Characterization of Chemically Activated Pyrolytic Carbon Black Derived from Waste Tires as a Candidate for Nanomaterial Precursor
Abstract
1. Introduction
2. Materials and Methods
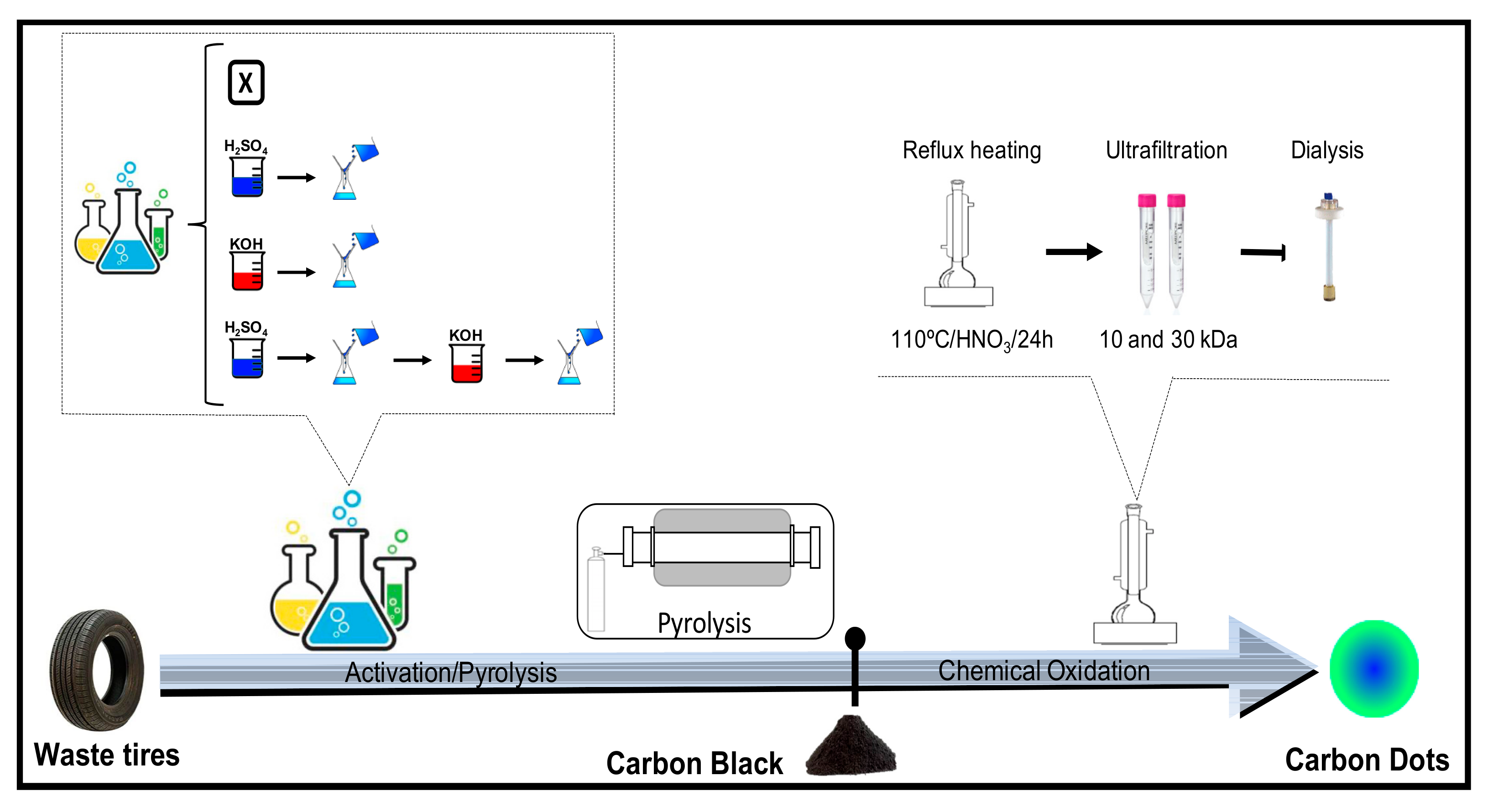
2.1. Carbon Black and Carbon Dots Synthesis
2.2. Waste Tire Powder Characterization
2.3. Pyrolytic Carbon Black Characterization and Preliminary Analysis of Carbon Dots
3. Results and Discussion
3.1. Waste Tire Powder Characterization
3.2. Pyrolytic Carbon Black Characterization
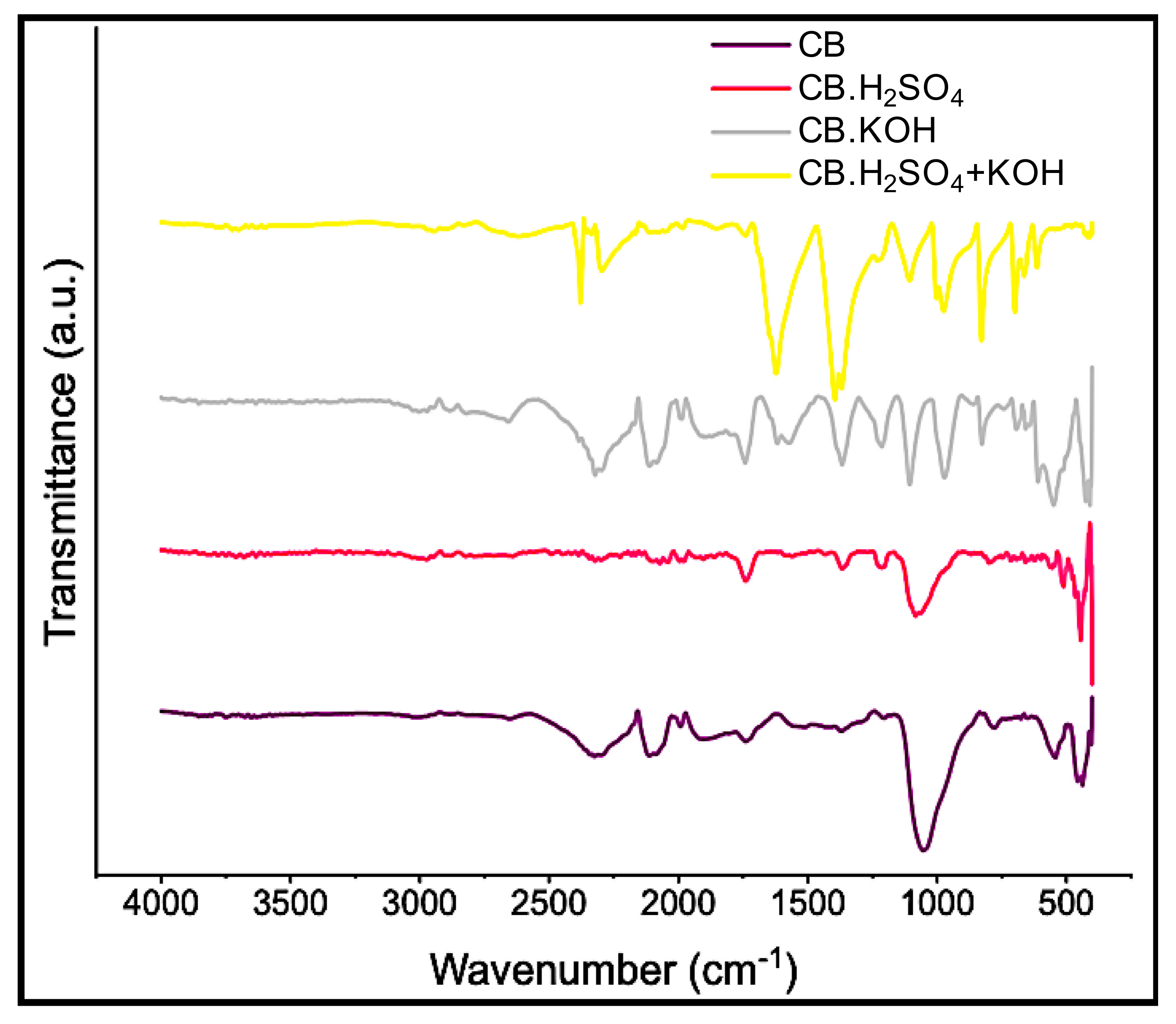
3.2.1. Chemical Composition
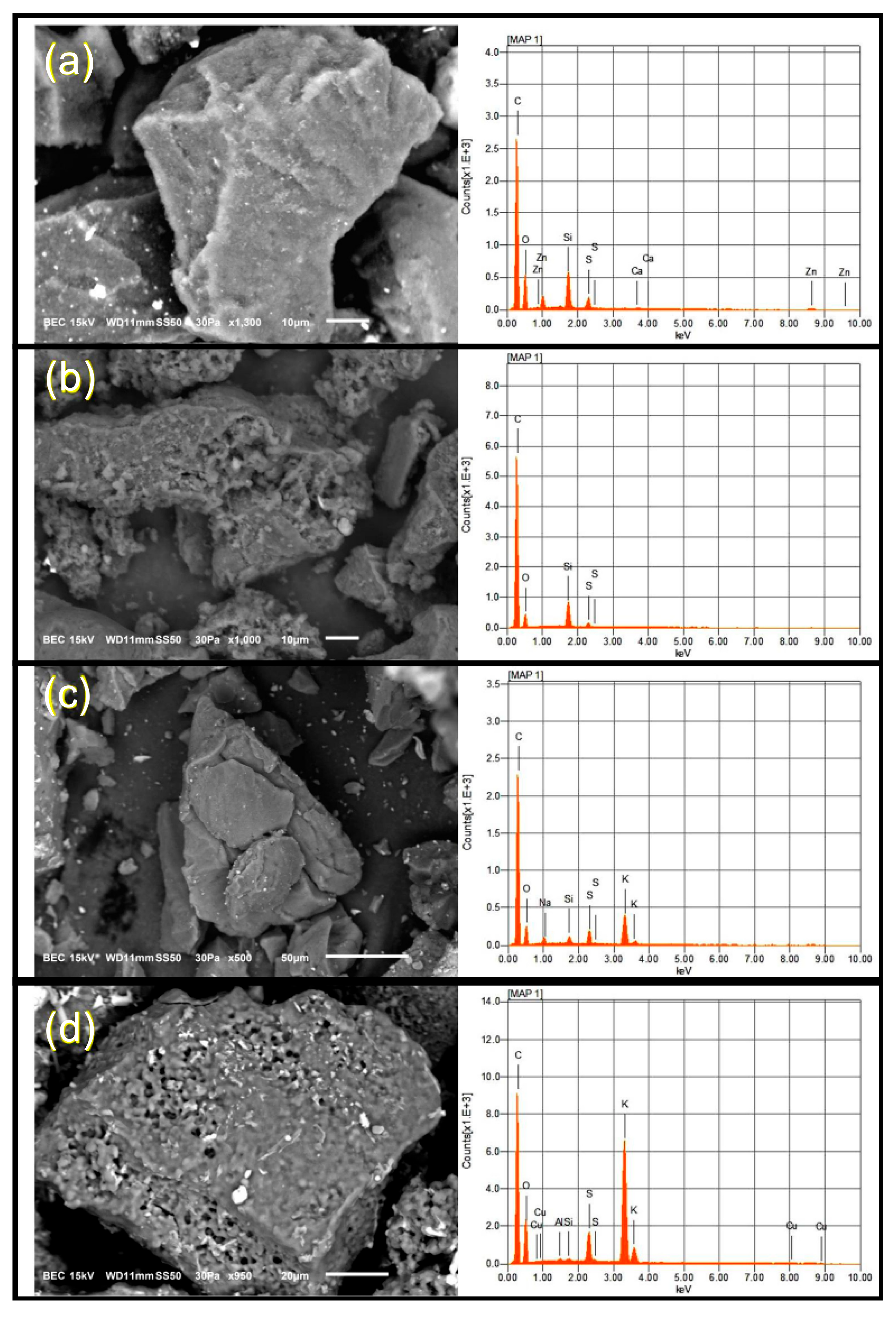
3.2.2. Surface Morphology
3.2.3. Structural Analysis
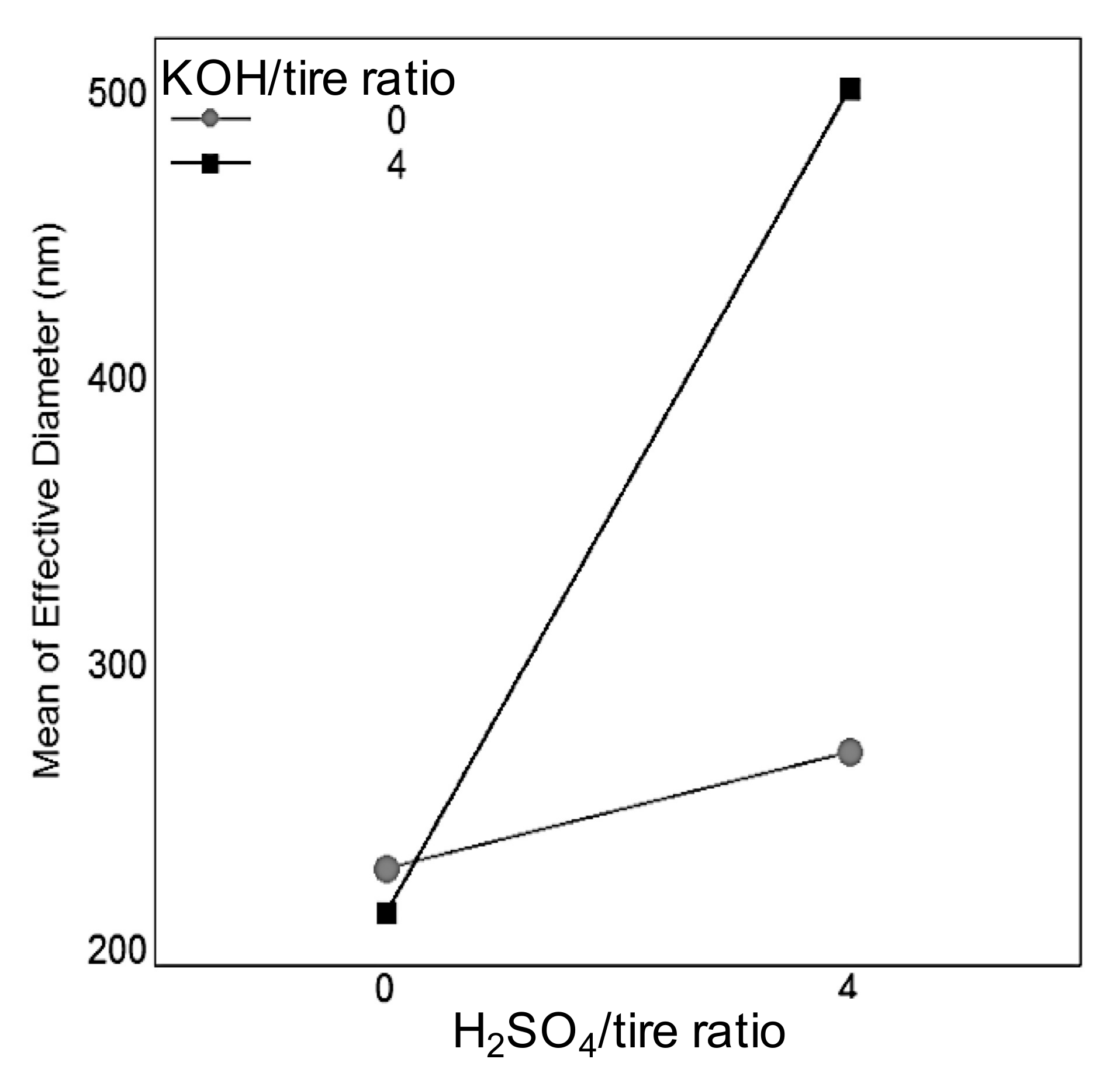
3.2.4. Particle Size and Dispersion Stability
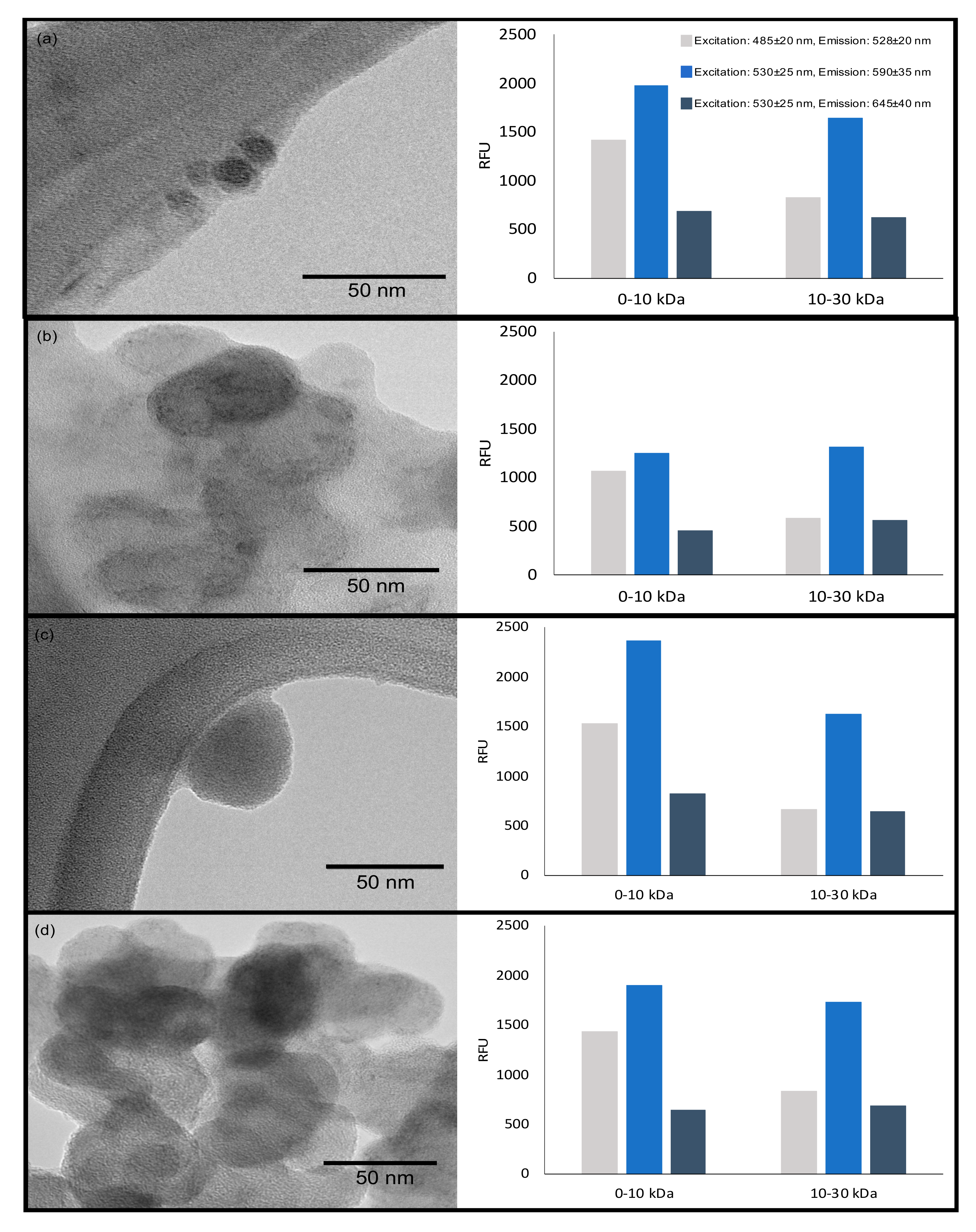
3.3. Preliminary Analysis of Carbon Dots
4. Conclusions
Author Contributions
Funding
Aknowledgments
Conflicts of Interest
References
- Ayoob, A.K.; Fadhil, A.B. Valorization of waste tires in the synthesis of an effective carbon based catalyst for biodiesel production from a mixture of non-edible oils. Fuel 2020, 264, 116754. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Peronard, J.P.; Ballantyne, A.G. Broadening the understanding of the role of consumer services in the circular economy: Toward a conceptualization of value creation processes. J. Clean. Prod. 2019, 239, 118010. [Google Scholar] [CrossRef]
- Taleb, D.A.; Hamid, H.A.; Deris, R.R.R.; Zulkifli, M.; Khalil, N.A.; Ahmad Yahaya, A.N. Insights into pyrolysis of waste tire in fixed bed reactor: Thermal behavior. Mater. Today Proc. 2020, 1–9. [Google Scholar] [CrossRef]
- Martínez, J.D.; Puy, N.; Murillo, R.; García, T.; Navarro, M.V.; Mastral, A.M. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef]
- Miliotti, E.; Casini, D.; Rosi, L.; Lotti, G.; Rizzo, A.M.; Chiaramonti, D. Lab-scale pyrolysis and hydrothermal carbonization of biomass digestate: Characterization of solid products and compliance with biochar standards. Biomass—Bioenergy 2020, 139, 105593. [Google Scholar] [CrossRef]
- Alsaleh, A.; Sattler, M.L. Waste Tire Pyrolysis: Influential Parameters and Product Properties. Curr. Sustain. Energy Rep. 2014, 1, 129–135. [Google Scholar] [CrossRef]
- Puccini, M.; Stefanelli, E.; Hiltz, M.; Seggiani, M.; Vitolo, S. Activated carbon from hydrochar produced by hydrothermal carbonization of wastes. Chem. Eng. Trans. 2017, 57, 169–174. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Aylón, E.; Fernández-Colino, A.; Murillo, R.; Navarro, M.V.; García, T.; Mastral, A.M. Valorisation of waste tyre by pyrolysis in a moving bed reactor. Waste Manag. 2010, 30, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Arabiourrutia, M.; Lopez, G.; Artetxe, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Waste tyre valorization by catalytic pyrolysis—A review. Renew. Sustain. Energy Rev. 2020, 129, 109932. [Google Scholar] [CrossRef]
- Martínez, J.D.; Cardona-Uribe, N.; Murillo, R.; García, T.; López, J.M. Carbon black recovery from waste tire pyrolysis by demineralization: Production and application in rubber compounding. Waste Manag. 2019, 85, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Piskorz, J.; Majerski, P.; Radlein, D.; Wik, T.; Scott, D.S. Recovery of carbon black from scrap rubber. Energy Fuels 1999, 13, 544–551. [Google Scholar] [CrossRef]
- López, F.A.; Centeno, T.A.; Rodríguez, O.; Alguacil, F.J. Preparation and characterization of activated carbon from the char produced in the thermolysis of granulated scrap tyres. J. Air Waste Manag. Assoc. 2013, 63, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, W.; Mennerich, C. Pyrolysis of synthetic tire rubber in a fluidised-bed reactor to yield 1,3-butadiene, styrene and carbon black. J. Anal. Appl. Pyrolysis 2001, 58–59, 803–811. [Google Scholar] [CrossRef]
- Ko, D.; Mui, E.; Lau, K.; McKay, G. Production of activated carbons from waste tire - Process design and economical analysis. Waste Manag. 2004, 24, 875–888. [Google Scholar] [CrossRef]
- Nieto-Márquez, A.; Atanes, E.; Morena, J.; Fernández-Martínez, F.; Valverde, J.L. Upgrading waste tires by chemical activation for the capture of SO2. Fuel Process. Technol. 2016, 144, 274–281. [Google Scholar] [CrossRef]
- López, G.; Olazar, M.; Artetxe, M.; Amutio, M.; Elordi, G.; Bilbao, J. Steam activation of pyrolytic tyre char at different temperatures. J. Anal. Appl. Pyrolysis 2009, 85, 539–543. [Google Scholar] [CrossRef]
- Bergna, D.; Varila, T.; Romar, H.; Lassi, U. Comparison of the Properties of Activated Carbons Produced in One-Stage and Two-Stage Processes. C J. Carbon Res. 2018, 4, 41. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Do, D.D. The preparation of active carbons from coal by chemical and physical activation. Carbon N.Y. 1996, 34, 471–479. [Google Scholar] [CrossRef]
- Naskar, A.K.; Bi, Z.; Li, Y.; Akato, S.K.; Saha, D.; Chi, M.; Bridges, C.A.; Paranthaman, M.P. Tailored recovery of carbons from waste tires for enhanced performance as anodes in lithium-ion batteries. RSC Adv. 2014, 4, 38213. [Google Scholar] [CrossRef]
- Sirimuangjinda, A.; Atong, D.; Pechyen, C. Comparison on pore development of activated carbon produced from scrap tire by hydrochloric acid and sulfuric acid. Adv. Mater. Res. 2013, 626, 706–710. [Google Scholar] [CrossRef]
- Dimpe, K.M.; Ngila, J.C.; Nomngongo, P.N. Application of waste tyre-based activated carbon for the removal of heavy metals in wastewater. Cogent Eng. 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Teng, H.; Lin, Y.C.; Hsu, L.Y. Production of activated carbons from pyrolysis of waste tires impregnated with potassium hydroxide. J. Air Waste Manag. Assoc. 2011, 50, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.; Jaseem, S.A.; Ahmed, M.; Singh, P.; Sesha, N. Studies on Activated Carbon Produced From Waste Tires. IOSR J. Electr. Electron. Eng. 2018, 11, 71–74. [Google Scholar] [CrossRef]
- Al-Rahbi, A.S.; Williams, P.T. Production of activated carbons from waste tyres for low temperature NOx control. Waste Manag. 2016, 49, 188–195. [Google Scholar] [CrossRef]
- Kumar, R; Sharma, A. Morphologically tailored activated carbon derived from waste tires as high-performance anode for Li-ion battery. J. Appl. Electrochem. 2018, 48. [Google Scholar] [CrossRef]
- Kumar, R.; Bhuvana, T.; Sharma, A. Tire Waste Derived Turbostratic Carbon as an Electrode for a Vanadium Redox Flow Battery. ACS Sustain. Chem. Eng. 2018, 6, 8238–8246. [Google Scholar] [CrossRef]
- Mui, E.L.K.; Ko, D.C.K.; McKay, G. Production of active carbons from waste tyres—A review. Carbon N.Y. 2004, 42, 2789–2805. [Google Scholar] [CrossRef]
- Juma, M.; Koreňová, Z.; Markoš, J.; Jelemensky, L.; Bafrnec, M. Experimental study of pyrolysis and combustion of scrap tire. Polym. Adv. Technol. 2007, 18, 144–148. [Google Scholar] [CrossRef]
- Hadi, P.; Yeung, K.Y.; Guo, J.; Wang, H.; McKay, G. Sustainable development of tyre char-based activated carbons with different textural properties for value-added applications. J. Environ. Manage. 2016, 170, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Paranthaman, M.P.; Akato, K.; Naskar, A.K.; Levine, A.M.; Lee, R.J.; Kim, S.O.; Zhang, J.; Dai, S.; Manthiram, A. Tire-derived carbon composite anodes for sodium-ion batteries. J. Power Sources 2016, 316, 232–238. [Google Scholar] [CrossRef]
- Maroufi, S.; Mayyas, M.; Sahajwalla, V. Nano-carbons from waste tyre rubber: An insight into structure and morphology. Waste Manag. 2017, 69, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hernández, R.; Panecatl-Bernal, Y.; Méndez-Rojas, M.Á. High yield and simple one-step production of carbon black nanoparticles from waste tires. Heliyon 2019, 5. [Google Scholar] [CrossRef]
- Chan, K.K.; Yap, S.H.K.; Yong, K.T. Biogreen Synthesis of Carbon Dots for Biotechnology and Nanomedicine Applications; Springer: Berlin/Heidelberg, Germany, 2018; Volume 10, ISBN 0123456789. [Google Scholar]
- Chatzimitakos, T.; Stalikas, C. Recent Advances in Carbon Dots. C J. Carbon Res. 2019, 5, 41. [Google Scholar] [CrossRef]
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef]
- Pudza, M.Y.; Abidin, Z.Z.; Rashid, S.A.; Yasin, F.M.; Noor, A.S.M.; Issa, M.A. Eco-friendly sustainable fluorescent carbon dots for the adsorption of heavy metal ions in aqueous environment. Nanomaterials 2020, 10. [Google Scholar] [CrossRef]
- Hoang, V.C.; Hassan, M.; Gomes, V.G. Coal derived carbon nanomaterials – Recent advances in synthesis and applications. Appl. Mater. Today 2018, 12, 342–358. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Singh, D.P. Natural and waste hydrocarbon precursors for the synthesis of carbon based nanomaterials: Graphene and CNTs. Renew. Sustain. Energy Rev. 2016, 58, 976–1006. [Google Scholar] [CrossRef]
- Mohammad, N.N.; Omer, K.M.; Baban, S. Valorization of tire wastes to carbon quantum dots (P-CDs) and photocatalytic degradation enhancement of organic wastes using ZnO-CDs nanocomposites. J. Mater. Sci. Mater. Electron. 2019, 30, 11598–11606. [Google Scholar] [CrossRef]
- Parra, J.; Silva, K.; Valezin, P.; Martins, R.; Gomes, R.; Pereira, R.; de Melo, F.; Morandim-Giannetti, A.; dos Santos, R.; Panzarini, L.C.; et al. Preparation of Fluorescent Carbon-Based Dots from Waste Tire Pyrolysis. J. Braz. Chem. Soc. 2020, 31, 2224–2231. [Google Scholar] [CrossRef]
- Chu, K.W.; Lee, S.L.; Chang, C.J.; Liu, L. Recent progress of carbon dot precursors and photocatalysis applications. Polymers 2019, 11. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Gruber, T.; Zerda, T.W.; Gerspacher, M. Raman studies of heat-treated carbon blacks. Carbon N.Y. 1994, 32, 1377–1382. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, N.; Lin, X.; Lin, J.; Chi, Y.; Chen, G. Extraction of electrochemiluminescent oxidized carbon quantum dots from activated carbon. Chem. Mater. 2010, 22, 5895–5899. [Google Scholar] [CrossRef]
- ASTM Standard D3173 Standard Test Method for Moisture in the Analysis Sample of Coal and Coke. ASTM Int. 2011, 3, 1–3. [CrossRef]
- ASTM Standard D3174 Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal. ASTM Int. 2000, 5, 1–6. [CrossRef]
- ASTM Standard D3175 Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM Int. 2007, 3, 1–3. [CrossRef]
- ASTM Standard D3172 Standard Practice for Proximate Analysis of Coal and Coke. ASTM Int. 2007, 89, 1–2. [CrossRef]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J. Physicochemical Properties of Activated Carbon: Their Effect on the Adsorption of Pharmaceutical Compounds and Adsorbate–Adsorbent Interactions. C J. Carbon Res. 2018, 4, 62. [Google Scholar] [CrossRef]
- Raj Shakya, P.; Shrestha, P.; Shova Tamrakar, C.; Bhattarai, P.K. Studies and Determination of Heavy Metals in Waste Tyres and their Impacts on the Environment. J. Anal. Envir. Chem 2006, 7, 70–76. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Seaton, N.A.; Walton, J.P.R.B.; Quirke, N. A new analysis method for the determination of the pore size distribution of porous carbons from nitrogen adsorption measurements. Carbon N.Y. 1989, 27, 853–861. [Google Scholar] [CrossRef]
- Aslam, N.; Khawaja, A.; Shahid, Y.; Ja, A. Acid Base Demineralization of Pyrolytic Carbon Black Obtained From Waste Rubber. J. Austin Chem. Eng. 2018, 5, 1–3. [Google Scholar]
- Susa, D.; Haydary, J. Sulphur distribution in the products of waste tire pyrolysis. Chem. Pap. 2013, 67, 1521–1526. [Google Scholar] [CrossRef]
- Liu, X.; Bi, X.T. Removal of inorganic constituents from pine barks and switchgrass. Fuel Process. Technol. 2011, 92, 1273–1279. [Google Scholar] [CrossRef]
- Mukherjee, S.; Borthakur, P.C. Effect of leaching high sulphur subbituminous coal by potassium hydroxide and acid on removal of mineral matter and sulphur. Fuel 2003, 82, 783–788. [Google Scholar] [CrossRef]
- Nieto-Márquez, A.; Pinedo-Flores, A.; Picasso, G.; Atanes, E.; Sun Kou, R. Selective adsorption of Pb2+, Cr3+ and Cd2+ mixtures on activated carbons prepared from waste tires. J. Environ. Chem. Eng. 2017, 5, 1060–1067. [Google Scholar] [CrossRef]
- Wacharasindhu, S.; Likitmaskul, S.; Punnakanta, L.; Chaichanwatanakul, K.; Angsusingha, K.; Tuchinda, C. Reporting Physisorption Data For Gas/solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure App. Chem 1985, 81, 603–619. [Google Scholar]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 122383. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Mikulova, Z.; Sedenkova, I.; Matejova, L.; Vecer, M.; Dombek, V. Study of carbon black obtained by pyrolysis of waste scrap tyres. J. Therm. Anal. Calorim. 2013, 111, 1475–1481. [Google Scholar] [CrossRef]
- Aranda, A.; Murillo, R.; García, T.; Callén, M.S.; Mastral, A.M. Steam activation of tyre pyrolytic carbon black: Kinetic study in a thermobalance. Chem. Eng. J. 2007, 126, 79–85. [Google Scholar] [CrossRef]
- González-Gonz#xE1;lez, R.B. Valorización del Negro de humo de Llantas de Desecho para Aplicaciones en Baterías de Litio. Master’s Thesis, Tecnológico de Monterrey, Monterrey, N.L., Mexico, 2016. [Google Scholar]
- Moreno-Castilla, C.; López-Ramón, M.V.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon N.Y. 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Wibawa, P.J.; Nur, M.; Asy’ari, M.; Nur, H. SEM, XRD and FTIR analyses of both ultrasonic and heat generated activated carbon black microstructures. Heliyon 2020, 6, e03546. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C J. Carbon Res. 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Knight, D.S.; White, W.B. Characterization of diamond films by Raman spectroscopy. J. Mater. Res. 1989, 4, 385–393. [Google Scholar] [CrossRef]
- Lespade, P.; Marchand, A.; Couzi, M.; Cruege, F. Caracterisation de materiaux carbones par microspectrometrie Raman. Carbon N.Y. 1984, 22, 375–385. [Google Scholar] [CrossRef]
- Oya, A.; Otani, S. Catalytic graphitization of carbons by various metals. Fuel 1979, 58, 495–500. [Google Scholar] [CrossRef]
- Kiciński, W.; Dyjak, S. Transition metal impurities in carbon-based materials: Pitfalls, artifacts and deleterious effects. Carbon N.Y. 2020, 168, 748–845. [Google Scholar] [CrossRef]
- Bakeev, K.A.; Chimenti, R.V. Pros and cons of using correlation versus multivariate algorithms for material identification via handheld spectroscopy. Eur. Pharm. Rev. 2013, 1–5. [Google Scholar]
- Matsukata, M.; Fujikawa, T.; Kikuchi, E.; Morita, Y. Interaction Between Potassium Carbonate and Carbon Substrate at Subgasification Temperatures. Migration of Potassium into the Carbon Matrix. Energy Fuels 1988, 2, 750–756. [Google Scholar] [CrossRef]
- Liu, T.; Jia, S.; Kowalewski, T.; Matyjaszewski, K.; Casado-Portilla, R.; Belmont, J. Water-dispersible carbon black nanocomposites prepared by surface-initiated atom transfer radical polymerization in protic media. Macromolecules 2006, 39, 548–556. [Google Scholar] [CrossRef]
- Roy, C.; Chaala, A.; Darmstadt, H. Vacuum pyrolysis of used tires end-uses for oil and carbon black products. J. Anal. Appl. Pyrolysis 1999, 51, 201–221. [Google Scholar] [CrossRef]
- Abbas, A.; Mariana, L.T.; Phan, A.N. Biomass-waste derived graphene quantum dots and their applications. Carbon N.Y. 2018, 140, 77–99. [Google Scholar] [CrossRef]
- Sharma, V.; Tiwari, P.; Mobin, S.M. Sustainable carbon-dots: Recent advances in green carbon dots for sensing and bioimaging. J. Mater. Chem. B 2017, 5, 8904–8924. [Google Scholar] [CrossRef] [PubMed]
- Mugadza, K.; Stark, A.; Ndungu, P.G.; Nyamori, V.O. Synthesis of Carbon Nanomaterials from Biomass Utilizing Ionic Liquids for Potential Application in Solar Energy Conversion and Storage. Materials 2020, 13, 3945. [Google Scholar] [CrossRef]
- Boruah, A.; Saikia, M.; Das, T.; Goswamee, R.L.; Saikia, B.K. Blue-emitting fluorescent carbon quantum dots from waste biomass sources and their application in fluoride ion detection in water. J. Photochem. Photobiol. B Biol. 2020, 209, 111940. [Google Scholar] [CrossRef]
- El Essawy, N.A.; Konsowa, A.H.; Elnouby, M.; Farag, H.A. A novel one-step synthesis for carbon-based nanomaterials from polyethylene terephthalate (PET) bottles waste. J. Air Waste Manag. Assoc. 2017, 67, 358–370. [Google Scholar] [CrossRef]
- Jayaweera, S.; Yin, K.; Ng, W.J. Nitrogen-Doped Durian Shell Derived Carbon Dots for Inner Filter Effect Mediated Sensing of Tetracycline and Fluorescent Ink. J. Fluoresc. 2019, 29, 221–229. [Google Scholar] [CrossRef]
- Jivani, A.; Moradiya, M.A. Economical, Green Synthesis of Fluorescent Carbon Quantum Dots from Lac Extract. J. Sci. Technol. 2020, 5, 213–217. [Google Scholar] [CrossRef]
- Favela-Camacho, S.E.; Samaniego-Benítez, E.J.; Godínez-García, A.; Avilés-Arellano, L.M.; Pérez-Robles, J.F. How to decrease the agglomeration of magnetite nanoparticles and increase their stability using surface properties. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 574, 29–35. [Google Scholar] [CrossRef]
- Youssry, M.; Kamand, F.Z.; Magzoub, M.I.; Nasser, M.S. Aqueous dispersions of carbon black and its hybrid with carbon nanofibers. RSC Adv. 2018, 8, 32119–32131. [Google Scholar] [CrossRef]
- Kawaraya, M.; Shimizu, S.; Hisashi, H.; Kamiya, H. Influence of the functional groups on carbon black particles on the zeta-potential. Ceram. Trans. 2010, 219, 255–261. [Google Scholar] [CrossRef]
- Xu, H.; Yang, X.; Li, G.; Zhao, C.; Liao, X. Green Synthesis of Fluorescent Carbon Dots for Selective Detection of Tartrazine in Food Samples. J. Agric. Food Chem. 2015, 63, 6707–6714. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.M.; Saifi, S.; Aslam, Z; Khan, S.A.; Zulfequar, M. A facile one step hydrothermal synthesis of carbon quantum dots for label -free fluorescence sensing approach to detect picric acid in aqueous solution. J. Photochem. Photobiol. A Chem. 2020, 388. [Google Scholar] [CrossRef]
- Yu, J.; Liu, C.; Yuan, K.; Lu, Z.; Cheng, Y.; Li, L.; Zhang, X.; Jin, P.; Meng, F.; Liu, H. Luminescence mechanism of carbon dots by tailoring functional groups for sensing Fe3+ ions. Nanomaterials 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Xu, Q.; Pu, P.; Zhao, J.; Dong, C.; Gao, C.; Chen, Y.; Chen, J.; Liu, Y.; Zhou, H. Preparation of highly photoluminescent sulfur-doped carbon dots for Fe(iii) detection. J. Mater. Chem. A 2015, 3, 542–546. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Leung, K.; Loffroy, R.; Lu, P.-X.; Wáng, J.Y. Opportunities and Challenges of Fluorescent Carbon Dots in Translational Optical Imaging. Curr. Pharm. Des. 2015, 21, 5401–5416. [Google Scholar] [CrossRef]
- Bayati, M.; Dai, J.; Zambrana, A.; Rees, C.; Fidalgo de Cortalezzi, M. Effect of water chemistry on the aggregation and photoluminescence behavior of carbon dots. J. Environ. Sci. (China) 2018, 65, 223–235. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Ghanbari, D.; Salavati-Niasari, M. Photoluminescence carbon dot as a sensor for detecting of Pseudomonas aeruginosa bacteria: Hydrothermal synthesis of magnetic hollow NiFe 2 O 4 -carbon dots nanocomposite material. Compos. Part. B Eng. 2019, 161, 564–577. [Google Scholar] [CrossRef]
- Ray, S.C.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent carbon nanoparticles: Synthesis, characterization, and bioimaging application. J. Phys. Chem. C 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Sivanesan, S.; Panneerselvam, P. Turn-On fluorescence sensor based detection of heavy metal ion using carbon dots@graphitic-carbon nitride nanocomposite probe. J. Photochem. Photobiol. A Chem. 2020, 389, 112204. [Google Scholar] [CrossRef]
- Barman, M.K.; Patra, A. Current status and prospects on chemical structure driven photoluminescence behaviour of carbon dots. J. Photochem. Photobiol. C Photochem. Rev. 2018, 37, 1–22. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Q.; Long, Y.; Zhang, H.; Huang, X.; Zhu, R. Enhancing the luminescence of carbon dots with a reduction pathway. Chem. Commun. 2011, 47, 10650–10652. [Google Scholar] [CrossRef]
- Romero, V.; Vila, V.; de la Calle, I.; Lavilla, I.; Bendicho, C. Turn–on fluorescent sensor for the detection of periodate anion following photochemical synthesis of nitrogen and sulphur co–doped carbon dots from vegetables. Sensors Actuators B Chem. 2019, 280, 290–297. [Google Scholar] [CrossRef]
- Costas-Mora, I.; Romero, V.; Lavilla, I.; Bendicho, C. In situ photochemical synthesis of fluorescent carbon dots for optical sensing of hydrogen peroxide and antioxidants. Talanta 2015, 144, 1308–1315. [Google Scholar] [CrossRef]



| Analysis | Waste Tire | Tire Activated with H2SO4 | Tire Activated with KOH | Tire Activated with H2SO4 and KOH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SD 1 | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | |
| Moisture (%) | 0.89 | ± | 0.01 | 3.06 | ± | 0.17 | 0.71 | ± | 0.00 | 8.96 | ± | 0.33 |
| Ash (%) | 9.99 | ± | 0.52 | 7.20 | ± | 0.51 | 9.75 | ± | 0.41 | 3.16 | ± | 0.29 |
| Volatile matter (%) | 66.95 | ± | 3.60 | 48.36 | ± | 0.49 | 66.07 | ± | 1.35 | 38.87 | ± | 0.16 |
| Fixed carbon 2 (%) | 22.16 | ± | 4.14 | 41.38 | ± | 0.83 | 23.47 | ± | 1.77 | 49.02 | ± | 0.46 |
| Nitrogen (%) | 1.59 | ± | 0.08 | 1.63 | ± | 0.08 | 1.57 | ± | 0.18 | 1.45 | ± | 0.01 |
| Carbon (%) | 82.25 | ± | 1.29 | 72.90 | ± | 0.13 | 81.49 | ± | 0.29 | 61.43 | ± | 0.15 |
| Hydrogen (%) | 7.34 | ± | 0.09 | 4.99 | ± | 0.00 | 7.14 | ± | 0.04 | 4.26 | ± | 0.06 |
| Sulfur (%) | 1.72 | ± | 0.05 | 5.18 | ± | 0.03 | 1.77 | ± | 0.04 | 4.24 | ± | 0.01 |
| Oxygen (%) | 2.21 | ± | 0.15 | 9.74 | ± | 0.04 | 3.74 | ± | 0.02 | 14.90 | ± | 4.42 |
| CB | CB.H2SO4 | CB.KOH | CB.H2SO4 + KOH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | |
| Nitrogen (%) | 0.90 | ± | 0.01 | 0.86 | ± | 0.15 | 0.97 | ± | 0.02 | 1.02 | ± | 0.02 |
| Carbon (%) | 79.46 | ± | 0.34 | 86.90 | ± | 1.08 | 70.38 | ± | 1.49 | 60.75 | ± | 0.94 |
| Hydrogen (%) | 0.30 | ± | 0.01 | 0.41 | ± | 0.05 | 0.44 | ± | 0.08 | 0.75 | ± | 0.02 |
| Sulfur (%) | 3.09 | ± | 0.06 | 1.36 | ± | 0.02 | 3.04 | ± | 0.04 | 4.45 | ± | 0.06 |
| Oxygen (%) | 0.70 | ± | 0.01 | 0.97 | ± | 0.06 | 8.79 | ± | 0.05 | 16.06 | ± | 0.39 |
| Cd (mg/kg) | 0.00 | ± | 0.01 | 0.30 | ± | 0.08 | 0.45 | ± | 0.21 | 0.11 | ± | 0.00 |
| Cr (mg/kg) | 8.83 | ± | 0.25 | 6.41 | ± | 0.15 | 9.29 | ± | 0.51 | 5.77 | ± | 0.30 |
| Fe (mg/kg) | 862.47 | ± | 2.50 | 147.93 | ± | 1.14 | 549.67 | ± | 2.15 | 80.98 | ± | 0.61 |
| Ni (mg/kg) | 7.36 | ± | 0.11 | 6.87 | ± | 0.06 | 3.74 | ± | 0.11 | 2.99 | ± | 0.14 |
| Pb (mg/kg) | 4.77 | ± | 0.18 | 1.99 | ± | 0.30 | 6.73 | ± | 0.23 | 3.16 | ± | 0.19 |
| Zn (mg/kg) | 37543.33 | ± | 565.89 | 1990.67 | ± | 4.51 | 8032.67 | ± | 74.90 | 158.13 | ± | 0.72 |
| Fitting Parameters | CB | CB.H2SO4 | CB.KOH | CB.H2SO4 + KOH |
|---|---|---|---|---|
| ID/IG | 0.96 | 1.01 | 0.94 | 0.93 |
| D band position (cm−1) | 1345 | 1345 | 1361 | 1360 |
| G band position (cm−1) | 1584 | 1579 | 1577 | 1574 |
| D band height | 0.79 | 0.84 | 0.70 | 0.37 |
| G band height | 0.82 | 0.83 | 0.74 | 0.39 |
| Sample | ζ Potential (mV) | |||
|---|---|---|---|---|
| Mean | ± | SD | ||
| CB | −23.88 | ± | 1.20 | A |
| CB.H2SO4 | −23.75 | ± | 2.15 | A |
| CB.KOH | −37.35 | ± | 1.42 | |
| CB.H2SO4 + KOH | −46.94 | ± | 1.90 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-González, R.B.; González, L.T.; Iglesias-González, S.; González-González, E.; Martinez-Chapa, S.O.; Madou, M.; Alvarez, M.M.; Mendoza, A. Characterization of Chemically Activated Pyrolytic Carbon Black Derived from Waste Tires as a Candidate for Nanomaterial Precursor. Nanomaterials 2020, 10, 2213. https://doi.org/10.3390/nano10112213
González-González RB, González LT, Iglesias-González S, González-González E, Martinez-Chapa SO, Madou M, Alvarez MM, Mendoza A. Characterization of Chemically Activated Pyrolytic Carbon Black Derived from Waste Tires as a Candidate for Nanomaterial Precursor. Nanomaterials. 2020; 10(11):2213. https://doi.org/10.3390/nano10112213
Chicago/Turabian StyleGonzález-González, Reyna Berenice, Lucy T. González, Sigfrido Iglesias-González, Everardo González-González, Sergio O. Martinez-Chapa, Marc Madou, Mario Moisés Alvarez, and Alberto Mendoza. 2020. "Characterization of Chemically Activated Pyrolytic Carbon Black Derived from Waste Tires as a Candidate for Nanomaterial Precursor" Nanomaterials 10, no. 11: 2213. https://doi.org/10.3390/nano10112213
APA StyleGonzález-González, R. B., González, L. T., Iglesias-González, S., González-González, E., Martinez-Chapa, S. O., Madou, M., Alvarez, M. M., & Mendoza, A. (2020). Characterization of Chemically Activated Pyrolytic Carbon Black Derived from Waste Tires as a Candidate for Nanomaterial Precursor. Nanomaterials, 10(11), 2213. https://doi.org/10.3390/nano10112213









