Synthesis of Mo2C by Thermal Decomposition of Molybdenum Blue Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Molybdenum Blue Dispersion
2.3. Molybdenum Blue Dispersion Characterization
2.4. Procedure of Synthesis of Mo2C
2.5. Mo2C Characterization
2.6. Catalyts Activity Test
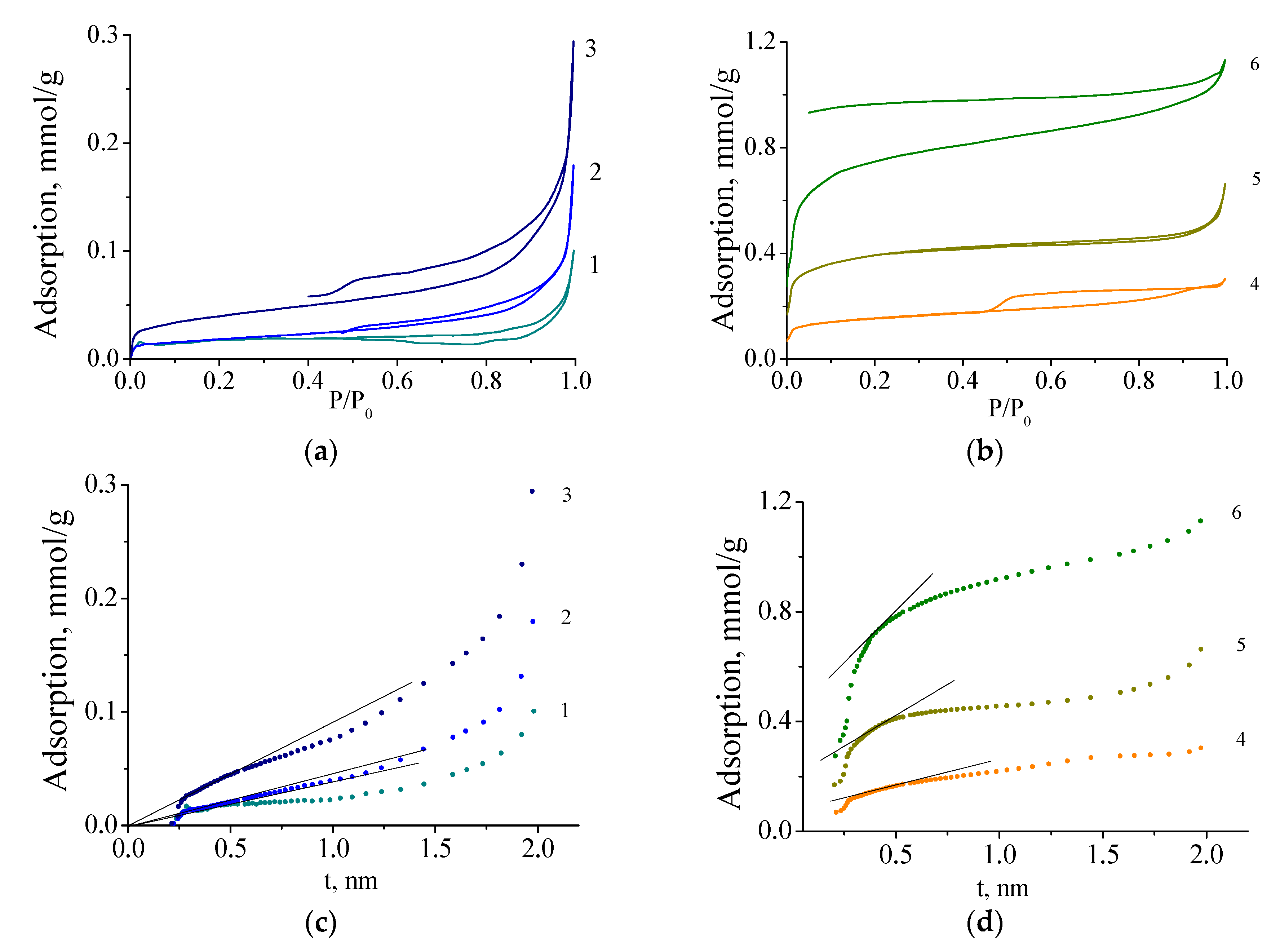
3. Results
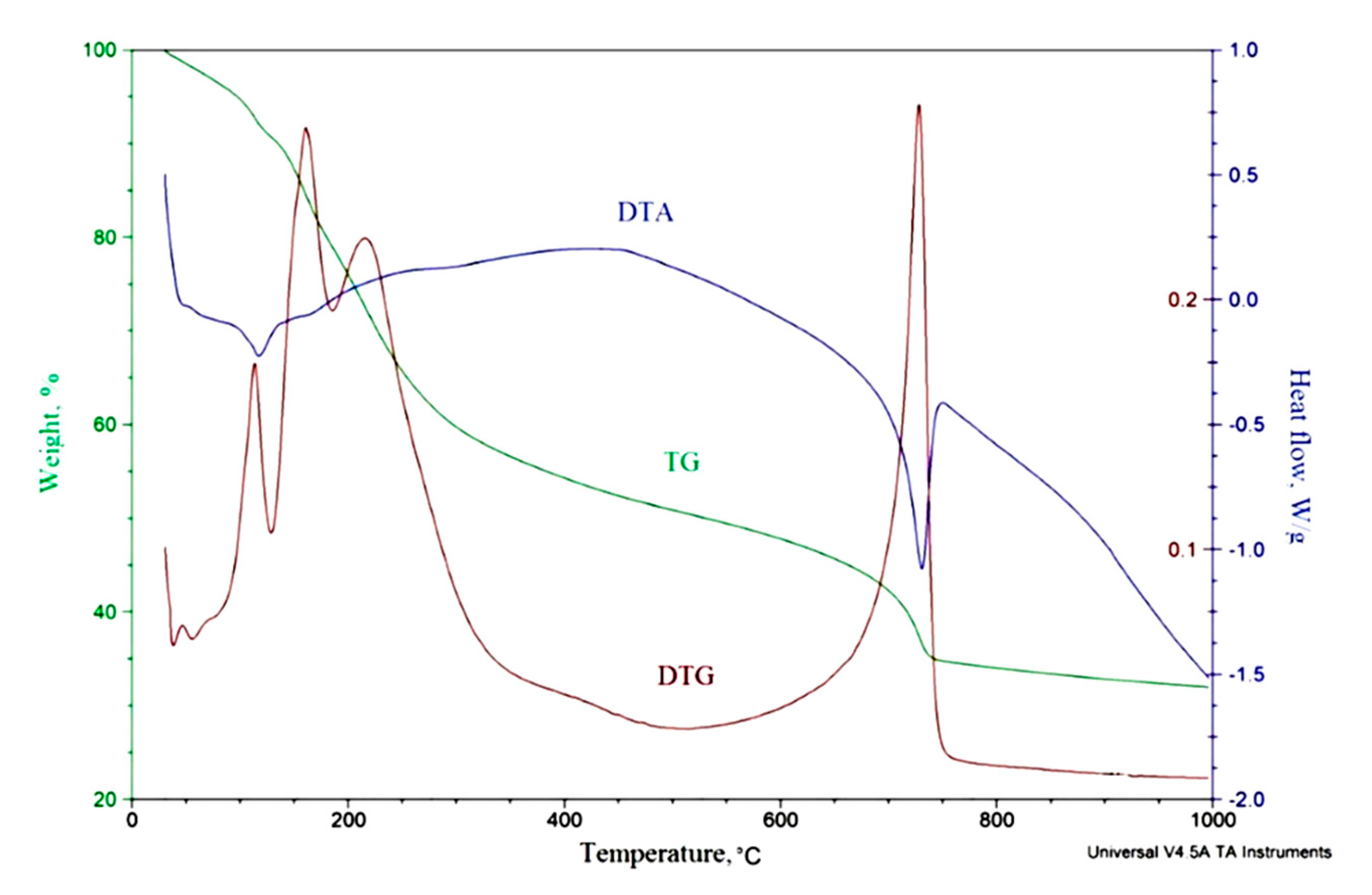
3.1. Molybdenum Blue Characterization
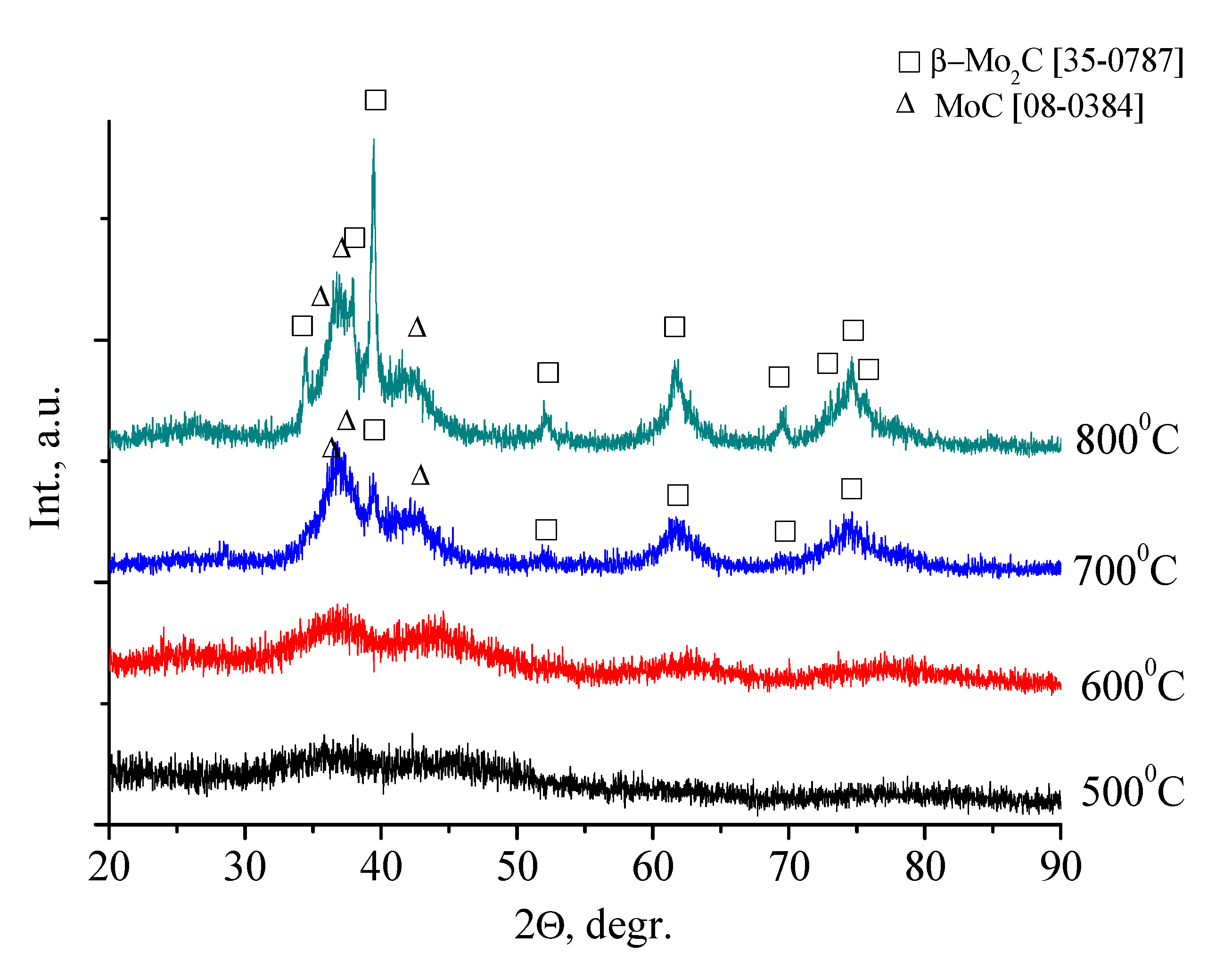
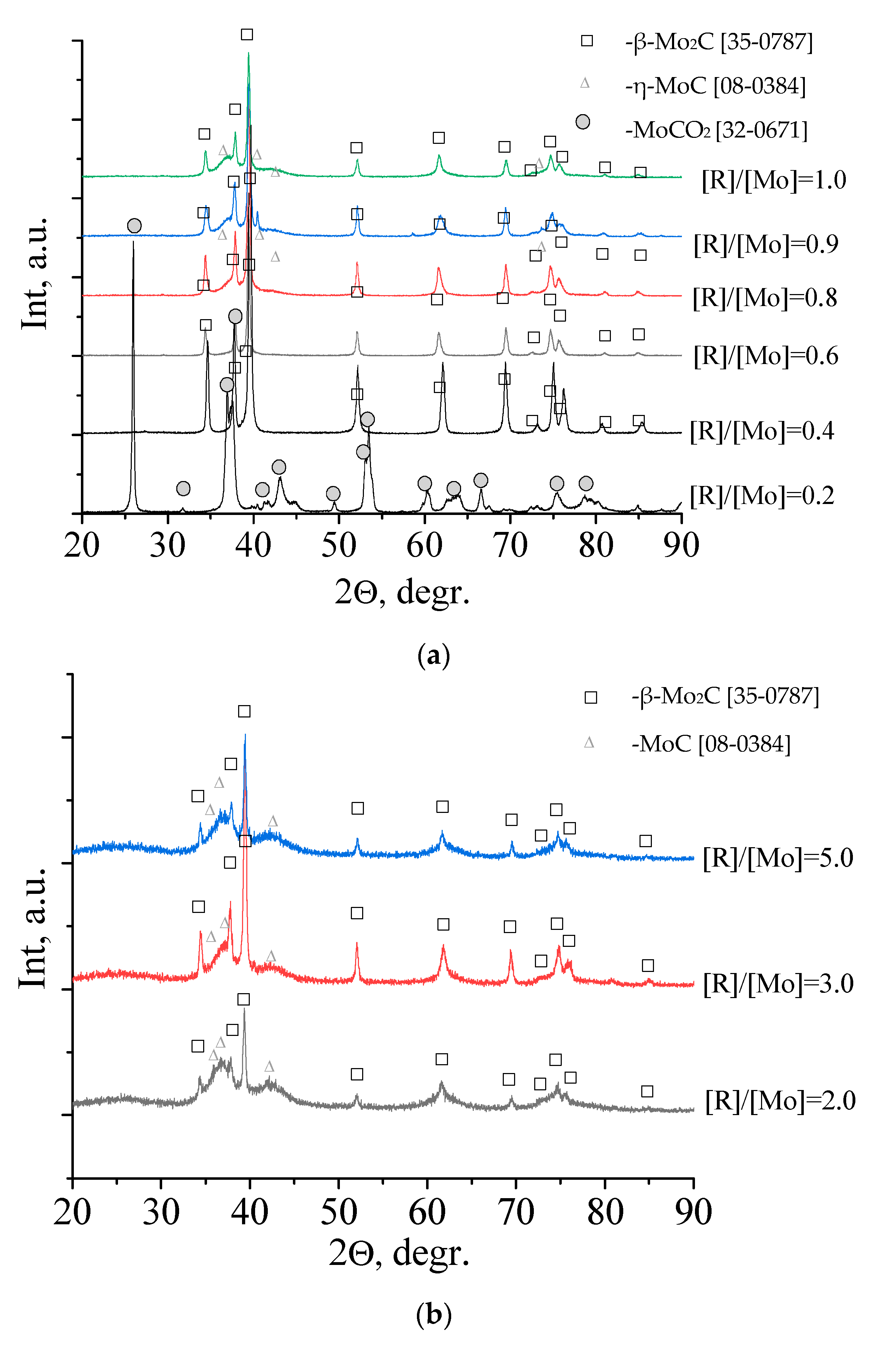
3.2. Mo2C Preparation
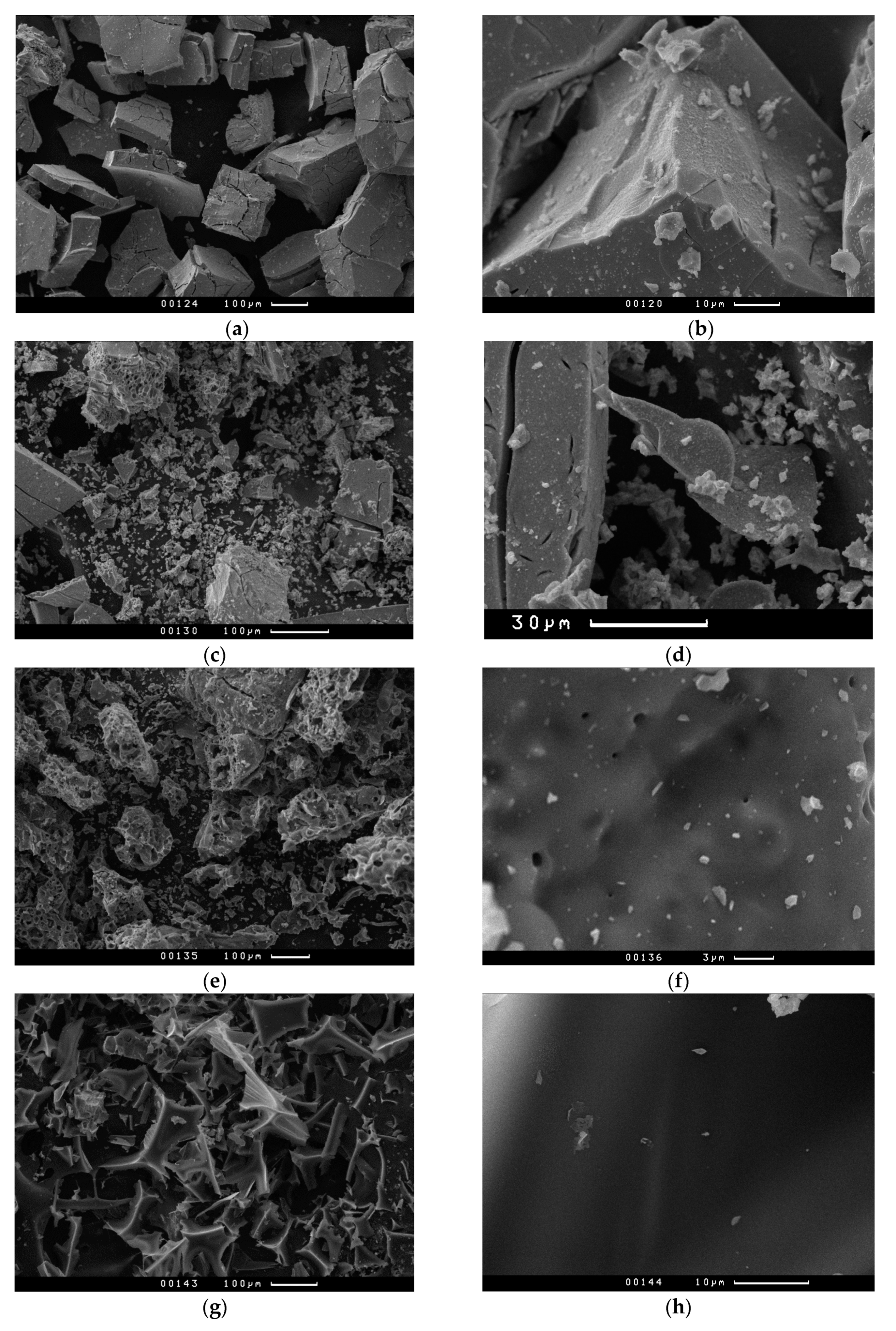
3.3. Mo2C Characterization
3.4. Mo2C Catalytic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Toth, L.E. Transition Metal Carbides and Nitrides; Academic Press: New York, NY, USA, 1971; p. 279. [Google Scholar]
- Levy, R.; Boudart, M. Platinum-like behavior of tungsten carbide in surface catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Aghoyan, A.M.; Mnatsakanyan, R.A.; Davtyan, D.H. Catalytic properties of supported mo2c synthesized by microwave irradiation in hydrazine hydrate decomposition reaction. Chem. J. Armen. 2019, 72, 419–426. [Google Scholar]
- Ji, N.; Zhang, T.; Zheng, M.; Wang, A.; Wang, H.; Wang, X.; Shu, Y.; Stottlemyer, A.L.; Chen, J.G. Catalytic conversion of cellulose into ethylene glycol over supported carbide catalysts. Catal. Today 2009, 147, 77–85. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, P.; Duanm, J.; Han, J.; Lou, H.; Zheng, X.; Hong, H. Carbon nanofibers supported molybdenum carbide catalysts for hydrodeoxygenation of vegetable oils. RSC Adv. 2013, 3, 17458–17491. [Google Scholar] [CrossRef]
- Frauwallner, M.-L.; López-Linares, F.; Lara-Romero, J.; Scott, C.E.; Ali, V.; Hernández, E.; Pereira-Almao, P. Toluene hydrogenation at low temperature using a molybdenum carbide catalyst. Appl. Catal. A Gen. 2011, 394, 62–70. [Google Scholar] [CrossRef]
- Tominaga, H.; Nagaim, M. Theoretical study of methane reforming on molybdenum carbide. Appl. Catal. A Gen. 2007, 328, 35–42. [Google Scholar] [CrossRef]
- Christofoletti, T.; Assaf, J.; Assaf, E. Methane steam reforming on supported and nonsupported molybdenum carbides. Chem. Eng. J. 2005, 106, 97–103. [Google Scholar] [CrossRef]
- La Mont, D.C.; Thomson, W.J. Dry reforming kinetics over a bulk molybdenum carbide catalyst. Chem. Eng. Sci. 2005, 60, 3553–3559. [Google Scholar] [CrossRef]
- Marin Flores, O.G.; Ha, S. Study of the performance of Mo2C for iso-octane steam reforming. Catal. Today 2008, 136, 235–242. [Google Scholar] [CrossRef]
- Roohi, P.; Alizadeh, R.; Fatehifar, E. Dry reforming of methane over nano-Mo2C/Al2O3 catalyst: Effect of carburization conditions on excess carbon deposition. Energy Sources A Recover. Util. Environ. Eff. 2016, 38, 3565–3571. [Google Scholar] [CrossRef]
- Lia, R.; Shahbazia, A.; Wanga, L.; Zhanga, B.; Chungb, C.-C.; Daytonc, D.; Yan, Q. Nanostructured molybdenum carbide on biochar for CO2 reforming of CH4. Fuel 2018, 225, 403–410. [Google Scholar] [CrossRef]
- Solymosi, F.; Németh, R.; Oszkó, A. The oxidative dehydrogenation of propane with CO2 over supported Mo2C catalyst. Stud. Surf. Sci. Catal. 2001, 136, 339–344. [Google Scholar]
- Zhang, Q.; Pastor-Pérez, L.; Jin, W.; Gu, S.; Reina, T.R. Understanding the promoter effect of Cu and Cs over highly effective β-Mo2C catalysts for the reverse water-gas shift reaction. Appl. Catal. B Environ. 2019, 244, 889–898. [Google Scholar] [CrossRef]
- Tominaga, H.; Nagai, M. Density functional theory of water-gas shift reaction on molybdenum carbide. J. Phys. Chem. B. 2005, 109, 20415–20423. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.J.; Rue, J.W. Molybdenum carbide water-gas shift catalyst for fuel cell- powered vehicles application. Catal. Lett. 2004, 92, 17–24. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Liu, P.; Takahashi, Y.; Nakamura, K.; Viñes, F.; Illas, F. Desulfurization reactions on surfaces of metal carbides: Photoemission and density–functional studies. Top. Catal. 2010, 53, 393–402. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, J.A.; Muckerman, J.T. Desulfurization of SO2 and thiophene on surfaces and nanoparticles of molybdenum carbide: Unexpected ligand and steric effects. J. Phys. Chem. B 2004, 108, 15662–15670. [Google Scholar] [CrossRef]
- Pasupulety, N.; Al-Zahrani, A.A.; Daous, M.A.; Driss, H.; Petrov, L.A. Studies on molybdenum carbide supported HZSM-5 (Si/Al = 23, 30, 50 and 80) catalysts for aromatization of methane. Arab. J. Chem. 2020, 13, 5199–5207. [Google Scholar] [CrossRef]
- Han, J.; Duan, J.; Chen, P.; Lou, H.; Zheng, X.; Hong, H. Nanostructured molybdenum carbides supported on carbon nanotubes as efficient catalysts for one-step hydrodeoxygenation and isomerization of vegetable oils. Green Chem. 2011, 13, 2561–2568. [Google Scholar] [CrossRef]
- Yang, W.; Haiyan, W.; Min, W.; Jun, M. Study on the isomerization of n-hexane over beta-zeolite supported molybdenum carbide catalyst. Pet. Process. Petrochem. 2008, 39, 16–25. [Google Scholar]
- Porosoff, M.D.; Yang, X.; Boscoboinik, J.A.; Chen, J.G. Molybdenum carbide as alternative catalysts to precious metals for highly selective reduction of CO2 to CO. Angew. Chem. Int. Ed. 2014, 126, 6823–6827. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Villa, A. Metal Carbides for Biomass Valorization. Appl. Sci. 2018, 8, 259. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Yu, W.; Salciccioli, M.; Chen, Y.; Huang, Y.; Xiong, K.; Vlachos, D.G.; Chen, J.G. Selective hydrodeoxygenation of biomass-derived oxygenates to unsaturated hydrocarbons using molybdenum carbide catalysts. ChemSusChem 2013, 6, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kelly, T.G.; Chen, J.G.; Mustain, W.E. Metal carbides as alternative electrocatalyst supports. ACS Catal. 2013, 3, 1184–1194. [Google Scholar] [CrossRef] [Green Version]
- Kelly, T.G.; Chen, J.G. Metal overlayer on metal carbide substrate: Unique bimetallic properties for catalysis and electrocatalysis. Chem. Soc. Rev. 2012, 41, 8021–8034. [Google Scholar] [CrossRef]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Trends in the chemical properties of early transition metal carbide surfaces: A density functional study. Catal. Today 2005, 105, 66–73. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, J.A. Catalytic properties of molybdenum carbide, nitride and phosphide: A theoretical study. Catal. Lett. 2003, 91, 247–252. [Google Scholar] [CrossRef]
- Chen, J.G. NEXAFS investigations of transition metal oxides, nitrides, carbides, sulfides and other interstitial compounds. Surf. Sci. Rep. 1997, 30, 1–152. [Google Scholar] [CrossRef]
- Hwu, H.H.; Chen, J.G. Surface chemistry of transition metal carbides. Chem. Rev. 2005, 105, 185–212. [Google Scholar] [CrossRef]
- Chen, J.G. Carbide and nitride overlayers on early transition metal surfaces: Preparation, characterization, and reactivities. Chem. Rev. 1996, 96, 1477–1498. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, G.; Hao, X.; Cao, J.; Abudula, A. Molybdenum carbide as alternative catalyst for hydrogen production—A review. Renew. Sustain. Energy Rev. 2017, 75, 1101–1129. [Google Scholar] [CrossRef]
- Neckel, A. Recent investigations on the electronic structure of the fourth and fifth group transition metal monocarbides, mononitrides, and monoxides. Int. J. Quantum Chem. 1983, 23, 1317–1353. [Google Scholar] [CrossRef]
- Wu, M.; Lin, X.; Hagfeldt, A.; Ma, T. Low-cost molybdenum carbide and tungsten carbide counter electrodes for dye-sensitized solar cells. Angew. Chem. Int. Ed. 2011, 50, 3520–3524. [Google Scholar] [CrossRef]
- Izhar, S.; Yoshida, M.; Nagai, M. Characterization of cobalt-tungsten and molybdenum-tungsten carbides an anode catalyst for PEFC. Electrochim. Acta 2009, 54, 1255–1262. [Google Scholar] [CrossRef]
- Szymanska-Kolasa, A.; Lewandowski, M.; Sayag, C.; Brodzki, D.; Djega-Mariadassou, G. Comparison between tungsten carbide and molybdenum carbide for the hydrogenitrogeneration of carbazole. Catal. Today 2007, 119, 35–38. [Google Scholar] [CrossRef]
- Darujati, A.D.S.; Thompson, W.J. Kinetic study of a ceria-promoted Mo2C/γ-Al2O3 catalyst in dry-methane reforming. Chem. Eng. Sci. 2006, 61, 4309–4315. [Google Scholar] [CrossRef]
- Pielazec, J.; Mierzwa, B.; Medjahdi, G.; Mareche, J.F.; Puricelli, S.; Celzard, A.; Furdin, G. Molybdenum carbide catalyst formation from precursors deposited on active carbons: XRD studies. Appl. Catal. A Gen. 2005, 296, 232–237. [Google Scholar]
- Xia, P.; Chen, Y.Q.; Shen, J.J.; Li, Z.Q. Mechanosynthesis of molybdenum carbides by ball milling at room temperature. J. Alloys Compd. 2008, 453, 185–190. [Google Scholar] [CrossRef]
- Knabbaz, S.; Honarbakhsh-Raouf, A.; Araie, A.; Saghafi, M. Effect of processing parameters on the mechanochemical synthesis of nanocrystalline molybdenum carbide. Int. J. Refract. Met. Hard Mater. 2013, 41, 402–407. [Google Scholar]
- Vitale, G.; Frauwallner, M.; Hernandez, E.; Scott, C.; Pereira-Almao, P. Low temperature synthesis of cubic molybdenum carbide catalysts via pressure induced crystallographic orientation of MoO3 precursor. Appl. Catal. A Gen. 2011, 400, 221–229. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, Q.; Yang, X.; Ke, D. A new method for the synthesis of molybdenum carbide. Mater. Lett. 2007, 61, 5173–5174. [Google Scholar] [CrossRef]
- Wang, X.-H.; Hao, H.-L.; Zhang, M.-H.; Li, W.; Tao, K.-Y. Synthesis and characterization of molybdenum carbides using propane as carbon source. Solid State Chem. 2006, 179, 538–543. [Google Scholar] [CrossRef]
- Xiao, T.C.; York, A.P.; Al-Megren, H.; Williams, C.V.; Wang, H.T.; Green, M.L. Preparation and characterisation of bimetallic cobalt and molybdenum carbides. J. Catal. 2001, 202, 100–109. [Google Scholar] [CrossRef]
- Zhang, A.; Zhu, A.; Chen, B.; Zhang, S.; Au, C.; Shi, C. In-situ synthesis of nickel modified molybdenum carbide catalyst for dry reforming of methane. Catal. Commun. 2011, 12, 803–807. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, C.; Chen, B.; Zhang, Y.; Zhu, Y.; Qiu, J.; Au, C. Catalytic role of β-Mo2C in DRM catalysts that contain Ni and Mo. Catal. Today 2015, 158, 254–258. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, G.; Phanthong, P.; Hao, X.; Huang, W.; Tsutsumi, A.; Kusakabe, K.; Abudula, A. Catalytic activity and stability of nickel-modified molybdenum carbide catalysts for steam reforming of methanol. J. Phys. Chem. C 2014, 118, 9485–9496. [Google Scholar] [CrossRef]
- Giordano, C.; Erpen, C.; Yao, W.; Antonietti, M. Synthesis of Mo and W carbide and nitride nanoparticles via a simple “urea glass” route. Nano Lett. 2008, 8, 4659–4663. [Google Scholar] [CrossRef]
- Giordano, C.; Antonietti, M. Synthesis of crystalline metal nitride and metal carbide nanostructures by sol-gel chemistry. Nano Today 2011, 6, 366–380. [Google Scholar] [CrossRef]
- Kirakosyan, H.V.; Nazaretyan, K.T.; Kirakosyan, K.G.; Tumanyan, M.E.; Aydinyan, S.V.; Kharatyan, S.L. Nanosize molybdenum carbide preparation by sol-gel combustion synthesis with subsequent fast heating. Chem. J. Armen. 2017, 70, 11–19. [Google Scholar]
- Muller, A.; Serain, C. Soluble molybdenum blues—“Des Pudels Kern”. Acc. Chem. Res. 2000, 33, 2–10. [Google Scholar] [CrossRef]
- Müller, A.; Roy, S. En route from the mystery of molybdenum blue via related manipulatable building blocks to aspects of materials science. Coord. Chem. Rev. 2003, 245, 153–166. [Google Scholar] [CrossRef]
- Nakamura, I.; Miras, H. Investigating the formation of “Molybdenum Blues” with gel electrophoresis and mass spectrometry. J. Am. Chem. Soc. 2015, 137, 6524–6530. [Google Scholar] [CrossRef]
- Long, D.L.; Burkholder, E.; Cronin, L. Polyoxometalate clusters, nanostructures and materials: From self-assembly to designer materials and devices. Chem. Soc. Rev. 2007, 36, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, N.N.; Nazarov, V.V.; Skudin, V.V. Synthesis of Membrane Catalysts Based on Mo2C. Kinet. Catal. 2015, 56, 670–680. [Google Scholar] [CrossRef]
- Bazhenova, М.D.; Gavrilova, N.N.; Krzhanovskiy, А.S.; Nazarov, V.V.; Skudin, V.V. ; Vityaz, P.А.; Sudnik, L.V. Synthesis and some properties of molybdenum carbide obtained on the base of molybdenum blue. Chim. Promyshlennost Segodnya 2014, 1, 4–10. [Google Scholar]
- Gavrilova, N.; Myachina, M.; Harlamova, D.; Nazarov, V. Synthesis of Molybdenum Blue Dispersions Using Ascorbic Acid as Reducing Agent. Colloids Interfaces 2020, 4, 24. [Google Scholar] [CrossRef]
- Il’in, E.G.; Beirakhov, A.G.; Teterin, Y.A.; Teterin, A.Y.; Maslakov, K.I. Surface morphology and composition of nanocrystalline MoO2 produced via the thermal decomposition of the MoO2(I-C3H7NHO)2 complex. Inorg. Mater. 2017, 53, 602–612. [Google Scholar] [CrossRef]
- Botar, B.; Ellern, A.; Kögerler, P. Mapping the formation areas of giant molybdenum blue clusters: A spectroscopic study. Dalton Trans. 2012, 41, 8951–8959. [Google Scholar] [CrossRef] [Green Version]
- Oshikawa, K.; Nagai, M.; Omi, S. Characterization of molybdenum carbides for methane reforming by TPR, XRD and XPS. J. Phys. Chem. 2001, 105, 9124–9131. [Google Scholar] [CrossRef]
- Vasilevich, A.V.; Baklanova, O.N.; Lavrenov, A.V.; Knyazheva, O.A.; Gulyaeva, T.I.; Trenikhin, M.V.; Likholobov, V.A. etc. Synthesis and characterization of massive molybdenum carbides and supported carbide-containing catalysts Mo2C/C prepared through mechanical activation. Catal. Ind. 2013, 6, 8–14. [Google Scholar] [CrossRef]
- Du, X.; France, L.J.; Kuznetsov, V.L.; Xiao, T.; Edwards, P.P.; AlMegren, H.; Bagabas, A. Dry reforming of methane over ZrO2-supported Co-Mo carbide catalyst. Appl. Pet. Res. 2014, 4, 137–144. [Google Scholar] [CrossRef] [Green Version]




| Molar Ratio [R]/[Mo] | Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Sa (BET), m2/g | Sa (t-plot), m2/g | ∑V, sm3/g | Vmeso (BJH), sm3/g | V0 (DR), sm3/g | Vt, (t-plot) sm3/g | dp, nm | |
| 0.6 | 1.4 | 1.4 | 0.0033 | 0.0023 | 0.0006 | - | 4 |
| 0.8 | 1.4 | 1.4 | 0.0039 | 0.0031 | 0.0008 | - | 4 |
| 0.9 | 1.4 | 1.4 | 0.0043 | 0.0032 | 0.0007 | - | 4 |
| 1.0 | 3.2 | 3.2 | 0.0099 | 0.0062 | 0.0020 | - | 4 |
| 2.0 | 12.4 | 7.1 | 0.1053 | 0.0067 | 0.0051 | 0.0022 | 4; 1 |
| 3.0 | 31.5 | 14.8 | 0.0227 | 0.0061 | 0.0130 | 0.0070 | 4; 1 |
| 5.0 | 63.0 | 27.7 | 0.0391 | 0.0046 | 0.0253 | 0.0137 | 4; 1 |
| Molar Ratio [R]/[Mo] | Parameters | |||
|---|---|---|---|---|
| Phase Composition | Sa (BET)*, m2/g | k900, s−1 | ks900, s−1 m−2 | |
| 0.6 | β-Mo2C | 0.6 | 2.64 | 8.00 |
| 1.0 | β-Mo2C, η-MoC | 1.4 | 8.20 | 22.44 |
| 2.0 | β-Mo2C, η-MoC, C | 1.6 | 0.45 | 0.50 |
| 5.0 | β-Mo2C, η-MoC, C | 16.0 | 0.60 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrilova, N.; Dyakonov, V.; Myachina, M.; Nazarov, V.; Skudin, V. Synthesis of Mo2C by Thermal Decomposition of Molybdenum Blue Nanoparticles. Nanomaterials 2020, 10, 2053. https://doi.org/10.3390/nano10102053
Gavrilova N, Dyakonov V, Myachina M, Nazarov V, Skudin V. Synthesis of Mo2C by Thermal Decomposition of Molybdenum Blue Nanoparticles. Nanomaterials. 2020; 10(10):2053. https://doi.org/10.3390/nano10102053
Chicago/Turabian StyleGavrilova, Natalia, Victor Dyakonov, Maria Myachina, Victor Nazarov, and Valery Skudin. 2020. "Synthesis of Mo2C by Thermal Decomposition of Molybdenum Blue Nanoparticles" Nanomaterials 10, no. 10: 2053. https://doi.org/10.3390/nano10102053
APA StyleGavrilova, N., Dyakonov, V., Myachina, M., Nazarov, V., & Skudin, V. (2020). Synthesis of Mo2C by Thermal Decomposition of Molybdenum Blue Nanoparticles. Nanomaterials, 10(10), 2053. https://doi.org/10.3390/nano10102053





