García, M. Antibacterial Character of Cationic Polymers Attached to Carbon-Based Nanomaterials. Nanomaterials 2020, 10, 1218. [Google Scholar] [CrossRef]
- Fu, J.; Chang, B.; Tian, Y.; Xi, F.; Dong, X. Novel C3N4–CdS composite photocatalysts with organic–inorganic heterojunctions: In situ synthesis, exceptional activity, high stability and photocatalytic mechanism. J. Mater. Chem. A 2013, 1, 3083–3090. [Google Scholar] [CrossRef]
- Guibal, E.; Cambe, S.; Bayle, S.; Taulemesse, J.-M.; Vincent, T. Silver/chitosan/cellulose fibers foam composites: From synthesis to antibacterial properties. J. Colloid Interface Sci. 2013, 393, 411–420. [Google Scholar] [CrossRef]
- Botelho, G.; Andres, J.; Gracia, L.; Matos, L.S.; Longo, E. Photoluminescence and photocatalytic properties of Ag3PO4 microcrystals: An experimental and theoretical investigation. ChemPlusChem 2016, 81, 202–212. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, C.; Zhai, W.; Wang, Z.; Sun, Y.; Chi, F.; Ran, S.; Liu, X.; Lv, Y. Novel Ag3PO4/CeO2 pn hierarchical heterojunction with enhanced photocatalytic performance. Mater. Res. 2016, 19, 673–679. [Google Scholar] [CrossRef]
- Sepahvand, S.; Farhadi, S. Fullerene-modified magnetic silver phosphate (Ag3PO4/Fe3O4/C60) nanocomposites: Hydrothermal synthesis, characterization and study of photocatalytic, catalytic and antibacterial activities. RSC Adv. 2018, 8, 10124–10140. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, H.; Cui, Z.; Zhang, H.; Wang, X. A novel Bi4Ti3O12/Ag3PO4 heterojunction photocatalyst with enhanced photocatalytic performance. Nanoscale Res. Lett. 2017, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Yang, H.; Zhao, X. Enhanced photocatalytic performance of g-C3N4/Bi4Ti3O12 heterojunction nanocomposites. Mater. Sci. Eng. B 2018, 229, 160–172. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Setayesh, S.R.; Gholami, M.R. Synthesis of the visible-light-driven Ag3VO4/Ag3PO4/Ag photocatalysts with enhanced photocatalytic activity. RSC Adv. 2016, 6, 14909–14915. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Kaveh, R. A new SnS2-BiFeO3/reduced graphene oxide photocatalyst with superior photocatalytic capability under visible light irradiation. J. Photochem. Photobiol. A Chem. 2018, 359, 11–22. [Google Scholar] [CrossRef]
- Bi, Y.; Ouyang, S.; Cao, J.; Ye, J. Facile synthesis of rhombic dodecahedral AgX/Ag3PO4 (X= Cl, Br, I) heterocrystals with enhanced photocatalytic properties and stabilities. Phys. Chem. Chem. Phys. 2011, 13, 10071–10075. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Y.; Wang, X. Methods and mechanism for improvement of photocatalytic activity and stability of Ag3PO4: A review. J. Alloy. Compd. 2015, 649, 910–932. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Mao, L. Recent progress in highly efficient Ag-based visible-light photocatalysts. RSC Adv. 2014, 4, 53649–53661. [Google Scholar] [CrossRef]
- Yang, Z.-M.; Huang, G.-F.; Huang, W.-Q.; Wei, J.-M.; Yan, X.-G.; Liu, Y.-Y.; Jiao, C.; Wan, Z.; Pan, A. Novel Ag3PO4/CeO2 composite with high efficiency and stability for photocatalytic applications. J. Mater. Chem. A 2014, 2, 1750–1756. [Google Scholar] [CrossRef]
- Tang, Y.; Jiang, Z.; Xing, G.; Li, A.; Kanhere, P.D.; Zhang, Y.; Sum, T.C.; Li, S.; Chen, X.; Dong, Z. Efficient Ag@AgCl cubic cage photocatalysts profit from ultrafast plasmon-induced electron transfer processes. Adv. Funct. Mater. 2013, 23, 2932–2940. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z.; Shang, L.; Liu, J.; Shen, J. Heterostructured Ag3PO4/TiO2 film with high efficiency for degradation of methyl orange under visible light. Thin Solid Film. 2014, 551, 8–12. [Google Scholar] [CrossRef]
- Cui, X.; Li, Y.; Zhang, Q.; Wang, H. Silver orthophosphate immobilized on flaky layered double hydroxides as the visible-light-driven photocatalysts. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, J.; Zhang, L. In situ synthesis of Ag3PO4/cellulose nanocomposites with photocatalytic activities under sunlight. Cellulose 2014, 21, 3371–3382. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, B.; Yu, J.; Liu, G.; Fan, W. Visible-light photocatalytic activity and deactivation mechanism of Ag3PO4 spherical particles. Chem. Asian J. 2012, 7, 1902–1908. [Google Scholar] [CrossRef]
- Dong, P.; Xi, X.; Hou, G. Typical non-TiO2-based visible-light photocatalysts. Semicond. Photocatal. Mater. Mech. Appl. 2016. [Google Scholar] [CrossRef][Green Version]
- Yan, T.; Zhang, H.; Liu, Y.; Guan, W.; Long, J.; Li, W.; You, J. Fabrication of robust M/Ag3PO4 (M= Pt, Pd, Au) Schottky-type heterostructures for improved visible-light photocatalysis. RSC Adv. 2014, 4, 37220–37230. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Jiang, Q.; Yuan, J.; Lin, C.; Shangguan, W. Promotion effect of nickel loaded on CdS for photocatalytic H2 production in lactic acid solution. Appl. Surf. Sci. 2014, 316, 590–594. [Google Scholar] [CrossRef]
- Cheng, F.; Liu, W.-S.; Juan, W.; Wang, Y.-K. Research progress of Ag3PO4-based photocatalyst: Fundamentals and performance enhancement. Trans. Nonferrous Met. Soc. China 2015, 25, 112–121. [Google Scholar]
- Thiyagarajan, S.; Singh, S.; Bahadur, D. Reusable sunlight activated photocatalyst Ag3PO4 and its significant antibacterial activity. Mater. Chem. Phys. 2016, 173, 385–394. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Sedaghat, S.; Tahvildari, K.; Derakhshi, P.; Ghaemi, A. Synthesis of silver nanoparticles using Peganum harmala extract as a green route. Green Chem. Lett. Rev. 2017, 10, 420–427. [Google Scholar] [CrossRef]
- Harish, R.; Nisha, K.; Prabakaran, S.; Sridevi, B.; Harish, S.; Navaneethan, M.; Ponnusamy, S.; Hayakawa, Y.; Vinniee, C.; Ganesh, M. Cytotoxicity assessment of chitosan coated CdS nanoparticles for bio-imaging applications. Appl. Surf. Sci. 2020, 499, 143817. [Google Scholar] [CrossRef]
- Majhi, D.; Das, K.; Mishra, A.; Dhiman, R.; Mishra, B. One pot synthesis of CdS/BiOBr/Bi2O2CO3: A novel ternary double Z-scheme heterostructure photocatalyst for efficient degradation of atrazine. Appl. Catal. B Environ. 2020, 260, 118222. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Chi, R.; Shi, J.; Yang, Y.; Zhang, X. Reduced graphene oxide loaded with MoS2 and Ag3PO4 nanoparticles/PVA interpenetrating hydrogels for improved mechanical and antibacterial properties. Mater. Des. 2019, 183, 108166. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.-H.; Yu, X.-H.; Wang, H.-J. Facile synthesis and antineoplastic activity of bovine serum albumin-conjugated Ag/Ca phosphate nanocomposites. Micro Nano Lett. 2012, 7, 489–491. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiani, M.; Bagherzadeh, M.; Kaveh, R.; Rabiee, N.; Fatahi, Y.; Dinarvand, R.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Novel Pt-Ag3PO4/CdS/Chitosan Nanocomposite with Enhanced Photocatalytic and Biological Activities. Nanomaterials 2020, 10, 2320. https://doi.org/10.3390/nano10112320
Kiani M, Bagherzadeh M, Kaveh R, Rabiee N, Fatahi Y, Dinarvand R, Jang HW, Shokouhimehr M, Varma RS. Novel Pt-Ag3PO4/CdS/Chitosan Nanocomposite with Enhanced Photocatalytic and Biological Activities. Nanomaterials. 2020; 10(11):2320. https://doi.org/10.3390/nano10112320
Chicago/Turabian StyleKiani, Mahsa, Mojtaba Bagherzadeh, Reyhaneh Kaveh, Navid Rabiee, Yousef Fatahi, Rassoul Dinarvand, Ho Won Jang, Mohammadreza Shokouhimehr, and Rajender S. Varma. 2020. "Novel Pt-Ag3PO4/CdS/Chitosan Nanocomposite with Enhanced Photocatalytic and Biological Activities" Nanomaterials 10, no. 11: 2320. https://doi.org/10.3390/nano10112320
APA StyleKiani, M., Bagherzadeh, M., Kaveh, R., Rabiee, N., Fatahi, Y., Dinarvand, R., Jang, H. W., Shokouhimehr, M., & Varma, R. S. (2020). Novel Pt-Ag3PO4/CdS/Chitosan Nanocomposite with Enhanced Photocatalytic and Biological Activities. Nanomaterials, 10(11), 2320. https://doi.org/10.3390/nano10112320








