CO2 Adsorption in Metal-Organic Framework Mg-MOF-74: Effects of Inter-Crystalline Space
Abstract
1. Introduction
2. Materials and Methods
3. Results
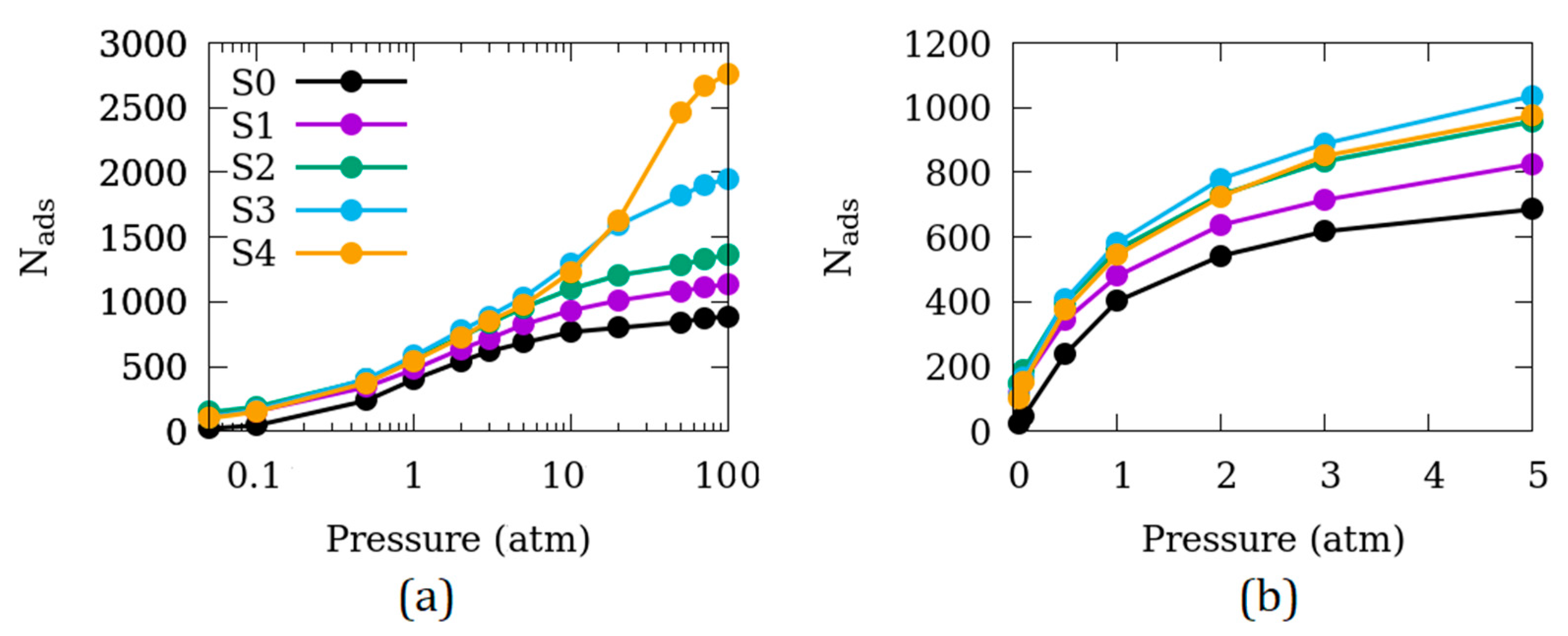
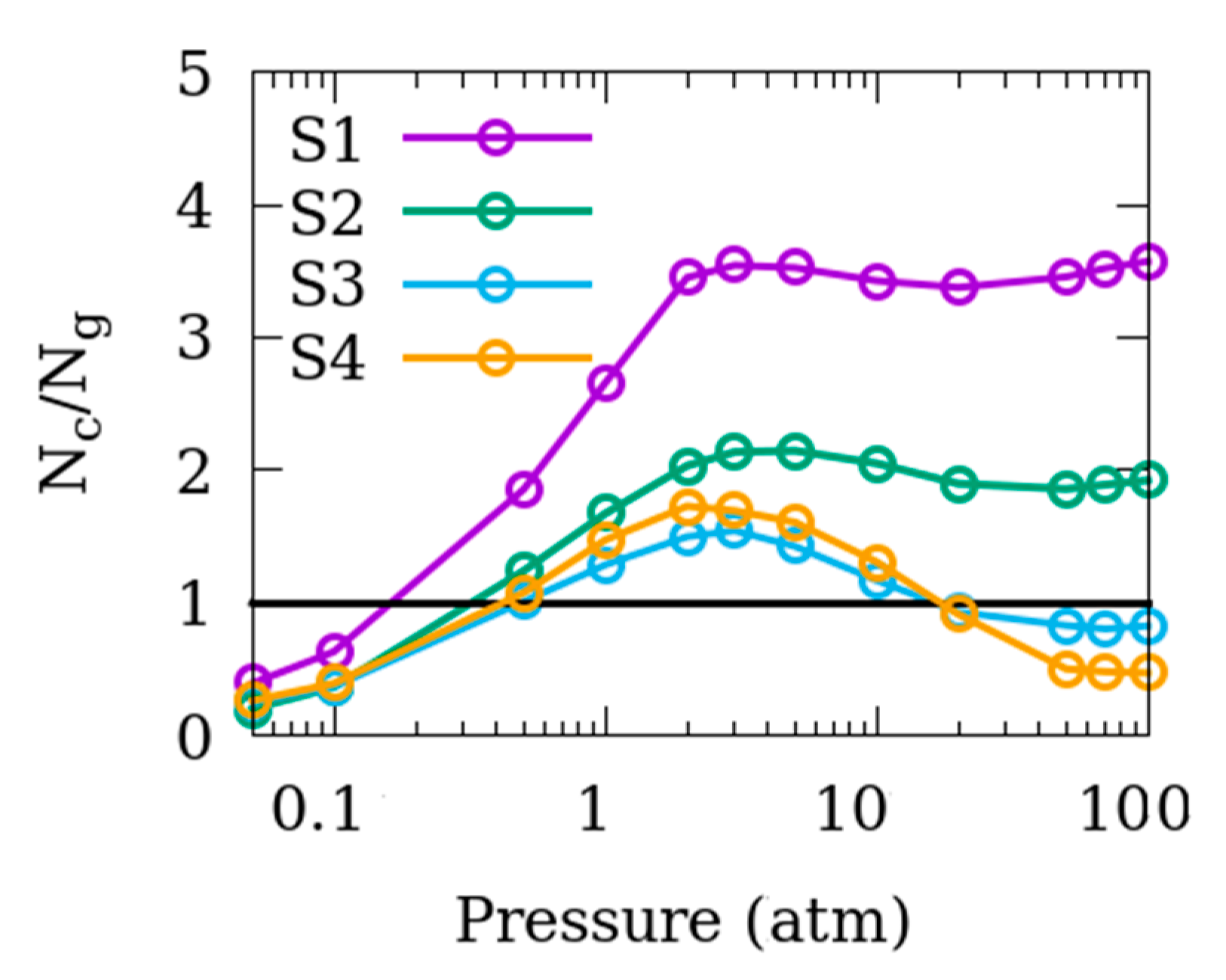
3.1. Effects of the Size of Inter-Crystalline Spacing
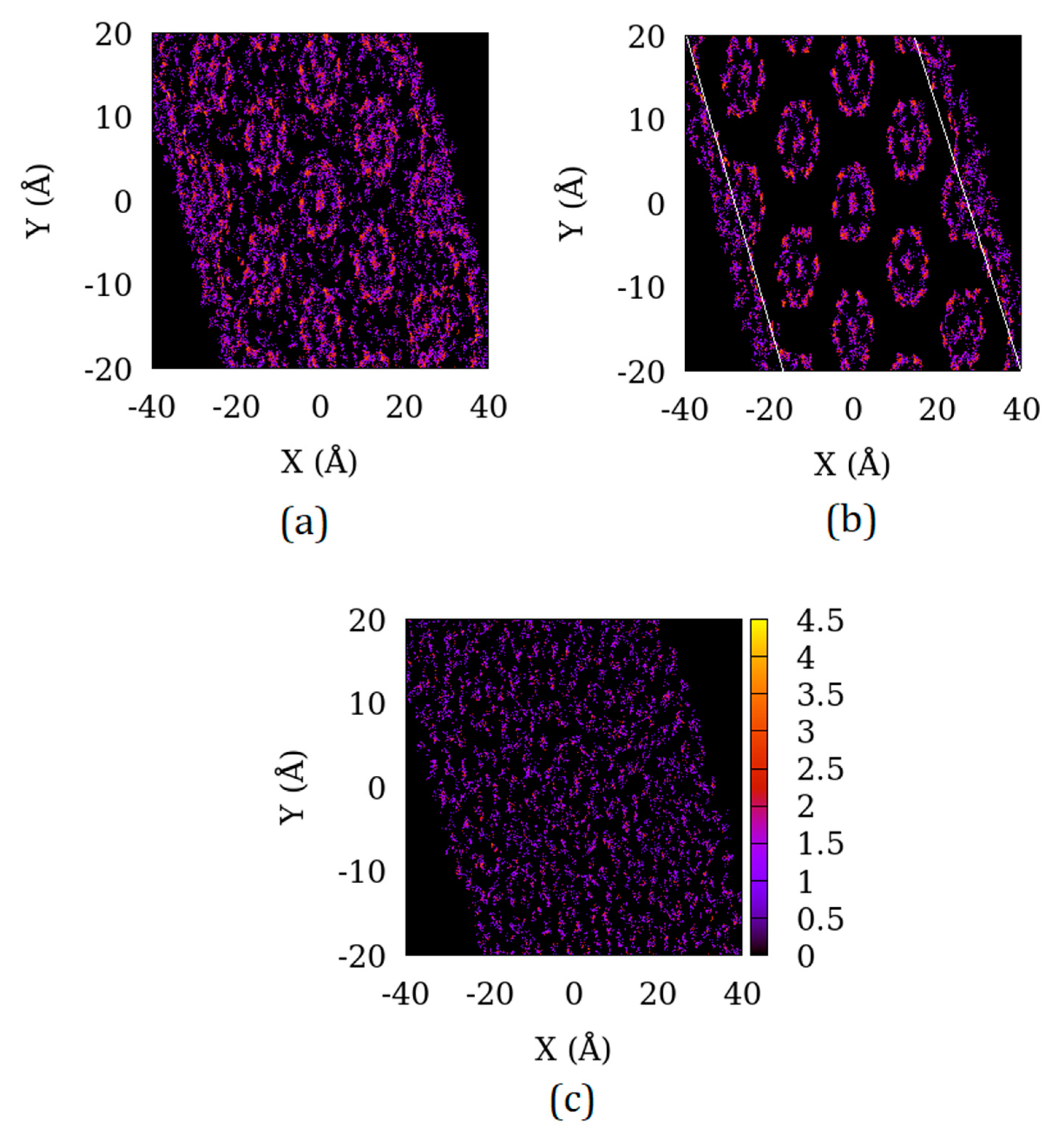
3.2. Difference in the Surface Exposed
3.3. Effects of Temperature
3.4. Effects of the Crystallite Size
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Anderson, T.R.; Hawkins, E.; Philip, D.J. CO2, the greenhouse effect and global warming: From the pioneering work of Arrhenius and Callendar to today’s Earth System Models. Endeavour 2016, 40, 178–187. [Google Scholar] [CrossRef]
- Fanchi, J.R.; Fanchi, C.J. Energy in the 21st Century; World Scientific Publishing Company: Singapore, 2016. [Google Scholar]
- Lackner, K.S. A guide to CO2 sequestration. Science 2003, 300, 1677–1678. [Google Scholar] [CrossRef]
- Arindam, M.; Subhra, J. Advances in Porous Adsorbents for CO2 Capture and Storage, Carbon Dioxide Chemistry, Capture and Oil Recovery, Iyad Karame´, Janah Shaya and Hassan Srour; IntechOpen: London, UK, 20 December 2017; Available online: https://www.intechopen.com/books/carbon-dioxide-chemistry-capture-and-oilrecovery/advances-in-porous-adsorbents-for-co2-capture-andstorage (accessed on 2 October 2020). [CrossRef]
- Regufe, M.J.; Ribeiro, A.M.; Ferreira, A.F.P. Rodrigues ACO2 Storage on Zeolites Other Adsorbents. In Nanoporous Materials for Gas Storage. Green Energy and Technology; Kaneko, K., Rodríguez-Reinoso, F., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.L.; Yaghi, O.M. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Yazaydın, A.O.; Snurr, R.Q.; Park, T.H.; Koh, K.; Liu, J.; LeVan, M.D.; Benin, A.I.; Jakubczak, P.; Lanuza, M.; Galloway, D.B.; et al. Screening of metal-organic frameworks for carbon dioxide capture from flue gas using a combined experimental and modeling approach. J. Am. Chem. Soc. 2009, 131, 18198–18199. [Google Scholar] [CrossRef] [PubMed]
- Herm, Z.R.; Swisher, J.A.; Smit, B.; Krishna, R.; Long, J.R. Metal-Organic frameworks as adsorbents for hydrogen purification and precombustion carbon dioxide capture. J. Am. Chem. Soc. 2011, 133, 5664–5667. [Google Scholar] [CrossRef] [PubMed]
- Dzubak, A.L.; Lin, L.C.; Kim, J.; Swisher, J.A.; Poloni, R.; Maximoff, S.N.; Smit, B.; Gagliardi, L. Ab initio carbon capture in open-site metal–organic frameworks. Nat. Chem. 2012, 4, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Qasem, N.A.; Ben-Mansour, R. Energy and productivity efficient vacuum pressure swing adsorption process to separate CO2 from CO2/N2 mixture using Mg-MOF-74: A CFD simulation. Appl. Energy 2018, 209, 190–202. [Google Scholar] [CrossRef]
- Bendt, S.; Dong, Y.; Keil, F.J. Diffusion of Water and Carbon Dioxide and Mixtures Thereof in Mg-MOF-74. J. Phys. Chem. C 2018, 123, 8212–8220. [Google Scholar] [CrossRef]
- Becker, T.M.; Heinen, J.; Dubbeldam, D.; Lin, L.C.; Vlugt, T.J. Polarizable force fields for CO2 and CH4 adsorption in M-MOF-74. J. Phys. Chem. C 2017, 121, 4659–4673. [Google Scholar] [CrossRef]
- Thomas, A.M.; Subramanian, Y. Bridging the gap between diffusivities from experiment and molecular dynamics: N-hexane and 2,2-dimethyl butane in zeolite BEA. Microporous Mesoporous Mater. 2019, 287, 124–134. [Google Scholar] [CrossRef]
- Thomas, A.M.; Subramanian, Y. Simulations on “Powder” Samples for Better Agreement with Macroscopic Measurements. J. Phys. Chem. C 2019, 123, 16172–16178. [Google Scholar] [CrossRef]
- Thomas, A.M.; Subramanian, Y. Diffusion processes in a poly-crystalline zeolitic material: A molecular dynamics study. J. Chem. Phys. 2018, 149, 064702. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Cole, D.R. Effects of inter-crystalline space on the adsorption of ethane and CO2 in silicalite: Implications for enhanced adsorption. Phys. Chem. Chem. Physics. 2020, 22, 13951–13957. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Liu, T.; Cole, D. Sorption, Structure and Dynamics of CO2 and Ethane in Silicalite at High Pressure: A Combined Monte Carlo and Molecular Dynamics Simulation Study. Molecules 2019, 24, 99. [Google Scholar] [CrossRef] [PubMed]
- Elola, M.D.; Rodriguez, J. Preferential Adsorption in Ethane/Carbon Dioxide Fluid Mixtures Confined within Silica Nanopores. J. Phys. Chem. C 2019, 123, 30937–30948. [Google Scholar] [CrossRef]
- Le, T.; Striolo, A.; Cole, D.R. CO2–C4H10 mixtures simulated in silica slit pores: Relation between structure and dynamics. J. Phys. Chem. C 2015, 119, 15274–15284. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mayadevi, S. Adsorption of methane, ethane, ethylene, and carbon dioxide on silicalite-I. Zeolites 1996, 17, 501–507. [Google Scholar] [CrossRef]
- García-Pérez, E.; Parra, J.B.; Ania, C.O.; García-Sánchez, A.; van Baten, J.M.; Krishna, R.; Dubbeldam, D.; Calero, S. A computational study of CO2, N2, and CH4 adsorption in zeolites. Adsorption 2007, 13, 469–476. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, B.; Wang, Z. Highly selective separation of CO2, CH4, and C2–C4 hydrocarbons in ultramicroporous semicycloaliphatic polyimides. ACS Appl. Mater. Interfaces 2018, 10, 26618–26627. [Google Scholar] [CrossRef]
- Duan, J.; Higuchi, M.; Horike, S.; Foo, M.L.; Rao, K.P.; Inubushi, Y.; Fukushima, T.; Kitagawa, S. High CO2/CH4 and C2 hydrocarbons/CH4 selectivity in a chemically robust porous coordination polymer. Adv. Funct. Mater. 2013, 23, 3525–3530. [Google Scholar] [CrossRef]
- Gautam, S.; Liu, T.; Rother, G.; Jalarvo, N.; Mamontov, E.; Welch, S.; Sheets, J.; Droege, M.; Cole, D.R. Dynamics of propane in nanoporous silica aerogel: A quasielastic neutron scattering study. J. Phys. Chem. C 2015, 119, 18188–18195. [Google Scholar] [CrossRef]
- Patankar, S.; Gautam, S.; Rother, G.; Podlesnyak, A.; Ehlers, G.; Liu, T.; Cole, D.R.; Tomasko, D.L. Role of confinement on adsorption and dynamics of ethane and an ethane–CO2 mixture in mesoporous CPG silica. J. Phys. Chem. C 2016, 120, 4843–4853. [Google Scholar] [CrossRef]
- Liu, T.; Gautam, S.; Cole, D.R.; Patankar, S.; Tomasko, D.; Zhou, W.; Rother, G. Structure and dynamics of ethane confined in silica nanopores in the presence of CO2. J. Chem. Phys. 2020, 152, 084707. [Google Scholar] [CrossRef] [PubMed]
- Chathoth, S.M.; He, L.; Mamontov, E.; Melnichenko, Y.B. Effect of Carbon Dioxide and Nitrogen on the Diffusivity of Methane Confined in Nano-Porous Carbon. Microporous Mesoporous Mater. 2012, 148, 101–106. [Google Scholar] [CrossRef]
- Purton, J.A.; Crabtree, J.C.; Parker, S.C. DL_Monte: A general purpose program for parallel Monte Carlo simulation. Mol. Simul. 2013, 39, 1240–1252. [Google Scholar] [CrossRef]
- Available online: https://github.com/iRASPA/RASPA2/tree/master/structures/mofs/cif (accessed on 28 September 2020).
- Dubbeldam, D.; Calero, S.; Ellis, D.E.; Snurr, R.Q. RASPA: Molecular simulation software for adsorption and diffusion in flexible nanoporous materials. Mol. Simul. 2016, 42, 81–101. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Greathouse, J.A.; Kinnibrugh, T.L.; Allendorf, M.D. Adsorption and separation of noble gases by IRMOF-1: Grand canonical Monte Carlo simulations. Ind. Eng. Chem. Res. 2009, 48, 3425–3431. [Google Scholar] [CrossRef]
- Potoff, J.J.; Siepmann, J.I. Vapor–liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen. Aiche J. 2001, 47, 1676–1682. [Google Scholar] [CrossRef]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A., III; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A generic force field for molecular simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Clarendon: Oxford, UK, 1987. [Google Scholar]
- Yang, J.; Zhao, Q.; Xu, H.; Li, L.; Dong, J.; Li, J. Adsorption of CO2, CH4, and N2 on Gas Diameter Grade Ion-exchange Small Pore Zeolites. J. Chem. Eng. Data 2012, 57, 3701–3709. [Google Scholar] [CrossRef]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.Y.; Guo, J.H.; Wang, P.; Liu, Y.; Guo, F.; Sun, W.Y. Controlled synthesis of micro/nanoscale Mg-MOF-74 materials and their adsorption property. Mater. Lett. 2018, 223, 174–177. [Google Scholar] [CrossRef]
- Campbell, J.; Tokay, B. Controlling the size and shape of Mg-MOF-74 crystals to optimise film synthesis on alumina substrates. Microporous Mesoporous Mater. 2017, 251, 190–199. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.Y.; Willman, J.; Zhou, H.C. Hierarchy in Metal–Organic Frameworks. ACS Cent. Sci. 2020, 6, 359–367. [Google Scholar] [CrossRef]
- Gosselin, E.J.; Rowland, C.A.; Bloch, E.D. Permanently Microporous Metal–Organic Polyhedra. Chem. Rev. 2020, 120, 8987–9014. [Google Scholar] [CrossRef]
- Lorzing, G.R.; Trump, B.A.; Brown, C.M.; Bloch, E.D. Selective gas adsorption in highly porous chromium (II)-based metal–organic polyhedra. Chem. Mater. 2017, 29, 8583–8587. [Google Scholar] [CrossRef]
- Williams, T.; Kelley, C.; Lang, R.; Kotz, D.; Campbell, J. Gnuplot 4.6: An Interactive Ploƫting Program. 2014. Available online: http://gnuplot.info (accessed on 16 September 2020).
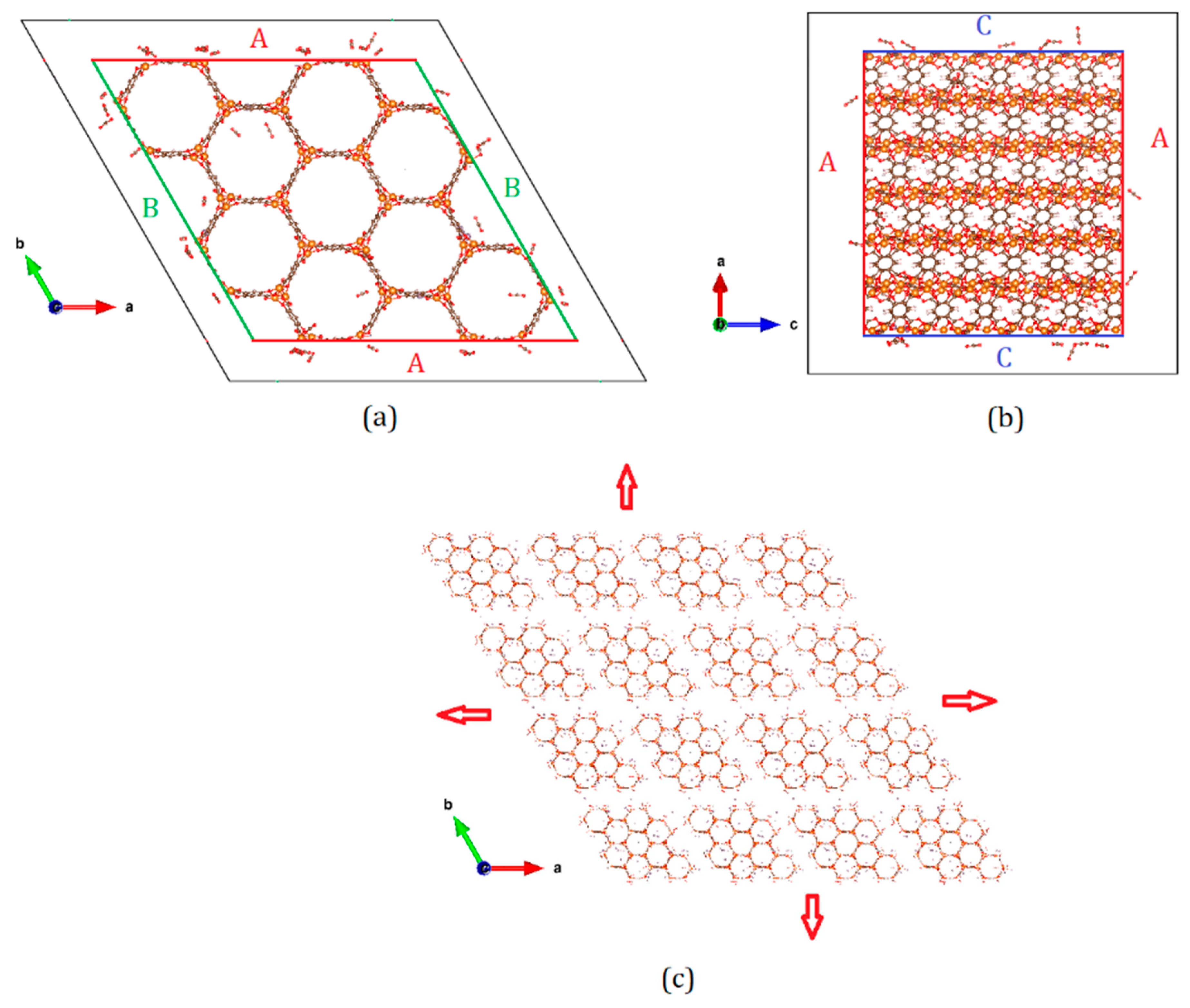
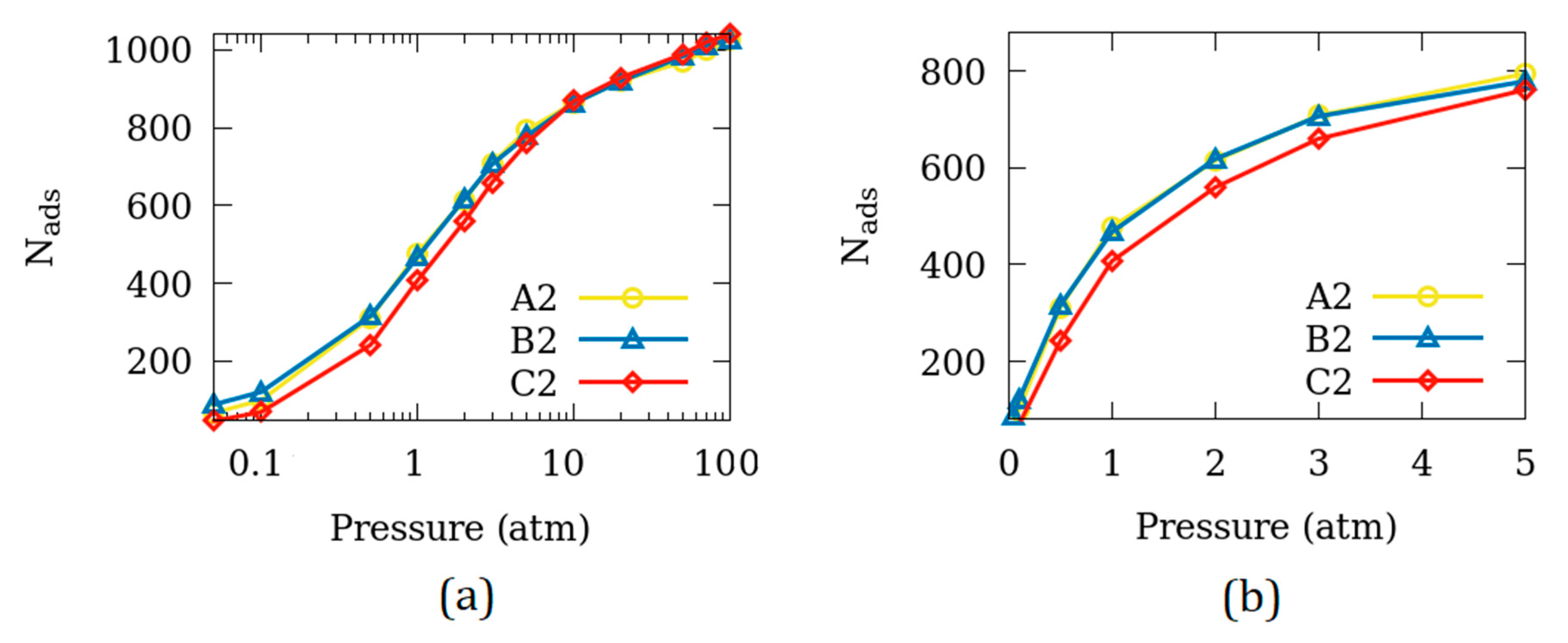
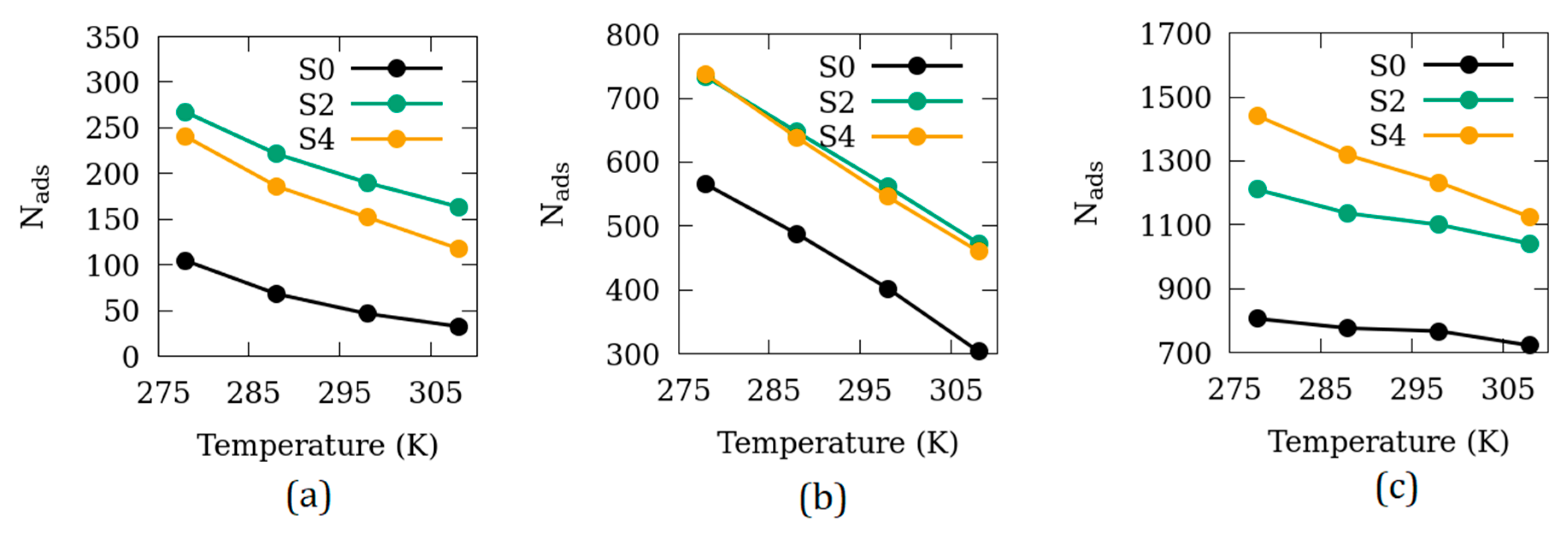
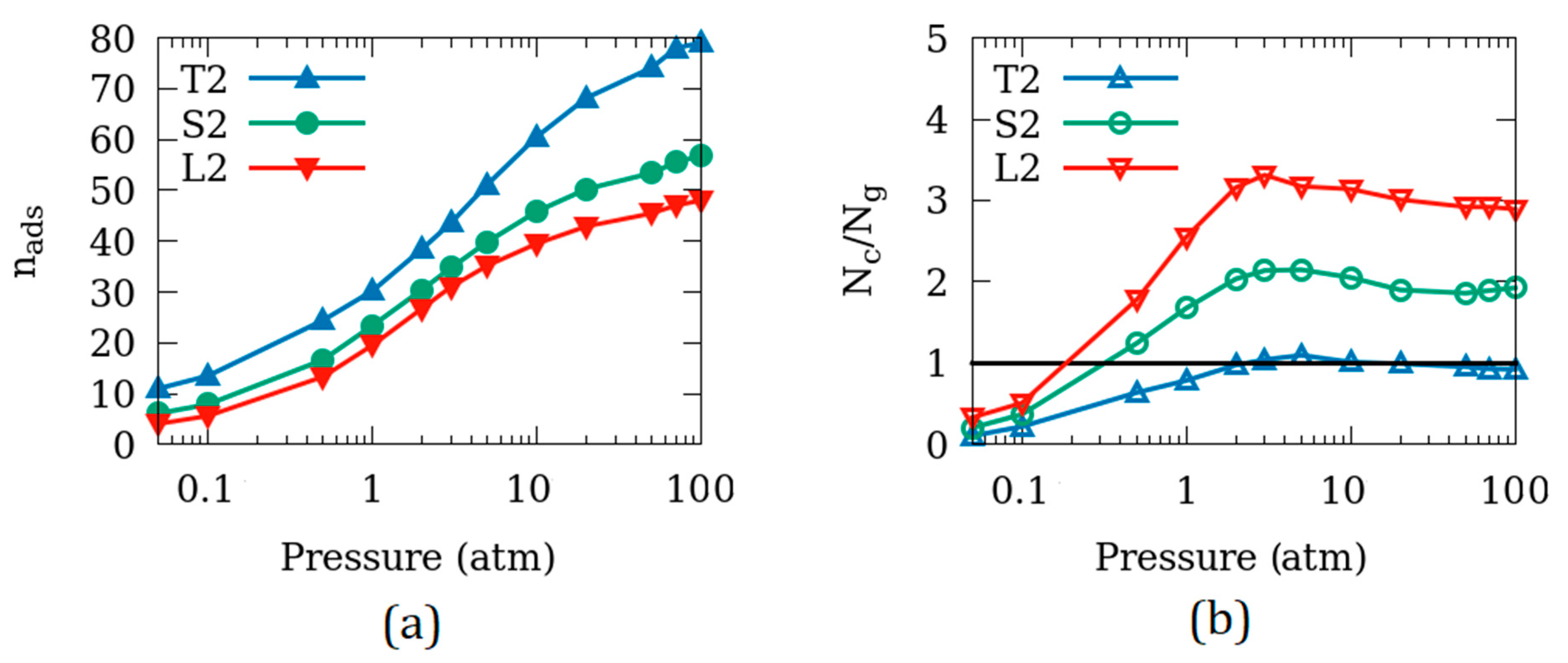
| Name of the Simulation Cell (System) | Number of Unit Cells in the Simulation Cell | Surfaces Exposed to Empty Space (Side Exposed*) | Size of the Empty Space (2× Space on Each Surface, in Å) |
|---|---|---|---|
| S0 | 2 × 2 × 6 | 0 | 0 |
| S1 | 2 × 2 × 6 | 6 | 3.0 |
| S2 | 2 × 2 × 6 | 6 | 5.0 |
| S3 | 2 × 2 × 6 | 6 | 10.0 |
| S4 | 2 × 2 × 6 | 6 | 15.0 |
| A2 | 2 × 2 × 6 | 2 (A*) | 5.0 |
| B2 | 2 × 2 × 6 | 2 (B*) | 5.0 |
| C2 | 2 × 2 × 6 | 2 (C*) | 5.0 |
| T2 | 1 × 1 × 4 | 6 | 5.0 |
| L2 | 3 × 3 × 9 | 6 | 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, S.; Cole, D. CO2 Adsorption in Metal-Organic Framework Mg-MOF-74: Effects of Inter-Crystalline Space. Nanomaterials 2020, 10, 2274. https://doi.org/10.3390/nano10112274
Gautam S, Cole D. CO2 Adsorption in Metal-Organic Framework Mg-MOF-74: Effects of Inter-Crystalline Space. Nanomaterials. 2020; 10(11):2274. https://doi.org/10.3390/nano10112274
Chicago/Turabian StyleGautam, Siddharth, and David Cole. 2020. "CO2 Adsorption in Metal-Organic Framework Mg-MOF-74: Effects of Inter-Crystalline Space" Nanomaterials 10, no. 11: 2274. https://doi.org/10.3390/nano10112274
APA StyleGautam, S., & Cole, D. (2020). CO2 Adsorption in Metal-Organic Framework Mg-MOF-74: Effects of Inter-Crystalline Space. Nanomaterials, 10(11), 2274. https://doi.org/10.3390/nano10112274







