On the Formation of Black Silicon Features by Plasma-Less Etching of Silicon in Molecular Fluorine Gas
Abstract
1. Introduction
2. Materials and Methods
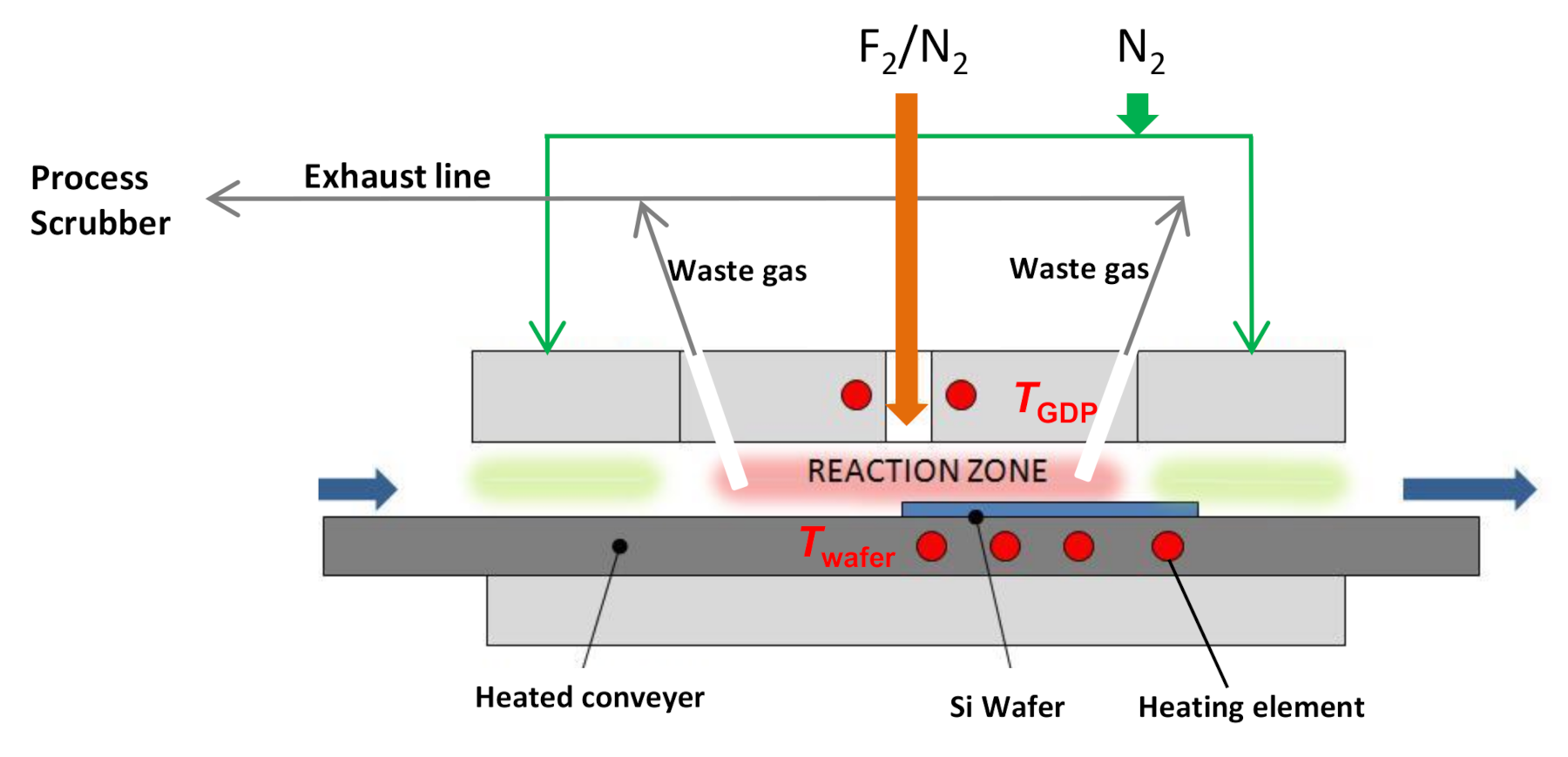
2.1. Experimental Tool
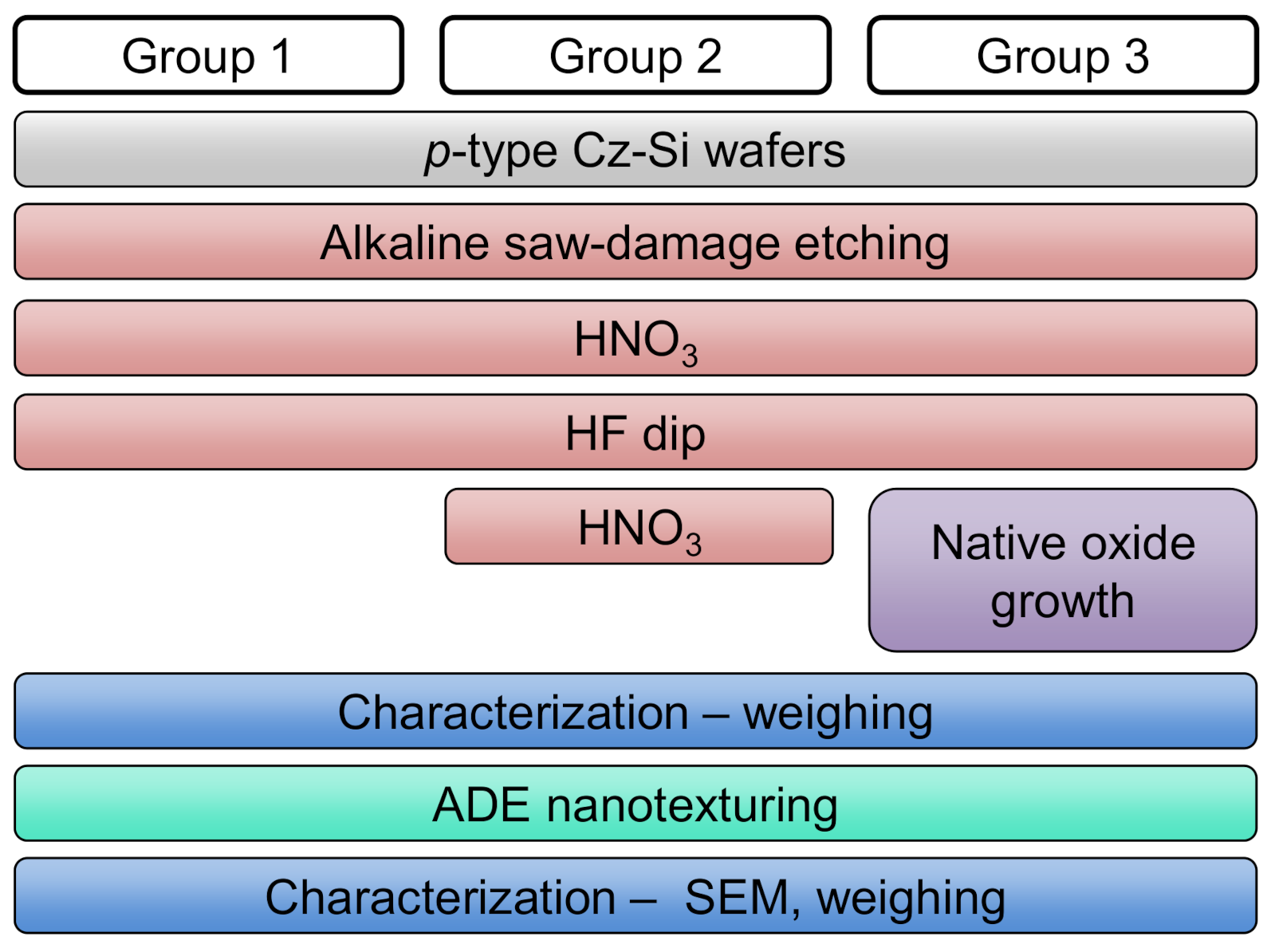
2.2. Design of Experiments
2.3. Estimating Activation Energy
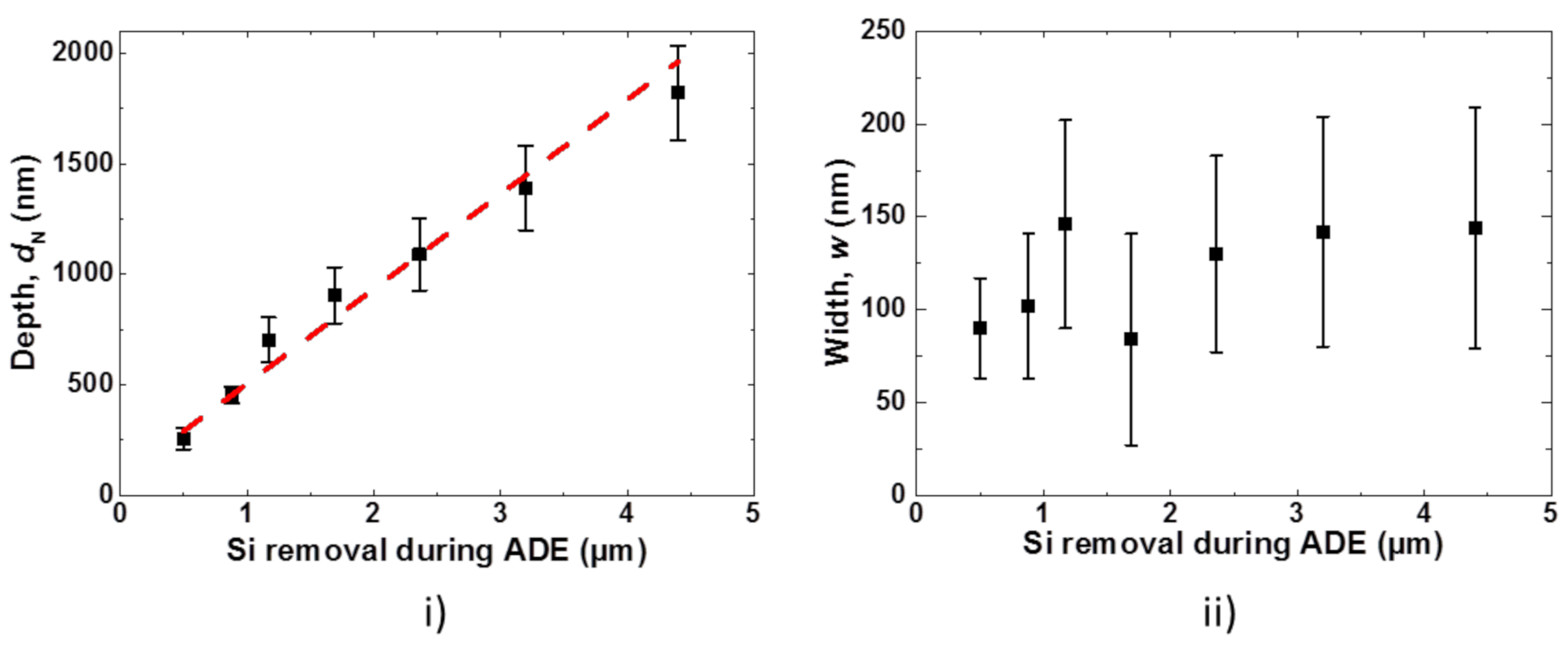
2.4. Characterization of Nanostructures
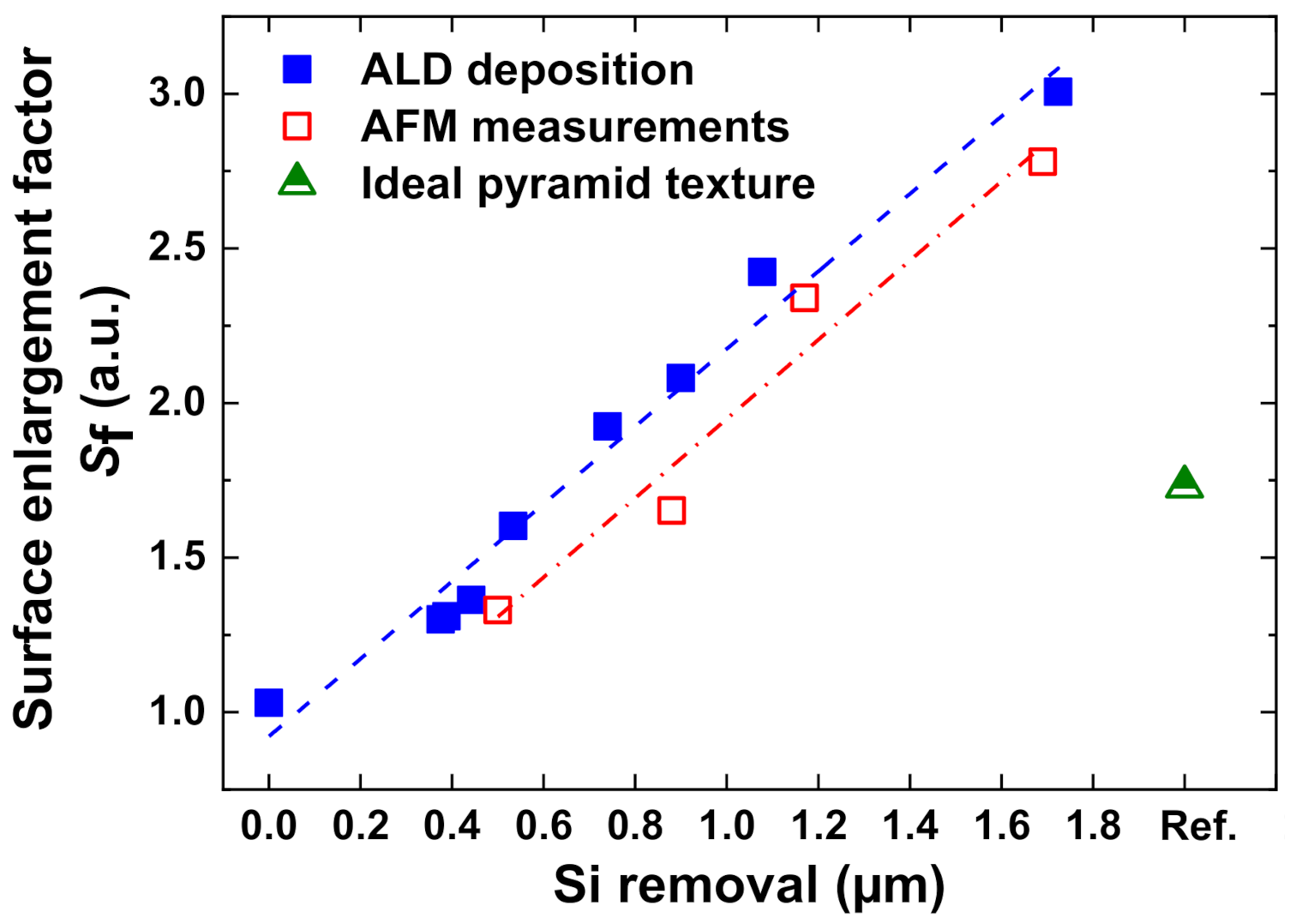
2.5. Estimation of Surface Enlargement Factor
3. Results
3.1. Statistical Variation of Process Parameters
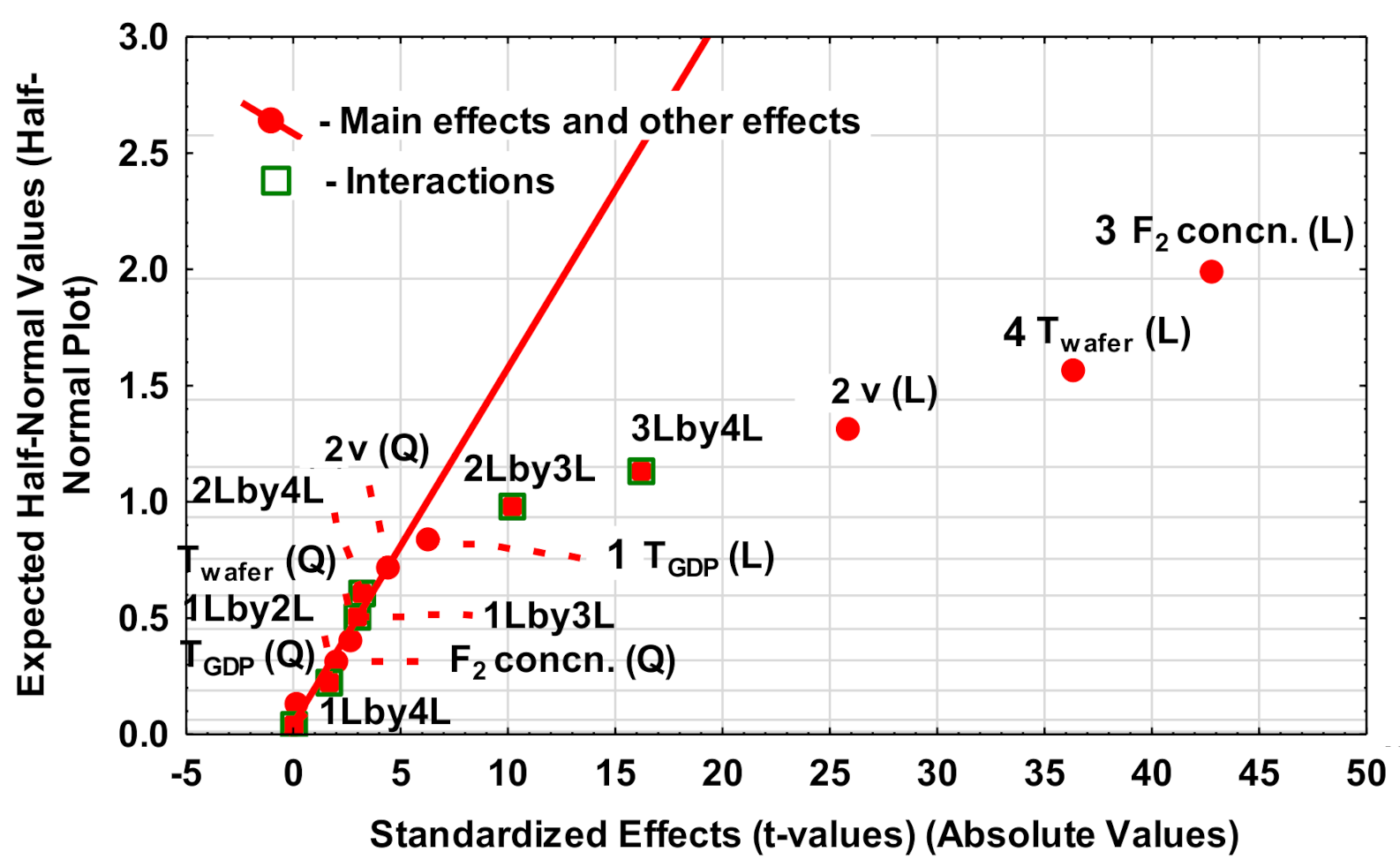
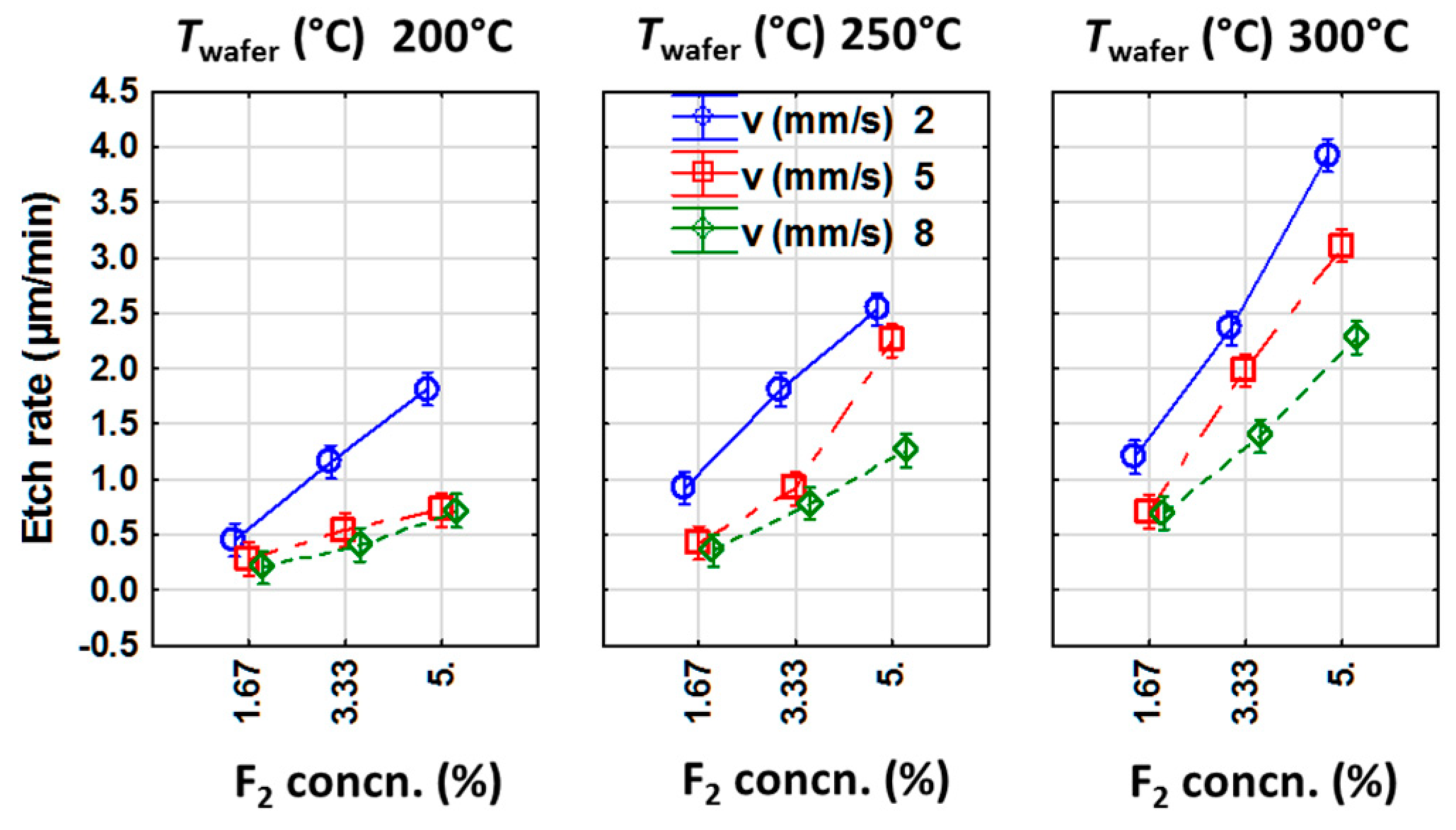
3.1.1. Analysis of Etch Rate as the Dependent Variable
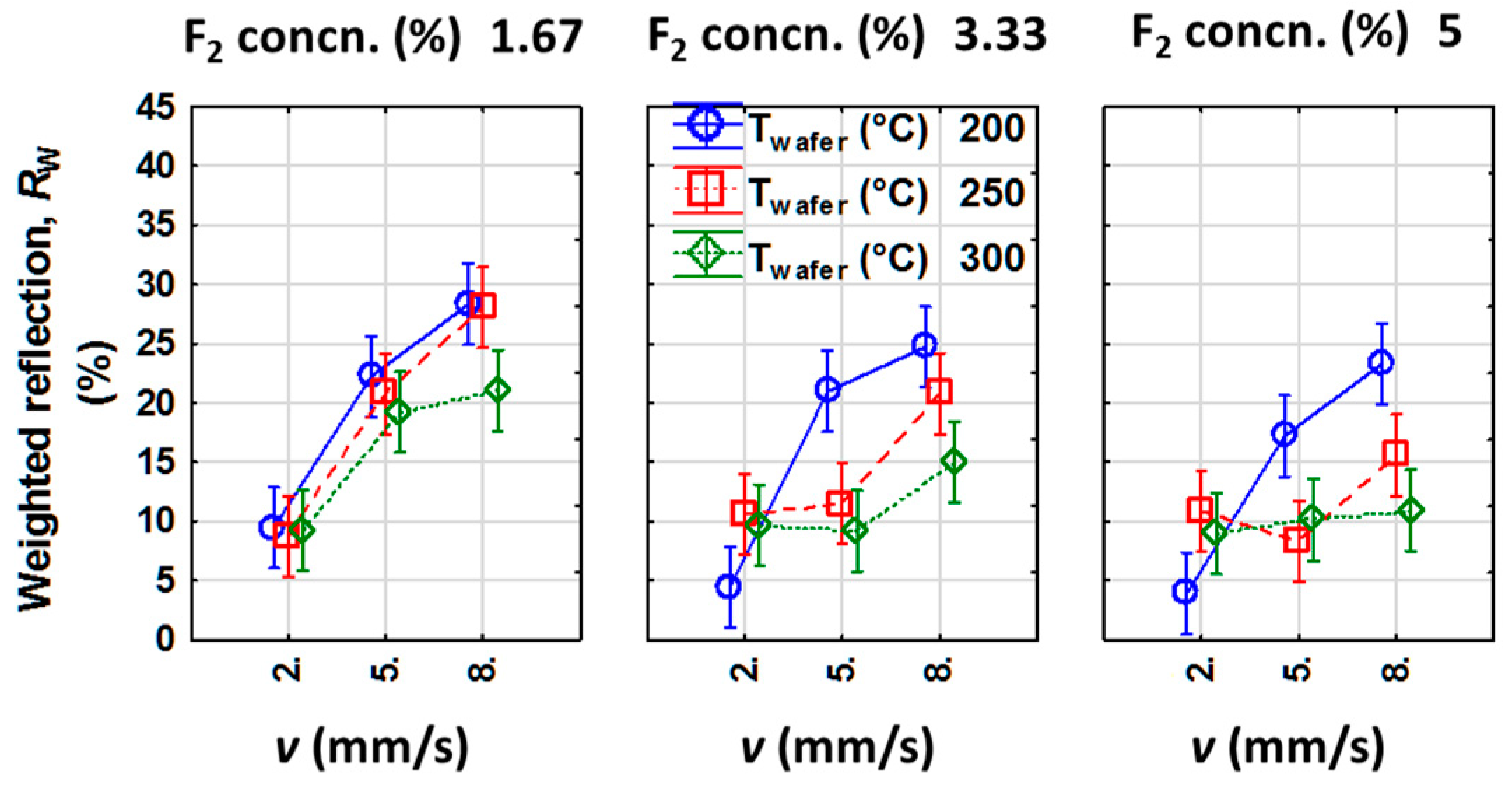
3.1.2. Analysis of Rw as the Dependent Variable
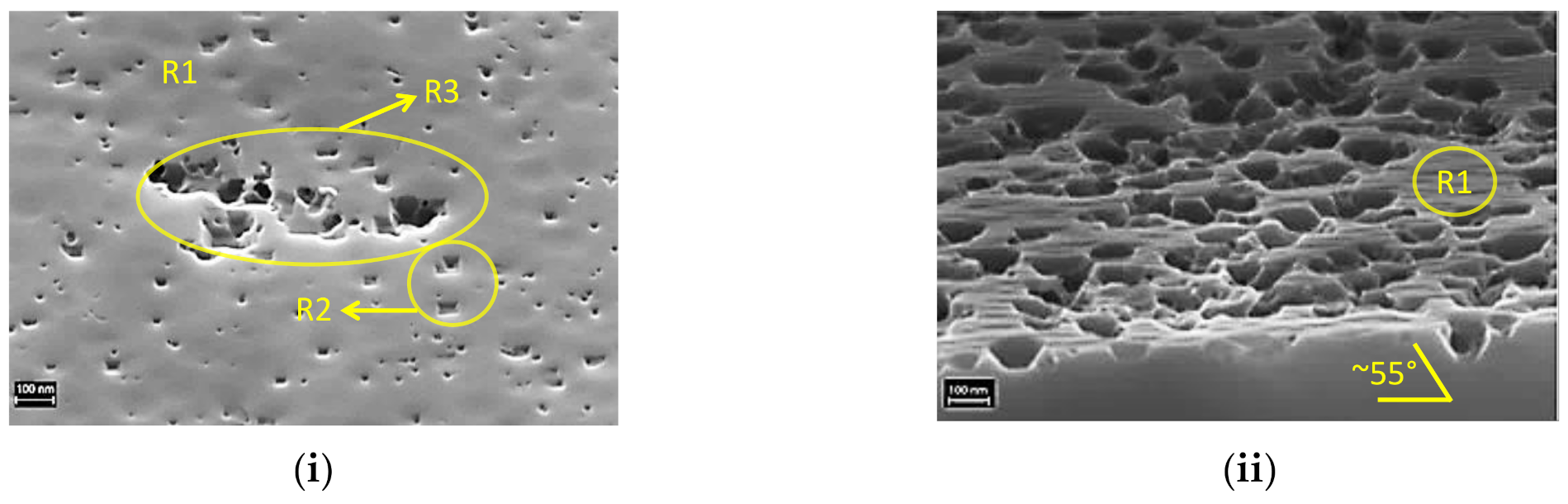
3.1.3. Change in Surface Morphology
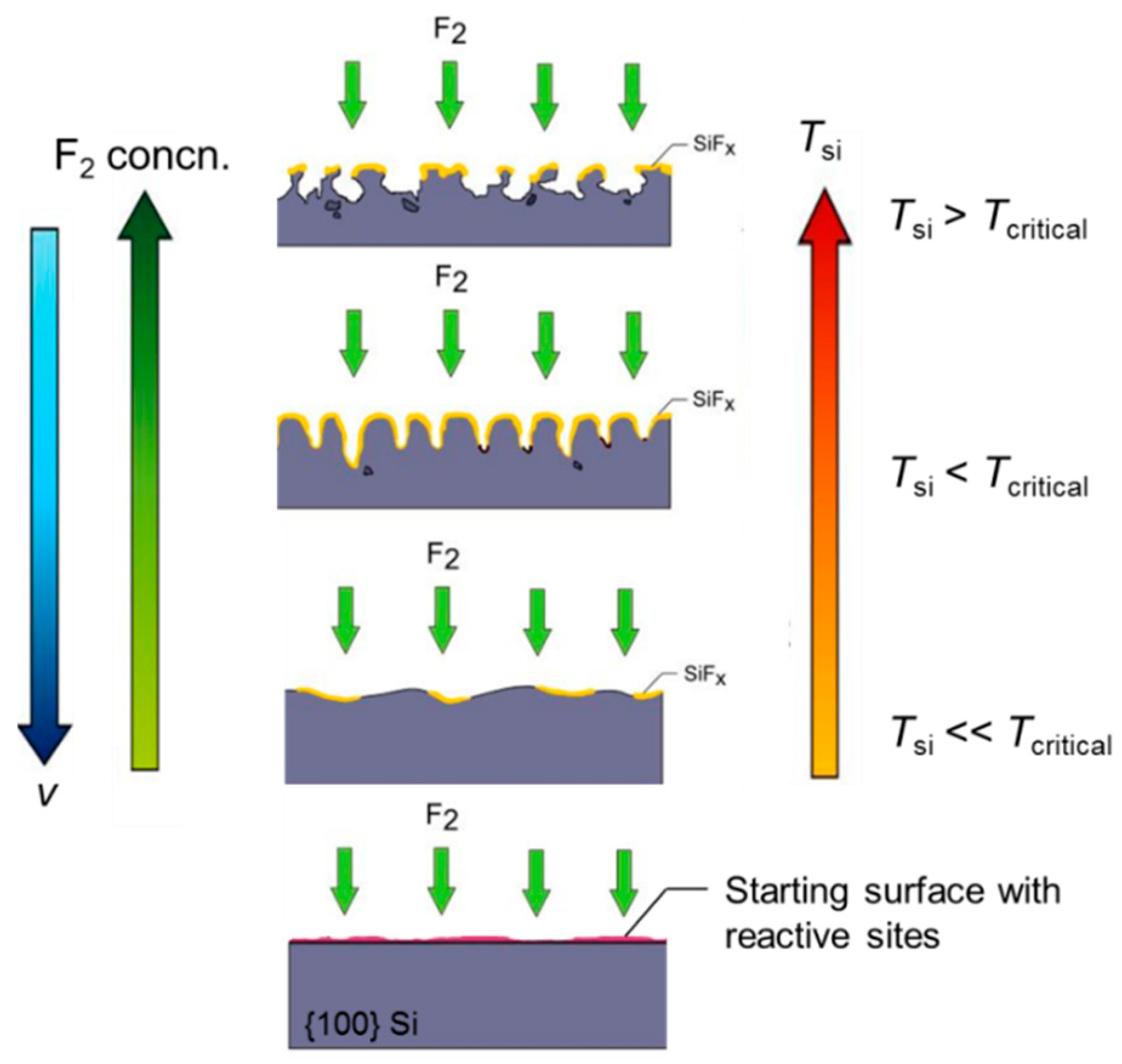
3.2. Initiation of F2-Si Etching
3.3. Influence of Surface Termination on Activation Energy
4. Discussion
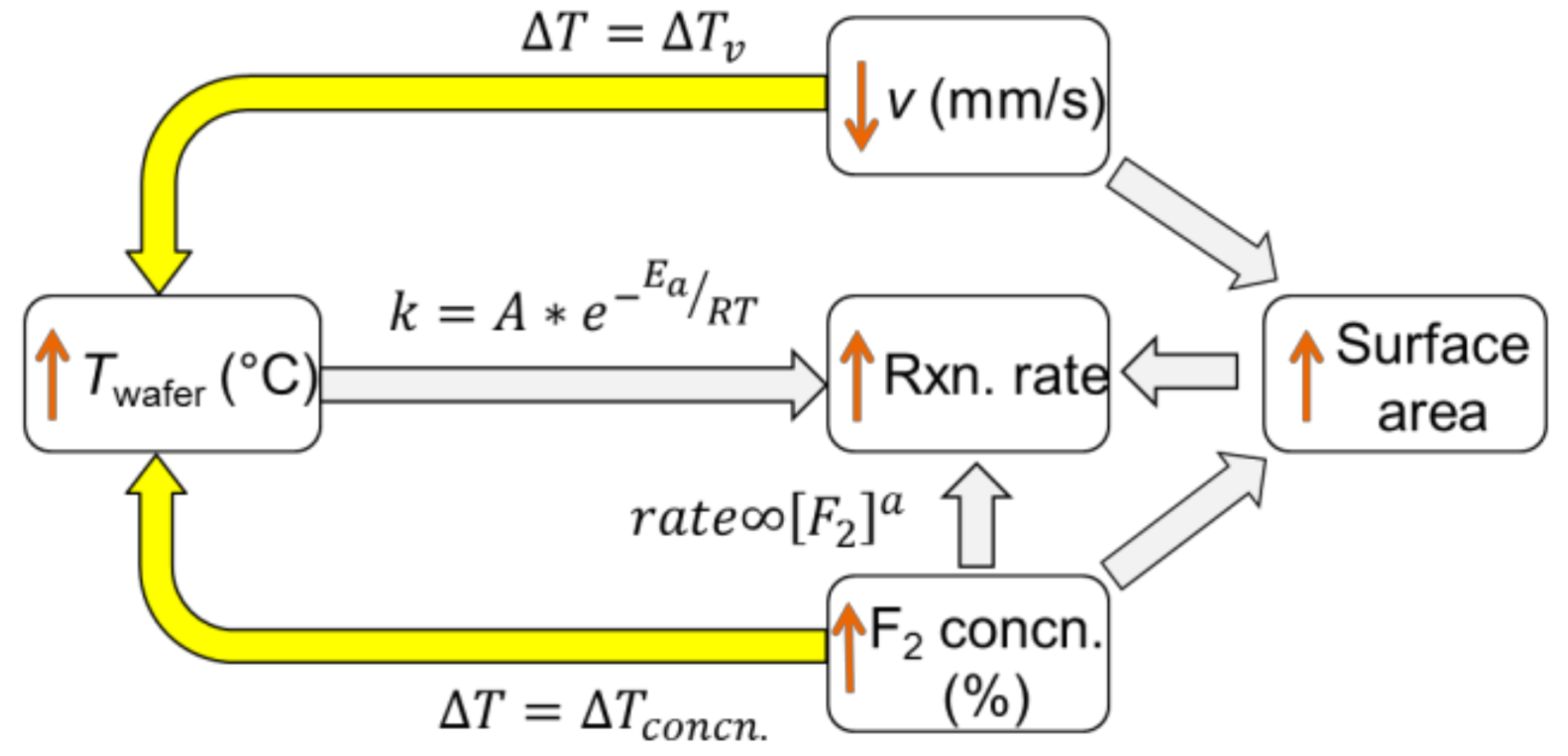
4.1. Etching Mechanism
4.2. Nanostructure Properties
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fukui, K.; Inomata, Y.; Shirasawa, K. Surface Texturing Using Reactive Ion Etching For Multicrystalline Silicon Solar Cells. In Proceedings of the 26th PVSC, Anaheim, CA, USA, 29 September–3 October 1997. [Google Scholar]
- Repo, P.; Haarahiltunen, A.; Sainiemi, L.; Yli-Koski, M.; Talvitie, H.; Schubert, M.C.; Savin, H. Effective Passivation of Black Silicon Surfaces by Atomic Layer Deposition. IEEE J. Photovolt. 2013, 3, 90–94. [Google Scholar] [CrossRef]
- Otto, M.; Kroll, M.; Käsebier, T.; Salzer, R.; Tünnermann, A.; Wehrspohn, R.B. Extremely low surface recombination velocities in black silicon passivated by atomic layer deposition. Appl. Phys. Lett. 2012, 100, 191603. [Google Scholar] [CrossRef]
- Jansen, H.; de Boer, M.; Legtenberg, R.; Elwenspoek, M. The black silicon method: A universal method for determining the parameter setting of a fluorine-based reactive ion etcher in deep silicon trench etching with profile control. J. Micromech. Microeng. 1995, 5, 115. [Google Scholar] [CrossRef]
- Pezoldt, J.; Kups, T.; Stubenrauch, M.; Fischer, M. Black luminescent silicon. Phys. Status Solidi 2011, 8, 1021–1026. [Google Scholar] [CrossRef]
- Arana, L.R.; Mas, N.d.; Schmidt, R.; Franz, A.J.; Schmidt, M.A.; Jensen, K.F. Isotropic etching of silicon in fluorine gas for MEMS micromachining. J. Micromech. Microeng. 2007, 17, 384–392. [Google Scholar] [CrossRef]
- Saito, Y. Plasmaless etching of silicon using chlorine trifluoride. J. Vac. Sci. Technol. B 1991, 9, 2503. [Google Scholar] [CrossRef]
- Winters, H.F.; Coburn, J.W. The etching of silicon with XeF2 vapor. Appl. Phys. Lett. 1979, 34, 70. [Google Scholar] [CrossRef]
- Ibbotson, D.E.; Mucha, J.A.; Flamm, D.L.; Cook, J.M. Plasmaless dry etching of silicon with fluorine-containing compounds. J. Appl. Phys. 1984, 56, 2939. [Google Scholar] [CrossRef]
- Kafle, B.; Seiffe, J.; Hofmann, M.; Clochard, L.; Duffy, E.; Rentsch, J. Nanostructuring of c-Si surface by F2 -based atmospheric pressure dry texturing process. Phys. Status Solidi A 2015, 212, 307–311. [Google Scholar] [CrossRef]
- Zhao, J.; Green, M.A. Optimized antireflection coatings for high-efficiency silicon solar cells. IEEE Trans. Electron Devices 1991, 38, 1925–1934. [Google Scholar] [CrossRef]
- Hibbs, A.R. Imaging Software. In Image Processing with ImageJ; Abramoff, M.D., Magalhaes, P.J., Ram, S.J., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2004; pp. 163–176. ISBN 978-1-4757-0983-4. [Google Scholar]
- Ralf Steudel. Chemistry of the Non-Metals. Translation of Chemie der Nichtmetalle; Walter de Gruyter & Co.: Berlin, Germany, 1976. [Google Scholar]
- Wicke, E.; Franck, E.U. Physikalisch-chemische Eigenschaften des Fluors. Angew. Chem. 1954, 66. [Google Scholar] [CrossRef]
- Branz, H.M.; Yost, V.E.; Ward, S.; Jones, K.M.; To, B.; Stradins, P. Nanostructured black silicon and the optical reflectance of graded-density surfaces. Appl. Phys. Lett. 2009, 94, 231121. [Google Scholar] [CrossRef]
- Ohmi, T.; Isagawa, T.; Kogure, M.; Imaoka, T. Native Oxide Growth and Organic Impurity Removal on Si Surface with Ozone-Injected Ultrapure Water. J. Electrochem. Soc. 1993, 140, 804–810. [Google Scholar] [CrossRef]
- Aldao, C.M.; Weaver, J.H. Halogen etching of Si via atomic-scale processes. Prog. Surf. Sci. 2001, 68, 189–230. [Google Scholar] [CrossRef][Green Version]
- Huff, H. Into the Nano Era: Moore’s Law Beyond Planar Silicon CMOS; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 9783540745594. [Google Scholar]
- Deal, B.E.; Helms, C.R. (Eds.) The Physics and Chemistry of SiO2 and the Si-SiO2 Interface; Springer: New York, NY, USA, 1988. [Google Scholar]
- Mucha, J.A.; Donnelly, V.M.; Flamm, D.L.; Webb, L.M. Chemiluminescence and the Reaction of Molecular Fluorine with Silicon. J. Phys. Chem. 1981, 85, 3529–3532. [Google Scholar] [CrossRef]
- Chen, M.; Minkiewicz, V.J.; Lee, K. Etching Silicon with Fluorine Gas. J. Electrochem. Soc. 1979, 126, 1946. [Google Scholar] [CrossRef]
- Flamm, D.L. The reaction of fluorine atoms with silicon. J. Appl. Phys. 1981, 52, 3633. [Google Scholar] [CrossRef]
- Winters, H.F.; Coburn, J.W. Surface science aspects of etching reactions. Surf. Sci. Rep. 1992, 14, 162–269. [Google Scholar] [CrossRef]
- Nakayama, K.S.; Weaver, J.H. Si (100) − (2 × 1) Etching with Fluorine: Planar Removal versus Three Dimensional Pitting. Phys. Rev. Lett. 1999, 83, 3210–3213. [Google Scholar] [CrossRef]





| Process Parameters | Units | Levels | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| TGDP | °C | 200 | 245 | 290 |
| Twafer | °C | 200 | 250 | 300 |
| σF | % | 1.67 | 3.33 | 5 |
| v | mm/s | 2 | 5 | 8 |
| Etching Species | Starting Surface | (kCal/mol) | Reactor Pressure | Reference |
|---|---|---|---|---|
| F2 | Si (100) after HF dip | 12.9 ± 0.1 | Atmospheric | This work |
| F2 | Si (100) with native oxide | 13.7 | Atmospheric | This work |
| F2 | Si (100) with HNO3 oxide | 23.0 | Atmospheric | This work |
| F2 | Si (100), Si(110), Si (111) | 8.0 | Vacuum | [16] |
| F2 | Si (100) | 9.3 ± 1.8 | Vacuum | [17] |
| F | Si (100) | 2.5 ± 0.1 | Vacuum | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kafle, B.; Ridoy, A.I.; Miethig, E.; Clochard, L.; Duffy, E.; Hofmann, M.; Rentsch, J. On the Formation of Black Silicon Features by Plasma-Less Etching of Silicon in Molecular Fluorine Gas. Nanomaterials 2020, 10, 2214. https://doi.org/10.3390/nano10112214
Kafle B, Ridoy AI, Miethig E, Clochard L, Duffy E, Hofmann M, Rentsch J. On the Formation of Black Silicon Features by Plasma-Less Etching of Silicon in Molecular Fluorine Gas. Nanomaterials. 2020; 10(11):2214. https://doi.org/10.3390/nano10112214
Chicago/Turabian StyleKafle, Bishal, Ahmed Ismail Ridoy, Eleni Miethig, Laurent Clochard, Edward Duffy, Marc Hofmann, and Jochen Rentsch. 2020. "On the Formation of Black Silicon Features by Plasma-Less Etching of Silicon in Molecular Fluorine Gas" Nanomaterials 10, no. 11: 2214. https://doi.org/10.3390/nano10112214
APA StyleKafle, B., Ridoy, A. I., Miethig, E., Clochard, L., Duffy, E., Hofmann, M., & Rentsch, J. (2020). On the Formation of Black Silicon Features by Plasma-Less Etching of Silicon in Molecular Fluorine Gas. Nanomaterials, 10(11), 2214. https://doi.org/10.3390/nano10112214






