Enhanced High-Temperature (600 °C) NO2 Response of ZnFe2O4 Nanoparticle-Based Exhaust Gas Sensors
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Zwolińska, E.; Chmielewski, A.G. Abatement technologies for high concentrations of NOx and SO2 removal from exhaust gases: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 119–142. [Google Scholar] [CrossRef]
- Air Quality Standards: 2008/50/EC Directive on Ambient Air Quality and Cleaner Air for Europe. Available online: https://www.eea.europa.eu/themes/air/air-quality-concentrations/air-quality-standards (accessed on 28 April 2020).
- Casquero-Vera, J.A.; Lyamani, H.; Titos, G.; Borrás, E.; Olmo, F.J.; Alados-Arboledas, L. Impact of primary NO2 emissions at different urban sites exceeding the European NO2 standard limit. Sci. Total Environ. 2019, 646, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.; Avdan, Z.Y.; Avdan, U. Spaceborne Nitrogen Dioxide Observations from the Sentinel-5P TROPOMI over Turkey. Proceedings 2019, 18, 4. [Google Scholar] [CrossRef]
- Afzal, A.; Andersson, M.; Di Franco, C.; Ditaranto, N.; Cioffi, N.; Scamarcio, G.; Lloyd Spetz, A.; Torsi, L. Electrochemical deposition of gold on indium zirconate (InZrOx with In/Zr atomic ratio 1.0) for high temperature automobile exhaust gas sensors. J. Solid State Electrochem. 2015, 19, 2859–2868. [Google Scholar] [CrossRef]
- Liu, F.; Wang, B.; Yang, X.; Guan, Y.; Sun, R.; Wang, Q.; Liang, X.; Sun, P.; Lu, G. High-temperature stabilized zirconia-based sensors utilizing MNb2O6 (M: Co, Ni and Zn) sensing electrodes for detection of NO2. Sens. Actuators B Chem. 2016, 232, 523–530. [Google Scholar] [CrossRef]
- Liu, F.; Wang, B.; Yang, X.; Guan, Y.; Wang, Q.; Liang, X.; Sun, P.; Wang, Y.; Lu, G. High-temperature NO2 gas sensor based on stabilized zirconia and CoTa2O6 sensing electrode. Sens. Actuators B Chem. 2017, 240, 148–157. [Google Scholar] [CrossRef]
- Dai, L.; Shi, M.; Han, W.; Meng, W.; He, Z.; Zhu, L.; Wang, L. High-temperature NO2 sensor based on aluminum/indium co-doped lanthanum silicate oxyapatite electrolyte and cobalt-free perovskite oxide sensing electrode. Sens. Actuators B Chem. 2017, 250, 629–640. [Google Scholar] [CrossRef]
- Ghosh, A.; Zhang, C.; Shi, S.Q.; Zhang, H. High-Temperature Gas Sensors for Harsh Environment Applications: A Review. CLEAN Soil Air Water 2019, 47, 1800491. [Google Scholar] [CrossRef]
- Afzal, A.; Cioffi, N.; Sabbatini, L.; Torsi, L. NOx sensors based on semiconducting metal oxide nanostructures: Progress and perspectives. Sens. Actuators B Chem. 2012, 171–172, 25–42. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Barsan, N.; Schierbaum, K. Gas Sensors Based on Conducting Metal Oxides: Basic Understanding, Technology and Applications; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-811225-0. [Google Scholar]
- Afzal, A. β-Ga2O3 nanowires and thin films for metal oxide semiconductor gas sensors: Sensing mechanisms and performance enhancement strategies. J. Mater. 2019, 5, 542–557. [Google Scholar] [CrossRef]
- Miura, N.; Zhuiykov, S.; Ono, T.; Hasei, M.; Yamazoe, N. Mixed potential type sensor using stabilized zirconia and ZnFe2O4 sensing electrode for NOx detection at high temperature. Sens. Actuators B Chem. 2002, 83, 222–229. [Google Scholar] [CrossRef]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, J.; Dun, M.; Huang, X. Porous ZnFe2O4 nanorods with net-worked nanostructure for highly sensor response and fast response acetone gas sensor. Sens. Actuators B Chem. 2017, 248, 85–91. [Google Scholar] [CrossRef]
- Lv, L.; Wang, Y.; Cheng, P.; Zhang, B.; Dang, F.; Xu, L. Ultrasonic spray pyrolysis synthesis of three-dimensional ZnFe2O4-based macroporous spheres for excellent sensitive acetone gas sensor. Sens. Actuators B Chem. 2019, 297, 126755. [Google Scholar] [CrossRef]
- Khurshid, R.; Ali, F.; Afzal, A.; Ali, Z.; Qureshi, M.T. Polyol-mediated coprecipitation and aminosilane grafting of superparamagnetic, spinel ZnFe2O4 nanoparticles for room-temperature ethanol sensors. J. Electrochem. Soc. 2019, 166, B258–B265. [Google Scholar] [CrossRef]
- Nemufulwi, M.I.; Swart, H.C.; Mdlalose, W.B.; Mhlongo, G.H. Size-tunable ferromagnetic ZnFe2O4 nanoparticles and their ethanol detection capabilities. Appl. Surf. Sci. 2020, 508, 144863. [Google Scholar] [CrossRef]
- Dong, C.; Liu, X.; Xiao, X.; Du, S.; Wang, Y. Monodisperse ZnFe2O4 nanospheres synthesized by a nonaqueous route for a highly slective low-ppm-level toluene gas sensor. Sens. Actuators B Chem. 2017, 239, 1231–1236. [Google Scholar] [CrossRef]
- Gao, X.; Sun, Y.; Zhu, C.; Li, C.; Ouyang, Q.; Chen, Y. Highly sensitive and selective H2S sensor based on porous ZnFe2O4 nanosheets. Sens. Actuators B Chem. 2017, 246, 662–672. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Meng, F.-N.; Liu, L.-Z.; Chen, Y.-J.; Wang, P.-J. Highly sensitive H2S sensor based on solvothermally prepared spinel ZnFe2O4 nanoparticles. J. Alloys Compd. 2018, 764, 147–154. [Google Scholar] [CrossRef]
- Fareed, S.; Jamil, A.; Afsar, F.; Sher, F.; Li, C.; Xu, X.; Rafiq, M.A. Selective Oxygen Sensor Prepared Using Ni-doped Zinc Ferrite Nanoparticles. J. Electron. Mater. 2019, 48, 5677–5685. [Google Scholar] [CrossRef]
- Runa, A.; Zhang, X.; Wen, G.; Zhang, B.; Fu, W.; Yang, H. Actinomorphic flower-like n-ZnO/p-ZnFe2O4 composite and its improved NO2 gas-sensing property. Mater. Lett. 2018, 225, 73–76. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the “Debye-Scherrer equation”. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Satalkar, M.; Kane, S.N. On the study of Structural properties and Cation distribution of Zn 0.75-x Ni x Mg 0.15 Cu 0.1 Fe 2 O 4 nano ferrite: Effect of Ni addition. J. Phys. Conf. Ser. 2016, 755, 012050. [Google Scholar] [CrossRef]
- Afzal, A.; Abuilaiwi, F.A.; Javaid, R.; Ali, F.; Habib, A. Solid-state synthesis of heterogeneous Ni0.5Cu0.5-xZnxFe2O4 spinel oxides with controlled morphology and tunable dielectric properties. J. Mater. Sci. Mater. Electron. 2020, 31, 14261–14270. [Google Scholar] [CrossRef]
- Gomes, J.A.; Azevedo, G.M.; Depeyrot, J.; Mestnik-Filho, J.; da Silva, G.J.; Tourinho, F.A.; Perzynski, R. ZnFe2O4 nanoparticles for ferrofluids: A combined XANES and XRD study. J. Magn. Magn. Mater. 2011, 323, 1203–1206. [Google Scholar] [CrossRef]
- Manikandan, A.; Judith Vijaya, J.; Sundararajan, M.; Meganathan, C.; Kennedy, L.J.; Bououdina, M. Optical and magnetic properties of Mg-doped ZnFe2O4 nanoparticles prepared by rapid microwave combustion method. Superlattices Microstruct. 2013, 64, 118–131. [Google Scholar] [CrossRef]
- Ferrari, S.; Kumar, R.S.; Grinblat, F.; Aphesteguy, J.C.; Saccone, F.D.; Errandonea, D. In-situ high-pressure x-ray diffraction study of zinc ferrite nanoparticles. Solid State Sci. 2016, 56, 68–72. [Google Scholar] [CrossRef]
- Chinnasamy, C.N.; Narayanasamy, A.; Ponpandian, N.; Chattopadhyay, K. The influence of Fe3+ ions at tetrahedral sites on the magnetic properties of nanocrystalline ZnFe2O4. Mater. Sci. Eng. A 2001, 304–306, 983–987. [Google Scholar] [CrossRef]
- Ehrhardt, H.; Campbell, S.J.; Hofmann, M. Structural evolution of ball-milled ZnFe2O4. J. Alloys Compd. 2002, 339, 255–260. [Google Scholar] [CrossRef]
- Fella, O.O.; Tamine, M.; Randrianantoandro, N.; Grenèche, J.M. Microstructural Studies of Milled and Annealed ZnFe2O4 Nanostructures Using X-Ray Diffraction and Mössbauer Spectroscopy. Nanosci. Nanoeng. 2013, 1, 1–6. [Google Scholar]
- Goldman, A. Crystal Structure of Ferrites. In Handbook of Modern Ferromagnetic Materials; Goldman, A., Ed.; The Springer International Series in Engineering and Computer Science; Springer US: Boston, MA, USA, 1999; pp. 207–227. ISBN 978-1-4615-4917-8. [Google Scholar]
- Qin, M.; Shuai, Q.; Wu, G.; Zheng, B.; Wang, Z.; Wu, H. Zinc ferrite composite material with controllable morphology and its applications. Mater. Sci. Eng. B 2017, 224, 125–138. [Google Scholar] [CrossRef]
- Fritsch, D. Electronic and optical properties of spinel zinc ferrite: Ab-initio hybrid functional calculations. J. Phys. Condens. Matter 2018, 30, 095502. [Google Scholar] [CrossRef]
- Li, G.; Zhu, X.; Song, W.; Yang, Z.; Dai, J.; Sun, Y.; Fu, Y. Annealing Effects on Semitransparent and Ferromagnetic ZnFe2O4 Nanostructured Films by Sol–Gel. J. Am. Ceram. Soc. 2011, 94, 2872–2877. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Urbánek, P.; Machovsky, M.; Masař, M.; Holek, M. Structural, magnetic, optical, dielectric, electrical and modulus spectroscopic characteristics of ZnFe2O4 spinel ferrite nanoparticles synthesized via honey-mediated sol-gel combustion method. J. Phys. Chem. Solids 2017, 110, 87–99. [Google Scholar] [CrossRef]
- Solano, E.; Frontera, C.; Puig, T.; Obradors, X.; Ricart, S.; Ros, J. Neutron and X-ray diffraction study of ferrite nanocrystals obtained by microwave-assisted growth. A structural comparison with the thermal synthetic route. J. Appl. Crystallogr. 2014, 47, 414–420. [Google Scholar] [CrossRef]
- Zhang, J.; Song, J.-M.; Niu, H.-L.; Mao, C.-J.; Zhang, S.-Y.; Shen, Y.-H. ZnFe2O4 nanoparticles: Synthesis, characterization, and enhanced gas sensing property for acetone. Sens. Actuators B Chem. 2015, 221, 55–62. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, L.; Haugen, N.O.; Wu, Z.; Jia, Y.; Zhong, W.; Zou, J. High humidity response property of sol–gel synthesized ZnFe2O4 films. Mater. Lett. 2018, 213, 266–268. [Google Scholar] [CrossRef]
- Sintering and Grain Growth. In Ceramic Materials: Science and Engineering; Carter, C.B., Norton, M.G., Eds.; Springer: New York, NY, USA, 2007; pp. 427–443. ISBN 978-0-387-46271-4. [Google Scholar]
- Ranjith Kumar, E.; Arunkumar, T.; Prakash, T. Heat treatment effects on structural and dielectric properties of Mn substituted CuFe2O4 and ZnFe2O4 nanoparticles. Superlattices Microstruct. 2015, 85, 530–535. [Google Scholar] [CrossRef]
- Van Hoang, N.; Hung, C.M.; Hoa, N.D.; Van Duy, N.; Van Hieu, N. Facile on-chip electrospinning of ZnFe2O4 nanofiber sensors with excellent sensing performance to H2S down ppb level. J. Hazard. Mater. 2018, 360, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Wilder, D.R.; Fitzsimmons, E.S. Further Study of Sintering Phenomena. J. Am. Ceram. Soc. 1955, 38, 66–71. [Google Scholar] [CrossRef]
- Yadav, R.S.; Havlica, J.; Masilko, J.; Tkacz, J.; Kuřitka, I.; Vilcakova, J. Anneal-tuned structural, dielectric and electrical properties of ZnFe2O4 nanoparticles synthesized by starch-assisted sol–gel auto-combustion method. J. Mater. Sci. Mater. Electron. 2016, 27, 5992–6002. [Google Scholar] [CrossRef]
- Kombaiah, K.; Vijaya, J.J.; Kennedy, L.J.; Bououdina, M. Studies on the microwave assisted and conventional combustion synthesis of Hibiscus rosa-sinensis plant extract based ZnFe2O4 nanoparticles and their optical and magnetic properties. Ceram. Int. 2016, 42, 2741–2749. [Google Scholar] [CrossRef]
- Kombaiah, K.; Vijaya, J.J.; Kennedy, L.J.; Bououdina, M. Optical, magnetic and structural properties of ZnFe2O4 nanoparticles synthesized by conventional and microwave assisted combustion method: A comparative investigation. Optik 2017, 129, 57–68. [Google Scholar] [CrossRef]
- Lemine, O.M.; Bououdina, M.; Sajieddine, M.; Al-Saie, A.M.; Shafi, M.; Khatab, A.; Al-Hilali, M.; Henini, M. Synthesis, structural, magnetic and optical properties of nanocrystalline ZnFe2O4. Phys. B Condens. Matter 2011, 406, 1989–1994. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Vasiljevic, Z.Z.; Lukovic, M.D.; Pavlovic, V.P.; Krstic, J.B.; Vujancevic, J.; Tadic, N.; Vlahovic, B.; Pavlovic, V.B. Investigation of ZnFe2O4 spinel ferrite nanocrystalline screen-printed thick films for application in humidity sensing. Int. J. Appl. Ceram. Technol. 2019, 16, 981–993. [Google Scholar] [CrossRef]
- Sivakumar, N.; Narayanasamy, A.; Ponpandian, N.; Govindaraj, G. Grain size effect on the dielectric behavior of nanostructured Ni0.5Zn0.5Fe2O4. J. Appl. Phys. 2007, 101, 084116. [Google Scholar] [CrossRef]
- Salcedo Rodríguez, K.L.; Stewart, S.J.; Mendoza Zélis, P.M.; Pasquevich, G.A.; Rodríguez Torres, C.E. Role of defects on the magnetic behaviour of the geometrically frustrated spinel ZnFe2O4. J. Alloys Compd. 2018, 752, 289–295. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Peng, S.; Wang, Z.; Liu, R.; Bi, J.; Wu, J. Controlled oxygen vacancies of ZnFe2O4 with superior gas sensing properties prepared via a facile one-step self-catalyzed treatment. Sens. Actuators B Chem. 2019, 288, 649–655. [Google Scholar] [CrossRef]
- Jeseentharani, V.; George, M.; Jeyaraj, B.; Dayalan, A.; Nagaraja, K.S. Synthesis of metal ferrite (MFe2O4, M = Co, Cu, Mg, Ni, Zn) nanoparticles as humidity sensor materials. J. Exp. Nanosci. 2013, 8, 358–370. [Google Scholar] [CrossRef]
- Valero, E.L. Advanced Nanomaterials for Inexpensive Gas Microsensors: Synthesis, Integration and Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-814828-0. [Google Scholar]
- Rathore, D.; Mitra, S.; Kurchania, R.; Pandey, R.K. Physicochemical properties of CuFe2O4 nanoparticles as a gas sensor. J. Mater. Sci. Mater. Electron. 2018, 29, 1925–1932. [Google Scholar] [CrossRef]
- Li, K.; Luo, Y.; Liu, B.; Gao, L.; Duan, G. High-performance NO2-gas sensing of ultrasmall ZnFe2O4 nanoparticles based on surface charge transfer. J. Mater. Chem. A 2019, 7, 5539–5551. [Google Scholar] [CrossRef]
- Waghmare, S.D.; Raut, S.D.; Ghule, B.G.; Jadhav, V.V.; Shaikh, S.F.; Al-Enizi, A.M.; Ubaidullah, M.; Nafady, A.; Thamer, B.M.; Mane, R.S. Pristine and palladium-doped perovskite bismuth ferrites and their nitrogen dioxide gas sensor studies. J. King Saud Univ. Sci. 2020, 32, 3125–3130. [Google Scholar] [CrossRef]
- Bagade, A.A.; Rajpure, K.Y. Development of CoFe2O4 thin films for nitrogen dioxide sensing at moderate operating temperature. J. Alloys Compd. 2016, 657, 414–421. [Google Scholar] [CrossRef]
- Wu, R.-A.; Wei Lin, C.; Tseng, W.J. Preparation of electrospun Cu-doped α-Fe2O3 semiconductor nanofibers for NO2 gas sensor. Ceram. Int. 2017, 43, S535–S540. [Google Scholar] [CrossRef]
- Yamada, Y.; Seno, Y.; Masuoka, Y.; Yamashita, K. Nitrogen oxides sensing characteristics of Zn2SnO4 thin film. Sens. Actuators B Chem. 1998, 49, 248–252. [Google Scholar] [CrossRef]
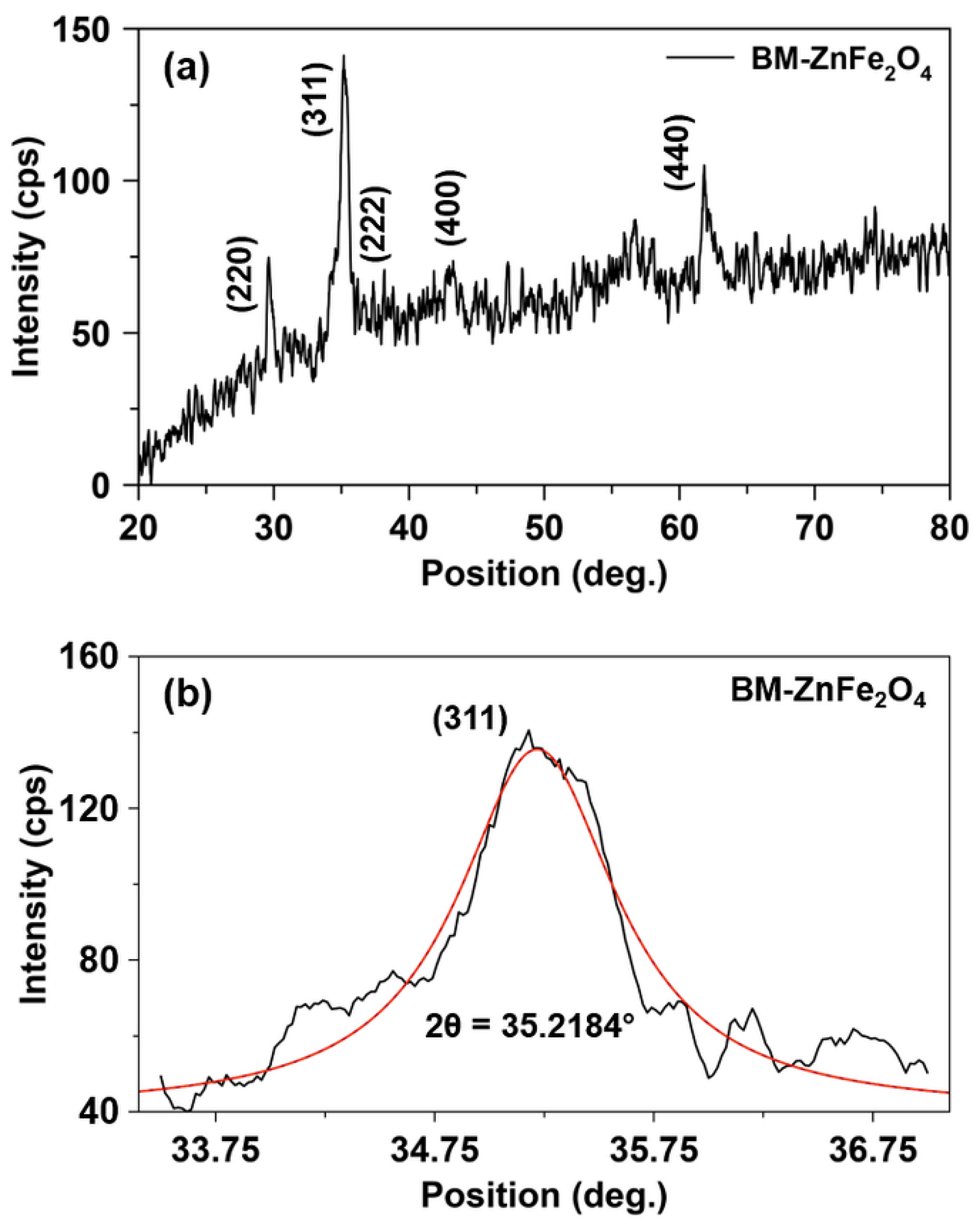
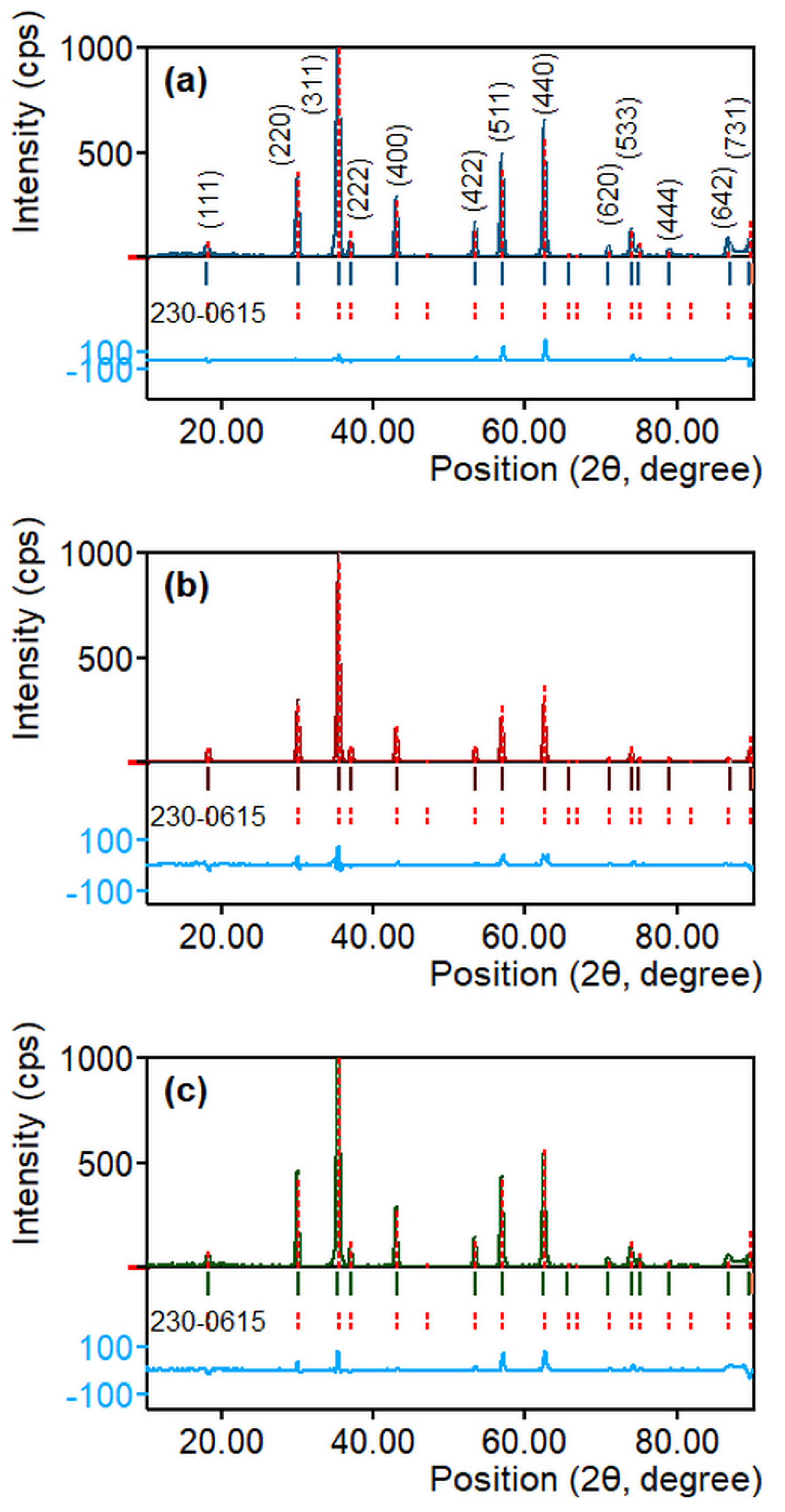

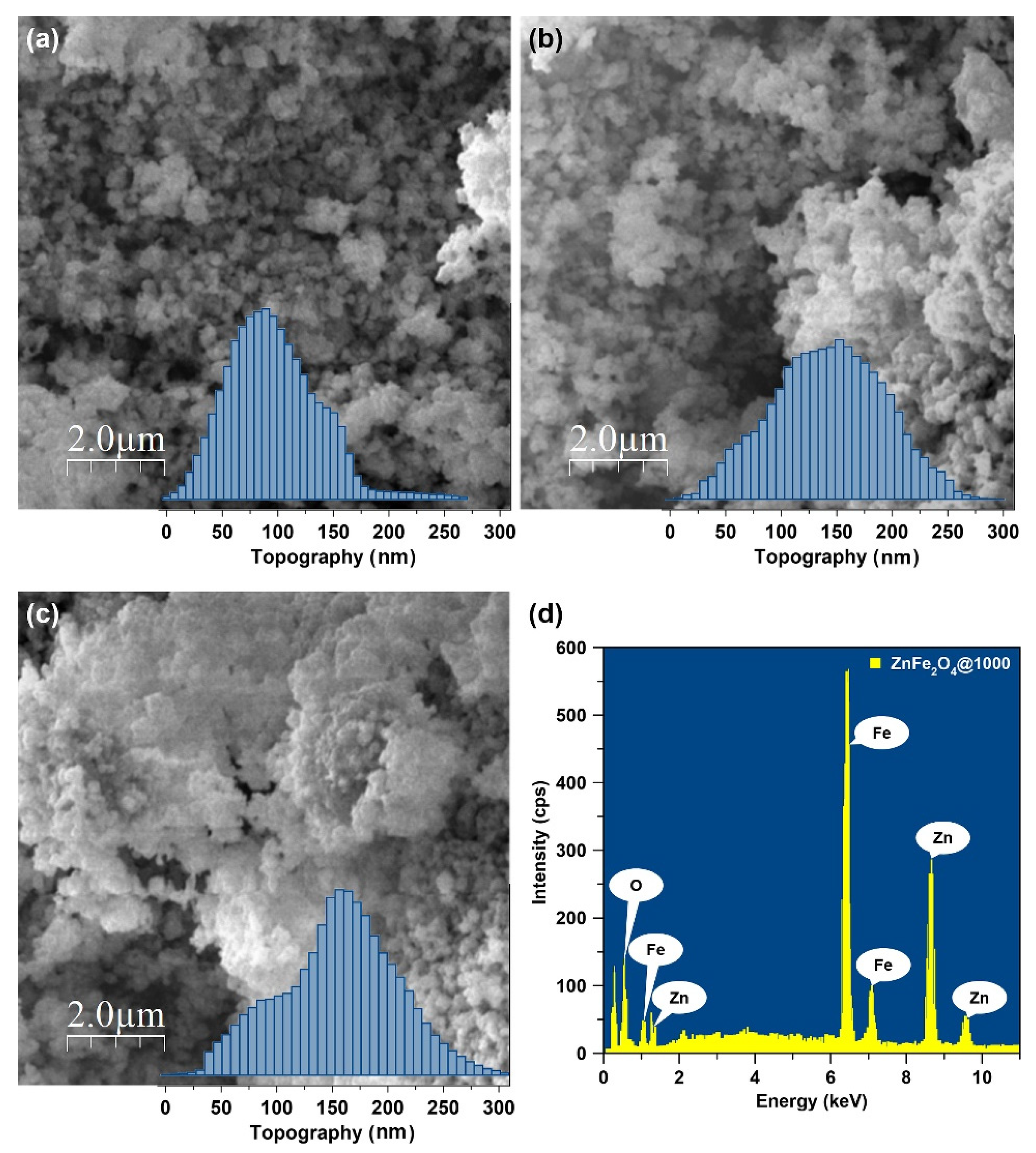
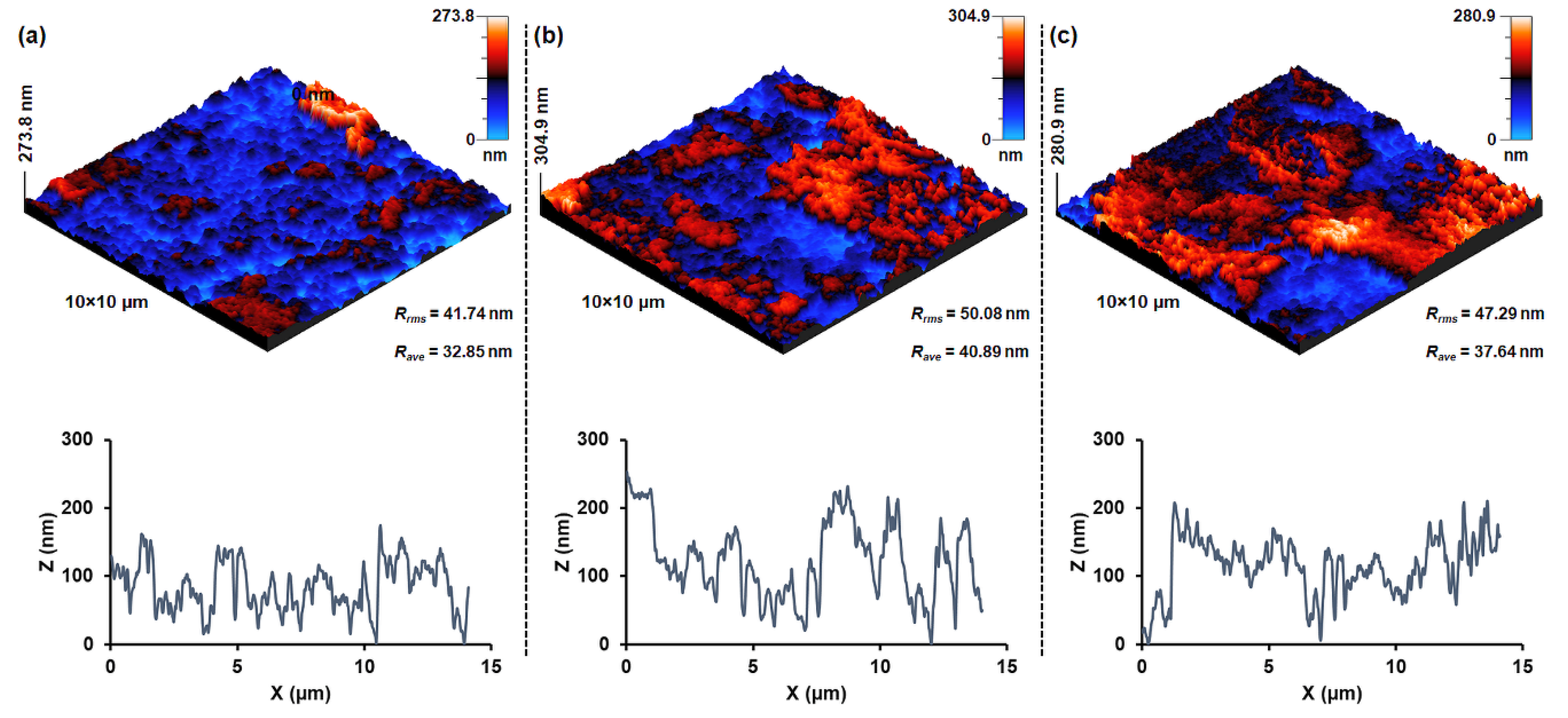


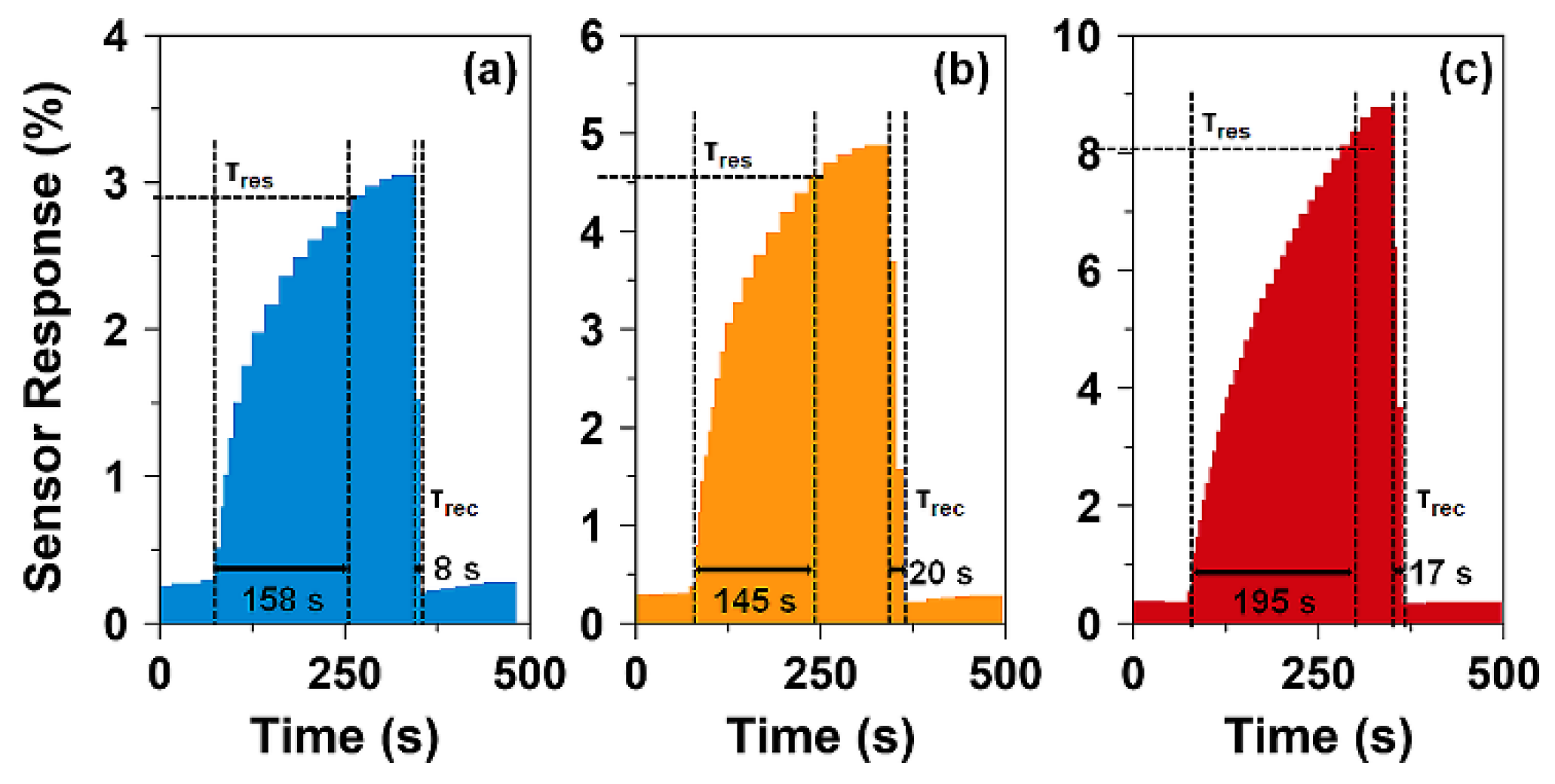
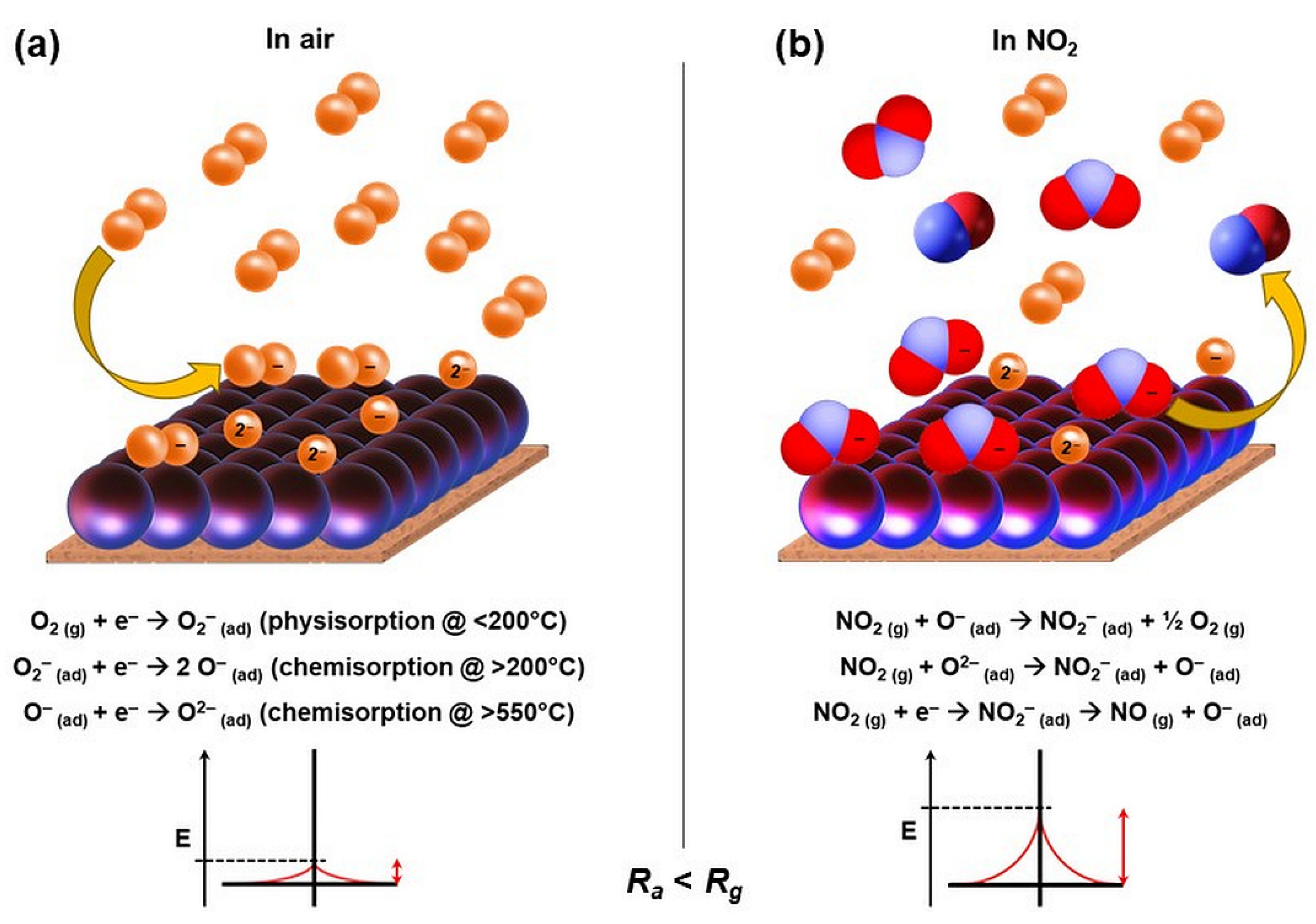
| Sample | T (°C) | D (nm) | a (Å) | V (Å3) | d311 (Å) | ρxrd (g/cm3) | S (m2/g) |
|---|---|---|---|---|---|---|---|
| BM-ZnFe2O4 | - | 9.30 | 8.445 | 602.3 | 2.546 | 5.321 | 121.24 |
| ZnFe2O4@600 | 600 | 18.71 | 8.430 | 599.0 | 2.542 | 5.350 | 59.94 |
| ZnFe2O4@800 | 800 | 23.03 | 8.424 | 597.8 | 2.540 | 5.361 | 48.59 |
| ZnFe2O4@1000 | 1000 | 23.25 | 8.420 | 597.0 | 2.539 | 5.368 | 48.07 |
| Sample | Zn | Fe | O | |||
|---|---|---|---|---|---|---|
| (wt.%) | (at.%) | (wt.%) | (at.%) | (wt.%) | (at.%) | |
| ZnFe2O4@600 | 26.61 | 14.08 | 47.15 | 29.17 | 26.24 | 56.75 |
| ZnFe2O4@800 | 25.60 | 13.51 | 48.04 | 29.64 | 26.36 | 56.85 |
| ZnFe2O4@1000 | 25.65 | 13.68 | 48.67 | 30.34 | 25.69 | 55.98 |
| Material | Fabrication Method | Temperature (°C) | Detection Range (ppm) | Response † (S) | Response Time (s) | Recovery Time (s) | Reference |
|---|---|---|---|---|---|---|---|
| CuFe2O4 | Coprecipitation | 27 | 20–240 | 72% | 8 | 5 | [58] |
| ZnFe2O4 | Hydrothermal | 125 | 1–10 | 248 ‡ | 6.5 | 11 | [59] |
| Pd-doped BiFeO3 | Sol-gel | 140 | 50–3500 | 93% | 60 | 100 | [60] |
| CoFe2O4 | Spray pyrolysis | 150 | 20–80 | 95% | 5 | 114 | [61] |
| ZnO/ZnFe2O4 | Wet chemical | 200 | 0.1–20 | ~ 300 ‡ | 7 | 15 | [25] |
| Cu-doped α-Fe2O3 | Electrospinning | 300 | 5–50 | 2 ‡ | 118 | 258 | [62] |
| ZnFe2O4 | Ball-milling | 600 | 100–400 | 11% | 195 | 17 | This work |
| Sb-doped Zn2SnO4 | Sputtering | 600 | 50–300 | ~ 4 ‡ | - | - | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, A.; Mujahid, A.; Iqbal, N.; Javaid, R.; Qazi, U.Y. Enhanced High-Temperature (600 °C) NO2 Response of ZnFe2O4 Nanoparticle-Based Exhaust Gas Sensors. Nanomaterials 2020, 10, 2133. https://doi.org/10.3390/nano10112133
Afzal A, Mujahid A, Iqbal N, Javaid R, Qazi UY. Enhanced High-Temperature (600 °C) NO2 Response of ZnFe2O4 Nanoparticle-Based Exhaust Gas Sensors. Nanomaterials. 2020; 10(11):2133. https://doi.org/10.3390/nano10112133
Chicago/Turabian StyleAfzal, Adeel, Adnan Mujahid, Naseer Iqbal, Rahat Javaid, and Umair Yaqub Qazi. 2020. "Enhanced High-Temperature (600 °C) NO2 Response of ZnFe2O4 Nanoparticle-Based Exhaust Gas Sensors" Nanomaterials 10, no. 11: 2133. https://doi.org/10.3390/nano10112133
APA StyleAfzal, A., Mujahid, A., Iqbal, N., Javaid, R., & Qazi, U. Y. (2020). Enhanced High-Temperature (600 °C) NO2 Response of ZnFe2O4 Nanoparticle-Based Exhaust Gas Sensors. Nanomaterials, 10(11), 2133. https://doi.org/10.3390/nano10112133







