Preparation and Characterization of Al/HTPB Composite for High Energetic Materials
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
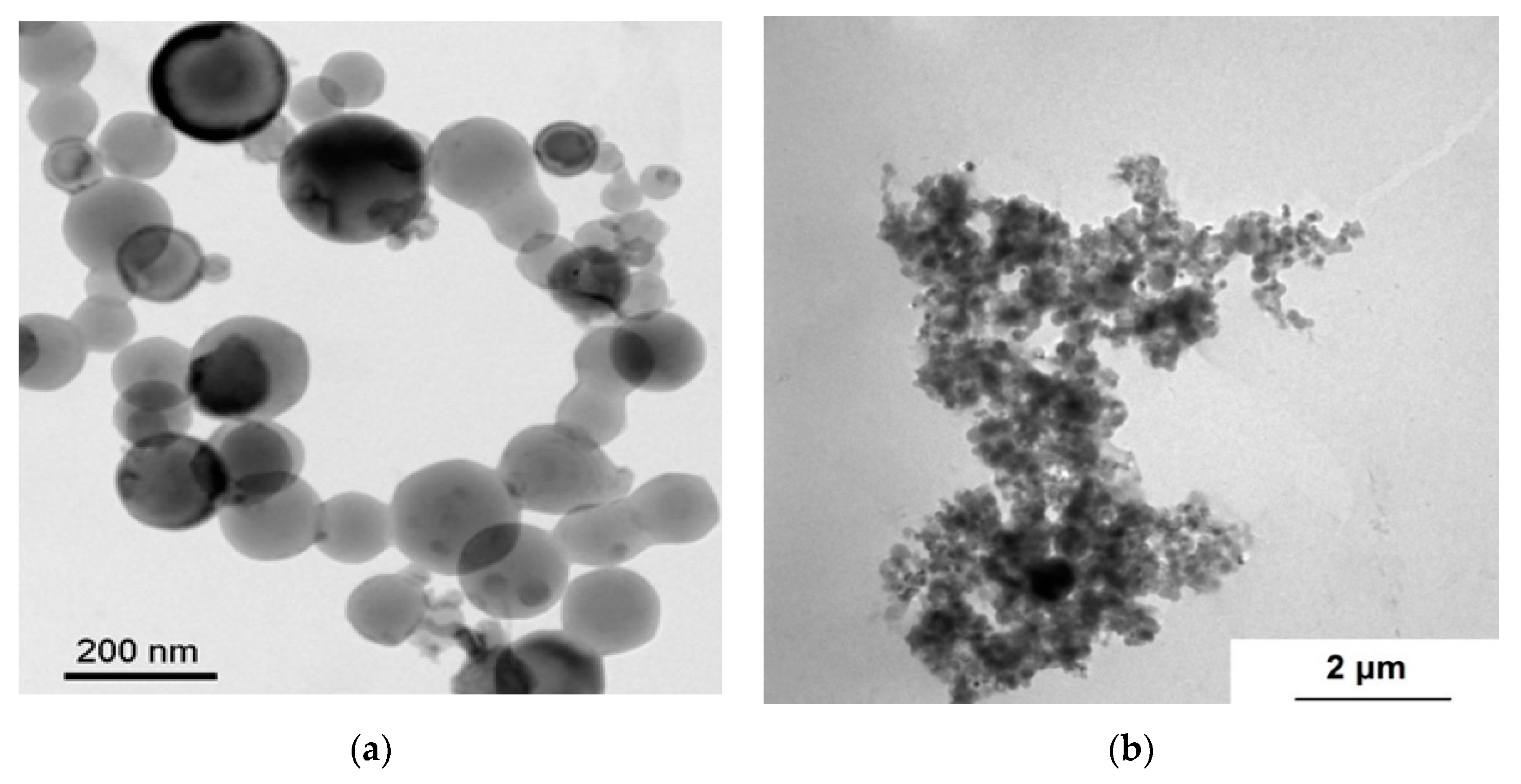
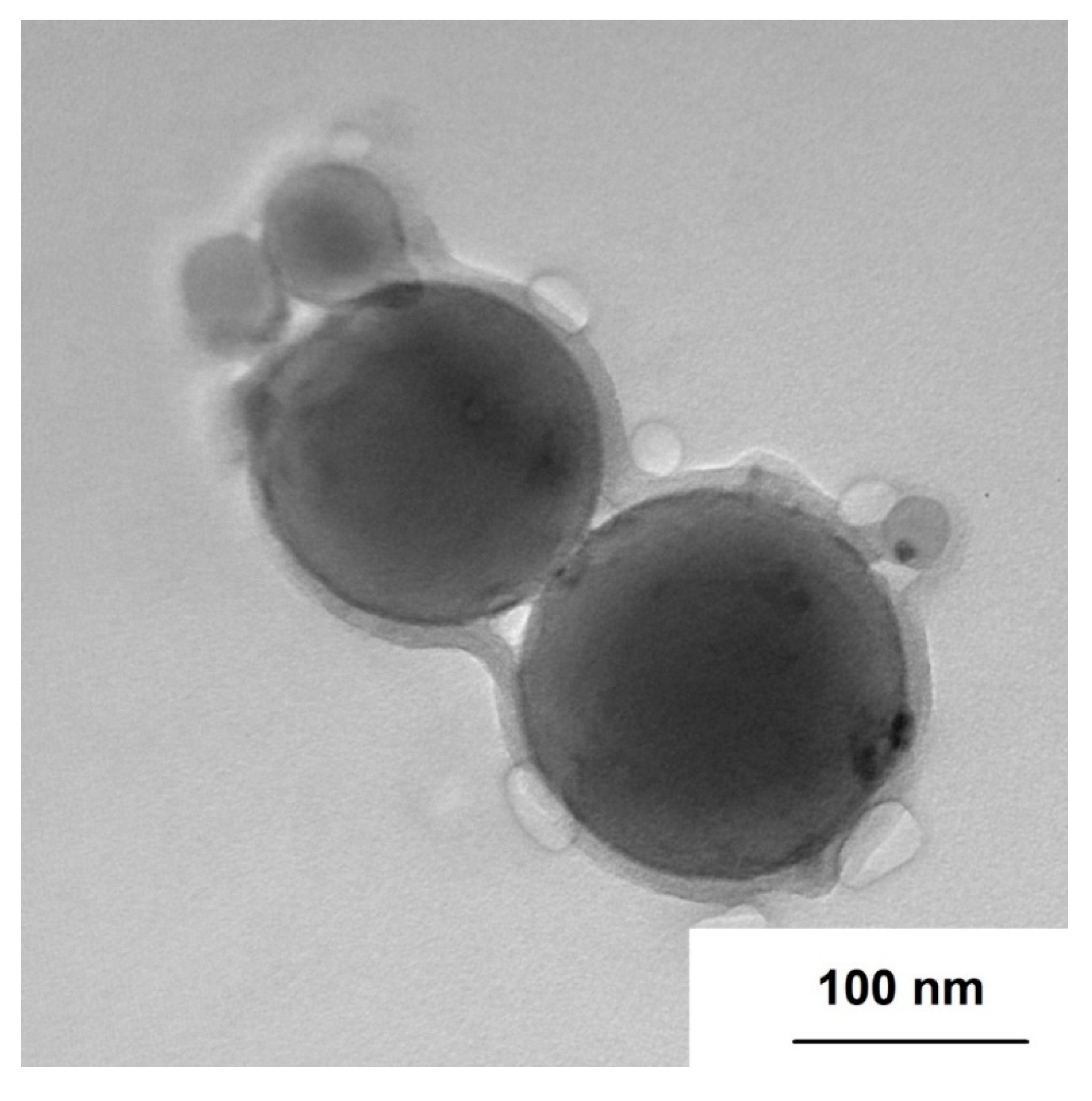
3.1. nAl Characterization
3.2. Characterization of the nAl/HTPB Pastes
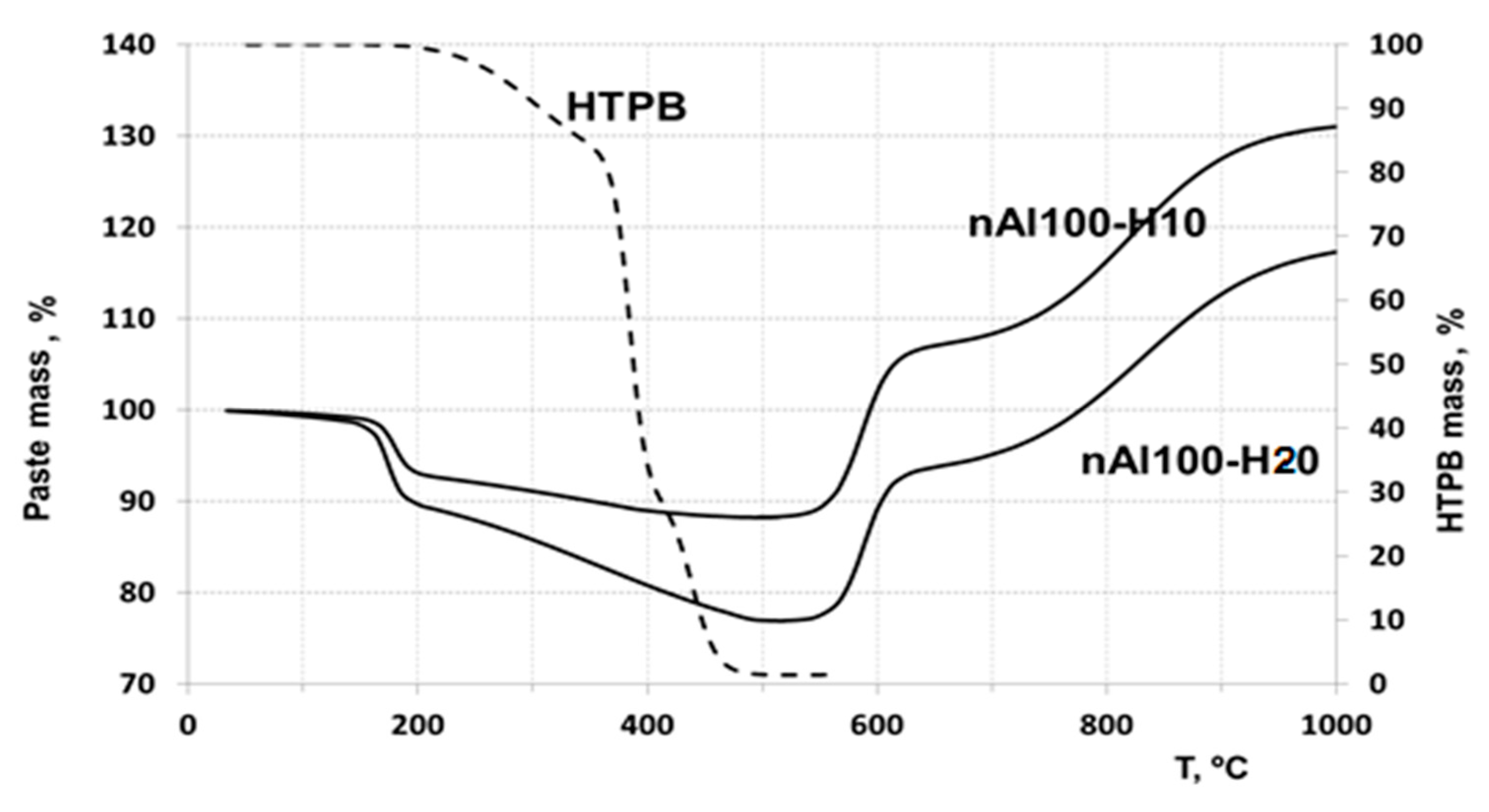
3.3. Thermogravimetric Analysis of the Pastes
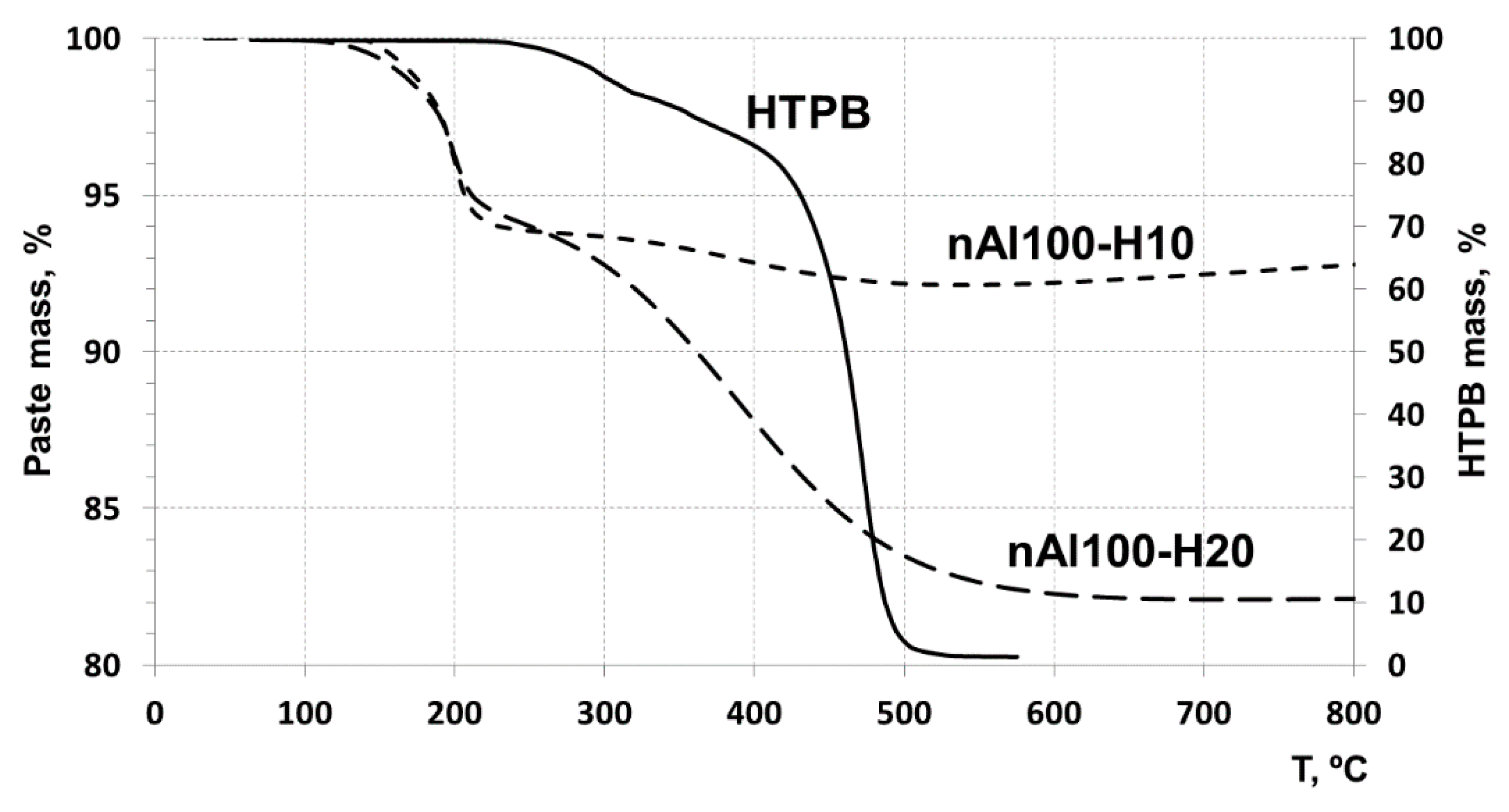
3.3.1. Thermogravimetric Analysis of the Pastes in Ar
3.3.2. Thermogravimetric Analysis of the Pastes in Air
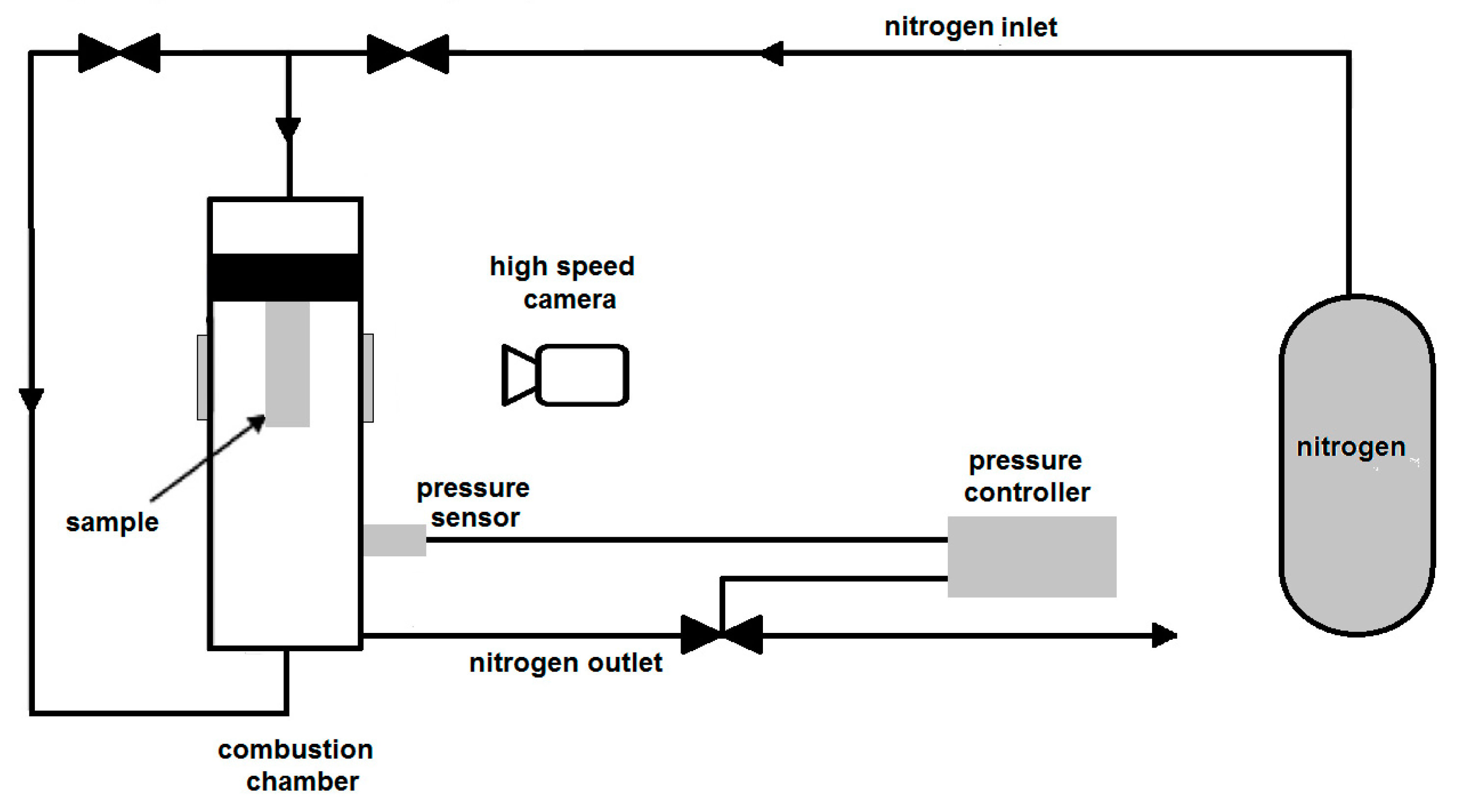
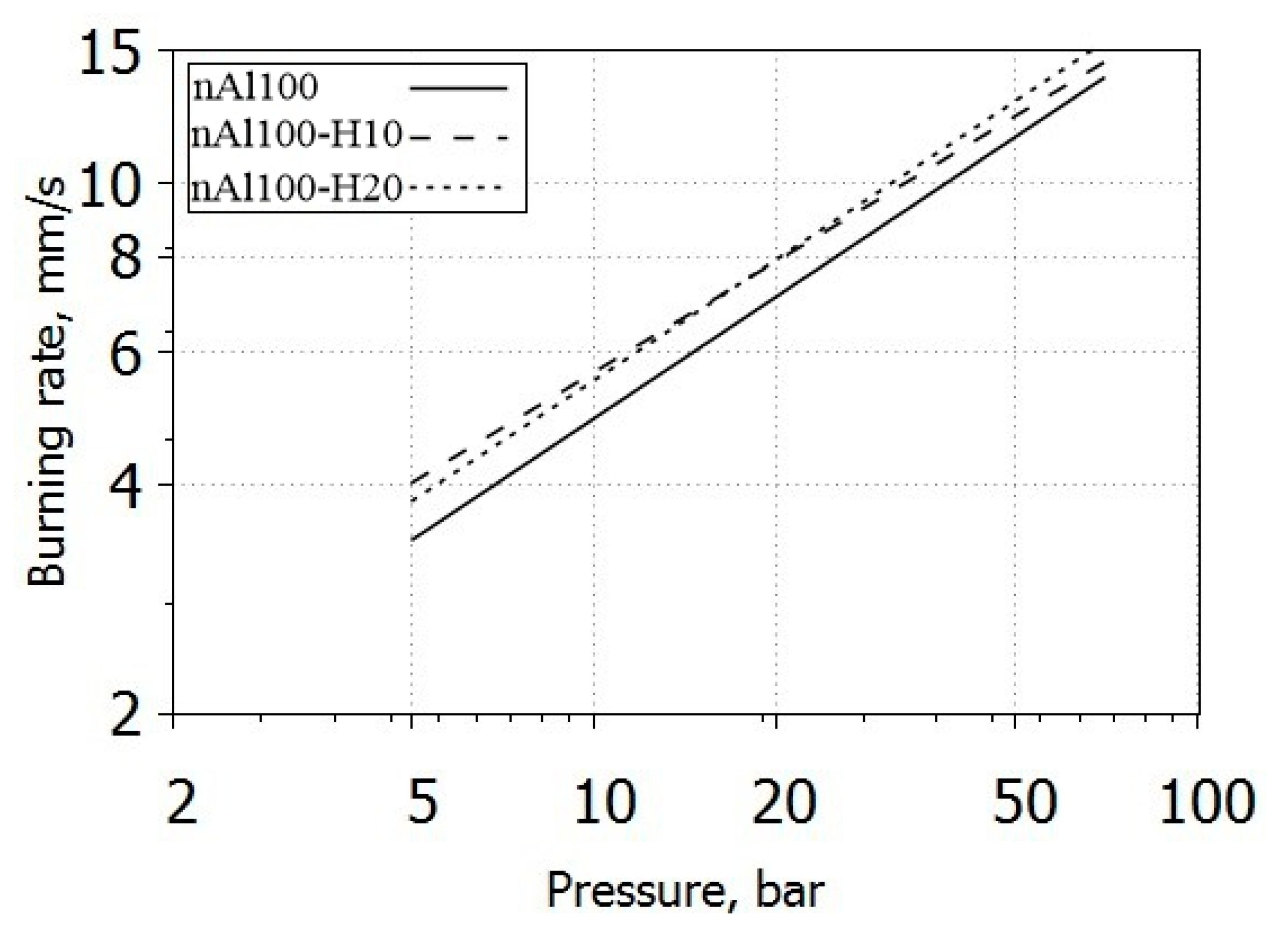
3.4. Burning Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yetter, R.A.; Risha, G.A.; Son, S.F. Metal particle combustion and nanotechnology. Proc. Combust. Inst. 2009, 32, 1819–1838. [Google Scholar] [CrossRef]
- Sundaram, D.S.; Yang, V.; Zarko, V.E. Combustion of nano aluminum particles (Review). Combust. Explos. Shock Waves 2015, 51, 173–196. [Google Scholar] [CrossRef]
- Sossi, A.; Duranti, E.; Manzoni, M.; Paravan, C.; De Luca, L.T.; Vorozhtsov, A.B.; Lerner, M.; Rodkevich, N.; Gromov, A.; Savin, N. Combustion of HTPB-based solid fuels loaded with coated nanoaluminum. Combust. Sci. Technol. 2013, 185, 17–36. [Google Scholar] [CrossRef]
- Meda, L.; Marra, G.; Galfetti, L.; Inchingalo, S.; Severini, F.; De Luca, L.T. Nanocomposites for rocket solid propellants. Compos. Sci. Technol. 2005, 65, 769–773. [Google Scholar] [CrossRef]
- Abraham, A.; Nie, H.; Schoenitz, M.; Vorozhtsov, A.B.; Lerner, M.; Pervikov, A.; Dreizin, E.L. Bimetal Al–Ni nano-powders for energetic formulations. Combust. Flame. 2016, 173, 179–186. [Google Scholar] [CrossRef]
- Noor, F.; Vorozhtsov, A.; Lerner, M.; Wen, D. Exothermic characteristics of aluminum-based nanomaterials. Powder Technol. 2015, 282, 19–24. [Google Scholar] [CrossRef]
- Sakovich, G.V.; Arkhipov, V.A.; Vorozhtsov, A.B.; Bondarchuk, S.S.; Pevchenko, B.V. Investigation of combustion of HEM with aluminum nanopowders. Nanotechnol. Russ. 2010, 5, 91–107. [Google Scholar] [CrossRef]
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167. [Google Scholar] [CrossRef]
- Young, G.; Wang, H.; Zachariah, M.R. Application of Nano-Aluminum/Nitrocellulose Mesoparticles in Composite Solid Rocket Propellants. Propellants Explos. Pyrotech. 2015, 40, 413–418. [Google Scholar] [CrossRef]
- Pang, W.; De Luca, L.; Huixang, X.; Xuezhong, F.; Fengqu, Z.; Fangli, L.; Wuxi, X.; Yonghong, L. Efects of nano-metric aluminum powder on the properties of composite solid propellants. Int. J. Energetic Mater. Chem. Propul. 2015, 14, 265–282. [Google Scholar] [CrossRef]
- Ivanov, Y.F.; Osmonoliev, M.N.; Sedoi, V.S.; Arkhipov, V.A.; Bondarchuk, S.S.; Vorozhtsov, A.B.; Kuznetsov, V.T. Productions of ultra-fine powders and their use in high energetic compositions. Propellants Explos. Pyrotech. 2003, 28, 319–333. [Google Scholar] [CrossRef]
- Vorozhtsov, A.B.; Rodkevich, N.G.; Lerner, M.I.; Zhukov, A.S.; Bondarchuk, S.S.; Dyachenko, N.N. Metal nanoparticles in high-energetic materials practice. Int. J. Energetic Mater. Chem. Propul. 2017, 16, 231–241. [Google Scholar] [CrossRef]
- Vorozhtsov, A.B.; Lerner, M.; Rodkevich, N.; Nie, H.; Abraham, A.; Schoenitz, M.; Dreizin, E.L. Oxidation of nano-sized aluminum powders. Thermochim. Acta 2016, 636, 48–56. [Google Scholar] [CrossRef]
- Paravan, C.; Verga, A.; Maggi, F.; Galfetti, L. Accelerated aging of micron- and nano-sized aluminum powders: Metal content, composition and non-isothermal reactivity. Acta Astron. 2019, 158, 397–406. [Google Scholar] [CrossRef]
- Ju, Z.Y.; An, J.L.; Guo, C.Y.; Li, T.R.; Jia, Z.Y.; Wu, R.F. The oxidation reaction and sensitivity of aluminum nanopowders coated by hydroxyl-terminated polybutadiene. J. Energetic Mater. 2020, 1–14. [Google Scholar] [CrossRef]
- Guo, L.; Song, W.; Hu, M.; Xie, C.; Chen, X. Preparation and reactivity of aluminum nanopowders coated by hydroxyl-terminated polybutadiene (HTPB). Appl. Surf. Sci. 2008, 254, 2413–2417. [Google Scholar] [CrossRef]
- Lerner, M.I.; Glazkova, E.A.; Vorozhtsov, A.B.; Rodkevich, N.G.; Volkov, S.A.; Ivanov, A.N. Passivation of aluminum nanopowders for use in energetic materials. Russ. J. Phys. Chem. B 2015, 9, 56–61. [Google Scholar] [CrossRef]
- Vorozhtsov, A.; Lerner, M.; Rodkevich, N.; Teplov, G.; Sokolov, S.; Perchatkina, E. Deagglomeration and Encapsulation of Metal and Bimetal Nanoparticles for Energetic Applications. In Innovative Energetic Materials: Properties, Combustion Performance and Application; Springer: Singapore, 2020; pp. 457–491. [Google Scholar]
- Lu, Y.C.; Kuo, K.K. Thermal decomposition study of hydroxyl-terminated polybutadiene (HTPB) solid fuel. Thermochim. Acta 1996, 275, 181–191. [Google Scholar] [CrossRef]
- Vorozhtsov, A.B.; DeLuca, L.T.; Reina, A.; Lerner, M.I.; Rodkevich, N.G. Effects of HTPB-coating on nano-sized aluminum in solid rocket propellant performance. Sci. Technol. Energ. Mater. 2015, 76, 105–109. [Google Scholar]
- Pang, W.; De Luca, L.T.; Wang, K.; Fu, X.; Li, J.; Xu, H.; Li, H. Performance of Composite Solid Propellant Containing Nanosized Metal Particles. In Nanomaterials in Rocket Propulsion Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–298. [Google Scholar]
- Dennis, C.; Bojko, B. On the combustion of heterogeneous AP/HTPB composite propellants: A review. Fuel 2019, 254, 115646. [Google Scholar] [CrossRef]
- Lerner, M.I.; Glazkova, E.A.; Lozhkomoev, A.S.; Svarovskaya, N.V.; Bakina, O.V.; Pervikov, A.V.; Psakhie, S.G. Synthesis of Al nanoparticles and Al/AlN composite nanoparticles by electrical explosion of aluminum wires in argon and nitrogen. Powder Technol. 2016, 295, 307–314. [Google Scholar] [CrossRef]
- Lerner, M.; Vorozhtsov, A.; Guseinov, S.; Storozhenko, P. Metal Nanopowders Production. In Metal Nanopowders: Production, Characterization, and Energetic Applications; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Chen, L.; Song, W.; Lv, J.; Chen, X.; Xie, C. Research on the methods to determine metallic aluminum content in aluminum nanoparticles. Mater. Chem. Phys. 2010, 120, 670–675. [Google Scholar] [CrossRef]
- Kuśnieruk, S.; Wojnarowicz, J.; Chodara, A.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Influence of hydrothermal synthesis parameters on the properties of hydroxyapatite nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 1586–1601. [Google Scholar]
- Zare, A.; Harriman, T.A.; Lucca, D.A.; Roncalli, S.; Kosowski, B.M.; Paravan, C.; DeLuca, L.T. Mapping of aluminum particle dispersion in solid rocket fuel formulations. In Chemical Rocket Propulsion; Springer: Cham, Switzerland, 2017; pp. 673–688. [Google Scholar]
- Maggi, F.; Dossi, S.; Paravan, C.; Galfetti, L.; Rota, R.; Cianfanelli, S.; Marra, G. Iron oxide as solid propellant catalyst: A detailed characterization. Acta Astronaut. 2019, 158, 416–424. [Google Scholar] [CrossRef]
- Vorozhtsov, A.B.; Zhukov, A.S.; Ziatdinov, M.K.; Bondarchuk, S.S.; Lerner, M.I.; Rodkevich, N.G. Novel micro- and nanofuels: Production, characterization, and applications for high-energy materials. In Chemical Rocket Propulsion; Springer: Cham, Switzerland, 2017; pp. 235–251. [Google Scholar]
- Lozhkomoev, A.S.; Rodkevich, N.G.; Vorozhtsov, A.B.; Lerner, M.I. Oxidation and oxidation products of encapsulated aluminum nanopowders. J. Nanopart. Res. 2020, 22, 1–13. [Google Scholar] [CrossRef]
- Vorozhtsov, A.B.; Rodkevich, N.G.; Bondarchuk, I.S.; Lerner, M.I.; Zhukov, A.S.; Glazkova, E.A.; Bondarchuk, S.S. Thermokinetic investigation of the aluminum nanoparticles oxidation. Int. J. Energetic Mater. Chem. Propul. 2017, 16, 309–320. [Google Scholar] [CrossRef]
| Parameter | APcoarse | APfine |
|---|---|---|
| D0.1, µm | 139 ± 1.39 | 1.70 ± 0.02 |
| D0.5, µm | 238 ± 2.38 | 11.9 ± 0.12 |
| D0.9, µm | 392 ± 3.92 | 60.9 ± 0.61 |
| D32, µm | 202 ± 2.02 | 3.57 ± 0.036 |
| D43, µm | 253 ± 2.53 | 23.8 ± 0.24 |
| Composite | Nominal Powder Composition | Notes |
|---|---|---|
| nAl | nAl100 (Al, Al2O3) | Initial powder, air-passivated, 100 nm (nominal size) |
| nAl-H10 | nAl100 (90 wt.%) + HTPB (10 wt.%) | Acetylacetone: 0.5 wt.% of nAl100 mass |
| nAl-H20 | nAl100 (80 wt.%) + HTPB (20 wt.%) | Acetylacetone: 0.5 wt.% of nAl100 mass |
| Ingredients | Mass Fraction, wt.% |
|---|---|
| AP (coarse Dnominal = 200 µm) | 65 |
| AP (fine D0.5 = 18 µm) | 10 |
| HTPB | 17 |
| nAl | 8 |
| Powder | CAl, wt.% | CAl, Expected a, wt.% |
|---|---|---|
| nAl100 | 85.9 ± 0.8 | - |
| nAl100-H10 | 74.7 ± 0.5 | 77.3 |
| nAl100-H20 | 63.4 ± 2.8 | 68.7 |
| Formulations | ar, mm/(s bar nr) | nr | R2 | rb (40 bar), mm/s |
|---|---|---|---|---|
| AP_nAl100 | 1.444 ± 0.045 | 0.531 ± 0.012 | 0.997 | 10.2 ± 0.2 |
| AP_nAl100-H10 | 1.856 ± 0.027 | 0.483 ± 0.005 | 0.999 | 11.0 ± 0.1 |
| AP_nAl100-H20 | 1.636 ± 0.080 | 0.527 ± 0.018 | 0.992 | 11.3 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorozhtsov, A.; Lerner, M.; Rodkevich, N.; Sokolov, S.; Perchatkina, E.; Paravan, C. Preparation and Characterization of Al/HTPB Composite for High Energetic Materials. Nanomaterials 2020, 10, 2222. https://doi.org/10.3390/nano10112222
Vorozhtsov A, Lerner M, Rodkevich N, Sokolov S, Perchatkina E, Paravan C. Preparation and Characterization of Al/HTPB Composite for High Energetic Materials. Nanomaterials. 2020; 10(11):2222. https://doi.org/10.3390/nano10112222
Chicago/Turabian StyleVorozhtsov, Alexander, Marat Lerner, Nikolay Rodkevich, Sergei Sokolov, Elizaveta Perchatkina, and Christian Paravan. 2020. "Preparation and Characterization of Al/HTPB Composite for High Energetic Materials" Nanomaterials 10, no. 11: 2222. https://doi.org/10.3390/nano10112222
APA StyleVorozhtsov, A., Lerner, M., Rodkevich, N., Sokolov, S., Perchatkina, E., & Paravan, C. (2020). Preparation and Characterization of Al/HTPB Composite for High Energetic Materials. Nanomaterials, 10(11), 2222. https://doi.org/10.3390/nano10112222






