Towards the Continuous Hydrothermal Synthesis of ZnO@Mg2Al-CO3 Core-Shell Composite Nanomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of Composite Nanomaterials
2.3. Materials Characterisation
3. Results and Discussion
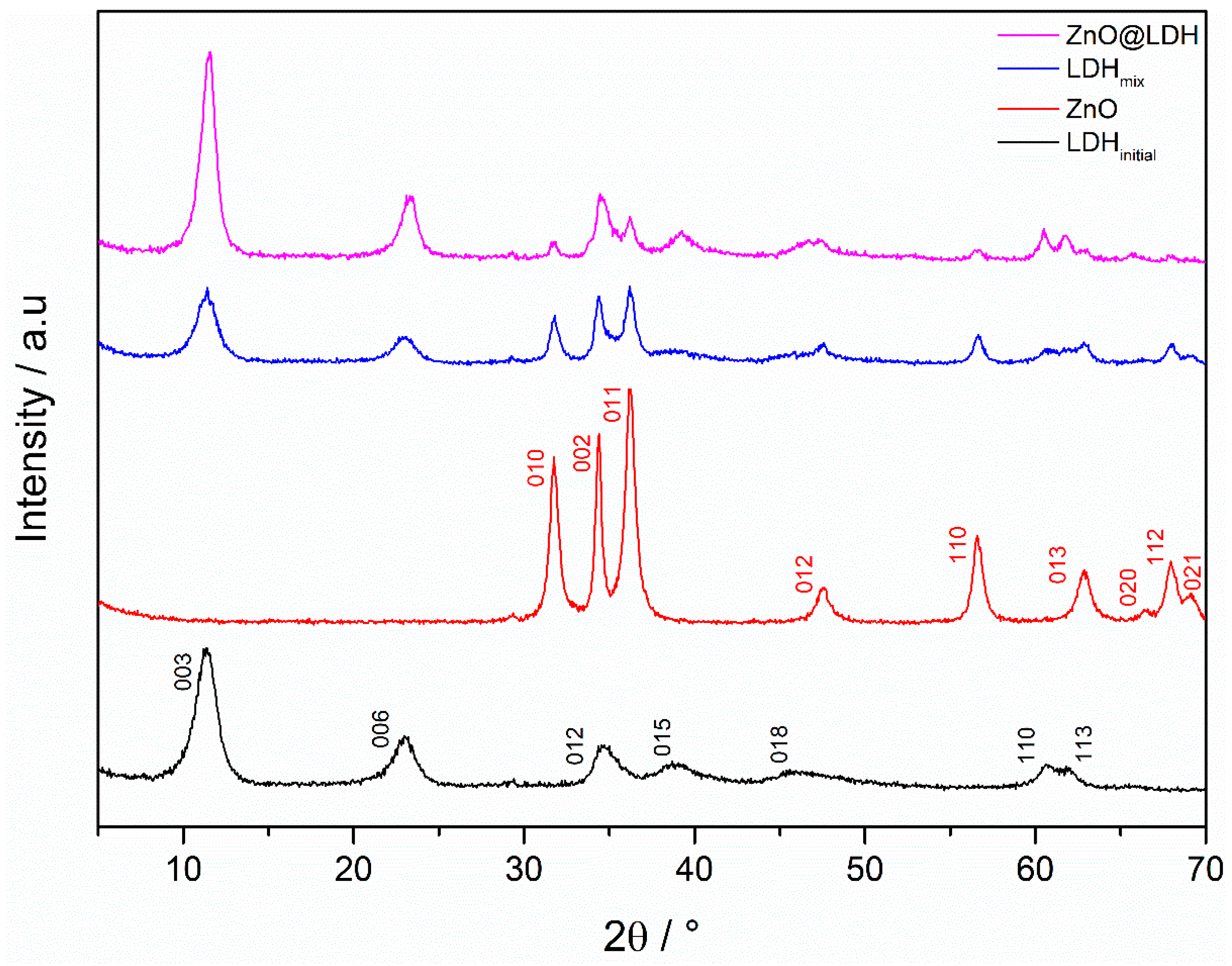
3.1. Crystal Characterisation of Composite and Single-Phase Materials
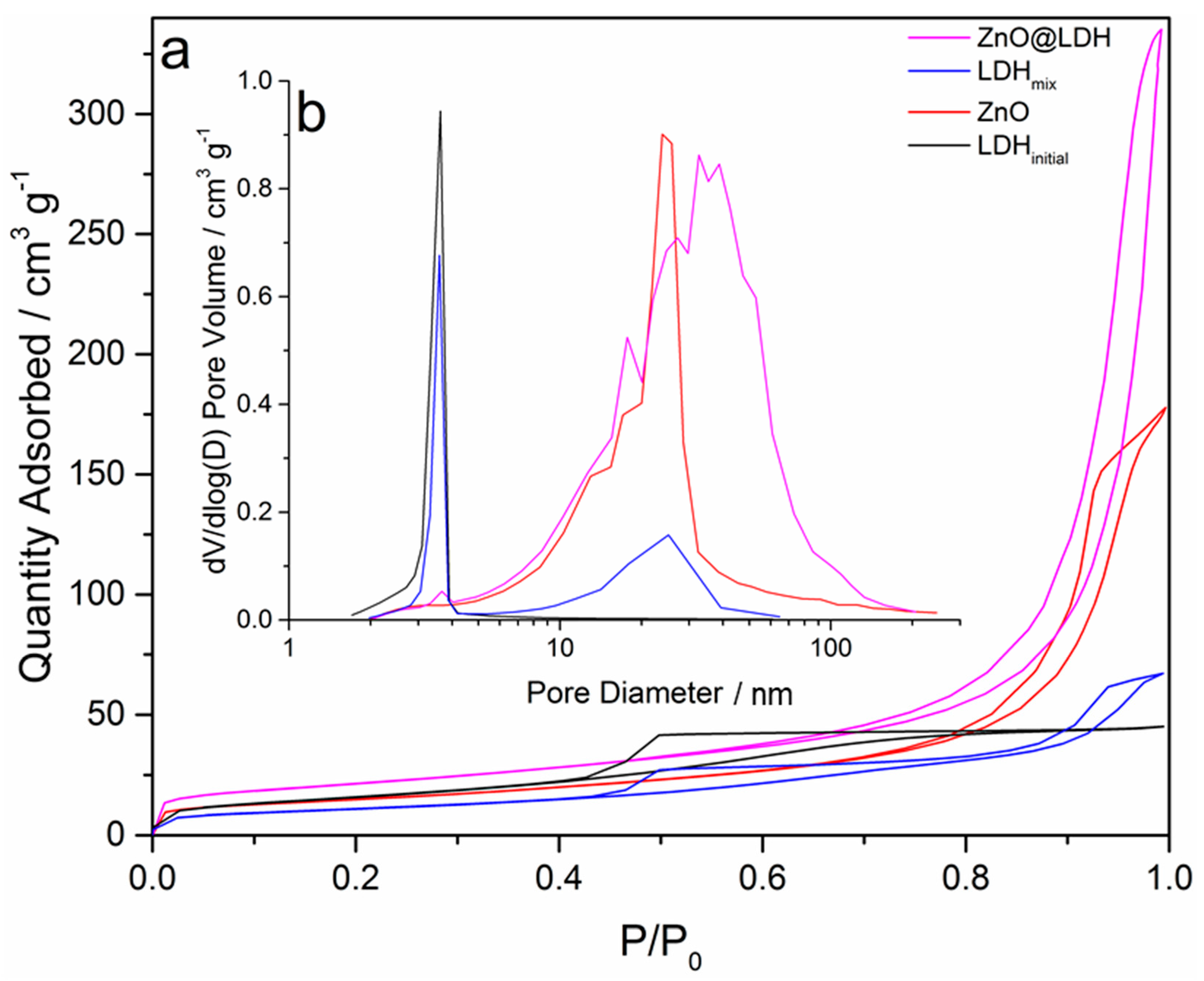
3.2. Specific Surface Area Analysis
3.3. Chemical Characterisation
3.4. Thermal Stability
3.5. Electronic Structure Characterisation
3.6. The Impact of Reactor 1 vs. Reactor 2
3.7. LDH Formation in Reactor 1 and in Reactor 2
3.8. The Impact of Residual Ions from ZnO Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Layered Materials; M. Dekker: New York, NY, USA, 2004; pp. 1–664. [Google Scholar]
- Xu, R.; Pang, W.; Huo, Q. Modern Inorganic Synthetic Chemistry; Elsevier Science: Amsterdam, The Netherlands, 2011; pp. 1–590. [Google Scholar]
- Bukhtiyarova, M.V. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Extremera, R.; Pavlovic, I.; Perez, M.R.; Barriga, C. Removal of acid orange 10 by calcined Mg/Al layered double hydroxides from water and recovery of the adsorbed dye. Chem. Eng. J. 2012, 213, 392–400. [Google Scholar] [CrossRef]
- Lv, L.; He, J.; Wei, M.; Evans, D.G.; Duan, X. Factors influencing the removal of fluoride from aqueous solution by calcined Mg-Al-CO3 layered double hydroxides. J. Hazard. Mater. 2006, 133, 119–128. [Google Scholar] [CrossRef]
- Lv, L.; He, J.; Wei, M.; Evans, D.G.; Zhou, Z.L. Treatment of high fluoride concentration water by MgAl-CO3 layered double hydroxides: Kinetic and equilibrium studies. Water Res. 2007, 41, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.A.; Wang, W.; Wei, M.; Cheng, H.J. Bromide ion removal from contaminated water by calcined and uncalcined MgAl-CO3 layered double hydroxides. J. Hazard. Mater. 2008, 152, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.I.; Lei, L.X.; Norquist, A.J.; O’Hare, D. Intercalation and controlled release of pharmaceutically active compounds from a layered double hydroxide. Chem. Commun. 2001, 22, 2342–2343. [Google Scholar] [CrossRef]
- Gao, Y.S.; Wu, J.W.; Wang, Q.; Wilkie, C.A.; O’Hare, D. Flame retardant polymer/layered double hydroxide nanocomposites. J. Mater. Chem. A 2014, 2, 10996–11016. [Google Scholar] [CrossRef]
- Wang, Q.; Undrell, J.P.; Gao, Y.S.; Cai, G.P.; Buffet, J.C.; Wilkie, C.A.; O’Hare, D. Synthesis of Flame-Retardant Polypropylene/LDH-Borate Nanocomposites. Macromolecules 2013, 46, 6145–6150. [Google Scholar] [CrossRef]
- Shao, M.F.; Han, J.B.; Wei, M.; Evans, D.G.; Duan, X. The synthesis of hierarchical Zn-Ti layered double hydroxide for efficient visible-light photocatalysis. Chem. Eng. J. 2011, 168, 519–524. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Li, B.; Wang, Q.; Gao, W.; Wang, C.L.J.; Wei, M.; Evans, D.G.; Duan, X.; O’Hare, D. NiTi-Layered double hydroxides nanosheets as efficient photocatalysts for oxygen evolution from water using visible Light. Chem. Sci. 2014, 5, 951–958. [Google Scholar] [CrossRef]
- Alejandre, A.; Medina, F.; Rodriguez, X.; Salagre, P.; Cesteros, Y.; Sueiras, J.E. Cu/Ni/Al layered double hydroxides as precursors of catalysts for the wet air oxidation of phenol aqueous solutions. Appl. Catal. B 2001, 30, 195–207. [Google Scholar] [CrossRef]
- Coq, B.; Tichit, D.; Ribet, S. Co/Ni/Mg/Al layered double hydroxides as precursors of catalysts for the hydrogenation of nitriles: Hydrogenation of acetonitrile. J. Catal. 2000, 189, 117–128. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, D.K.; Zou, K.; He, J.; Duan, X. A novel core-shell structured magnetic organic-inorganic nanohybrid involving drug-intercalated layered double hydroxides coated on a magnesium ferrite core for magnetically controlled drug release. J. Mater. Chem. 2009, 19, 3069–3077. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, D.K.; Duan, X. Synthesis, Characterization, and Magnetically Controlled Release Behavior of Novel Core-Shell Structural Magnetic Ibuprofen-Intercalated LDH Nanohybrids. J. Phys. Chem. C 2009, 113, 12140–12148. [Google Scholar] [CrossRef]
- Chen, C.P.; Felton, R.; Buffet, J.C.; O’Hare, D. Core-shell SiO2@LDHs with tuneable size, composition and morphology. Chem. Commun. 2015, 51, 3462–3465. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.P.; Wang, P.H.; Lim, T.T.; Liu, L.H.; Liu, S.M.; Xu, R. A facile synthesis of monodispersed hierarchical layered double hydroxide on silica spheres for efficient removal of pharmaceuticals from water. J. Mater. Chem. A 2013, 1, 3877–3880. [Google Scholar] [CrossRef]
- Chen, C.P.; Byles, C.F.H.; Buffet, J.C.; Rees, N.H.; Wu, Y.; O’Hare, D. Core-shell zeolite@aqueous miscible organic-layered double hydroxides. Chem. Sci. 2016, 7, 1457–1461. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Zhang, X.; Chen, Q.; Ruan, C.; Leng, Y. Enhanced removal performance by the core-shell zeolites/MgFe-layered double hydroxides (LDHs) for municipal wastewater treatment. Environ. Sci. Pollut. Res. 2016, 23, 6749–6757. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.Y.; Shao, M.F.; Zhang, C.L.; Xu, S.M.; Wei, M.; Duan, X. Co3O4@layered double hydroxide core/shell hierarchical nanowire arrays for enhanced supercapacitance performance. Nano Energy 2014, 7, 134–142. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.C.; Qi, W.; Li, Y.T.; Wu, Y.; Zhou, S.X.; Huang, S.M.; Wei, J.; Li, H.J.; Yao, P. Binder-free Co3O4@NiCoAl-layered double hydroxide core-shell hybrid architectural nanowire arrays with enhanced electrochemical performance. Appl. Surf. Sci. 2016, 363, 381–388. [Google Scholar] [CrossRef]
- Shao, M.F.; Ning, F.Y.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical Nanowire Arrays Based on ZnO Core-Layered Double Hydroxide Shell for Largely Enhanced Photoelectrochemical Water Splitting. Adv. Funct. Mater. 2014, 24, 580–586. [Google Scholar] [CrossRef]
- Trang, N.T.H.; Ngoc, H.V.; Lingappan, N.; Kang, D.J. A comparative study of supercapacitive performances of nickel cobalt layered double hydroxides coated on ZnO nanostructured arrays on textile fibre as electrodes for wearable energy storage devices. Nanoscale 2014, 6, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.S.; Zhang, Q.H.; Wang, H.Z.; Liu, Z.F.; Zhao, Z. Core-shell structured ZnO@Cu-Zn-Al layered double hydroxides with enhanced photocatalytic efficiency for CO2 reduction. Catal. Commun. 2016, 77, 118–122. [Google Scholar] [CrossRef]
- Hadnadjev-Kostic, M.; Vulic, T.; Marinkovic-Neducin, R. Solar light induced rhodamine B degradation assisted by TiO2-Zn-Al LDH based photocatalysts. Adv. Powder Technol. 2014, 25, 1624–1633. [Google Scholar] [CrossRef]
- Dou, Y.B.; Zhang, S.T.; Pan, T.; Xu, S.M.; Zhou, A.W.; Pu, M.; Yan, H.; Han, J.B.; Wei, M.; Evans, D.G.; et al. TiO2@ Layered Double Hydroxide Core-Shell Nanospheres with Largely Enhanced Photocatalytic Activity Toward O-2 Generation. Adv. Funct. Mater. 2015, 25, 2243–2249. [Google Scholar] [CrossRef]
- Seftel, E.M.; Niarchos, M.; Mitropoulos, C.; Mertens, M.; Vansant, E.F.; Cool, P. Photocatalytic removal of phenol and methylene-blue in aqueous media using TiO2@LDH clay nanocomposites. Catal. Today 2015, 252, 120–127. [Google Scholar] [CrossRef]
- Yan, R.-Q.; Liu, G.-H.; Wang, Q.-F.; Liu, W.; Song, C.-L. Fast Photodegradation of Malachite Green using Nano-ZnO on Ceramic MgAl Carbonate Layered Double Hydroxides Support. Chin. J. Chem. Phys. 2016, 29, 241–244. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Akbari, M. ZnO/Mg-Al Layered Double Hydroxides as a Photocatalytic Bleaching of Methylene Orange - A Black Box Modeling by Artificial Neural Network. Bull. Chem. React. Eng. Catal. 2016, 11. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Zhang, J.; Zhou, J.Z.; Guo, Y.; Liu, H. Magnetic Fe3O4/ZnCr-layered double hydroxide composite with enhanced adsorption and photocatalytic activity. Chem. Eng. J. 2012, 185, 120–126. [Google Scholar] [CrossRef]
- Lester, E.; Blood, P.; Denyer, J.; Giddings, D.; Azzopardi, B.; Poliakoff, M. Reaction engineering: The supercritical water hydrothermal synthesis of nano-particles. J. Supercrit. Fluids 2006, 37, 209–214. [Google Scholar] [CrossRef]
- Cheng, B.; Samulski, E.T. Hydrothermal synthesis of one-dimensional ZnO nanostructures with different aspect ratios. Chem. Commun. 2004, 986–987. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, F.; Zhang, R.; Evans, D.G.; Duan, X. Preparation of layered double-hydroxide nanomaterials with a uniform crystallite size using a new method involving separate nucleation and aging steps. Chem. Mater. 2002, 14, 4286–4291. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, S.; Lester, E.; O’Hare, D. Synthesis of ultrafine layered double hydroxide (LDHs) nanoplates using a continuous-flow hydrothermal reactor. Nanoscale 2013, 5, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Okada, A. SYNTHESIS OF HYDROTALCITE-LIKE COMPOUNDS AND THEIR PHYSICOCHEMICAL PROPERTIES - SYSTEMS MG2+-AL3+-SO42- AND MG2+-AL3+-CRO42. Clays Clay Miner. 1977, 25, 14–18. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Marsalek, R. Particle Size and Zeta Potential of ZnO. APCBEE Proc. 2014, 9, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Clark, I. Continuous Synthesis and Characterisation of Layered Double Hydroxide Nanomaterials for their Application for Dye Wastewater Remediation; University of Nottingham: Nottingham, UK, 2018. [Google Scholar]
- Rives, V. Layered Double Hydroxides: Present and Future; Nova Science Publishers: Huntington, NY, USA, 2001; pp. 1–439. [Google Scholar]
- Islam, M.; Patel, R. Synthesis and physicochemical characterization of Zn/Al chloride layered double hydroxide and evaluation of its nitrate removal efficiency. Desalination 2010, 256, 120–128. [Google Scholar] [CrossRef]
- Ogawa, M.; Kaiho, H. Homogeneous precipitation of uniform hydrotalcite particles. Langmuir 2002, 18, 4240–4242. [Google Scholar] [CrossRef]
- Miyata, S. Physico-chemical Properties of Synthetic Hydrotalcites in Relation to Composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Parker, L.M.; Milestone, N.B.; Newman, R.H. The use of hydrotalcite as an anion absorbent. Ind. Eng. Chem. Res. 1995, 34, 1196–1202. [Google Scholar] [CrossRef]


| Sample | Reactor | Material | [NaOH] (mol L−1) | Down-Flow (mL min−1) | [MII + MIII] (mol L−1) | Up-Flow (mL min−1) |
|---|---|---|---|---|---|---|
| ZnO | 1 | ZnO | 0.050 | 20 | 0.05 | 10 |
| LDHinitial | 1 | Mg2Al-CO3 | 0.125 | 20 | 0.10 | 10 |
| † LDHmix | - | mixture of ZnO and LDHinitial | - | - | - | - |
| ZnO-LDH | 1 - 2 | ZnO - Mg2Al-CO3 | 0.05 - 0.15 | 40 | 0.05 - 0.05 | 20 |
| LDH-only experiments | ||||||
| LDH3 | 2 | Mg2Al-CO3 | 0.25 | 40 | 0.05 | 20 |
| * LDH4 | 2 | Mg2Al-CO3 | 0.15 | 40 | 0.05 | 20 |
| LDH5 | 2 | Mg2Al-CO3 | 0.15 | 40 | 0.05 | 20 |
| LDH6 | 2 | Mg2Al-CO3 | 0.25 | 40 | 0.05 | 20 |
| LDH7 | 2 | Mg2Al-CO3 | 0.15 | 40 | 0.05 | 20 |
| LDH8 | 2 | Mg2Al-CO3 | 0.25 | 40 | 0.05 | 20 |
| Sample | Miller Indices | a Lattice Parameter a (nm) | Miller Indices | a Lattice Parameter c (nm) |
|---|---|---|---|---|
| ZnO | 010 | 0.33 | 002 | 0.52 |
| LDHinitial | 110 | 0.30 | 003 | 2.32 |
| ZnO-LDH | 010 110 | 0.32 a 0.31 b | 002 003 | 0.52 a 2.28 b |
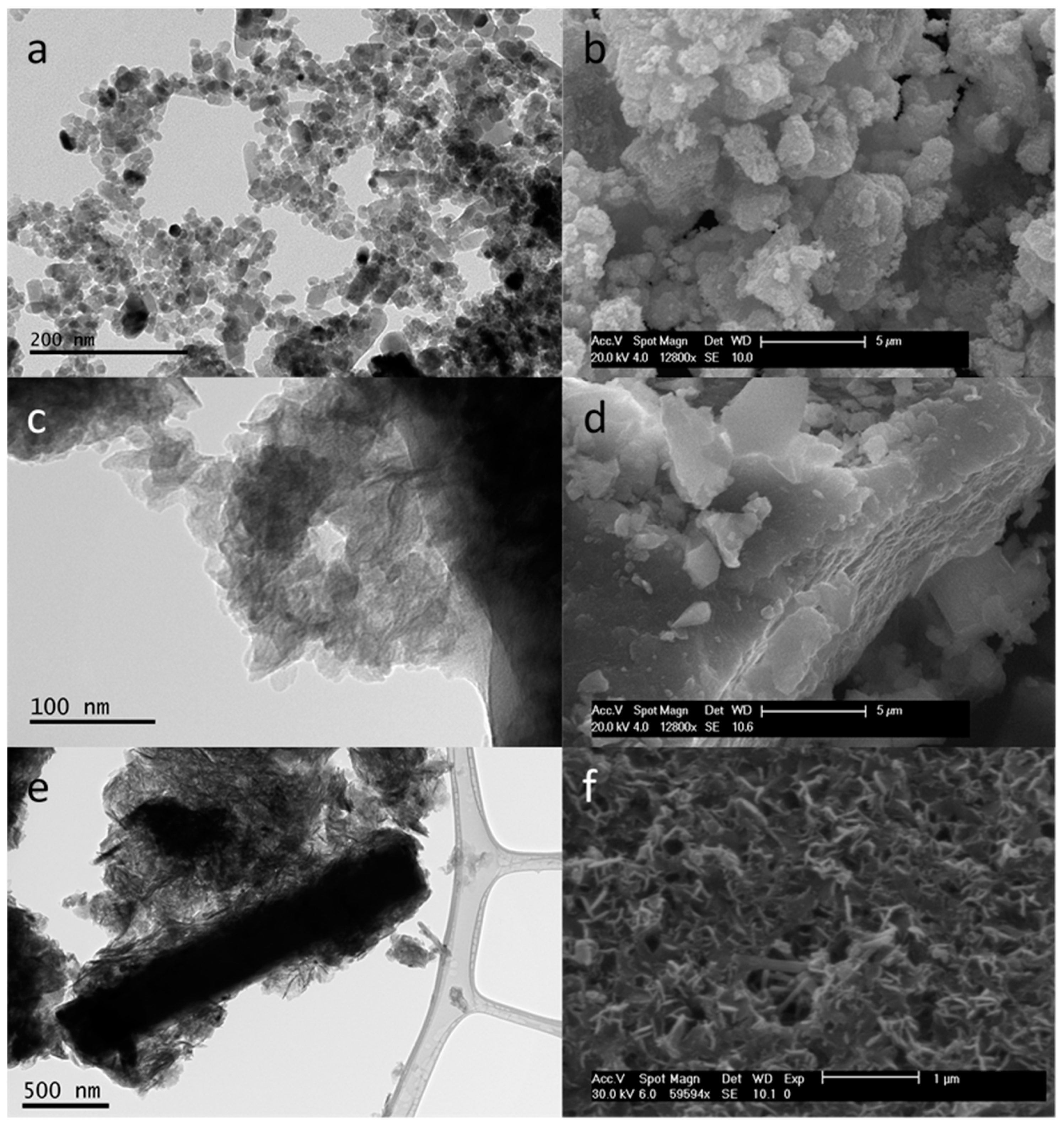
| Sample | XRD | TEM | |||
|---|---|---|---|---|---|
| CDL (012) (nm) | CDL (003) (nm) | CDL (110) (nm) | b Particle Size (nm) | b Particle Size (nm) | |
| ZnO | 25 | - | - | 22 ± 14 | - |
| LDHinitial | - | 8 | 25 | - | 34 ± 34 |
| ZnO-LDH | 42 | 12 | 27 | 53 ± 40 | 51 ± 22 |
| Sample | SBET (m2 g−1) | Pore Diameter (nm) | Pore Volume (cm−3 g−1) |
|---|---|---|---|
| ZnO | 53.0 ± 0.1 | 17.6 | 0.3 |
| LDHinitial | 58.2 ± 0.3 | 4.0 | 0.1 |
| LDHmix | 39.6 ± 0.1 | 7.2 | 0.1 |
| ZnO-LDH | 76.2 ± 0.1 | 17.0 | 0.7 |
| Sample | Band Gap (eV) |
|---|---|
| ZnO | 3.21 |
| LDHinitial | 5.24 |
| LDHmix | 3.22 |
| ZnO-LDH | 3.31 |
| Sample | Reactor | Flow Rate (mL min−1) | Re | CDL (003) (nm) | CDL (110) (nm) |
|---|---|---|---|---|---|
| LDHinitial | 1 | 30 | 235 | 8 | 25 |
| LDH3 | 2 | 60 | 297 | 7 | 30 |
| Sample | SBET (m2 g−1) | BJH (Barrett, Joyner, and Halenda) Pore Diameter (nm) | BJH Pore Volume (cm3 g−1) |
|---|---|---|---|
| LDHinitial | 58.2 ± 0.3 | 3.5 | 0.07 |
| LDH3 | 20.1 ± 0.1 | 4.2 | 0.04 |
| Sample | CDL (003)/nm | CDL (110)/nm |
|---|---|---|
| LDH4 | 6 | 25 |
| LDH5 | 6 | 26 |
| LDH6 | 7 | 25 |
| LDH7 | 5 | 27 |
| LDH8 | 8 | 27 |
| Sample | SBET (m2 g−1) | Pore Diameter (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|
| LDH4 | 266.8 ± 0.4 | 16.85 | 1.36 |
| LDH5 | 270.2 ± 0.5 | 15.87 | 1.29 |
| LDH6 | 22.6 ± 0.1 | 3.89 | 0.06 |
| LDH7 | 271.7 ± 0.5 | 14.55 | 1.20 |
| LDH8 | 26.1 ± 0.2 | 4.42 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, I.; Smith, J.; Gomes, R.L.; Lester, E. Towards the Continuous Hydrothermal Synthesis of ZnO@Mg2Al-CO3 Core-Shell Composite Nanomaterials. Nanomaterials 2020, 10, 2052. https://doi.org/10.3390/nano10102052
Clark I, Smith J, Gomes RL, Lester E. Towards the Continuous Hydrothermal Synthesis of ZnO@Mg2Al-CO3 Core-Shell Composite Nanomaterials. Nanomaterials. 2020; 10(10):2052. https://doi.org/10.3390/nano10102052
Chicago/Turabian StyleClark, Ian, Jacob Smith, Rachel L. Gomes, and Edward Lester. 2020. "Towards the Continuous Hydrothermal Synthesis of ZnO@Mg2Al-CO3 Core-Shell Composite Nanomaterials" Nanomaterials 10, no. 10: 2052. https://doi.org/10.3390/nano10102052
APA StyleClark, I., Smith, J., Gomes, R. L., & Lester, E. (2020). Towards the Continuous Hydrothermal Synthesis of ZnO@Mg2Al-CO3 Core-Shell Composite Nanomaterials. Nanomaterials, 10(10), 2052. https://doi.org/10.3390/nano10102052






