Silicon-Based Ag Dendritic Nanoforests for Light-Assisted Bacterial Inhibition
Abstract
1. Introduction
2. Experimental Setup
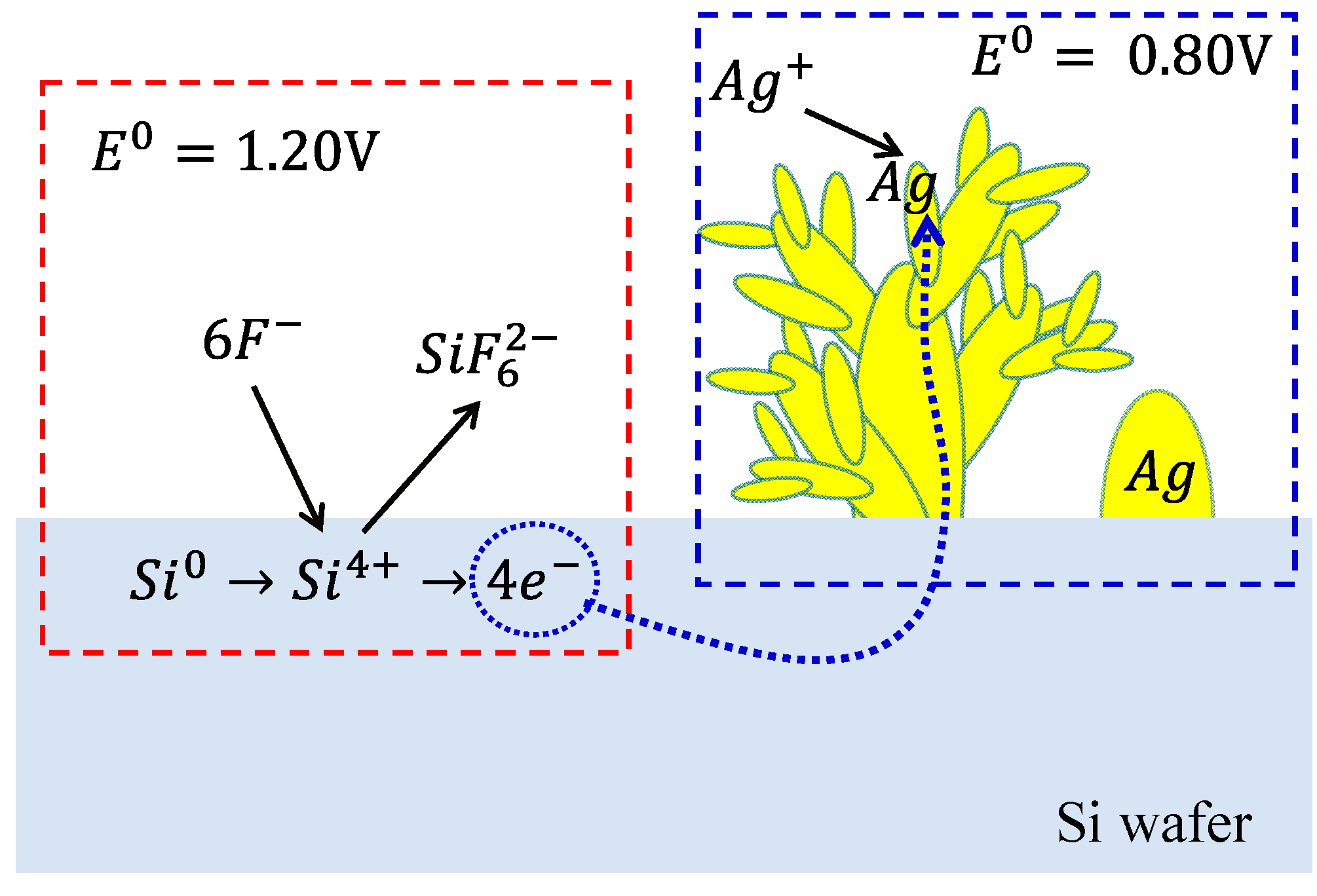
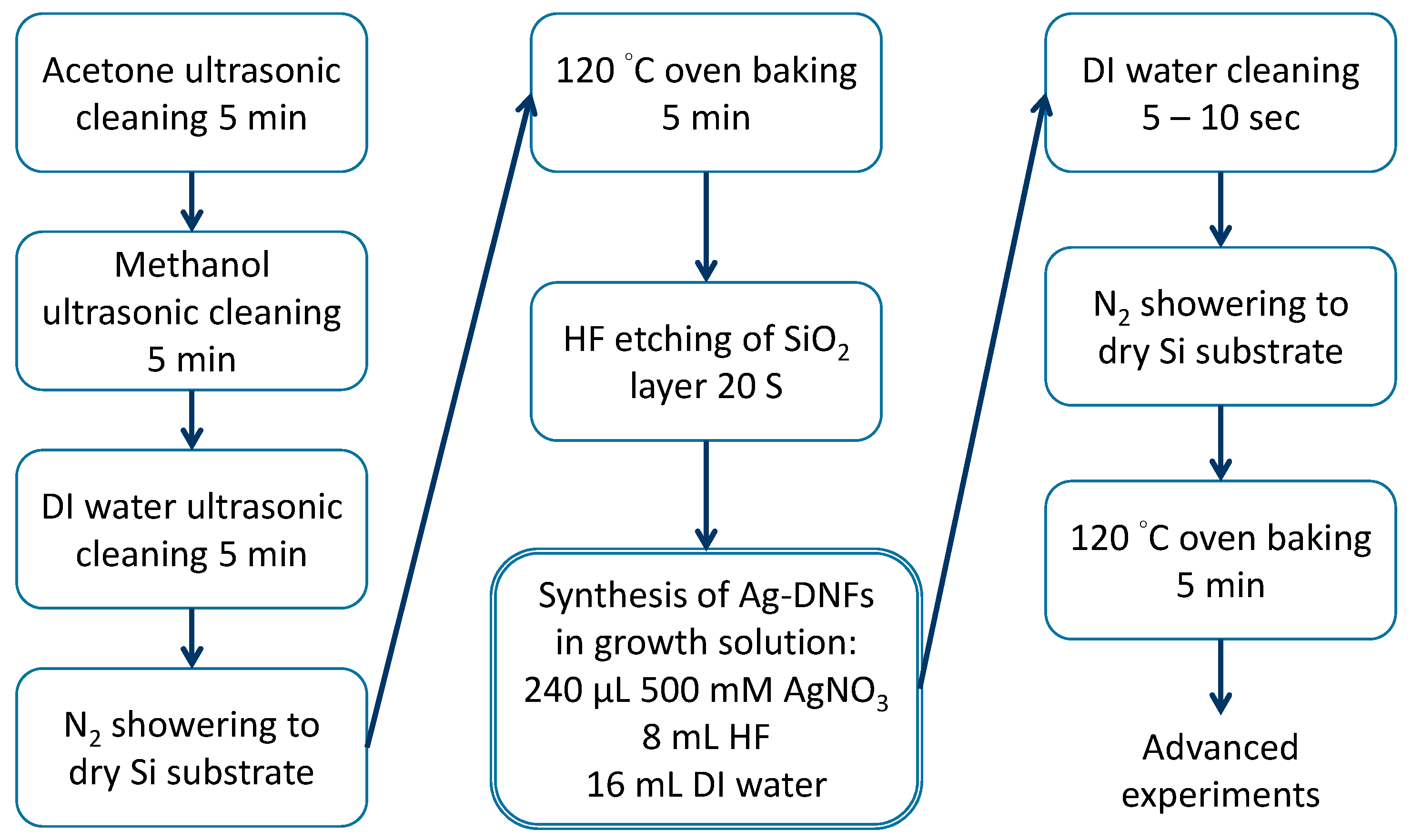
2.1. Preparation of Ag-DNFs/Si Substrate
2.2. Characterization
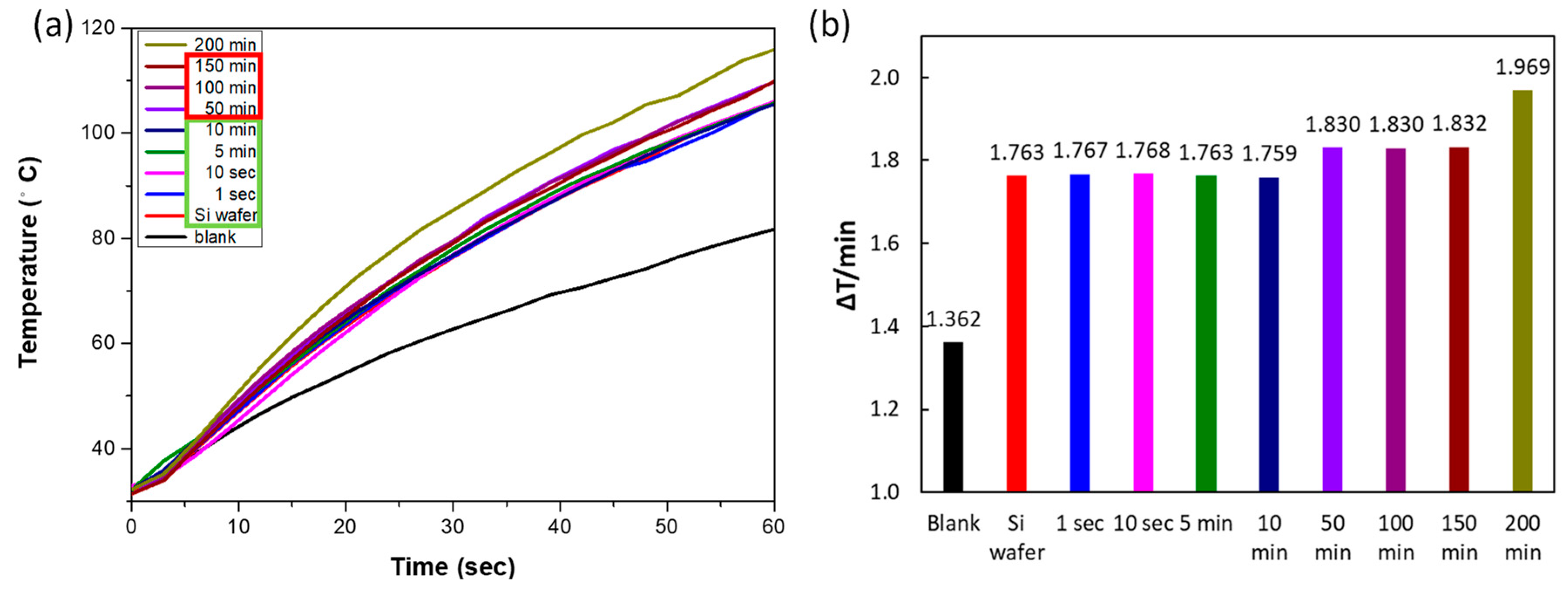
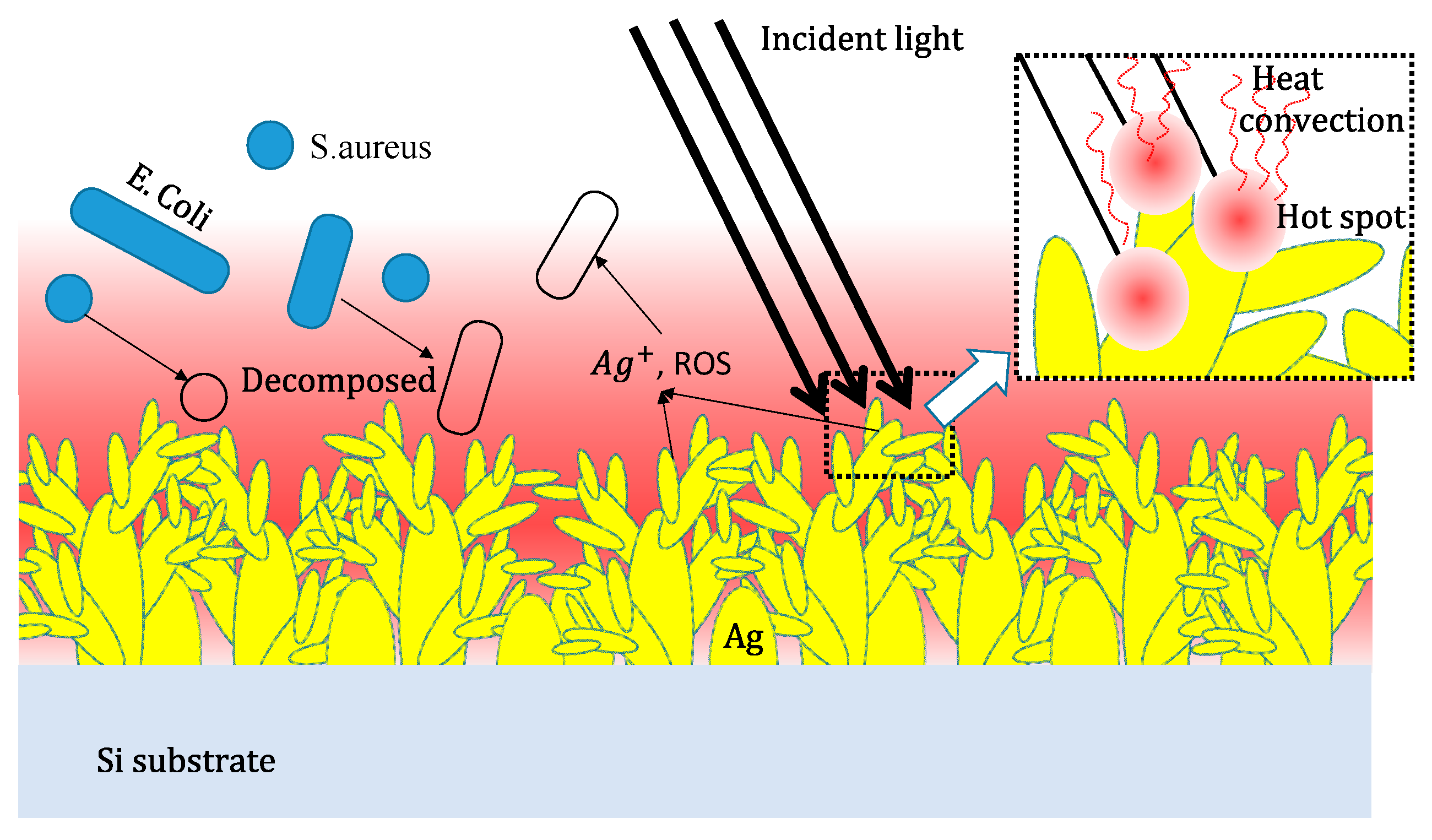
2.3. Light-to-Heat Energy Conversion and SERS Analysis
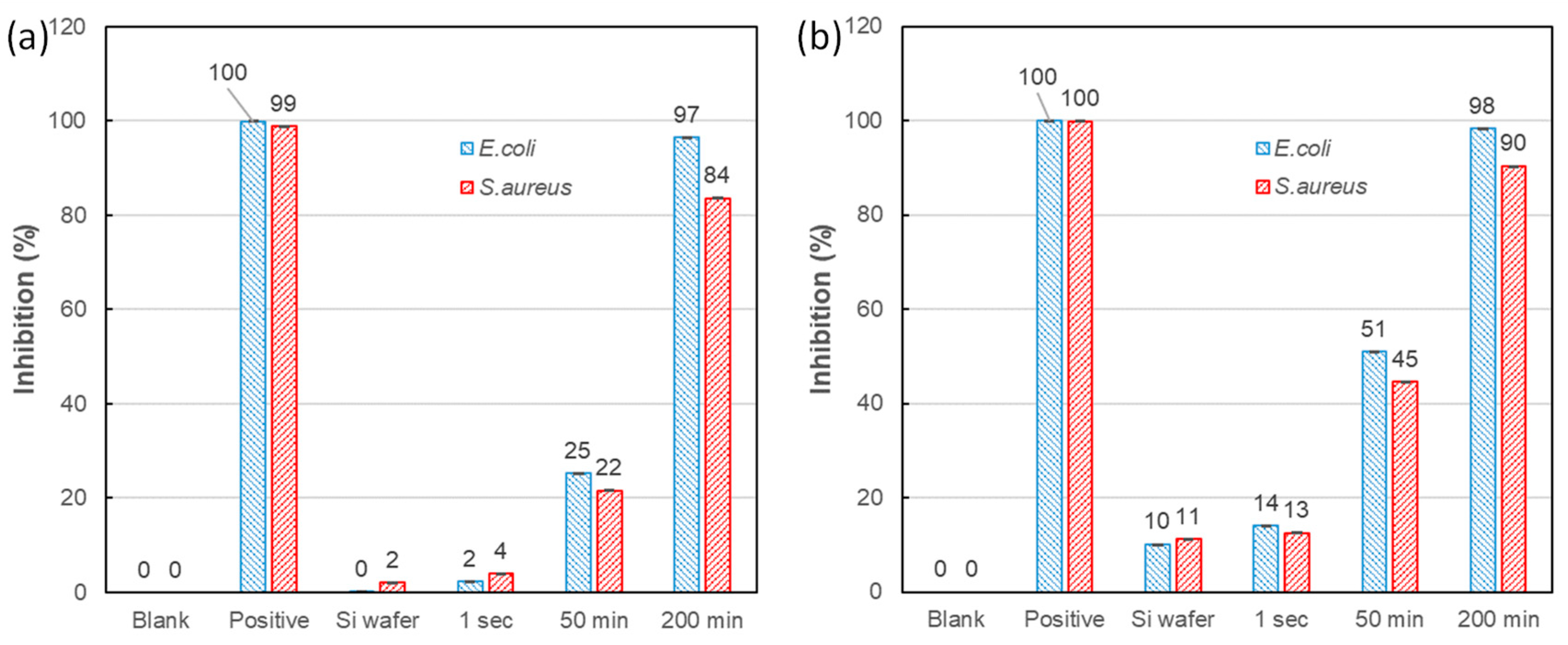
2.4. Antibacterial Performance
3. Results and Discussion
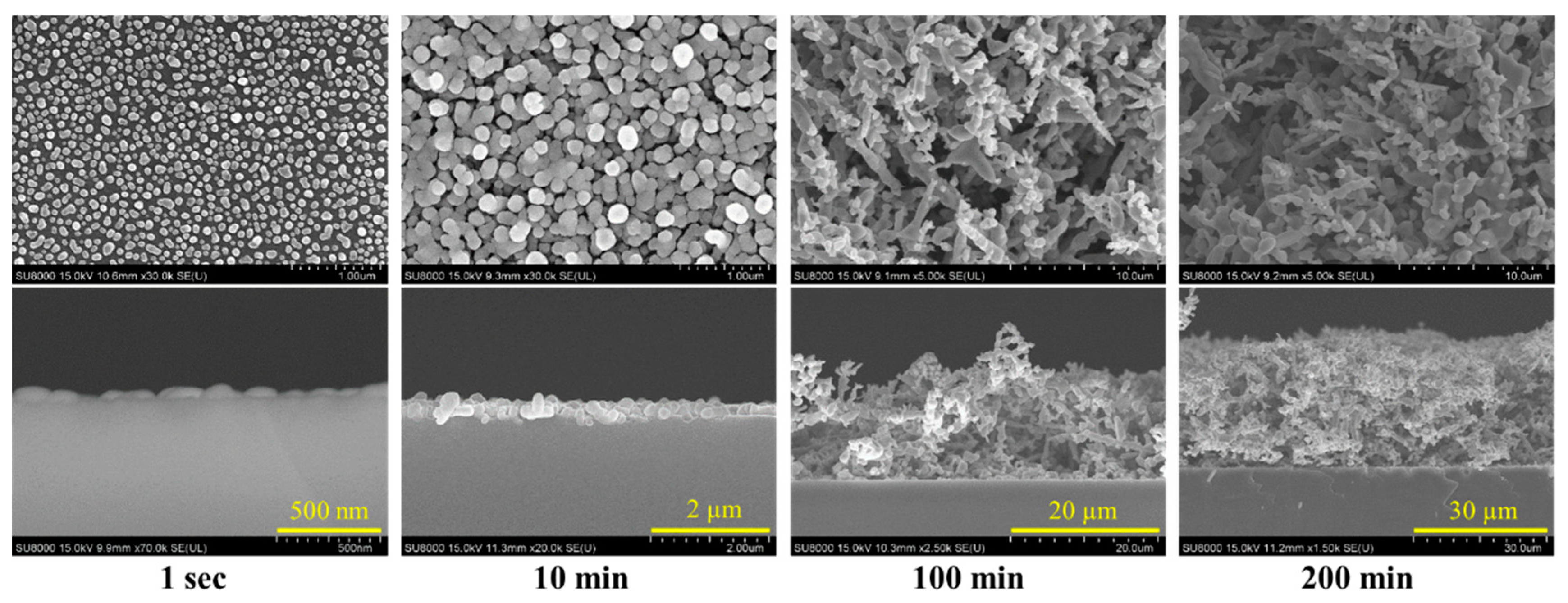
Morphology and Crystal Phase of Ag-DNFs/Si Substrate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duval, R.E.; Gouyau, J.; Lamouroux, E. Limitations of recent studies dealing with the antibacterial properties of silver nanoparticles: Fact and opinion. Nanomaterials 2019, 9, 1775. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.; Zbořil, R. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Kvítek, L.; Panáček, A.; Soukupová, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Kong, H.; Jang, J. Antibacterial properties of novel poly(methyl methacrylate) nanofiber containing silver nanoparticles. Langmuir 2008, 24, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Ghodake, G.; Kim, M.; Sung, J.-S.; Shinde, S.; Yang, J.; Hwang, K.; Kim, D.-Y. Extracellular synthesis and characterization of silver nanoparticles—Antibacterial activity against multidrug-resistant bacterial strains. Nanomaterials 2020, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Diniz, F.R.; Maia, R.C.A.P.; Rannier Andrade, L.; Andrade, L.N.; Vinicius Chaud, M.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; Pereira da Costa, L.; Shin, S.R.; et al. Silver nanoparticles-composing alginate/gelatine hydrogel improves wound healing in vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; OU-Yang, Y.-S.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Stoehr, L.C.; Gonzalez, E.; Stampfl, A.; Casals, E.; Duschl, A.; Puntes, V.; Oostingh, G.J. Shape matters: Effects of silver nanospheres and wires on human alveolar epithelial cells. Part Fibre Toxicol. 2011, 8, 36. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Zille, A.; Fernandes, M.M.; Francesko, A.; Tzanov, T.; Fernandes, M.; Oliveira, F.R.; Almeida, L.; Amorim, T.; Carneiro, N.; Esteves, M.F.; et al. Size and Aging effects on antimicrobial efficiency of silver nanoparticles coated on polyamide fabrics activated by amospheric DBD plasma. ACS Appl. Mater. Interfaces 2015, 7, 13731–13744. [Google Scholar] [CrossRef]
- Wen, X.; Xie, Y.-T.; Mak, W.C.; Cheung, K.Y.; Li, X.-Y.; Renneberg, R.; Yang, S. Dendritic nanostructures of silver: facile synthesis, structural characterizations, and sensing applications. Langmuir 2006, 22, 4836–4842. [Google Scholar] [CrossRef]
- Fang, J.; You, H.; Kong, P.; Yi, Y.; Song, X.; Ding, B. Dendritic silver nanostructure growth and evolution in replacement reaction. Cryst. Growth Des. 2007, 7, 864–867. [Google Scholar] [CrossRef]
- Alhmoud, H.; Delalat, B.; Ceto, X.; Elnathan, R.; Cavallaro, A.; Vasilev, K.; Voelcker, N.H. Antibacterial properties of silver dendrite decorated silicon nanowires. RSC Adv. 2016, 6, 65976–65987. [Google Scholar] [CrossRef]
- Vasil’kov, A.Y.; Dovnar, R.I.; Smotryn, S.M.; Iaskevich, N.N.; Naumkin, A.V. Plasmon resonance of silver nanoparticles as a method of increasing their antibacterial action. Antibiotics 2018, 7, 80. [Google Scholar] [CrossRef]
- Sarathi Kannan, D.; Mahboob, S.; Al-Ghanim, K.A.; Venkatachalam, P. Antibacterial, antibiofilm and photocatalytic activities of biogenic silver nanoparticles from Ludwigia octovalvis. J. Clust. Sci. 2020. [Google Scholar] [CrossRef]
- Podasca, V.E.; Buruiana, T.; Buruiana, E.C. Photocatalytic degradation of Rhodamine B dye by polymeric films containing ZnO, Ag nanoparticles and polypyrrole. J. Photochem. Photobiol. A 2019, 371, 188–195. [Google Scholar] [CrossRef]
- Guo, L.; Yin, H.; Xu, M.; Zheng, Z.; Fang, X.; Chong, R.; Zhou, Y.; Xu, L.; Xu, Q.; Li, J.; et al. In situ generated plasmonic silver nanoparticle-sensitized amorphous titanium dioxide for ultrasensitive photoelectrochemical sensing of formaldehyde. ACS Sens. 2019, 4, 2724–2729. [Google Scholar] [CrossRef] [PubMed]
- Ogundare, S.A.; van Zyl, W.E. Amplification of SERS “hot spots” by silica clustering in a silver-nanoparticle/nanocrystalline-cellulose sensor applied in malachite green detection. Colloid. Surf. A 2019, 570, 156–164. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Rodríguez-Argüelles, M.C.; Prado-López, S.; Lastra, M.; Grimaldi, M.; Cavazza, A.; Nasi, L.; Salviati, G.; Bigi, F. Macroalgae to nanoparticles: Study of Ulva lactuca L. role in biosynthesis of gold and silver nanoparticles and of their cytotoxicity on colon cancer cell lines. Mater. Sci. Eng. C 2019, 97, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Joseph, E.; Singhvi, G. Chapter 4—Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. In Nanomaterials for Drug Delivery and Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 91–116. ISBN 978-0-12-816505-8. [Google Scholar]
- Shiao, M.-H.; Lin, C.-T.; Zeng, J.-J.; Lin, Y.-S. Novel gold dendritic nanoforests combined with titanium nitride for visible-light-enhanced chemical degradation. Nanomaterials 2018, 8, 285. [Google Scholar] [CrossRef]
- Shiao, M.-H.; Lin, C.-T.; Huang, H.J.; Chen, P.-H.; Liao, B.-H.; Tseng, F.-G.; Lin, Y.-S. Novel gold dendritic nanoflowers deposited on titanium nitride for photoelectrochemical cells. J. Solid State Electrochem. 2018, 22, 3077–3084. [Google Scholar] [CrossRef]
- Shiao, M.-H.; Zeng, J.-J.; Huang, H.J.; Liao, B.-H.; Tang, Y.-H.; Lin, Y.-S. Growth of gold dendritic nanoforests on titanium nitride-coated silicon substrates. JoVE 2019, e59603. [Google Scholar] [CrossRef]
- Huang, H.J.; Chiang, Y.-C.; Hsu, C.-H.; Chen, J.-J.; Shiao, M.-H.; Yeh, C.-C.; Huang, S.-L.; Lin, Y.-S. Light energy conversion surface with gold dendritic nanoforests/Si chip for plasmonic polymerase chain reaction. Sensors 2020, 20, 1293. [Google Scholar] [CrossRef]
- Vimalraj, S.; Ashokkumar, T.; Saravanan, S. Biogenic gold nanoparticles synthesis mediated by Mangifera indica seed aqueous extracts exhibits antibacterial, anticancer and anti-angiogenic properties. Biomed. Pharmacother. 2018, 105, 440–448. [Google Scholar] [CrossRef]
- Chen, M.-N.; Chan, C.-F.; Huang, S.-L.; Lin, Y.-S. Green biosynthesis of gold nanoparticles using Chenopodium formosanum shell extract and analysis of the particles’ antibacterial properties. J. Sci. Food Agric. 2019, 99, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, X.; Tang, J.; Su, Y.; Peng, F.; Lu, Y.; Peng, R.; He, Y. A silicon-based antibacterial material featuring robust and high antibacterial activity. J. Mater. Chem. B 2014, 2, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.-R.S.; Tamer, T.M.; El-Meligy, M.A.; Mohy Eldin, M.S. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arab. J. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Carraro, C.; Maboudian, R.; Magagnin, L. Metallization and nanostructuring of semiconductor surfaces by Galvanic displacement processes. Surf. Sci. Rep. 2007, 62, 499–525. [Google Scholar] [CrossRef]
- Arzumanyan, G.; Doroshkevich, N.; Mamatkulov, K.; Shashkov, S.; Girel, K.; Bandarenka, H.; Borisenko, V. Phospholipid detection by surface-enhanced Raman scattering using silvered porous silicon substrates. Phys. Status Solidi A 2017, 214, 1600915. [Google Scholar] [CrossRef]
- Barman, B.; Dhasmana, H.; Verma, A.; Kumar, A.; Singh, D.; Jain, V. Fabrication of silver nanoparticles on glass substrate using low-temperature rapid thermal annealing. Energy Environ. 2018, 29, 358–371. [Google Scholar] [CrossRef]
- He, X.N.; Gao, Y.; Mahjouri-Samani, M.; Black, P.N.; Allen, J.; Mitchell, M.; Xiong, W.; Zhou, Y.S.; Jiang, L.; Lu, Y.F. Surface-enhanced Raman spectroscopy using gold-coated horizontally aligned carbon nanotubes. Nanotechnology 2012, 23, 205702. [Google Scholar] [CrossRef]
- Elbourne, A.; Coyle, V.E.; Truong, V.K.; Sabri, Y.M.; Kandjani, A.E.; Bhargava, S.K.; Ivanova, E.P.; Crawford, R.J. Multi-directional electrodeposited gold nanospikes for antibacterial surface applications. Nanoscale Adv. 2019, 1, 203–212. [Google Scholar] [CrossRef]
- Huang, H.J.; Wu, J.C.-S.; Chiang, H.-P.; Chou Chau, Y.-F.; Lin, Y.-S.; Wang, Y.H.; Chen, P.-J. Review of experimental setups for plasmonic photocatalytic reactions. Catalysts 2020, 10, 46. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S.; Prasad, S.; Gambhir, I.S. Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Dig. J. Nanomater. Biostruct. 2008, 3, 115–122. [Google Scholar]
- Sonseca, A.; Madani, S.; Rodríguez, G.; Hevilla, V.; Echeverría, C.; Fernández-García, M.; Muñoz-Bonilla, A.; Charef, N.; López, D. Multifunctional PLA blends containing chitosan mediated silver nanoparticles: Thermal, mechanical, antibacterial, and degradation properties. Nanomaterials 2020, 10, 22. [Google Scholar] [CrossRef]
- Huang, J.-R.; Cheng, Y.-C.; Huang, H.J.; Chiang, H.-P. Confocal mapping of myelin figures with micro-Raman spectroscopy. Appl. Phys. A 2017, 124, 58. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.J.; Chang, H.-W.; Lin, Y.-W.; Chuang, S.-Y.; Lin, Y.-S.; Shiao, M.-H. Silicon-Based Ag Dendritic Nanoforests for Light-Assisted Bacterial Inhibition. Nanomaterials 2020, 10, 2244. https://doi.org/10.3390/nano10112244
Huang HJ, Chang H-W, Lin Y-W, Chuang S-Y, Lin Y-S, Shiao M-H. Silicon-Based Ag Dendritic Nanoforests for Light-Assisted Bacterial Inhibition. Nanomaterials. 2020; 10(11):2244. https://doi.org/10.3390/nano10112244
Chicago/Turabian StyleHuang, Hung Ji, Han-Wei Chang, Yang-Wei Lin, Shao-Yi Chuang, Yung-Sheng Lin, and Ming-Hua Shiao. 2020. "Silicon-Based Ag Dendritic Nanoforests for Light-Assisted Bacterial Inhibition" Nanomaterials 10, no. 11: 2244. https://doi.org/10.3390/nano10112244
APA StyleHuang, H. J., Chang, H.-W., Lin, Y.-W., Chuang, S.-Y., Lin, Y.-S., & Shiao, M.-H. (2020). Silicon-Based Ag Dendritic Nanoforests for Light-Assisted Bacterial Inhibition. Nanomaterials, 10(11), 2244. https://doi.org/10.3390/nano10112244






