Novel Bionanocompounds: Outer Membrane Protein A and Laccase Co-Immobilized on Magnetite Nanoparticles for Produced Water Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein Production
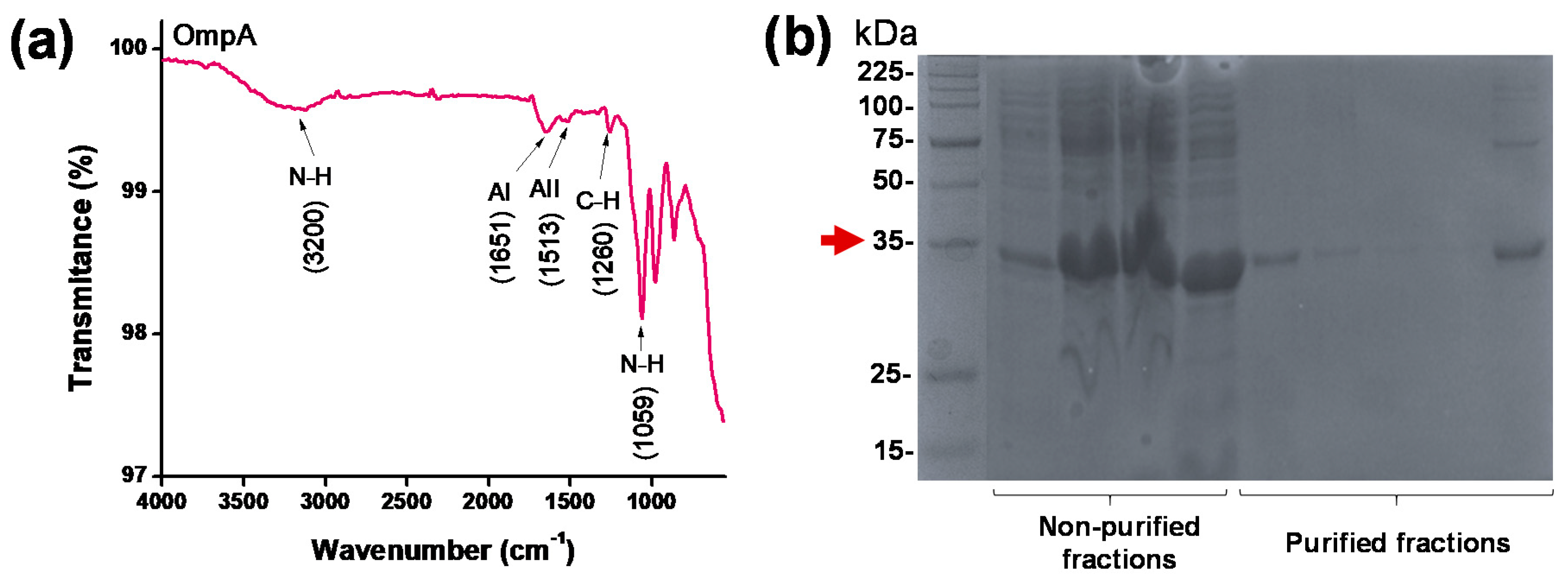
2.3. Protein Characterization
2.4. Polyetheramine (PEA) Oxidation
2.5. Synthesis of Magnetite Nanoparticles
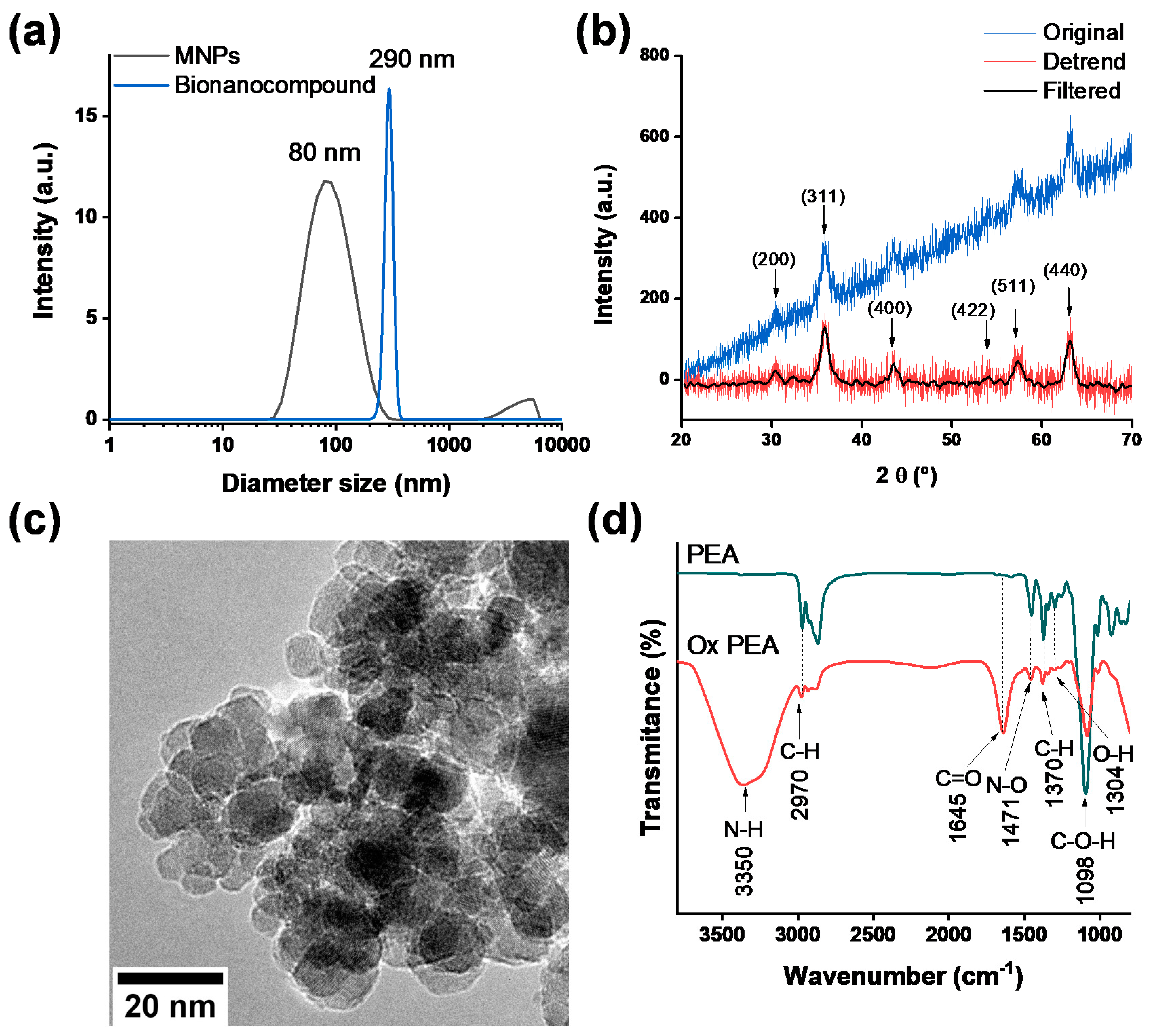
2.6. MNP Characterizations
2.7. OmpA and Laccase Immobilization: Magnetite-PEA-OmpA-Laccase Bionanocompounds
2.8. Characterization of Magnetite-PEA-OmpA-Laccase Bionanocompounds
2.9. Crude Oil in Water (O/W) Emulsion Preparation
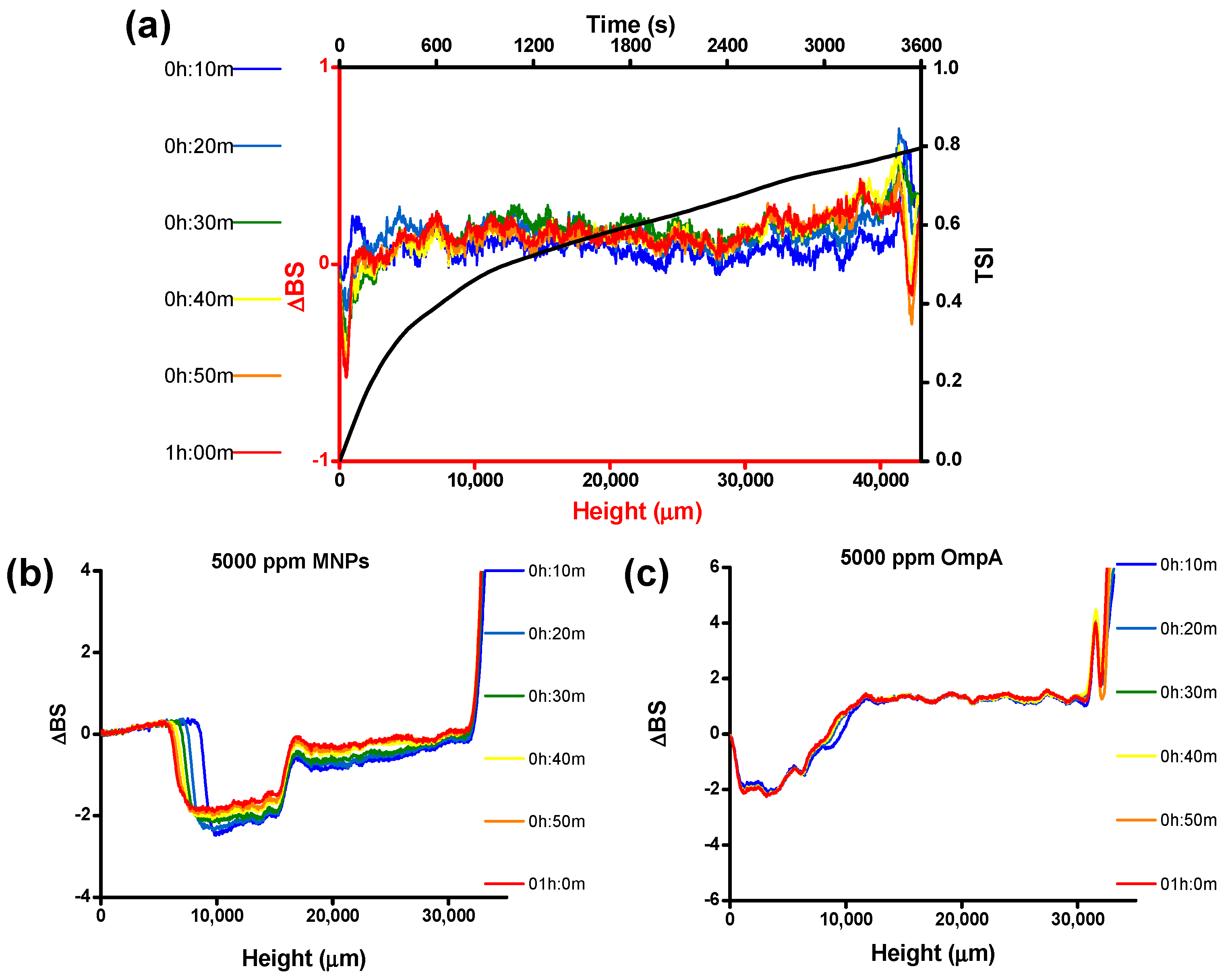
2.10. Physical Stability of O/W Emulsions
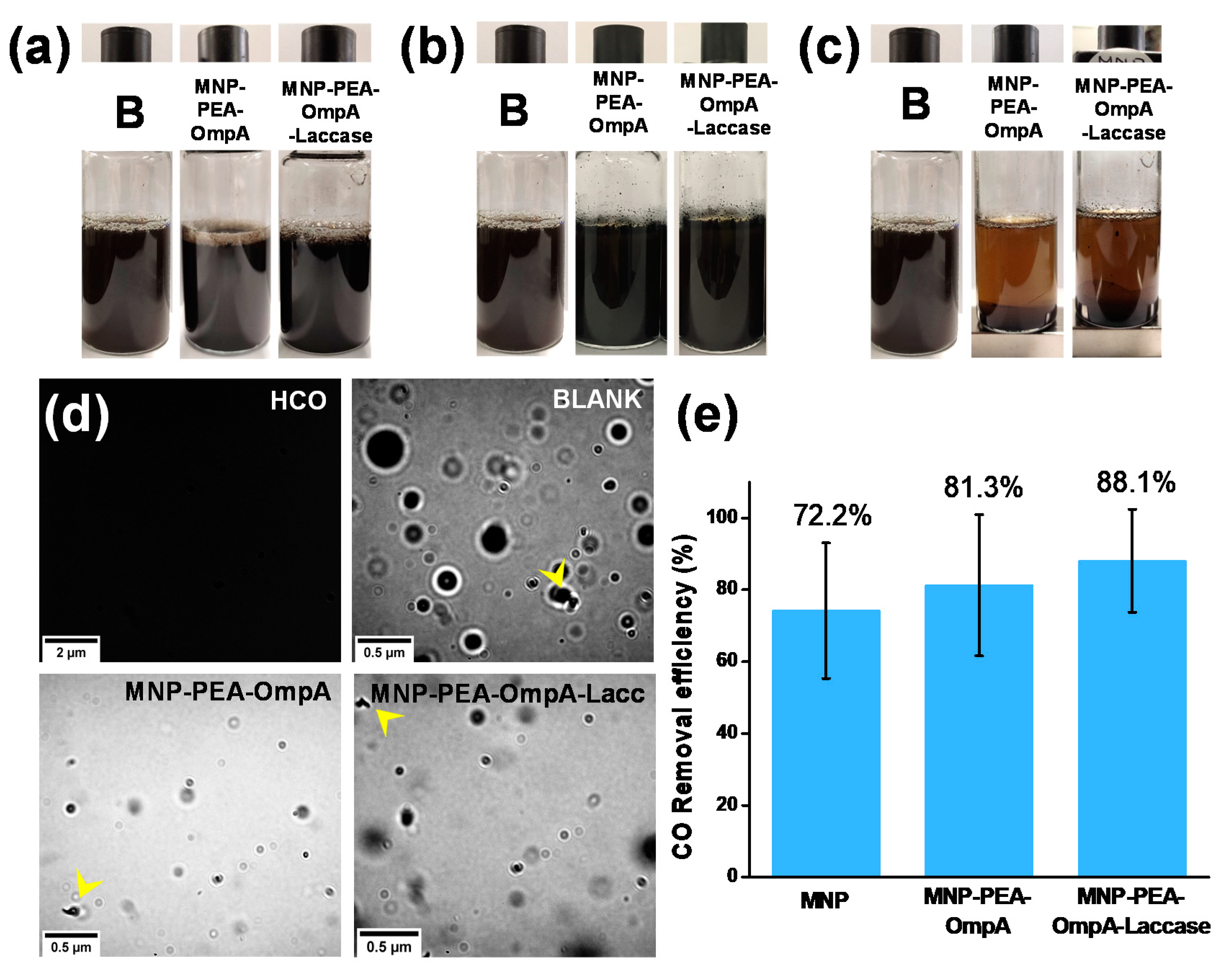
2.11. Demulsification Effect of MNP and OmpA in O/W Emulsion and Bottle Test
2.12. O/W Emulsion Separation and Treatment
3. Results and Discussion
3.1. Proteins, PEA and MNPs Characterization
3.2. Bionanocompounds Characterization
3.3. Immobilization Yield Determination
3.4. O/W Emulsion Stability and Characterization
3.5. Demulsification Effect of Free MNP and OmpA
3.6. Removal Efficiency
3.7. Gas Chromatography-Mass Spectrometry (GC-MS) Characterization of Residual Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yew, Y.P.; Shameli, K.; Miyake, M.; Ahmad Khairudin, N.B.B.; Mohamad, S.E.B.; Naiki, T.; Lee, K.X. Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: A review. Arab. J. Chem. 2020, 13, 2287–2308. [Google Scholar] [CrossRef]
- Lopez-Barbosa, N.; Suárez-Arnedo, A.; Cifuentes, J.; Gonzalez Barrios, A.F.; Silvera Batista, C.A.; Osma, J.F.; Munõz-Camargo, C.; Cruz, J.C. Magnetite-OmpA Nanobioconjugates as Cell-Penetrating Vehicles with Endosomal Escape Abilities. ACS Biomater. Sci. Eng. 2020, 6, 415–424. [Google Scholar] [CrossRef]
- Liu, L.L.; Li, X.T.; Zhang, N.; Tang, C.H. Novel soy β-conglycinin nanoparticles by ethanol-assisted disassembly and reassembly: Outstanding nanocarriers for hydrophobic nutraceuticals. Food Hydrocoll. 2019, 91, 246–255. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zhou, S.; Feng, H.; Liu, Y.; Jia, G. Review of health safety aspects of titanium dioxide nanoparticles in food application. NanoImpact 2020, 100224. [Google Scholar] [CrossRef]
- López-Naranjo, E.J.; Hernández-Rosales, I.P.; Bueno-Durán, A.Y.; Martínez-Aguilar, M.L.; González-Ortiz, L.J.; Pérez-Fonseca, A.A.; Robledo-Ortiz, J.R.; Sánchez-Peña, M.J.; Manzano-Ramírez, A. Biosynthesis of silver nanoparticles using a natural extract obtained from an agroindustrial residue of the tequila industry. Mater. Lett. 2018, 213, 278–281. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Fazal, H.; Ahmad, N.; Ali, M.; Giglioli-Guivarch, N.; Hano, C. Nanomaterials for Cosmeceuticals: Nanomaterials-Induced Advancement in Cosmetics, Challenges, and Opportunities; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128222867. [Google Scholar]
- Osma, J.F.; Stoytcheva Stilianova, M. Biosensors: Recent Advances and Mathematical Challenges; OmniaScience: Terrassa, Barcelona, Spain, 2014; ISBN 978-84-941872-0-9. [Google Scholar]
- Zhang, G.; Quin, M.B.; Schmidt-Dannert, C. Self-Assembling Protein Scaffold System for Easy in Vitro Coimmobilization of Biocatalytic Cascade Enzymes. ACS Catal. 2018, 8, 5611–5620. [Google Scholar] [CrossRef]
- Asal, M.; Özen, Ö.; Şahinler, M.; Baysal, H.T.; Polatoğlu, İ. An overview of biomolecules, immobilization methods and support materials of biosensors. Sens. Rev. 2018, 39. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Kim, M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Acharya, A. Nanomaterial-Based Biomedical Applications in Molecular Imaging, Diagnostics and Therapy. In Biomolecules Immobilized Nanomaterials and Their Biological Applications; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 978-981-15-4280-0. [Google Scholar]
- IPIECA. Petroleum refinery waste management and minimization. In An IPIECA Good Practice Guide; IPIECA: Londond, UK, 2014. [Google Scholar]
- Wong, S.F.; Lim, J.S.; Dol, S.S. Crude oil emulsion: A review on formation, classification and stability of water-in-oil emulsions. J. Pet. Sci. Eng. 2015, 135, 498–504. [Google Scholar] [CrossRef]
- Liang, J.; Du, N.; Song, S.; Hou, W. Magnetic demulsification of diluted crude oil-in-water nanoemulsions using oleic acid-coated magnetite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 197–202. [Google Scholar] [CrossRef]
- Xu, H.; Jia, W.; Ren, S.; Wang, J. Magnetically responsive multi-wall carbon nanotubes as recyclable demulsifier for oil removal from crude oil-in-water emulsion with different pH levels. Carbon N.Y. 2019, 145, 229–239. [Google Scholar] [CrossRef]
- Wang, Q.; Puerto, M.C.; Warudkar, S.; Buehler, J.; Biswal, S.L. Recyclable amine-functionalized magnetic nanoparticles for efficient demulsification of crude oil-in-water emulsions. Environ. Sci. Water Res. Technol. 2018, 4, 1553–1563. [Google Scholar] [CrossRef]
- Martínez-Palou, R.; Cerón-Camacho, R.; Chávez, B.; Vallejo, A.A.; Villanueva-Negrete, D.; Castellanos, J.; Karamath, J.; Reyes, J.; Aburto, J. Demulsification of heavy crude oil-in-water emulsions: A comparative study between microwave and thermal heating. Fuel 2013, 113, 407–414. [Google Scholar] [CrossRef]
- Isabel Briceno, M.; Salager, J.-L.; Luis Bracho, C. Heavy Hydrocarbon Emulsions Making Use of the State of the Art in Formulation Engineering. In Encyclopedic Handbook of Emulsion Technology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Martínez-Palou, R.; Reyes, J.; Cerón-Camacho, R.; Ramírez-de-Santiago, M.; Villanueva, D.; Vallejo, A.A.; Aburto, J. Study of the formation and breaking of extra-heavy-crude-oil-in-water emulsions-A proposed strategy for transporting extra heavy crude oils. Chem. Eng. Process. Process Intensif. 2015, 98, 112–122. [Google Scholar] [CrossRef]
- Borah, D.; Yadav, R.N.S. Bioremediation of petroleum based contaminants with biosurfactant produced by a newly isolated petroleum oil degrading bacterial strain. Egypt. J. Pet. 2017, 26, 181–188. [Google Scholar] [CrossRef]
- Sokół, W. Treatment of refinery wastewater in a three-phase fluidised bed bioreactor with a low density biomass support. Biochem. Eng. J. 2003, 15, 1–10. [Google Scholar] [CrossRef]
- Baycan Parilti, N. Treatment of a Petrochemical Industry Wastewater by a Solar Oxidation Process Using The Box-Wilson Experimental Design Method. Ekoloji 2010, 19, 9–15. [Google Scholar] [CrossRef]
- Volke, T.L.; Mulas, R.; Ercoli, E.; Fallis, A.; Vargas, P.; Cuéllar, R.; Dussán, J.; Cortón, E.; Viale, A.; Marta, M.; et al. Biorremediación de suelos: Desde el concepto a su aplicación. aplicación. J. Chem. Inf. Model. 2006, 23, 45. [Google Scholar] [CrossRef]
- Parthipan, P.; Elumalai, P.; Sathishkumar, K.; Sabarinathan, D.; Murugan, K.; Benelli, G.; Rajasekar, A. Biosurfactant and enzyme mediated crude oil degradation by Pseudomonas stutzeri NA3 and Acinetobacter baumannii MN3. 3 Biotech 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Parhamfar, M.; Bayat, Z.; Parhamfar, M.; Hassanshahian, M.; Hosseini, S.S. Investigation of Oil-in-Water Emulsions Treatment by Crude Oil Degrading Bacteria and Coagulation with Cationic Polyacrylamide. J. Pet. Environ. Biotechnol. 2018, 9. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, D.G.; Soares Da Silva, R.de.C.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising molecules for petroleum biotechnology advances. Front. Microbiol. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Otzen, D.E. Proteins in a brave new surfactant world. Curr. Opin. Colloid Interface Sci. 2015, 20, 161–169. [Google Scholar] [CrossRef]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef]
- Patel, G.J. The Lipid Bilayer-Inserted Membrane Protein BamA of Escherichia coli Facilitates Insertion and Folding of Outer Membrane Protein A from Its Complex with Skp. Biochemistry 2013, 52, 3974–3986. [Google Scholar] [CrossRef]
- Cardona Jaramillo, J.E.C.; Achenie, L.E.; Álvarez, O.A.; Carrillo Bautista, M.P.; González Barrios, A.F. The multiscale approach to the design of bio-based emulsions. Curr. Opin. Chem. Eng. 2020, 27, 65–71. [Google Scholar] [CrossRef]
- Ishida, H.; Garcia-Herrero, A.; Vogel, H.J. The periplasmic domain of Escherichia coli outer membrane protein A can undergo a localized temperature dependent structural transition. Biochim. Biophys. Acta—Biomembr. 2014, 1838, 3014–3024. [Google Scholar] [CrossRef]
- Wang, Y. The Function of OmpA in Escherichia coli. Biochem. Biophys. Res. Commun. 2002, 401, 396–401. [Google Scholar] [CrossRef]
- Tatiana, E.; Poveda, A.; Fernando, R.; Saiz, C. Emulsiones Agua-en-Crudo Pesado: Estudio de Separación de Fases a Través de la Proteína OmpA Pura e Inmovilizada en Nanopartículas de Magnetita; Uniandes: Bogotá, Colombia, 2018. [Google Scholar]
- Aguilera-Segura, S.M.; Núñez Vélez, V.; Achenie, L.; Álvarez Solano, O.; Torres, R.; González Barrios, A.F. Peptides design based on transmembrane Escherichia coli’s OmpA protein through molecular dynamics simulations in water–dodecane interfaces. J. Mol. Graph. Model. 2016, 68, 216–223. [Google Scholar] [CrossRef]
- Tibaquirá Martinez, M.A.; Gonzalez Barrios, A. Exploración de la proteína transmembranal OmpA para la recuperación mejorada de hidrocarburos presentes en sistemas porosos. Bachelor’s Thesis, Universidad de Los Andes, Bogotá, Colombia, 2014. [Google Scholar]
- Umar, A.A.; Saaid, I.B.M.; Sulaimon, A.A.; Pilus, R.B.M. A review of petroleum emulsions and recent progress on water-in-crude oil emulsions stabilized by natural surfactants and solids. J. Pet. Sci. Eng. 2018, 165, 673–690. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Li, X.; Jia, W.; Zhao, Y.; Ren, S. Recyclable magnetic graphene oxide for rapid and efficient demulsification of crude oil-in-water emulsion. Fuel 2017, 189, 79–87. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, R.; Sun, D.; Wu, T.; Li, Y. Fabrication of Magnetite-Graphene Oxide/MgAl-Layered Double Hydroxide Composites for Efficient Removal of Emulsified Oils from Various Oil-in-Water Emulsions. J. Chem. Eng. Data 2018, 63, 4689–4702. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Y.; Graff, R.W.; Lee, D.; Gao, H. Developing recyclable pH-responsive magnetic nanoparticles for oil-water separation. Polymer (Guildf) 2015, 72, 361–367. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, C.; Qi, D.; Lü, T.; Zhang, D. One-Step Synthesis of Polyethylenimine-Coated Magnetic Nanoparticles and its Demulsification Performance in Surfactant-Stabilized Oil-in-Water Emulsion. J. Dispers. Sci. Technol. 2019, 40, 231–238. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, X.; Liu, J.; Huang, Z. Application of magnetic nanoparticles in petroleum industry: A review. J. Pet. Sci. Eng. 2020, 188, 106943. [Google Scholar] [CrossRef]
- Kannel, P.R.; Gan, T.Y. Naphthenic acids degradation and toxicity mitigation in tailings wastewater systems and aquatic environments: A review. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2012, 47, 1–21. [Google Scholar] [CrossRef]
- Kilpatrick, P.K. Water-in-crude oil emulsion stabilization: Review and unanswered questions. Energy Fuels 2012, 26, 4017–4026. [Google Scholar] [CrossRef]
- Ikehata, K.; El-Din, M.G. Degradation of recalcitrant surfactants in wastewater by ozonation and advanced oxidation processes: A review. Ozone Sci. Eng. 2004, 26, 327–343. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Younesi, H.; Zinatizadeh, A.A. Preparation, characterization and photocatalytic application of TiO2/Fe-ZSM-5 nanocomposite for the treatment of petroleum refinery wastewater: Optimization of process parameters by response surface methodology. Chemosphere 2016, 159, 552–564. [Google Scholar] [CrossRef]
- Gargouri, B.; Mhiri, N.; Karray, F.; Aloui, F.; Sayadi, S. Isolation and Characterization of Hydrocarbon-Degrading Yeast Strains from Petroleum Contaminated Industrial Wastewater. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Van Do, T.; Le, C.T.N.; Do, L.T.; Dong, Q. Van Degradation of hydrocarbon components contaminated in oily waste-water collected in Doxa petroleum storage, Hanoi by microbial biofilm attached on coconut fiber. VNU J. Sci. Nat. Sci. Technol. 2017, 33. [Google Scholar] [CrossRef]
- Agarwal, P.; Gupta, R.; Agarwal, N. A Review on Enzymatic Treatment of Phenols in Wastewater. J. Biotechnol. Biomater. 2016, 6. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, Y.; Yin, Z.; Chen, D.; Bao, M.; Lu, J. Insights into the effect of different levels of crude oil on hydrolyzed polyacrylamide biotransformation in aerobic and anoxic biosystems: Bioresource production, enzymatic activity, and microbial function. Bioresour. Technol. 2019, 293, 122023. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Singh, B.; Pandey, A.K.; Badruddin, I.J. Application of Microbial Enzymes in Industrial Waste Water Treatment. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1243–1254. [Google Scholar] [CrossRef]
- Zhang, D.; Deng, M.; Cao, H.; Zhang, S.; Zhao, H. Laccase immobilized on magnetic nanoparticles by dopamine polymerization for 4-chlorophenol removal. Green Energy Environ. 2017, 2, 393–400. [Google Scholar] [CrossRef]
- García-Morales, R.; García-García, A.; Orona-Navar, C.; Osma, J.F.; Nigam, K.D.P.; Ornelas-Soto, N. Biotransformation of emerging pollutants in groundwater by laccase from P. sanguineus CS43 immobilized onto titania nanoparticles. J. Environ. Chem. Eng. 2018, 6, 710–717. [Google Scholar] [CrossRef]
- Vera, M.; Nyanhongo, G.S.; Guebitz, G.M.; Rivas, B.L. Polymeric microspheres as support to co-immobilized Agaricus bisporus and Trametes versicolor laccases and their application in diazinon degradation. Arab. J. Chem. 2020, 13, 4218–4227. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, S.; Miao, S.; Suo, H.; Xu, H.; Hu, Y. Co-immobilization of laccase and ABTS onto amino-functionalized ionic liquid-modified magnetic chitosan nanoparticles for pollutants removal. J. Hazard. Mater. 2020, 401, 123353. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Shriver-Lake, L.C.; Anderson, G.P.; Taitt, C.R. Effect of Linker Length on Cell Capture by Poly(ethylene glycol)-Immobilized Antimicrobial Peptides. Langmuir 2017, 33, 2878–2884. [Google Scholar] [CrossRef]
- Deb, A.; Vimala, R. Camptothecin loaded graphene oxide nanoparticle functionalized with polyethylene glycol and folic acid for anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 333–342. [Google Scholar] [CrossRef]
- Acosta, M.; Reyes, L.H.; Cruz, J.C.; Pradilla, D. Demulsification of Colombian Heavy Crude Oil (W/O) Emulsions: Insights into the Instability Mechanisms, Chemical Structure, and Performance of Different Commercial Demulsifiers. Energy Fuels 2020, 34, 5665–5678. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Hong, R.Y.; Wang, L.S.; Guo, L.; Li, H.Z.; Ding, J.; Zheng, Y.; Wei, D.G. Synthesis of Fe3O4/APTES/PEG diacid functionalized magnetic nanoparticles for MR imaging. Colloids Surf. A Physicochem. Eng. Asp. 2008, 328, 52–59. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Bannwart, A.C.; Briceño, M.I.; Loh, W. Physico-chemical properties of heavy crude oil-in-water emulsions stabilized by mixtures of ionic and non-ionic ethoxylated nonylphenol surfactants and medium chain alcohols. Chem. Eng. Res. Des. 2011, 89, 957–967. [Google Scholar] [CrossRef]
- Eftekhardadkhah, M.; Kløcker, K.N.; Trapnes, H.H.; Gawełe, B.; Øye, G. Composition and Dynamic Adsorption of Crude Oil Components Dissolved in Synthetic Produced Water at Different pH Values. Ind. Eng. Chem. Res. 2016, 55, 3084–3090. [Google Scholar] [CrossRef]
- Eftekhardadkhah, M.; Øye, G. Correlations between crude oil composition and produced water quality: A multivariate analysis approach. Ind. Eng. Chem. Res. 2013, 52, 17315–17321. [Google Scholar] [CrossRef]
- Tamm, L.K.; Tatulian, S.A. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q. Rev. Biophys. 1997, 30, 365–429. [Google Scholar] [CrossRef]
- Gonzalez Barrios, A.F.; Zuo, R.; Ren, D.; Wood, T.K. Hha, YbaJ, and OmpA regulate Escherichia coli K12 biofilm formation and conjugation plasmids abolish motility. Biotechnol. Bioeng. 2005, 93, 188–200. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Bruce, J.; Berne, C. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; Courier Corporation; Dover Publications: Garden City, NY, USA, 2000.
- Hepziba Suganthi, S.; Swathi, K.V.; Biswas, R.; Basker, S.; Ramani, K. Co-immobilization of multiple enzymes onto surface-functionalized magnetic nanoparticle for the simultaneous hydrolysis of multiple substrates containing industrial wastes. Appl. Nanosci. 2019, 9, 1439–1457. [Google Scholar] [CrossRef]
- Fan, L.; Wang, J.; Zhang, X.; Sadeghzadeh, S.M.; Zhiani, R.; Shahroudi, M.; Amarloo, F. Co-immobilization of Laccase and TEMPO onto Glycidyloxypropyl Functionalized Fibrous Phosphosilicate Nanoparticles for Fixing CO2 into β-Oxopropylcarbamatesin. Catal. Lett. 2019, 149, 3465–3475. [Google Scholar] [CrossRef]
- Li, C.; Jiang, S.; Zhao, X.; Liang, H. Co-immobilization of enzymes and magnetic nanoparticles by metal-nucleotide hydrogelnanofibers for improving stability and recycling. Molecules 2017, 22, 179. [Google Scholar] [CrossRef] [PubMed]
- Gür, S.D.; İdil, N.; Aksöz, N. Optimization of Enzyme Co-Immobilization with Sodium Alginate and Glutaraldehyde-Activated Chitosan Beads. Appl. Biochem. Biotechnol. 2017, 1–15. [Google Scholar] [CrossRef]
- Campaña, A.L.; Florez, S.L.; Noguera, M.J.; Fuentes, O.P.; Puentes, P.R.; Cruz, J.C.; Osma, J.F. Enzyme-based electrochemical biosensors for microfluidic platforms to detect pharmaceutical residues in wastewater. Biosensors 2019, 9, 41. [Google Scholar] [CrossRef]
- Zdarta, J.; Bachosz, K.; Degórska, O.; Zdarta, A.; Kaczorek, E.; Pinelo, M.; Meyer, A.S.; Jesionowski, T. Co-immobilization of glucose dehydrogenase and xylose dehydrogenase as a new approach for simultaneous production of gluconic and xylonic acid. Materials (Basel) 2019, 12, 3167. [Google Scholar] [CrossRef]
- Caparco, A.A.; Bommarius, B.R.; Bommarius, A.S.; Champion, J.A. Protein-inorganic calcium-phosphate supraparticles as a robust platform for enzyme co-immobilization. Biotechnol. Bioeng. 2020. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Mendez-Sanchez, C.; Rios, N.S.; Ortiz, C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. New applications of glyoxyl-octyl agarose in lipases co-immobilization: Strategies to reuse the most stable lipase. Int. J. Biol. Macromol. 2019, 131, 989–997. [Google Scholar] [CrossRef]
- Simonsen, G.; Strand, M.; Øye, G. Potential applications of magnetic nanoparticles within separation in the petroleum industry. J. Pet. Sci. Eng. 2018, 165, 488–495. [Google Scholar] [CrossRef]
- Wu, E.; Li, Y.; Huang, Q.; Yang, Z.; Wei, A.; Hu, Q. Laccase immobilization on amino-functionalized magnetic metal organic framework for phenolic compound removal. Chemosphere 2019, 233, 327–335. [Google Scholar] [CrossRef]
- Kandasamy, R. A novel single step synthesis and surface functionalization of iron oxide magnetic nanoparticles and thereof for the copper removal from pigment industry effluent. Sep. Purif. Technol. 2017. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Kang, W.; Xu, B.; An, F.; Shan, X.; Liu, J. Stability mechanism of W/O crude oil emulsion stabilized by polymer and surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 555–560. [Google Scholar] [CrossRef]
- Formulaction SAS Printing Status. Turbiscan LAB Instrum. Users Guide. 2013, p. 96. Available online: https://www.formulaction.com/en/our-solutions/stability-and-shelf-life (accessed on 7 September 2020).
- Chevalier, Y.; Bolzinger, M.A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Peng, J.; Zhang, Y.; Du, G.; Fang, Y. Smart magnetic ionic liquid-based Pickering emulsions stabilized by amphiphilic Fe3O4 nanoparticles: Highly efficient extraction systems for water purification. J. Colloid Interface Sci. 2017, 485, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Use of nanoparticles and microparticles in the formation and stabilization of food emulsions. Trends Food Sci. Technol. 2012, 24, 4–12. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F. Nano-emulsions. Handb. Nanopart. 2015, 10, 93–116. [Google Scholar] [CrossRef]
- Ko, S.; Kim, E.S.; Park, S.; Daigle, H.; Milner, T.E.; Huh, C.; Bennetzen, M.V.; Geremia, G.A. Oil droplet removal from produced water using nanoparticles and their magnetic separation. In Proceedings of the Proceedings—SPE Annual Technical Conference and Exhibition, Dubai, UAE, 26–28 September 2016. [Google Scholar]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef]
- Maity, D.; Chandrasekharan, P.; Feng, S.S.; Jun, D. Synthesis and studies of APTES functionalized magnetite nanoparticles. In Proceedings of the ICONN 2010—2010 International Conference on Nanoscience and Nanotechnology, Sydney, NSW, Australia, 22–26 February 2010; pp. 94–97. [Google Scholar] [CrossRef]
- Pradilla, D.; Ramírez, J.; Zanetti, F.; Álvarez, O. Demulsifier Performance and Dehydration Mechanisms in Colombian Heavy Crude Oil Emulsions. Energy Fuels 2017, 31, 10369–10377. [Google Scholar] [CrossRef]
- Rocha e Silva, N.M.P.; Meira, H.M.; Almeida, F.C.G.; Soares da Silva, R.D.C.F.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Natural Surfactants and Their Applications for Heavy Oil Removal in Industry. Sep. Purif. Rev. 2019, 48, 267–281. [Google Scholar] [CrossRef]
- Karlapudi, A.P.; Venkateswarulu, T.C.; Tammineedi, J.; Kanumuri, L.; Ravuru, B.K.; ramu Dirisala, V.; Kodali, V.P. Role of biosurfactants in bioremediation of oil pollution-a review. Petroleum 2018, 4, 241–249. [Google Scholar] [CrossRef]


| Ion | Concentration [ppm] |
|---|---|
| Brine with All Ions | |
| CL− | 62,819 |
| Na+ | 35,393 |
| Ca2+ | 3253 |
| Mg2+ | 909 |
| HCO3− | 218 |
| SO42− | 49 |
| Relative Area | Removal Efficiency | |||||
|---|---|---|---|---|---|---|
| Peak N° | Compound | Blank | MNP-PEA-OmpA | MNP-PEA-OmpA-Laccase | MNP-PEA-OmpA | MNP-PEA-OmpA-Laccase |
| 5 | Pentadecane | 100.00 | 97.43 | 94.44 | 2.57 | 5.56 |
| 7 | Naphthalene, 1,6,7-trimethyl | 34.91 | 33.77 | 31.84 | 3.28 | 8.79 |
| 9 | Heptadecane | 73.24 | 58.18 | 53.25 | 20.56 | 27.30 |
| 10 | Octadecane | 61.85 | 66.01 | 53.80 | 0.00 | 13.01 |
| 11 | Hexadecane, 2,6,10,14-tetramethyl | 48.41 | 25.31 | 23.90 | 47.71 | 50.62 |
| 12 | Nonadecane | 53.35 | 50.12 | 63.84 | 6.04 | 0.00 |
| 14 | Eicosane | 53.17 | 49.18 | 44.04 | 7.51 | 17.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel-Muñoz, N.; González-Barrios, A.F.; Pradilla, D.; Osma, J.F.; Cruz, J.C. Novel Bionanocompounds: Outer Membrane Protein A and Laccase Co-Immobilized on Magnetite Nanoparticles for Produced Water Treatment. Nanomaterials 2020, 10, 2278. https://doi.org/10.3390/nano10112278
Rangel-Muñoz N, González-Barrios AF, Pradilla D, Osma JF, Cruz JC. Novel Bionanocompounds: Outer Membrane Protein A and Laccase Co-Immobilized on Magnetite Nanoparticles for Produced Water Treatment. Nanomaterials. 2020; 10(11):2278. https://doi.org/10.3390/nano10112278
Chicago/Turabian StyleRangel-Muñoz, Nathaly, Andres Fernando González-Barrios, Diego Pradilla, Johann F. Osma, and Juan C. Cruz. 2020. "Novel Bionanocompounds: Outer Membrane Protein A and Laccase Co-Immobilized on Magnetite Nanoparticles for Produced Water Treatment" Nanomaterials 10, no. 11: 2278. https://doi.org/10.3390/nano10112278
APA StyleRangel-Muñoz, N., González-Barrios, A. F., Pradilla, D., Osma, J. F., & Cruz, J. C. (2020). Novel Bionanocompounds: Outer Membrane Protein A and Laccase Co-Immobilized on Magnetite Nanoparticles for Produced Water Treatment. Nanomaterials, 10(11), 2278. https://doi.org/10.3390/nano10112278








