Effect of TiO2 Nanotube Pore Diameter on Human Mesenchymal Stem Cells and Human Osteoblasts
Abstract
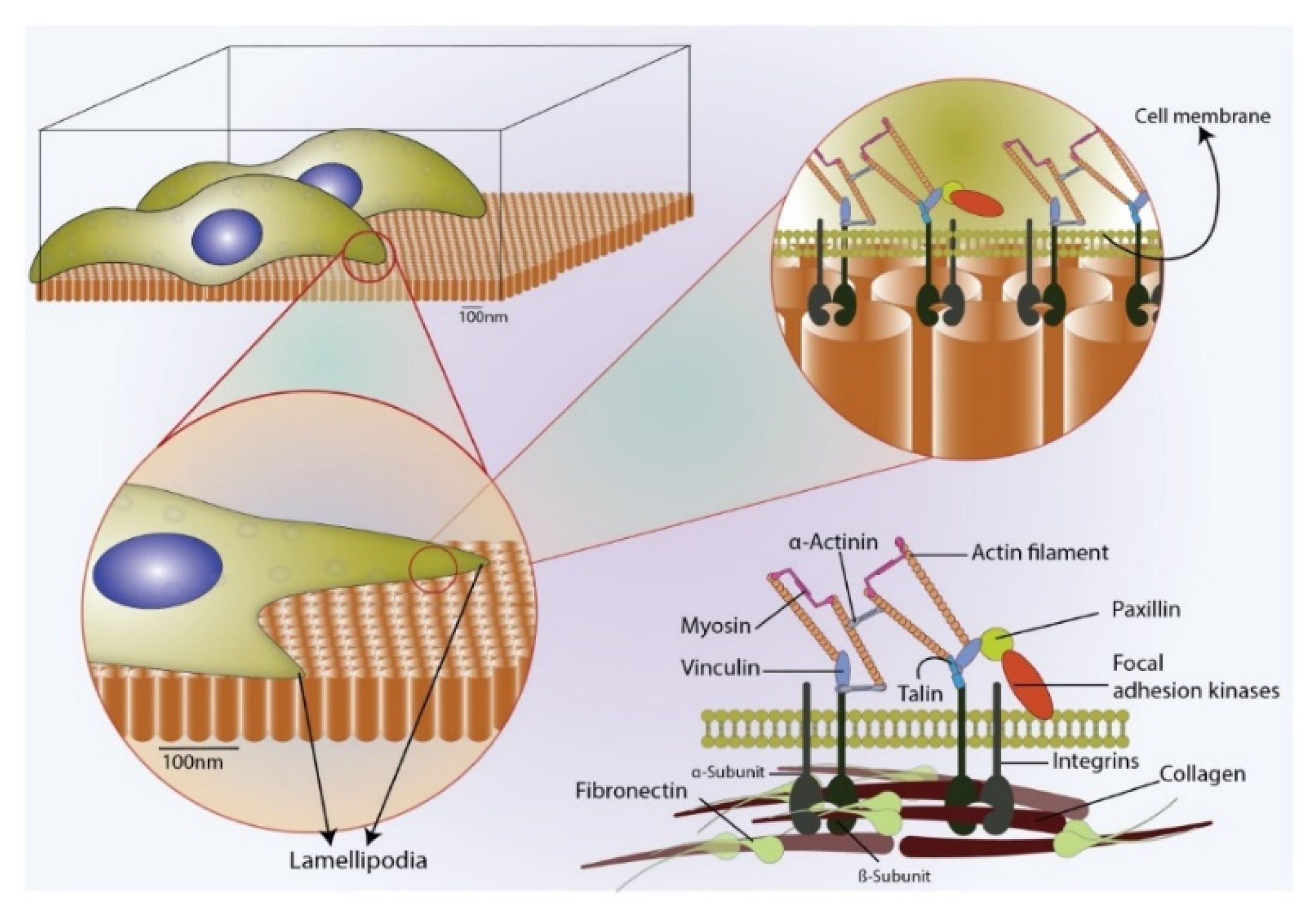
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Characterisation
2.2. Cell Culture
2.3. AlamarBlueTM Assay
2.4. LIVE/DEAD Cell Viability Assay
2.5. Cell lysate Preparation
2.6. PicoGreen DNA Quantification Assay
2.7. Alkaline Phosphatase (ALP) Assay
2.8. Immunofluorescence Staining
2.9. Data Presentation and Statistical Analysis
3. Results
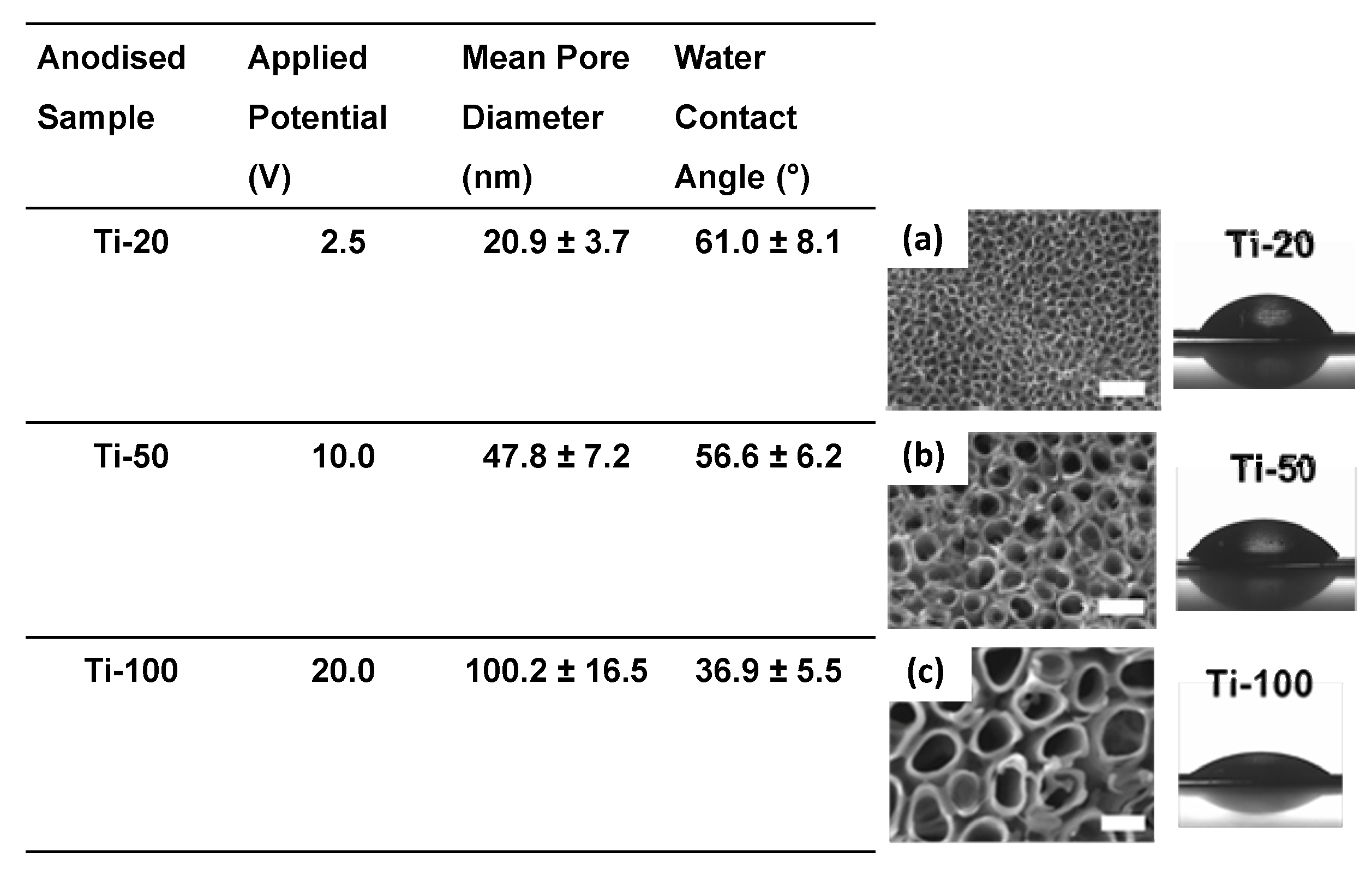
3.1. Surface Characterisation
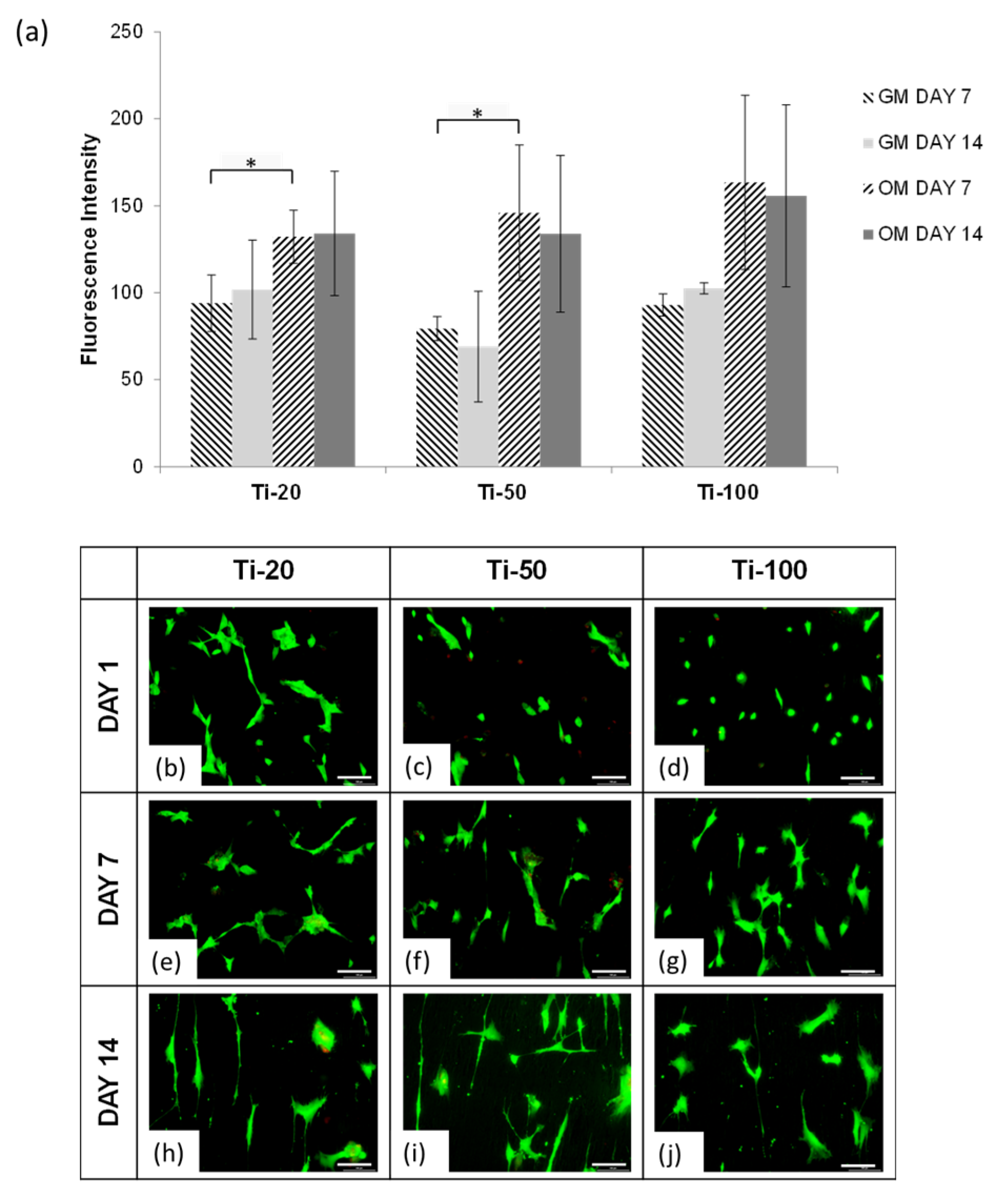
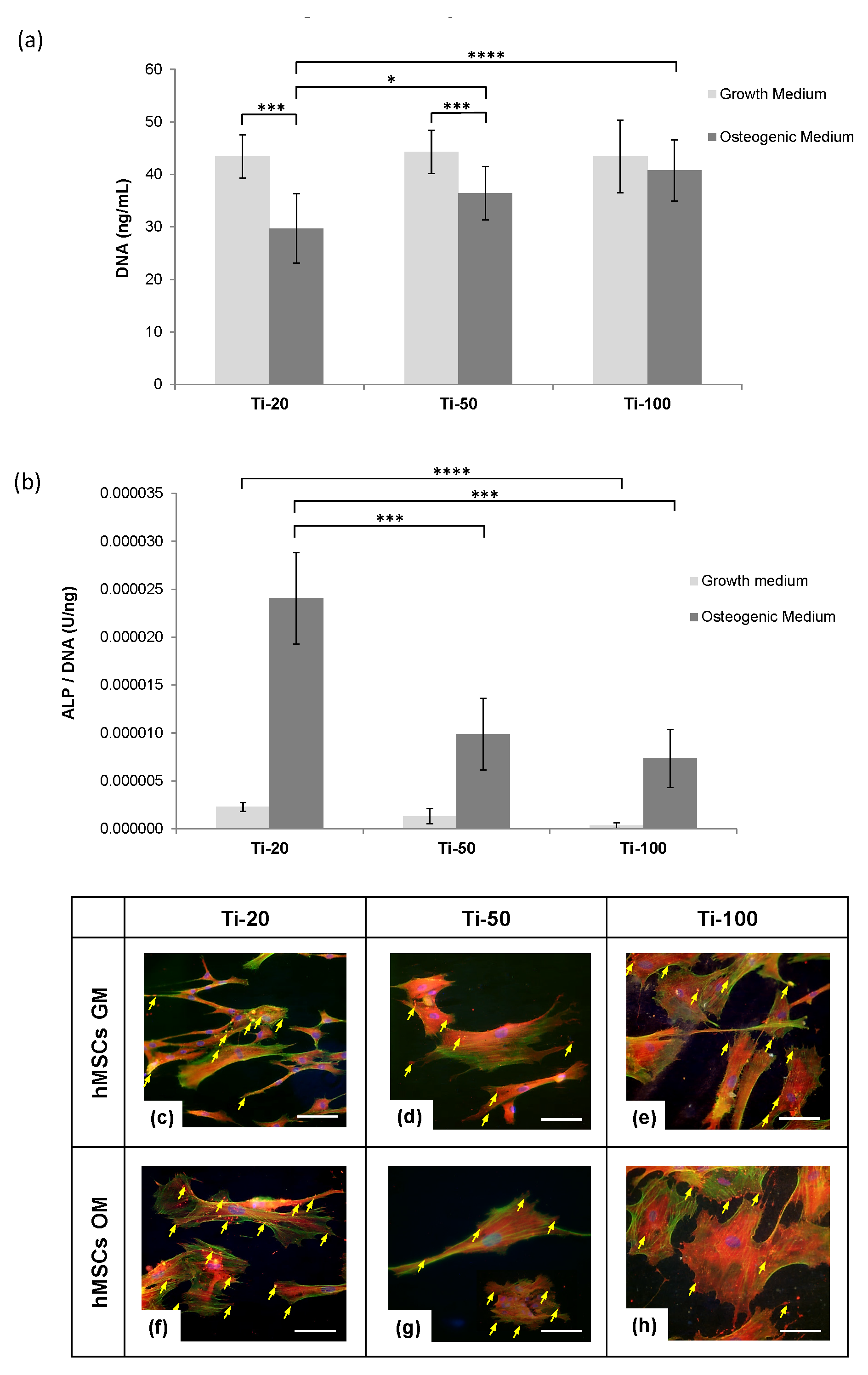
3.2. Human Mesenchymal Stem Cells Responses
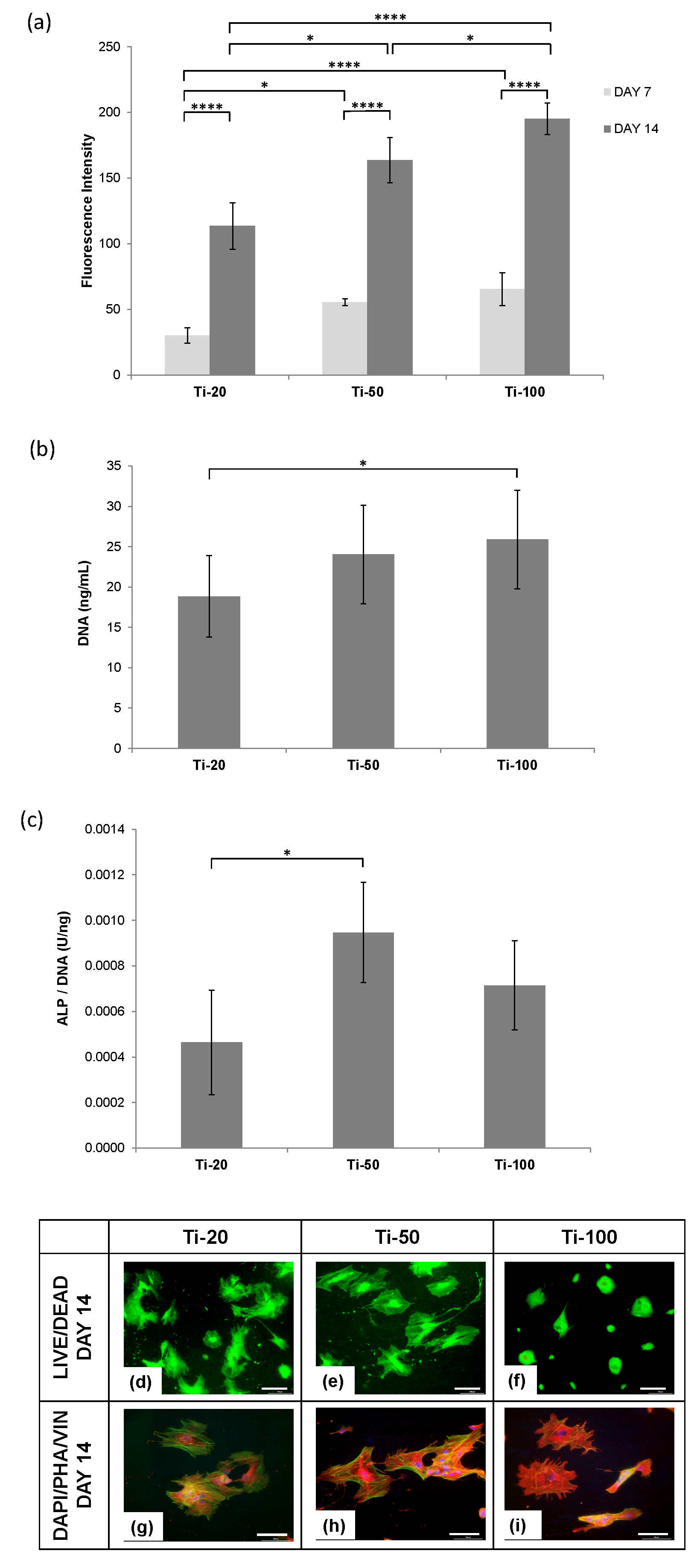
3.3. Human Osteoblast Cells Responses
4. Discussion
4.1. Human Mesenchymal Stem Cells
4.2. Human Osteoblasts
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Oshida, Y. Surface Modifications. In Bioscience and Bioengineering of Titanium Materials, 1st ed.; Elsevier Science: Indianapolis, IN, USA, 2007; Chapter 11; pp. 311–379. [Google Scholar]
- De Jonge, L.T.; Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Jansen, J.A. Organic-inorganic surface modifications for titanium implant surfaces. Pharm. Res. 2008, 25, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, C.; Mattmann, M.; Von Marttens, A.; Caviedes, P.; Arriagada, C.; Valenzuela, F.; Rodríguez, J.P.; Corral, C. Osseointegration properties of titanium dental implants modified with a nanostructured coating based on ordered porous silica and bioactive glass nanoparticles. Appl. Surf. Sci. 2016, 363, 286–295. [Google Scholar] [CrossRef]
- Brammer, K.S.; Oh, S.; Frandsen, C.J.; Jin, S. TiO2 nanotube structures for enhanced cell and biological functionality. JOM 2010, 62, 50–55. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Ni, M.; Rui, Y.; Cheng, T.; Cheng, C.; Pan, X.; Li, G.; Lin, C. Enhanced osteointegration of medical titanium implant with surface modifications in micro/nanoscale structures. J. Orthop. Transl. 2014, 2, 35–42. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Stevens, R.; Allsopp, D.; Jaroenworaluck, A. Anodised TiO2 nanotubes: Voltage ramp influence on the nano-structured oxide and investigation of phase changes promoted by thermal treatments. Phys. Status Solidi Appl. Mater. Sci. 2007, 204, 1814–1819. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, T.; Zhang, Z.; Li, G. Fabrication of highly ordered TiO2 nanotube arrays via anodization of Ti-6Al-4V alloy sheet. J. Nanosci. Nanotechnol. 2010, 10, 8312–8321. [Google Scholar] [CrossRef]
- Mingthong, P.; Veerasai, W.; Aeimbhu, A. Fabrication of titanium oxide nanotube arrays on titanium implants: The effect of electrolytes conditions. In Proceedings of the 6th World Congress of Biomechanics, Singapore, 1–6 August 2010; pp. 1208–1211. [Google Scholar]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, X.; Zhang, F.; Xu, L. The effect of anatase TiO2 nanotube layers on MC3T3-E1 preosteoblast adhesion, proliferation, and differentiation. J. Biomed. Mater. Res. Part A 2010, 94A, 1012–1022. [Google Scholar] [CrossRef]
- Filova, E.; Fojt, J.; Kryslova, M.; Moravec, H.; Joska, L.; Bacakova, L. The diameter of nanotubes formed on Ti-6Al-4V alloy controls the adhesion and differentiation of SaOS-2 cells. Int. J. Nanomed. 2015, 10, 7145–7163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, L.; Wu, Z.; Zhang, Y.; Chu, P.K. Effects of micropitted/nanotubular titania topographies on bone mesenchymal stem cell osteogenic differentiation. Biomaterials 2012, 33, 2629–2641. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Walters, N.J.; Gentleman, E. Evolving insights in cell-matrix interactions: Elucidating how non-soluble properties of the extracellular niche direct stem cell fate. Acta Biomater. 2015, 11, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Park, J.; Faltenbacher, J.; Berger, S.; von der Mark, K.; Schmuki, P. Size selective behavior of mesenchymal stem cells on ZrO2 and TiO2 nanotube arrays. Integr. Biol. (Camb.) 2009, 1, 525–532. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Schlegel, K.A.; Neukam, F.W.; von der Mark, K.; Schmuki, P. TiO2 nanotube surfaces: 15 nm—An optimal length scale of surface topography for cell adhesion and differentiation. Small 2009, 5, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Huo, K.; Zhang, X.; Wang, W.; Zhang, Y.; Wu, Z.; Chu, P.K. The osteogenic activity of strontium loaded titania nanotube arrays on titanium substrates. Biomaterials 2013, 34, 19–29. [Google Scholar] [CrossRef]
- Huang, H.H.; Wu, C.P.; Sun, Y.S.; Huang, H.M.; Lee, T.H. Enhanced corrosion resistance and biocompatibility of β-type Ti–25Nb–25Zr alloy by electrochemical anodization. Thin Solid Films 2013, 549, 87–92. [Google Scholar] [CrossRef]
- Wu, C.P.; Sun, Y.S.; Yang, W.E.; Lin, M.C.; Lee, T.H. Surface nanoporosity of β-type Ti–25Nb–25Zr alloy for the enhancement of protein adsorption and cell response. Surf. Coat. Technol. 2014, 259, 206–212. [Google Scholar]
- Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef]
- Ren, N.; Zhang, S.; Li, Y.; Shen, S.; Niu, Q.; Zhao, Y.; Kong, L. Bone mesenchymal stem cell functions on the hierarchical micro/nanotopographies of the Ti-6Al-7Nb alloy. Br. J. Oral Maxillofac. Surg. 2014, 52, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Brammer, K.S.; Julie Li, Y.S.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Lavenus, S.; Trichet, V.; Le Chevalier, S.; Hoornaert, A.; Louarn, G.; Layrolle, P. Cell differentiation and osseointegration influenced by nanoscale anodized titanium surfaces. Nanomedicine (Lond.) 2012, 7, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.Q.; Li, S.J.; Hao, Y.L.; Zhao, Y.K.; Ai, H.J. Effect of nanotube diameters on bioactivity of a multifunctional titanium alloy. Appl. Surf. Sci. 2013, 268, 44–51. [Google Scholar] [CrossRef]
- Yang, W.; Xi, X.; Shen, X.; Liu, P.; Hu, Y.; Cai, K. Titania nanotubes dimensions-dependent protein adsorption and its effect on the growth of osteoblasts. J. Biomed. Mater. Res. A 2014, 102, 3598–3608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, R.; Tan, J.; Wang, J.; Lu, X.; Qu, S.; Weng, J.; Feng, B. Osteoblast behaviors on titania nanotube and mesopore layers. Regen. Biomater. 2016, 4, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Minagar, S.; Li, Y.; Berndt, C.C.; Wen, C. The influence of titania-zirconia-zirconium titanate nanotube characteristics on osteoblast cell adhesion. Acta Biomater. 2015, 12, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, S.; Han, X.; Hao, Y.; Ai, H. Effects of the surface characteristics of nanoporous titanium oxide films on Ti-24Nb-4Zr-8Sn alloy on the initial adhesion of osteoblast-like MG-63 cells. Exp. Ther. Med. 2013, 6, 241–247. [Google Scholar] [CrossRef]
- Minagar, S.; Li, Y.; Berndt, C.C.; Wen, C. Cell response and bioactivity of titania–zirconia–zirconium titanate nanotubes with different nanoscale topographies fabricated in a non-aqueous electrolyte. Biomater. Sci. 2015, 3, 636–644. [Google Scholar] [CrossRef]
- Grigorescu, S.; Pruna, V.; Titorencu, I.; Jinga, V.V.; Mazare, A.; Schmuki, P.; Demetrescu, I. The two step nanotube formation on TiZr as scaffolds for cell growth. Bioelectrochemistry 2014, 98, 39–45. [Google Scholar] [CrossRef]
- Roguska, A.; Pisarek, M.; Belcarz, A.; Marcon, L.; Hołdyński, M.; Andrzejczuk, M.; Janik-Czachor, M. Improvement of the bio-functional properties of TiO2 nanotubes. Appl. Surf. Sci. 2016, 388, 775–785. [Google Scholar] [CrossRef]
- Liu, P.; Hao, Y.; Zhao, Y.; Yuan, Z.; Ding, Y.; Cai, K. Surface modification of titanium substrates for enhanced osteogenetic and antibacterial properties. Colloids Surf. B Biointerfaces 2017, 160, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Slamovich, E.B.; Webster, T.J. Enhanced osteoblast functions on anodized titanium with nanotube-like structures. J. Biomed. Mater. Res. A 2008, 85, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hashimoto, T.; Tsai, I.L.; Thompson, G.E.; Skeldon, P.; Liu, H. Scanning transmission electron microscopy technique for morphology analysis of anodic oxide film formed on titanium. Vacuum 2015, 115, 19–22. [Google Scholar] [CrossRef][Green Version]
- Rawsterne, R.E.; Gough, J.E.; Rutten, F.J.; Pham, N.T.; Poon, W.C.; Flitsch, S.L.; Maltman, B.; Alexander, M.R.; Ulijn, R.V. Controlling protein retention on enzyme-responsive surfaces. Surf. Interface Anal. 2006, 38, 1505–1511. [Google Scholar] [CrossRef]
- Frosch, K.H.; Barvencik, F.; Lohmann, C.H.; Viereck, V.; Siggelkow, H.; Breme, J.; Dresing, K.; Stürmer, K.M. Migration, matrix production and lamellar bone formation of human osteoblast-like cells in porous titanium implants. Cells. Tissues. Organs 2002, 170, 214–227. [Google Scholar] [CrossRef]
- Das, K.; Bose, S.; Bandyopadhyay, A. TiO2 nanotubes on Ti: Influence of nanoscale morphology on bone cell-materials interaction. J. Biomed. Mater. Res. A 2009, 90, 225–237. [Google Scholar] [CrossRef]
- Ferreira, L.; Karp, J.M.; Nobre, L.; Langer, R. New Opportunities: The use of nanotechnologies to manipulate and track stem cells. Cell Stem Cell 2008, 3, 136–146. [Google Scholar] [CrossRef]
- Arnold, M.; Cavalcanti-Adam, E.A.; Glass, R.; Blümmel, J.; Eck, W.; Kantlehner, M.; Kessler, H.; Spatz, J.P. Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem 2004, 5, 383–388. [Google Scholar] [CrossRef]
- Li, J.R.; Shi, L.; Deng, Z.; Lo, S.H.; Liu, G. Nanostructures of designed geometry and functionality enable regulation of cellular signaling processes. Biochemistry 2012, 51, 5876–5893. [Google Scholar] [CrossRef]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Sjöström, T.; Dalby, M.J.; Hart, A.; Tare, R.; Oreffo, R.O.C.; Su, B. Fabrication of pillar-like titania nanostructures on titanium and their interactions with human skeletal stem cells. Acta Biomater. 2009, 5, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Oberdörster, G.; Elder, A.; Gelein, R.; Mercer, P.; Biswas, P. Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology 2008, 2, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Gong, L.; Cui, X.; Zhang, Z.; Bajpai, A.; Liu, C.; Castillo, A.B.; Teo, J.; Chen, W. Nanotopographic regulation of human mesenchymal stem cell osteogenesis. ACS Appl. Mater. Interfaces 2017, 9, 41794–41806. [Google Scholar] [CrossRef]
- Puklin-Faucher, E.; Sheetz, M.P. The mechanical integrin cycle. J. Cell Sci. 2009, 122, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lee, H.; Zhu, C. Molecular mechanisms of mechanotransduction in integrin-mediated cell-matrix adhesion. Exp. Cell Res. 2016, 349, 85–94. [Google Scholar] [CrossRef]
- Ohashi, K.; Fujiwara, S.; Mizuno, K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J. Biochem. 2017, 161, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Biggs, M.J.; Richards, R.; McFarlane, S.; Wilkinson, C.D.; Oreffo, R.O.; Dalby, M. Adhesion formation of primary human osteoblasts and the functional response of mesenchymal stem cells to 330 nm deep microgrooves. J. R. Soc. Interface 2008, 5, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Herzyk, P.; Sutherland, D.; Agheli, H.; Wilkinson, C.D.W.; Curtis, A.S.G. Nanomechanotransduction and interphase nuclear organization influence on genomic control. J. Cell. Biochem. 2007, 102, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Cellular tensegrity: Defining new rules of biological design that govern the cytoskeleton. J. Cell Sci. 1993, 104, 613–627. [Google Scholar] [PubMed]
- Ge, J.; Guo, L.; Wang, S.; Zhang, Y.; Cai, T.; Zhao, R.C.; Wu, Y. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Rev. Rep. 2014, 10, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Wheeless, C.R. Osteoblasts—Wheeless’ Textbook of Orthopaedics, 3rd ed.; Duke Orthopaedics: Durham, NC, USA, 2016. [Google Scholar]
- Tsimbouri, P.; Gadegaard, N.; Burgess, K.; White, K.; Reynolds, P.; Herzyk, P.; Oreffo, R.; Dalby, M.J. Nanotopographical effects on mesenchymal stem cell morphology and phenotype. J. Cell. Biochem. 2014, 115, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Hue, K.A.; Mayes, A.M.; Griffith, L.G. Simulations of cell-surface integrin binding to nanoscale-clustered adhesion ligands. Biophys. J. 2002, 82, 120–132. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.; Oreffo, R.O. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Oyefusi, A.; Olanipekun, O.; Neelgund, G.M.; Peterson, D.; Stone, J.M.; Williams, E.; Carson, L.; Regisford, G.; Oki, A. Hydroxyapatite grafted carbon nanotubes and graphene nanosheets: Promising bone implant materials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 410–416. [Google Scholar] [CrossRef]
- Harris, S.A.; Zhang, M.; Kidder, L.S.; Evans, G.L.; Spelsberg, T.C.; Turner, R.T. Effects of orbital spaceflight on human osteoblastic cell physiology and gene expression. Bone 2000, 26, 325–331. [Google Scholar] [CrossRef][Green Version]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces. Part I: Materials and surfaces. Prog. Mater. Sci. 2012, 58, 261–326. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Siedlecki, C.A. Hemocompatibility of Biomaterials for Clinical Applications: Blood-Biomaterials Interactions, 1st ed.; Woodhead Publishing: Philadelphia, PA, USA, 2018; pp. 457–469. [Google Scholar]
- de Mel, A.; Cousins, B.G.; Seifalian, A.M. Surface modification of biomaterials: A quest for blood compatibility. Int. J. Biomater. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Solouk, A.; Cousins, B.G.; Mirzadeh, H.; Seifalian, A.M. Application of plasma surface modification techniques to improve hemocompatibility of vascular grafts: A review. Biotechnol. Appl. Biochem. 2011, 58, 311–327. [Google Scholar] [CrossRef]
- Movafaghi, S.; Leszczak, V.; Wang, W.; Sorkin, J.A.; Dasi, L.P.; Popat, K.C.; Kota, A.K. Hemocompatibility of superhemophobic titania surfaces. Adv. Healthc. Mater. 2017, 6, 201600717. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Tamjid, E.; Dinari, A. Enhanced cell attachment and hemocompatibility of titanium by nanoscale surface modification through severe plastic integration of magnesium-rich islands and porosification. Sci. Rep. 2017, 7, 12965. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaw, J.S.; Bowen, C.R.; Cartmell, S.H. Effect of TiO2 Nanotube Pore Diameter on Human Mesenchymal Stem Cells and Human Osteoblasts. Nanomaterials 2020, 10, 2117. https://doi.org/10.3390/nano10112117
Khaw JS, Bowen CR, Cartmell SH. Effect of TiO2 Nanotube Pore Diameter on Human Mesenchymal Stem Cells and Human Osteoblasts. Nanomaterials. 2020; 10(11):2117. https://doi.org/10.3390/nano10112117
Chicago/Turabian StyleKhaw, Juan Shong, Christopher R. Bowen, and Sarah H. Cartmell. 2020. "Effect of TiO2 Nanotube Pore Diameter on Human Mesenchymal Stem Cells and Human Osteoblasts" Nanomaterials 10, no. 11: 2117. https://doi.org/10.3390/nano10112117
APA StyleKhaw, J. S., Bowen, C. R., & Cartmell, S. H. (2020). Effect of TiO2 Nanotube Pore Diameter on Human Mesenchymal Stem Cells and Human Osteoblasts. Nanomaterials, 10(11), 2117. https://doi.org/10.3390/nano10112117





