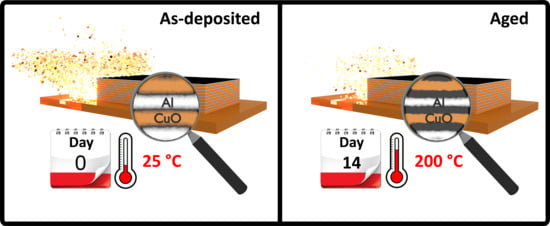
How Thermal Aging Affects Ignition and Combustion Properties of Reactive Al/CuO Nanolaminates: A Joint Theoretical/Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Details
2.2. Nanolaminate Fabrication
2.3. Experiments
2.3.1. Initiation Time Experiments
2.3.2. Burn-Rate Experiments
2.3.3. Thermal Analysis
3. Results and Discussion
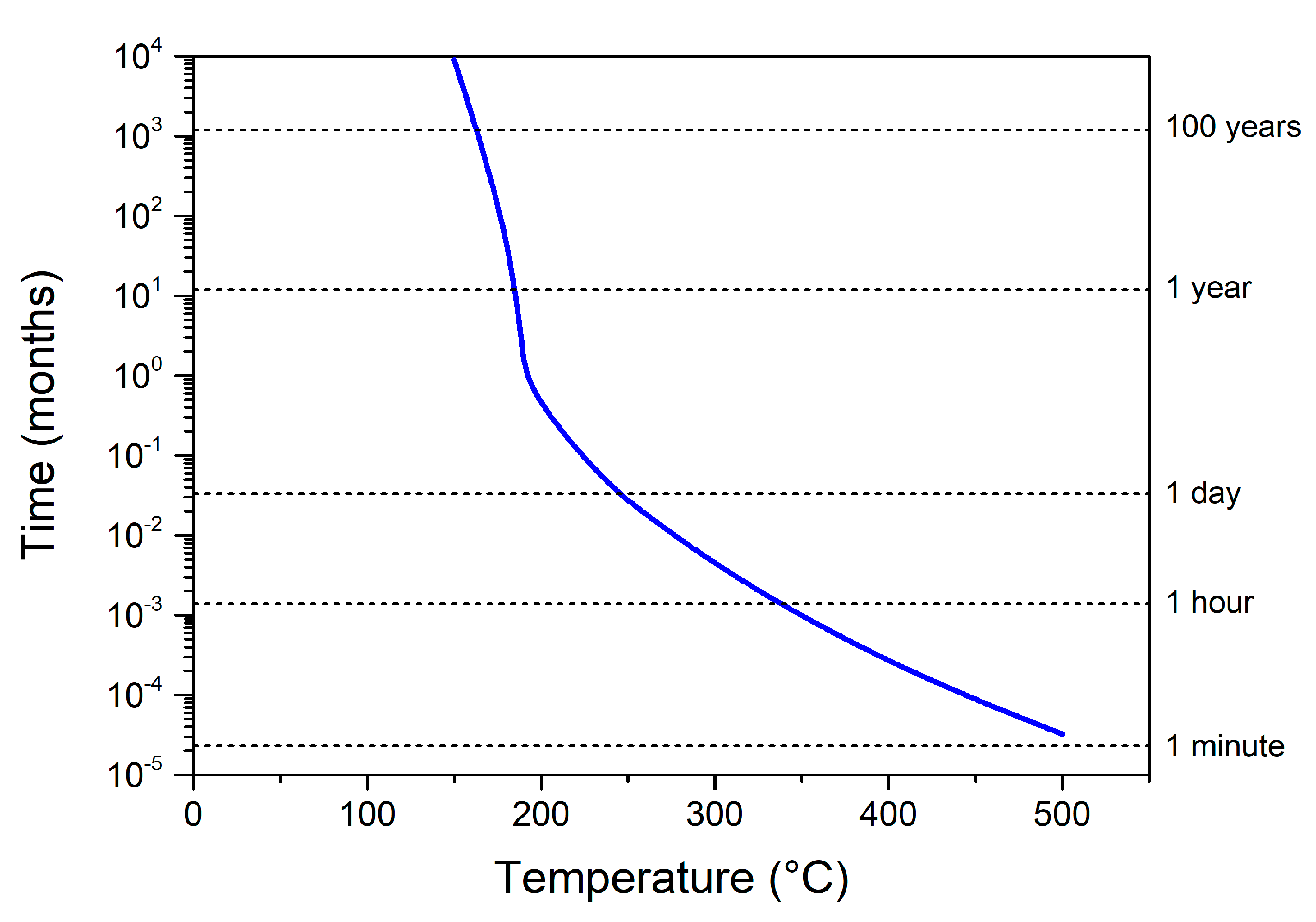
3.1. Modelling the Long-Term Aging at the Ambient Temperature
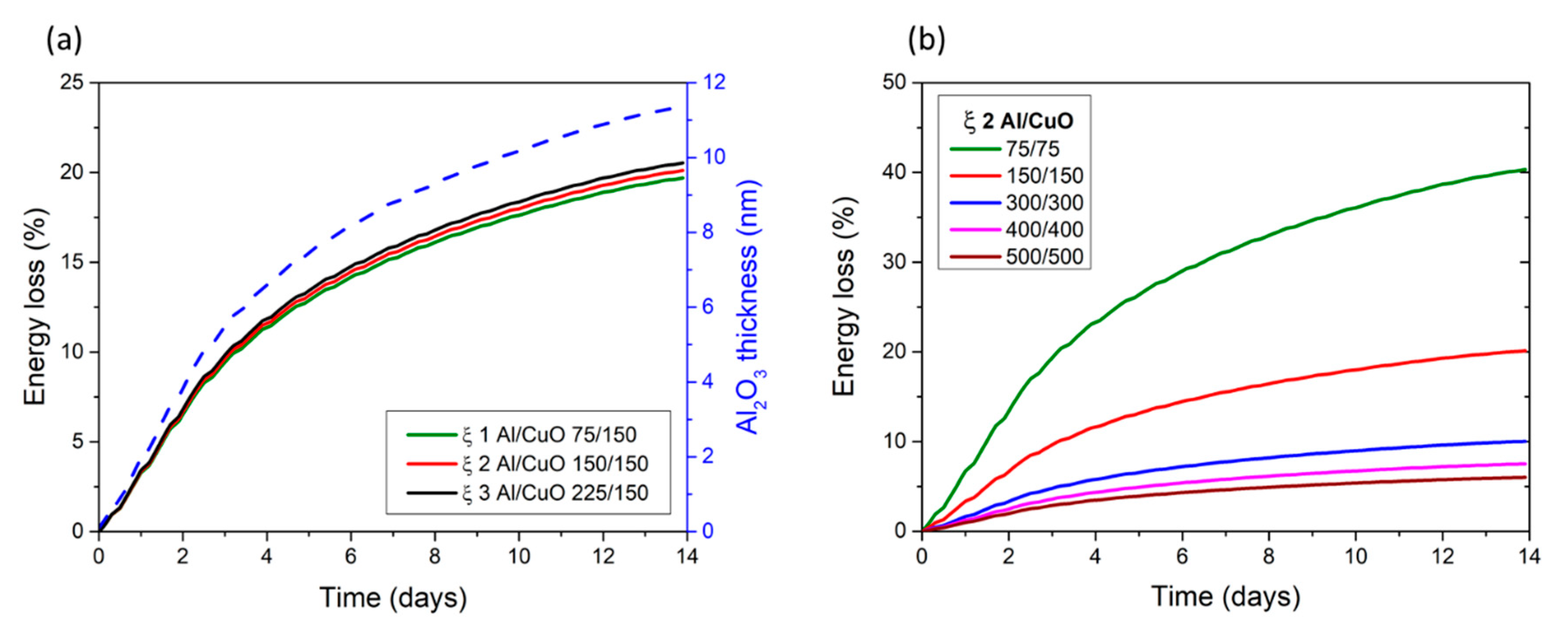
3.2. Accelerated Thermal Aging
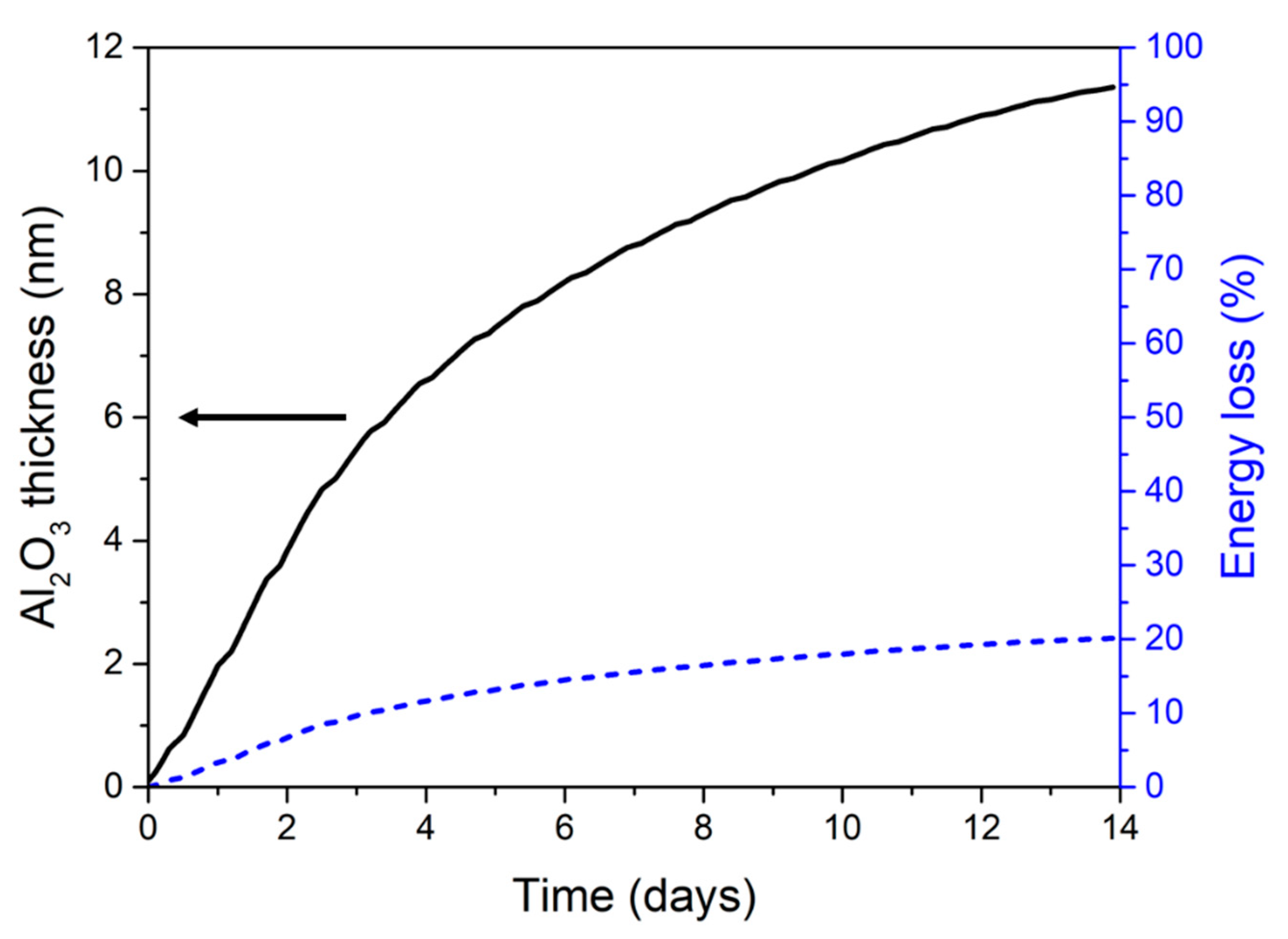
3.3. Modelling the Structural Modifications and Associated Loss of Energy Reservoir Upon Thermal Aging
3.4. Impact of Aging on Nanolaminate Performance
3.4.1. Initiation
3.4.2. Burn Rate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bezmelnitsyn, A.; Thiruvengadathan, R.; Barizuddin, S.; Tappmeyer, D.; Apperson, S.; Gangopadhyay, K.; Gangopadhyay, K.; Redner, P.; Donadio, M.; Kapoor, D.; et al. Modified Nanoenergetic Composites with Tunable Combustion Characteristics for Propellant Applications. Propellants Explos. Pyrotech. 2010, 35, 384–394. [Google Scholar] [CrossRef]
- Staley, C.S.; Raymond, K.E.; Thiruvengadathan, R.; Apperson, S.J.; Gangopadhyay, K.; Swaszek, S.M.; Taylor, R.J.; Gangopadhyay, S. Fast-Impulse Nanothermite Solid-Propellant Miniaturized Thrusters. J. Propuls. Power 2013, 29, 1400–1409. [Google Scholar] [CrossRef]
- Zhou, X.; Torabi, M.; Lu, J.; Shen, R.; Zhang, K. Nanostructured Energetic Composites: Synthesis, Ignition/Combustion Modeling, and Applications. ACS Appl. Mater. Interfaces 2014, 6, 3058–3074. [Google Scholar] [CrossRef] [PubMed]
- Stamatis, D.; Dreizin, E.L.; Higa, K. Thermal Initiation of Al-MoO3 Nanocomposite Materials Prepared by Different Methods. J. Propuls. Power 2011, 27, 1079–1087. [Google Scholar] [CrossRef]
- Duckham, A.; Spey, S.J.; Wang, J.; Reiss, M.E.; Weihs, T.; Besnoin, E.; Knio, O.M. Reactive nanostructured foil used as a heat source for joining titanium. J. Appl. Phys. 2004, 96, 2336–2342. [Google Scholar] [CrossRef]
- Rossi, C.; Zhang, K.; Esteve, D.; Alphonse, P.; Tailhades, P.; Vahlas, C. Nanoenergetic Materials for MEMS: A Review. J. Microelectromech. Syst. 2007, 16, 919–931. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Shen, Y.; Wang, C.; Dai, J.; Tai, Y.; Ye, Y.; Shen, R.; Wang, H.; Zachariah, M.R. Controlling the energetic characteristics of micro energy storage device by in situ deposition Al/MoO3 nanolaminates with varying internal structure. Chem. Eng. J. 2019, 373, 345–354. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, R.; Ye, Y.; Zhu, P.; Hu, Y.; Wu, L. Influence of Al/CuO reactive multilayer films additives on exploding foil initiator. J. Appl. Phys. 2011, 110, 094505. [Google Scholar] [CrossRef]
- Zhu, P.; Shen, R.; Ye, Y.; Fu, S.; Li, D. Characterization of Al/CuO nanoenergetic multilayer films integrated with semiconductor bridge for initiator applications. J. Appl. Phys. 2013, 113, 184505. [Google Scholar] [CrossRef]
- Zhu, P.; Shen, R.; Fiadosenka, N.N.; Ye, Y.; Hu, Y. Dielectric structure pyrotechnic initiator realized by integrating Ti/CuO-based reactive multilayer films. J. Appl. Phys. 2011, 109, 084523. [Google Scholar] [CrossRef]
- Nicollet, A.; Lahiner, G.; Belisario, A.; Assié-Souleille, S.; Rouhani, M.D.; Estève, A.; Rossi, C. Investigation of Al/CuO multilayered thermite ignition. J. Appl. Phys. 2017, 121, 034503. [Google Scholar] [CrossRef] [Green Version]
- Taton, G.; Lagrange, D.; Conedera, V.; Renaud, L.; Rossi, C. Micro-chip initiator realized by integrating Al/CuO multilayer nanothermite on polymeric membrane. J. Micromech. Microeng. 2013, 23, 105009. [Google Scholar] [CrossRef]
- Fu, S.; Shen, R.; Zhu, P.; Ye, Y. Metal–interlayer–metal structured initiator containing Al/CuO reactive multilayer films that exhibits improved ignition properties. Sens. Actuators A Phys. 2019, 292, 198–204. [Google Scholar] [CrossRef]
- Korampally, M.; Apperson, S.J.; Staley, C.S.; Castorena, J.A.; Thiruvengadathan, R.; Gangopadhyay, K.; Mohan, R.R.; Ghosh, A.; Polo-Parada, L.; Gangopadhyay, S. Transient pressure mediated intranuclear delivery of FITC-Dextran into chicken cardiomyocytes by MEMS-based nanothermite reaction actuator. Sens. Actuators B Chem. 2012, 171, 1292–1296. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Piekiel, N.W.; Chowdhury, S.; Wu, C.; Zachariah, M.R.; Johnson, C.E. Ignition and Combustion Characteristics of Nanoscale Al/AgIO3: A Potential Energetic Biocidal System. Combust. Sci. Technol. 2010, 183, 285–302. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Wu, C.; Piekiel, N.W.; Gaskell, K.; Zachariah, M.R. Synthesis and reactivity of nano-Ag2O as an oxidizer for energetic systems yielding antimicrobial products. Combust. Flame 2013, 160, 438–446. [Google Scholar] [CrossRef]
- Rossi, C. Engineering of Al/CuO Reactive Multilayer Thin Films for Tunable Initiation and Actuation. Propellants Explos. Pyrotech. 2018, 44, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167. [Google Scholar] [CrossRef]
- Egan, G.C.; Mily, E.J.; Maria, J.-P.; Zachariah, M.R. Probing the Reaction Dynamics of Thermite Nanolaminates. J. Phys. Chem. C 2015, 119, 20401–20408. [Google Scholar] [CrossRef]
- Manesh, N.A.; Basu, S.; Kumar, R. Experimental flame speed in multi-layered nano-energetic materials. Combust. Flame 2010, 157, 476–480. [Google Scholar] [CrossRef]
- Mily, E.; Oni, A.; Lebeau, J.; Liu, Y.; Brown-Shaklee, H.; Ihlefeld, J.F.; Maria, J.-P. The role of terminal oxide structure and properties in nanothermite reactions. Thin Solid Films 2014, 562, 405–410. [Google Scholar] [CrossRef]
- Zhu, P.; Shen, R.Q.; Ye, Y.H.; Zhou, X.; Hu, Y.; Wu, L.Z. Energetic Igniters Based on Al/CuO/B/Ti Reactive Multilayer Films. Theory and Practice of Energetic Materials (Vol Ix). In Proceedings of the 2011 International Autumn Seminar on Propellants, Explosives and Pyrotechnics, Nanjing, China, 20–23 September 2011; pp. 756–760. [Google Scholar]
- Nie, H.; Chan, H.Y.; Pisharath, S.; Hng, H.H. Combustion characteristic and aging behavior of bimetal thermite powders. Def. Technol. 2020. [Google Scholar] [CrossRef]
- Wang, C.-A.; Xu, J.-B.; Shen, Y.; Wang, Y.-T.; Yang, T.-L.; Zhang, Z.-H.; Li, F.-W.; Shen, R.-Q.; Ye, Y. Thermodynamics and performance of Al/CuO nanothermite with different storage time. Def. Technol. 2020. [Google Scholar] [CrossRef]
- Abdallah, I.; Zapata, J.; Lahiner, G.; Warot-Fonrose, B.; Cure, J.; Chabal, Y.J.; Esteve, A.; Rossi, C. Structure and Chemical Characterization at the Atomic Level of Reactions in Al/CuO Multilayers. ACS Appl. Energy Mater. 2018, 1, 1762–1770. [Google Scholar] [CrossRef]
- Lahiner, G.; Zapata, J.; Cure, J.; Richard, N.; Djafari-Rouhani, M.; Estève, A.; Rossi, C. A redox reaction model for self-heating and aging prediction of Al/CuO multilayers. Combust. Theory Model. 2019, 23, 700–715. [Google Scholar] [CrossRef] [Green Version]
- Deal, B.E.; Grove, A.S. General Relationship for the Thermal Oxidation of Silicon. J. Appl. Phys. 1965, 36, 3770–3778. [Google Scholar] [CrossRef] [Green Version]
- Fischer, S.; Grubelich, M. Theoretical energy release of thermites, intermetallics, and combustible metals. In Proceedings of the Twenty-Fourth International Pyrotechnics Seminar, Monterey, CA, USA, 27–31 July 1998; pp. 231–286. [Google Scholar]
- Nabatame, T.; Yasuda, T.; Nishizawa, M.; Ikeda, M.; Horikawa, T.; Toriumi, A. Comparative studies on oxygen diffusion coefficients for amorphous and gamma-Al2O3 films using O-18 isotope. Jpn. J. Appl. Phys. 2003, 42, 7205–7208. [Google Scholar] [CrossRef]
- Julien, B.; Cure, J.; Salvagnac, L.; Josse, C.; Esteve, A.; Rossi, C. Integration of Gold Nanoparticles to Modulate the Ignitability of Nanothermite Films. ACS Appl. Nano Mater. 2020, 3, 2562–2572. [Google Scholar] [CrossRef]
- Zapata, J.; Nicollet, A.; Julien, B.; Lahiner, G.; Esteve, A.; Rossi, C. Self-propagating combustion of sputter-deposited Al/CuO nanolaminates. Combust. Flame 2019, 205, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Lahiner, G.; Nicollet, A.; Zapata, J.; Marín, L.; Richard, N.; Rouhani, M.D.; Rossi, C.; Estève, A. A diffusion–reaction scheme for modeling ignition and self-propagating reactions in Al/CuO multilayered thin films. J. Appl. Phys. 2017, 122, 155105. [Google Scholar] [CrossRef]
- Wang, H.; Julien, B.; Kline, D.; Alibay, Z.; Rehwoldt, M.C.; Rossi, C.; Zachariah, M.R. Probing the Reaction Zone of Nanolaminates at ∼μs Time and ∼μm Spatial Resolution. J. Phys. Chem. C 2020, 124, 13679–13687. [Google Scholar] [CrossRef]
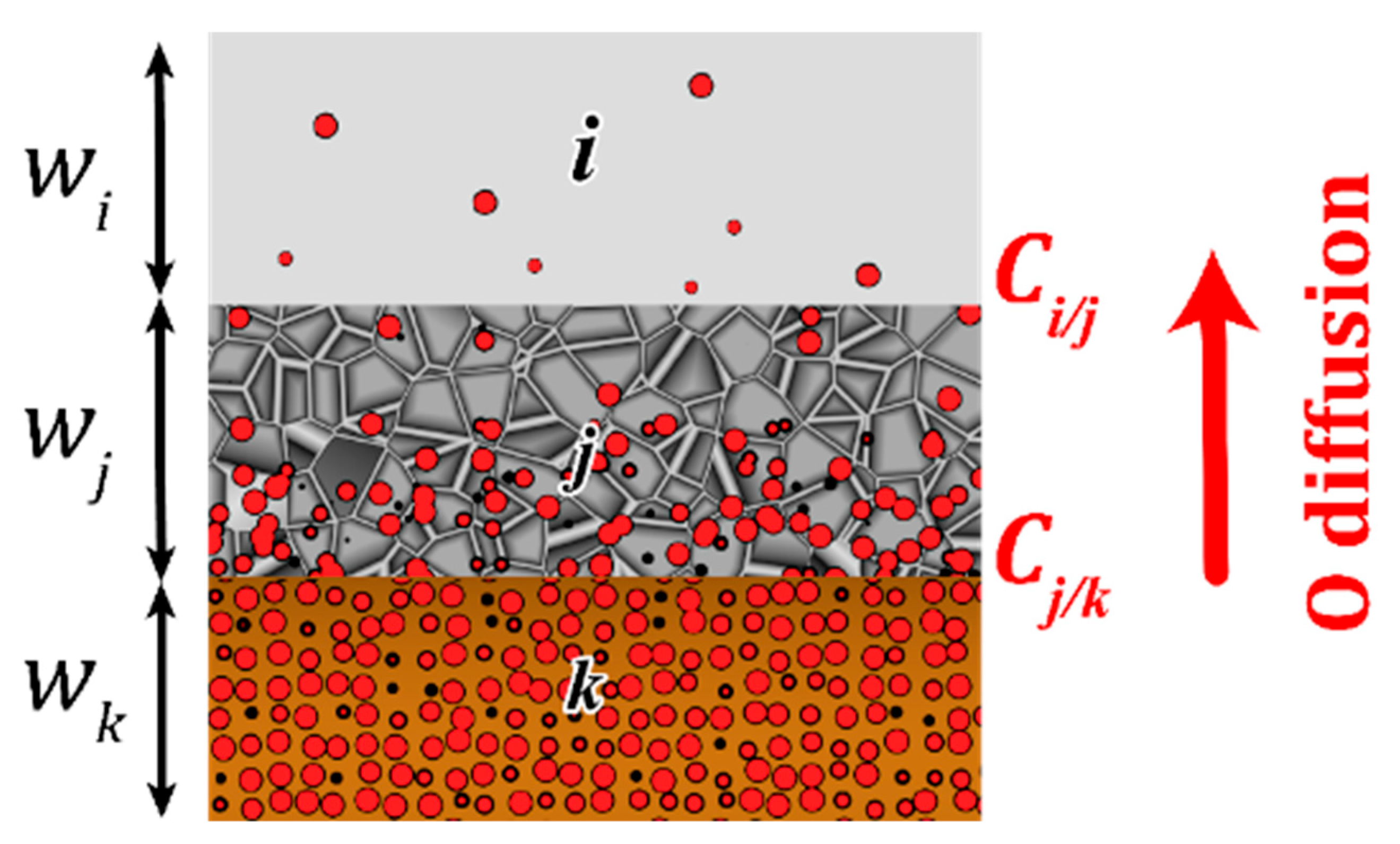




| Mechanisms | Arrhenius Parameters | Source |
|---|---|---|
| Oxygen diffusion through natural AlxCuyOz | = 1 m2·s−1 = 100 kJ·mol−1 | Calibrated from DSC experiments, see Ref. [26] |
| Oxygen diffusion through amorphous | = 1.67 m2·s−1 = 120 kJ·mol−1 | Isotopic labelling, see Ref. [29] |
| Nanolaminate Configuration | Grown Interfacial Alumina Thickness | Energy Loss in % |
|---|---|---|
| ξ 1 Al/CuO 75/150 | 0.3 nm | 0.023% |
| ξ 1 Al/CuO 100/200 | 0.017% | |
| ξ 1 Al/CuO 150/300 | 0.008% | |
| ξ 2 Al/CuO 75/75 | 0.048% | |
| ξ 2 Al/CuO 100/100 | 0.036% | |
| ξ 2 Al/CuO 150/150 | 0.018% | |
| ξ 3 Al/CuO 75/50 | 0.072% | |
| ξ 3 Al/CuO 100/67 | 0.054% | |
| ξ 3 Al/CuO 150/100 | 0.027% |
| Experiments Macroscopic Burn Rate m/s | From Model [32] m/s | |
|---|---|---|
| ξ 1 Al/CuO 75/150 | ||
| 15 bilayers | ||
| As-deposited | 4.6 ± 0.5 | 4.2 |
| Aged 14 days at 200 °C | 2.7 ± 0.5 | 3.0 |
| ξ 2 Al/CuO 150/150 | ||
| 11 bilayers | ||
| As-deposited | 11.7 ± 0.5 | 4.6 |
| Aged 14 days at 200 °C | 5.5 ± 0.5 | 3.8 |
| ξ 3 Al/CuO 225/150 | ||
| 9 bilayers | ||
| As-deposited | 10.1 ± 0.5 | 4.7 |
| Aged 14 days at 200 °C | 3.3 ± 0.5 | 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estève, A.; Lahiner, G.; Julien, B.; Vivies, S.; Richard, N.; Rossi, C. How Thermal Aging Affects Ignition and Combustion Properties of Reactive Al/CuO Nanolaminates: A Joint Theoretical/Experimental Study. Nanomaterials 2020, 10, 2087. https://doi.org/10.3390/nano10102087
Estève A, Lahiner G, Julien B, Vivies S, Richard N, Rossi C. How Thermal Aging Affects Ignition and Combustion Properties of Reactive Al/CuO Nanolaminates: A Joint Theoretical/Experimental Study. Nanomaterials. 2020; 10(10):2087. https://doi.org/10.3390/nano10102087
Chicago/Turabian StyleEstève, A., G. Lahiner, B. Julien, S. Vivies, N. Richard, and C. Rossi. 2020. "How Thermal Aging Affects Ignition and Combustion Properties of Reactive Al/CuO Nanolaminates: A Joint Theoretical/Experimental Study" Nanomaterials 10, no. 10: 2087. https://doi.org/10.3390/nano10102087
APA StyleEstève, A., Lahiner, G., Julien, B., Vivies, S., Richard, N., & Rossi, C. (2020). How Thermal Aging Affects Ignition and Combustion Properties of Reactive Al/CuO Nanolaminates: A Joint Theoretical/Experimental Study. Nanomaterials, 10(10), 2087. https://doi.org/10.3390/nano10102087