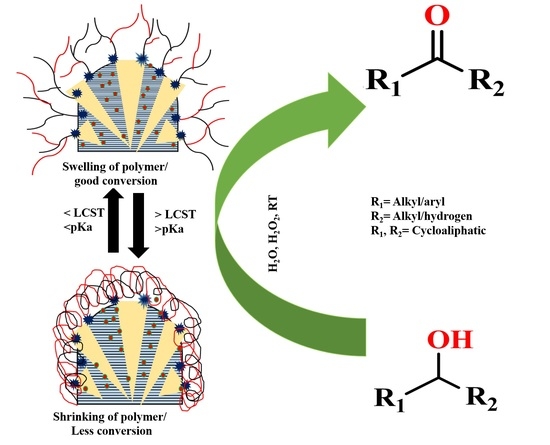
Dual Stimuli-Responsive Copper Nanoparticles Decorated SBA-15: A Highly Efficient Catalyst for the Oxidation of Alcohols in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of p(DMAEMA-co-TBA)/TSBA Hybrid Particles
2.2. Loading of Copper Nanoparticles (CuNPs) into p(DMAEMA-co-TBA)/TSBA
2.3. Catalysis—Oxidation of Alcohols
3. Results and Discussion
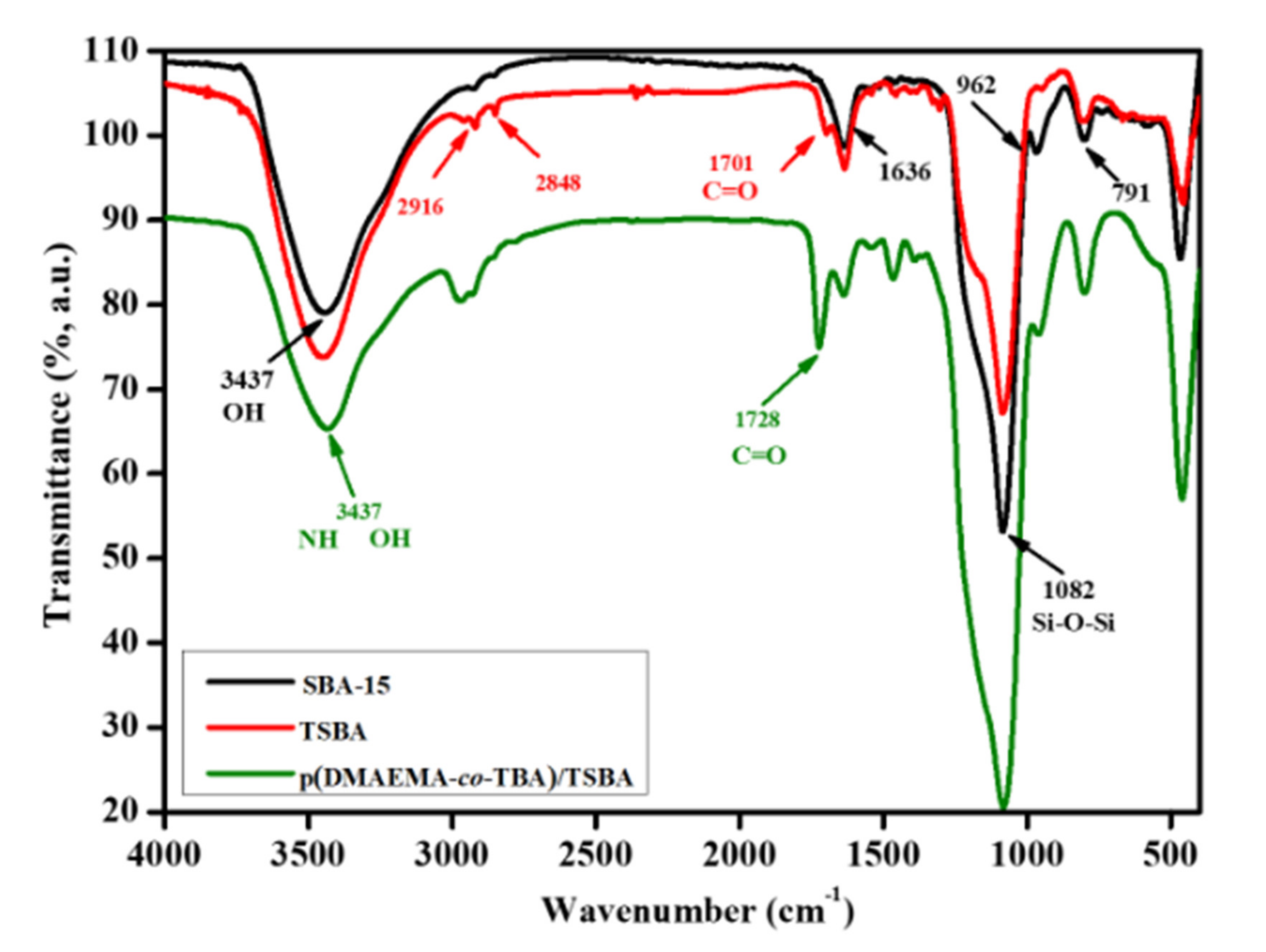
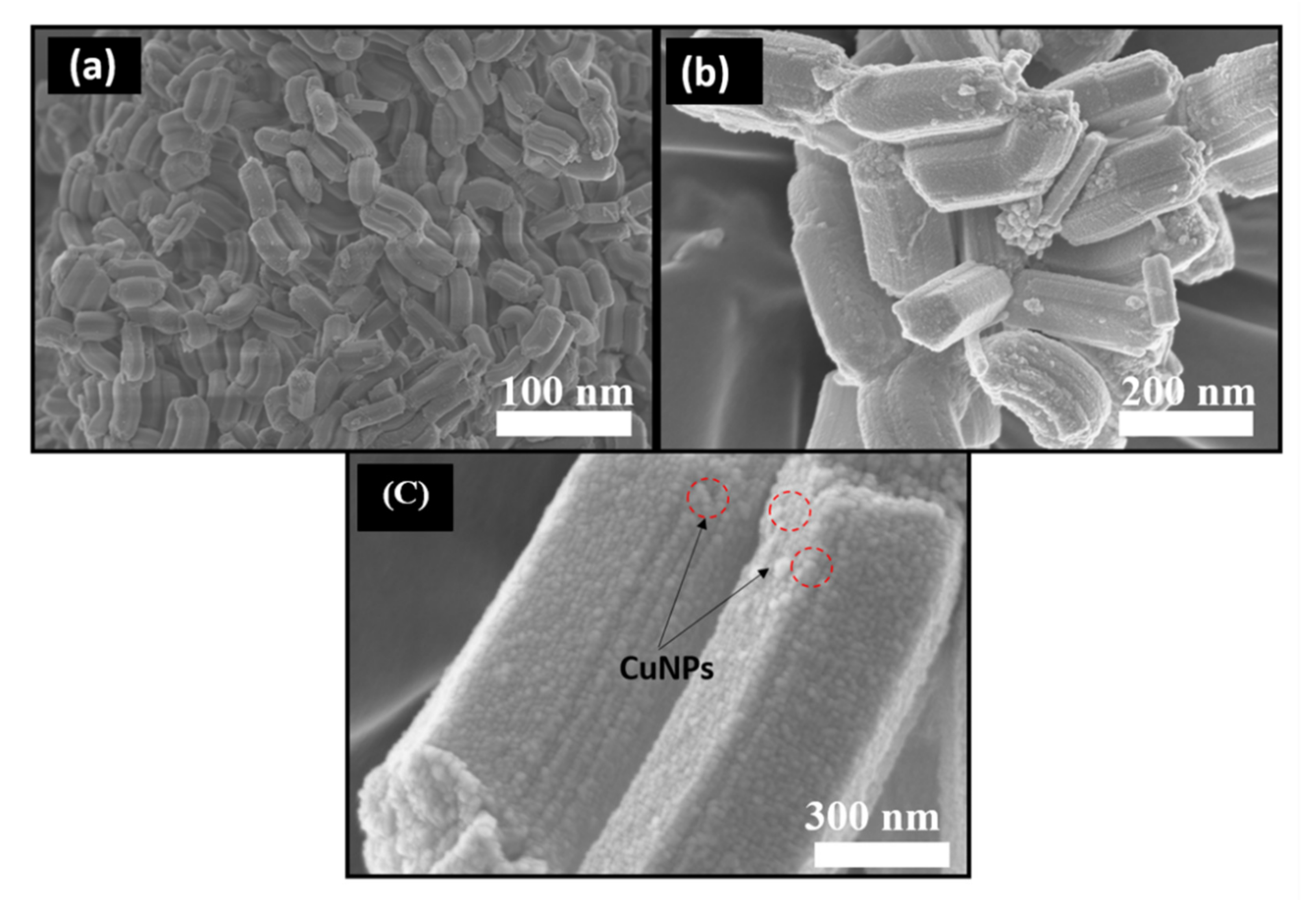
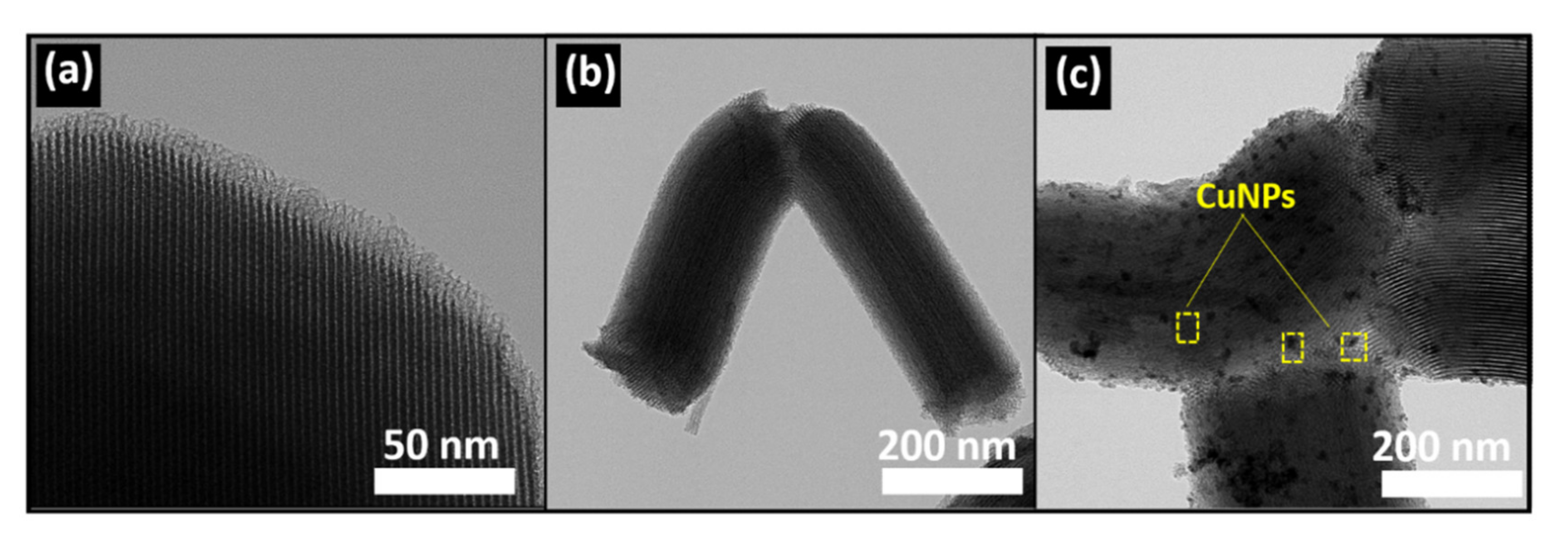
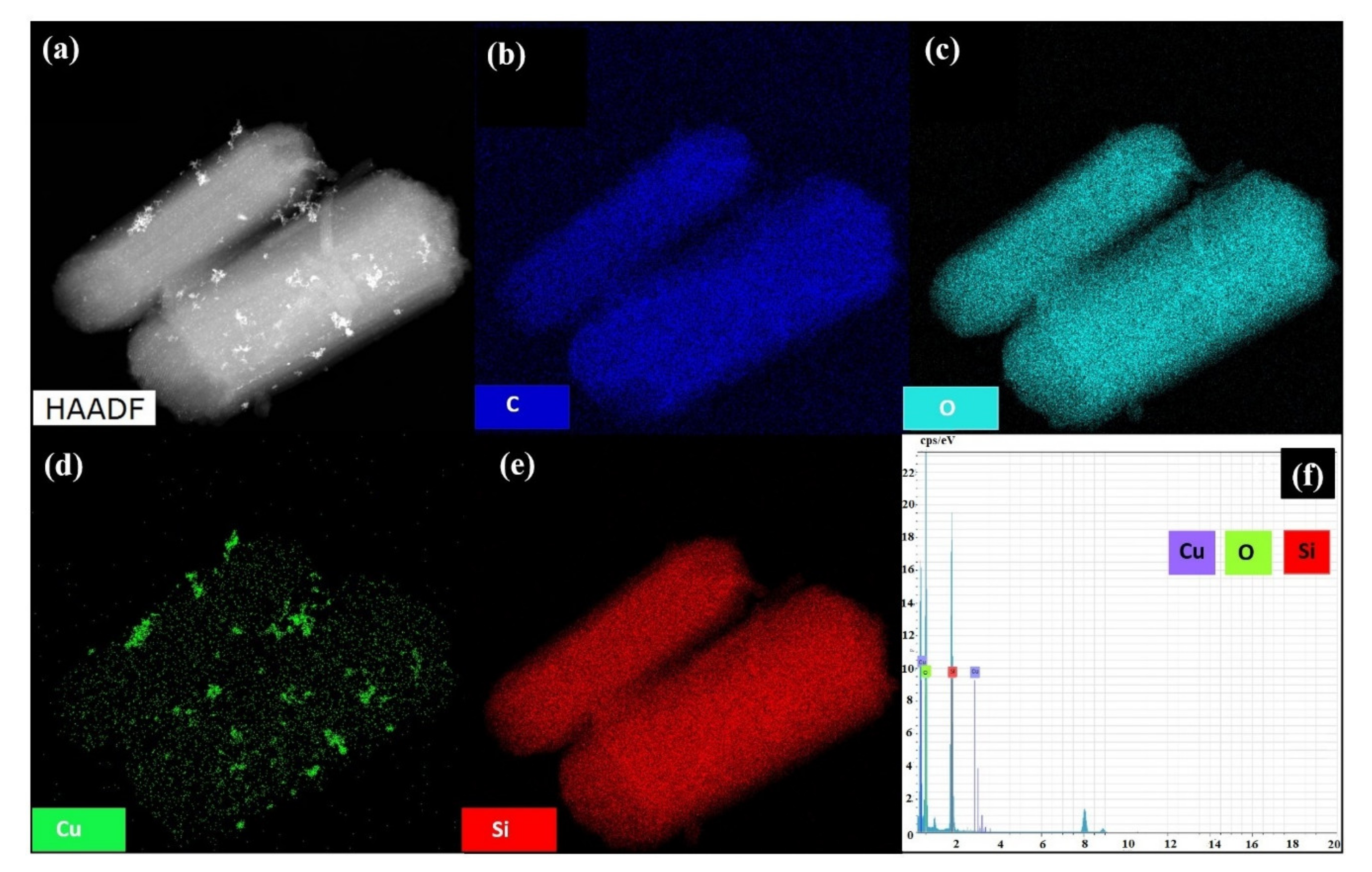
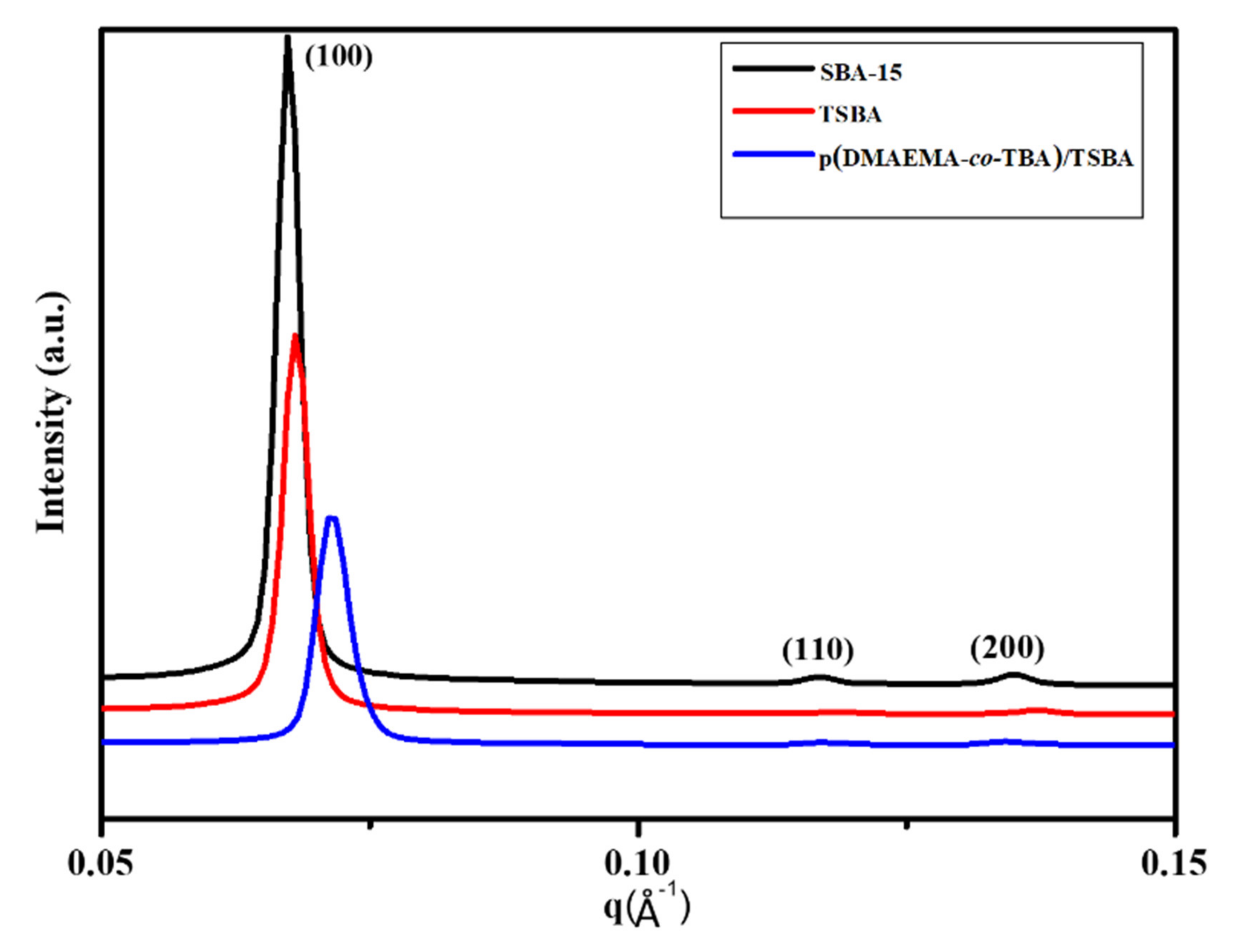
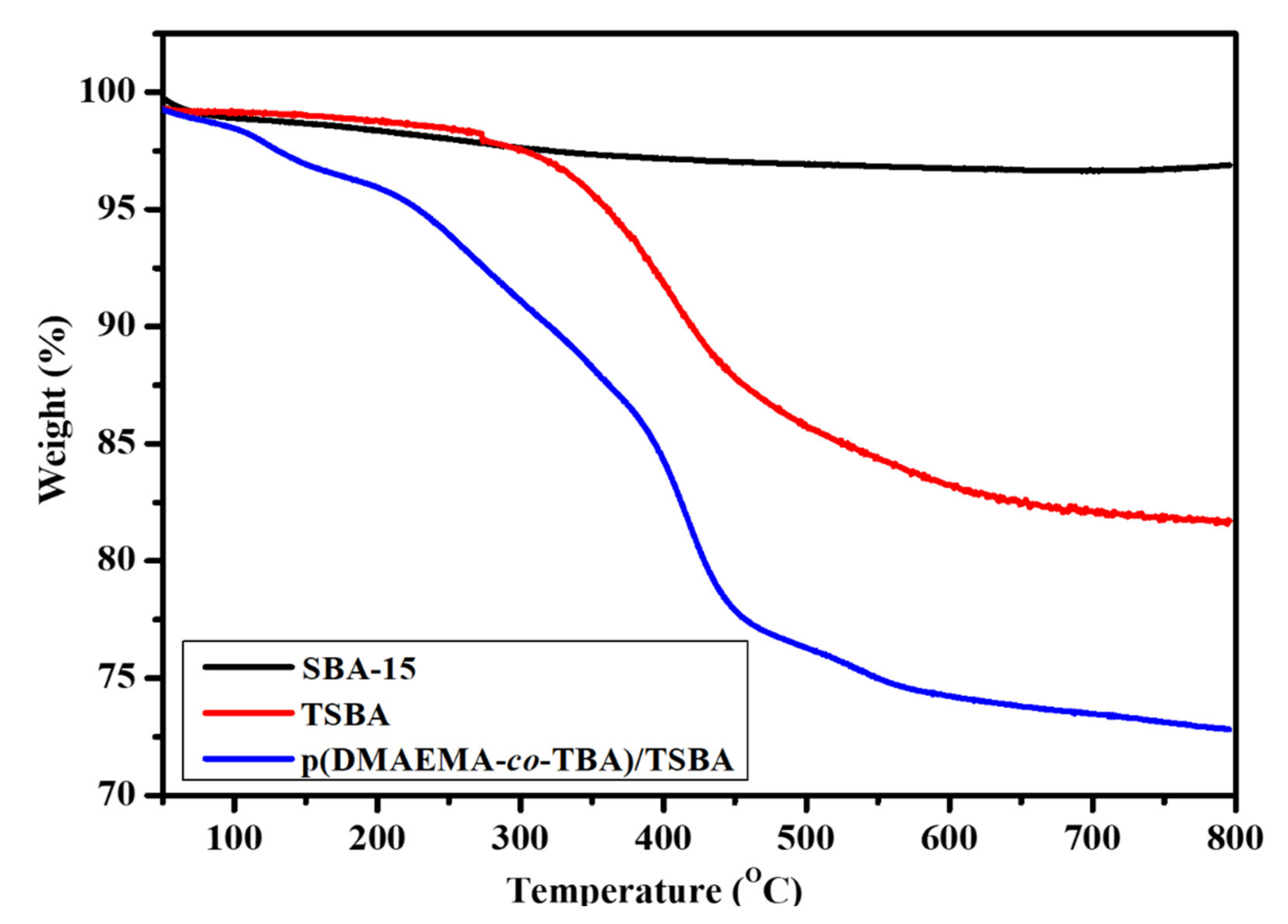
3.1. Characterization of Materials
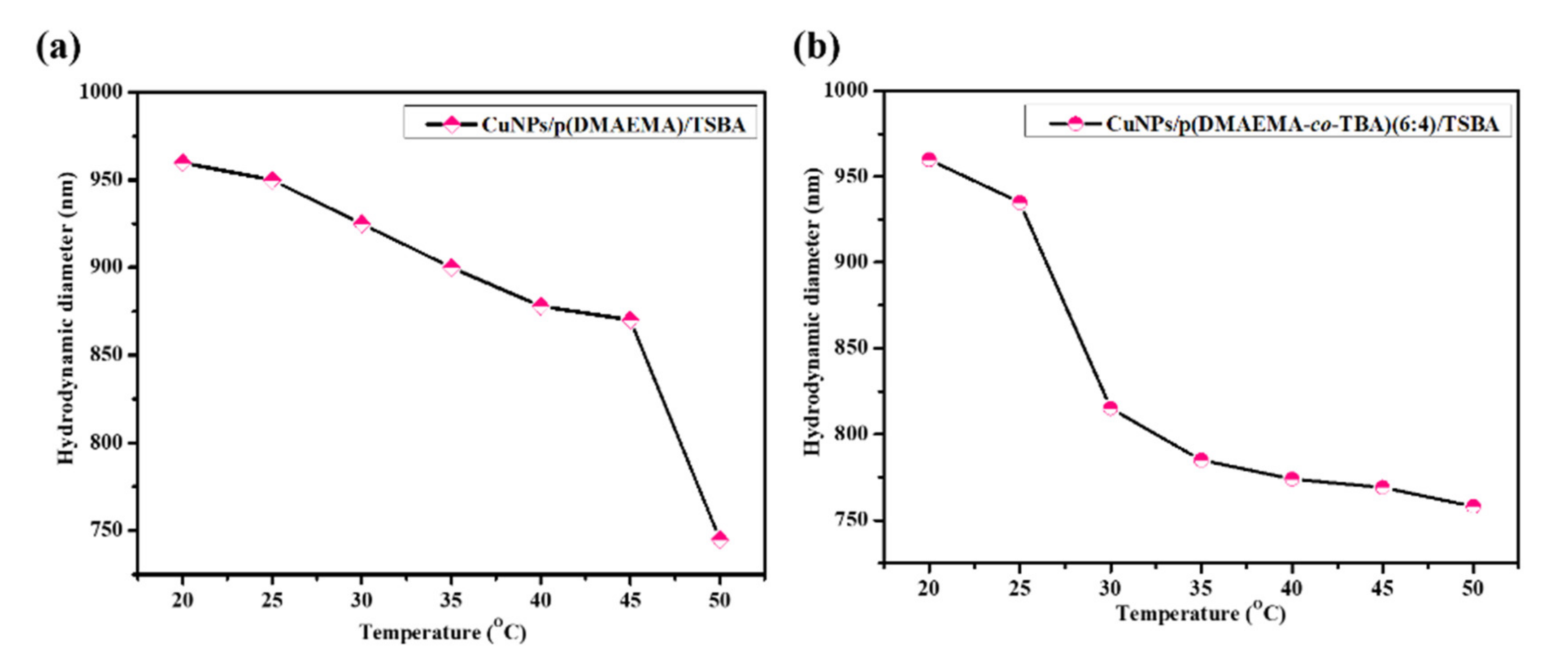
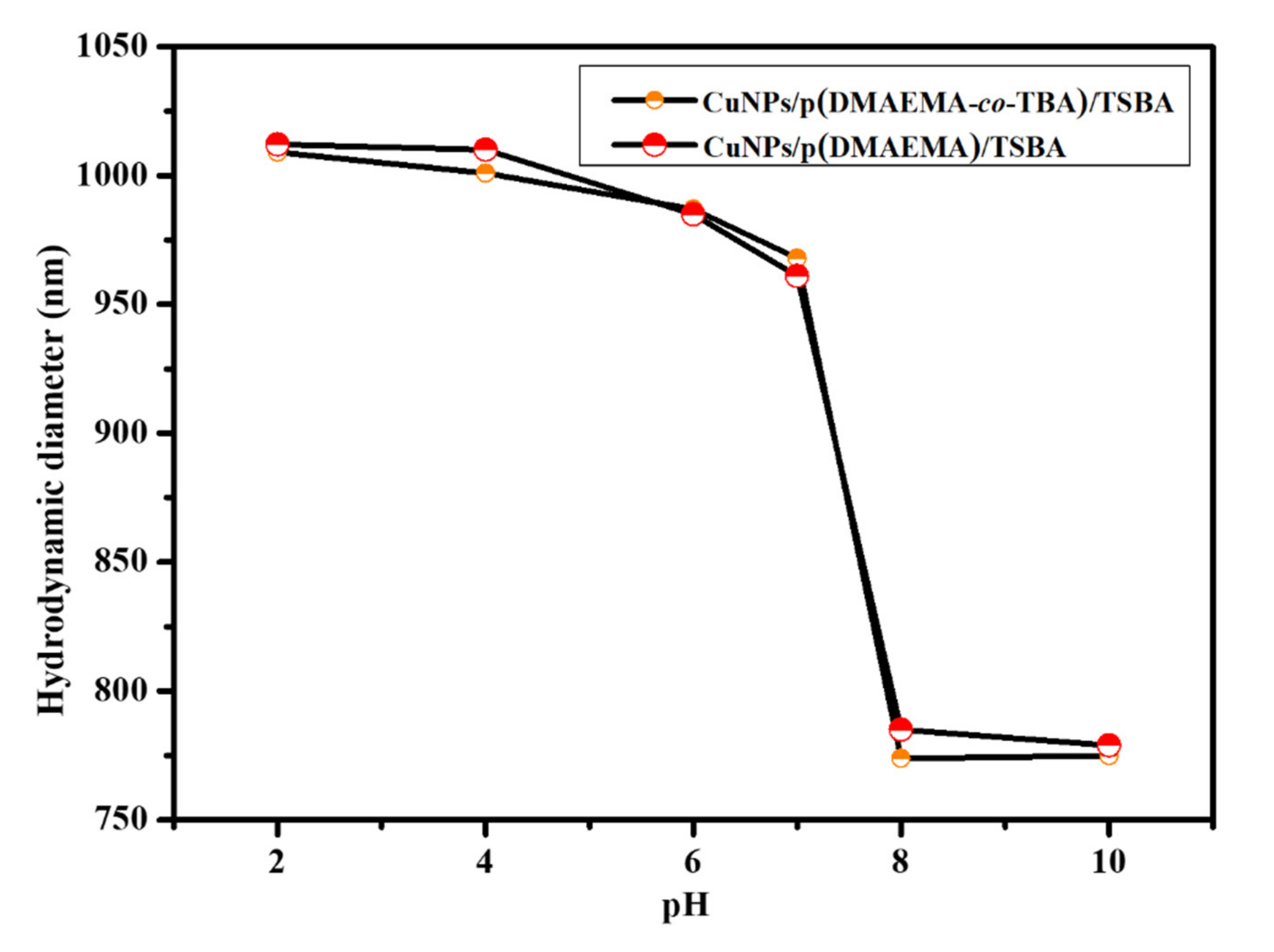
3.2. Stimuli-Responsive Performance
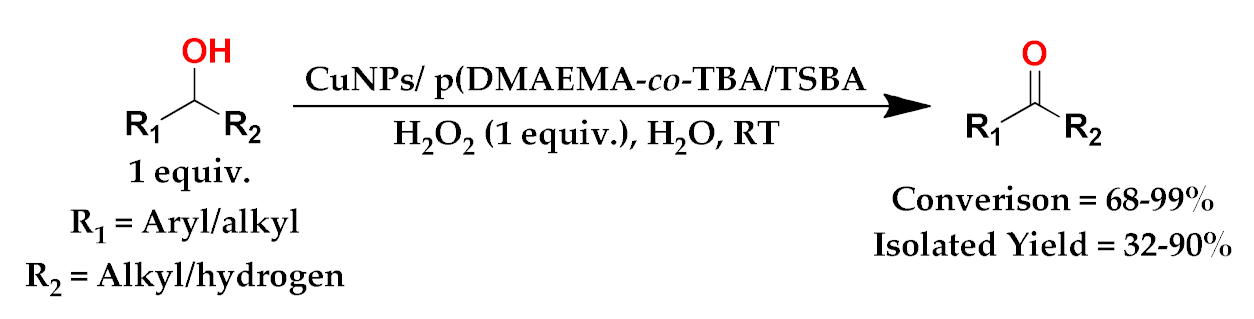
3.3. Catalysis
3.3.1. Optimization
3.3.2. Extension of Scope
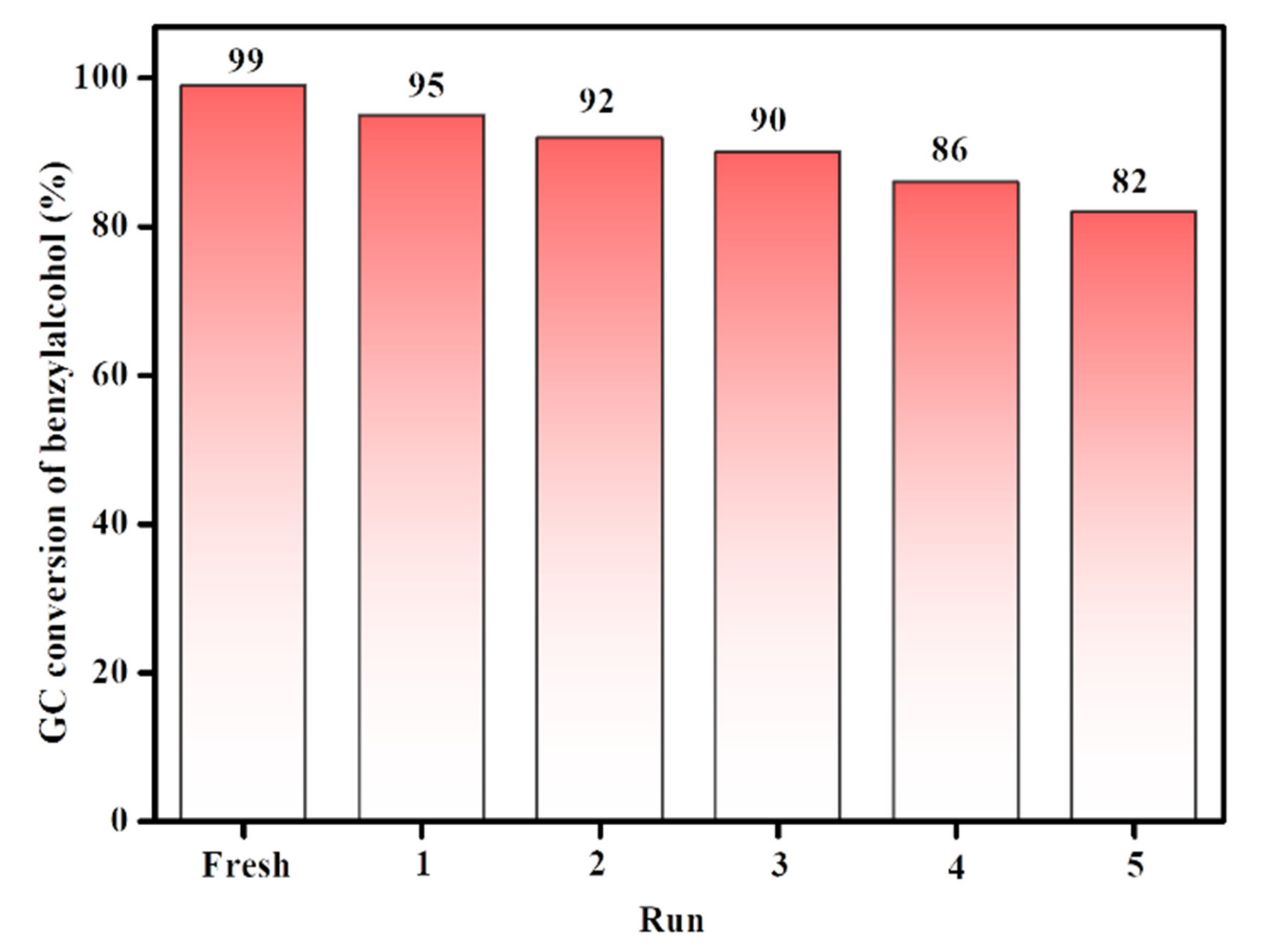
3.4. Reusability of the Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ragupathi, C.; Vijaya, J.J.; Narayanan, S.; Jesudoss, S.K.; Kennedy, L.J. Highly Selective Oxidation of Benzyl Alcohol to Benzaldehyde with Hydrogen Peroxide by Cobalt Aluminate Catalysis: A Comparison of Conventional and Microwave Methods. Ceram. Int. 2015, 41, 2069–2080. [Google Scholar] [CrossRef]
- Xu, J.; Shang, J.K.; Chen, Y.; Wang, Y.; Li, Y.X. Palladium Nanoparticles Supported on Mesoporous Carbon Nitride for Efficiently Selective Oxidation of Benzyl Alcohol with Molecular Oxygen. Appl. Catal. A Gen. 2017, 542, 380–388. [Google Scholar] [CrossRef]
- Ali, R.; Nour, K.; Warthan, A.A.; Siddiqui, M.R.H. Selective Oxidation of Benzylic Alcohols Using Copper-Manganese Mixed Oxide Nanoparticles as Catalyst. Arab. J. Chem. 2015, 8, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Nozaki, A.; Yasuku, T.; Kuwahara, Y.; Ohmichi, T.; Mori, K.; Nagase, T.; Yasuda, H.Y.; Yamashita, H. Oxidation of Benzyl Alcohol over Nanoporous Au−CeO2 Catalysts Prepared from Amorphous Alloys and Effect of Alloying Au with Amorphous Alloys. Ind. Eng. Chem. Res. 2018, 57, 5599–5605. [Google Scholar] [CrossRef]
- Tian, T.; Liu, Y.; Zhang, X. Bimetallic Synergistic Au/CuO-Hydroxyapatite Catalyst for Aerobic Oxidation of Alcohols. Chin. J. Catal. 2015, 36, 1358–1364. [Google Scholar] [CrossRef]
- Williams, R.M.; Medlin, J.W. Benzyl Alcohol Oxidation on Pd(111): Aromatic Binding Effects on Alcohol Reactivity. Langmuir 2014, 30, 4642–4653. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Dumbre, D.K.; Bhargava, S.K. Oxidation of Benzyl Alcohol to Benzaldehyde by tert-Butyl Hydroperoxide Over Nanogold Supported S supported on TiO2 and Other Transition and Rare-Earth Metal Oxides. Ind. Eng. Chem. Res. 2009, 48, 9471–9478. [Google Scholar] [CrossRef]
- Perez, Y.; Ballesteros, R.; Fajardo, M.; Sierra, I.; Hierro, I.D. Copper-Containing Catalysts for Solvent-Free Selective Oxidation of Benzyl Alcohol. J. Mol. Catal. A Chem. 2012, 352, 45–56. [Google Scholar] [CrossRef]
- Mirsafaei, R.; Heravi, M.M.; Hosseinnejad, T.; Ahmadi, S. Copper (II) Nanoparticles: An Efficient and Reusable Catalyst in Green Oxidation of Benzyl Alcohols to Benzaldehydes in Water. Appl. Organometal. Chem. 2016, 30, 823–830. [Google Scholar] [CrossRef]
- Feng, X.; Lv, P.; Sun, W.; Han, X.; Gao, L.; Zheng, G. Reduced Graphene Oxide-Supported Cu Nanoparticles for the Selective Oxidation of Benzyl Alcohol to Aldehyde with Molecular Oxygen. Catal. Commun. 2017, 99, 105–109. [Google Scholar] [CrossRef]
- Varma, S.V.; Dahiya, R. Copper (II) Nitrate on Clay (claycop)-Hydrogen Peroxide: Selective and Solvent-Free Oxidations Using Microwaves. Tetrahedron Lett. 1998, 39, 1307–1308. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracia, D.D.; Ardiles, P.R.; Prashar, S.; Rodriguez, D.A.; Paez, P.L.; Ruiz, S.G. Preparation and Study of the Antibacterial Applications and Oxidative Stress Induction of Copper Maleamate-Functionalized Mesoporous Silica Nanoparticle. Pharmaceutics 2019, 11, 1–18. [Google Scholar]
- Saikia, D.; Huang, Y.Y.; Wu, C.E.; Kao, H.M. Size Dependence of Silver Nanoparticles in Carboxylic Acid Functionalized Mesoporous Silica SBA-15 for Catalytic Reduction of 4-Nitrophenol. RSC Adv. 2016, 6, 35167–35176. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Chai, K.; Wang, Y.Q.; Zhang, H.D.; Xu, W.; Li, W.; Shi, Y. Mesoporous Silica-Supported CuCo2O4 Mixed-Metal Oxides for the Aerobic Oxidation of Alcohols. ACS Appl. Nano Mater. 2019, 2, 4435–4442. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, S.; Gu, F.; Ping, Y.; Guo, X.; Zhong, Z.; Su, F. Highly Selective Gas-Phase Oxidation of Benzyl Alcohol to Benzaldehyde Over Silver-Containing Hexagonal Mesoporous Silica. Microporous Mesoporous Mater. 2012, 149, 158–165. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Kuila, D.; Salomon, C.; Konarova, M.; Eguchi, M.; Na, J.; Yamauchi, Y. Metal Incorporated Mesoporous Oxides: Synthesis and Applications. J. Hazard. Mater. 2020, 401, 123348–123362. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.J.; Gao, M.R.; Liu, C.H.; Li, S.L.; Jiang, H.L.; Lan, Y.Q.; Han, M.; Yu, S.H. Porous Molybdenum-Based Hybrid Catalysts for Highly Efficient Hydrogen Evolution. Angew. Chem. Int. Ed. 2015, 54, 12928–12932. [Google Scholar] [CrossRef]
- Yano, K. Hollow Metal-Incorporated Monodispersed Mesoporous Silica Spheres. Langmuir 2015, 31, 8774–8779. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zou, G.; Yang, B.; Luo, X.; Xu, S. Amine-Functionalized Mesoporous Silica @ Reduced Graphene Sandwichlike Structure Composites for CO2 Adsorption. ACS Appl. Nano Mater. 2018, 1, 4695–4702. [Google Scholar] [CrossRef]
- Barczak, M.; Dobrowolski, R.; Borowski, P.; Giannakoudakis, D.A. Pyridine-, Thiol- and Amine-Functionalized Mesoporous Silicas for Adsorptive Removal of Pharmaceuticals. Microporous Mesoporous Mater. 2020, 299, 110132. [Google Scholar] [CrossRef]
- Wu, S.H.; Mou, C.Y.; Lin, H.P. Synthesis of Mesoporous Silica Nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Galet, V.M.; Esteve, E.P.; Rico, M.R.; Manez, R.M.; Barat, J.M.; Munoz, P.H.; Gavara, R. Anchoring Gated Mesoporous Silica Particles to Ethylene Vinyl Alcohol Films for Smart Packaging Applications. Nanomaterials 2018, 8, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Tang, X.; Pei, M. Facile synthesis of PDMAEMA-coated Hollow Mesoporous Silica Nanoparticles and their pH-Responsive Controlled Release. Microporous Mesoporous Mater. 2013, 173, 64–69. [Google Scholar] [CrossRef]
- Brilmayer, R.; Hess, C.; Brunsen, A.A. Influence of Chain Architecture on Nanopore Accessibility in Polyelectrolyte Block-Co-Oligomer Functionalized Mesopores. Small 2019, 15, 1902710–1902717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, A.M.; Mohan, A.; Rout, L.; Nagappan, S.; Park, S.S.; Ha, C.S. Pd Nanoparticle Incorporated Mesoporous Silicas with Excellent Catalytic Activity and Dual Responsivity. Colloid Surf. A Physciochem. Eng. Asp. 2020, 585, 124074–124084. [Google Scholar] [CrossRef]
- Lambert, B.D.; Charreyre, M.T.; Chaix, C.; Pichot, C. Poly(N-tert-butyl acrylamide-b-N-acryloylmorpholine) Amphiphilic Block Copolymers Via Polymerization: Synthesis, Purification and Characterization. Polymer 2006, 28, 437–447. [Google Scholar] [CrossRef]
- Zhao, D.; Li, X.; Shi, X.; Ye, K.; Liu, W.; Qiu, G.; Lu, X. In Situ Synthesis of Magnetic Poly(N-tert-butyl acrylamide-co-acrylic acid)/Fe3O4 Nanogels for Magnetic Resonance Imaging. RSC Adv. 2016, 6, 61001–61005. [Google Scholar] [CrossRef]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; Hasan, M.M.U. Investigations into the Antibacterial Behavior of Copper Nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Cruz, P.; Perez, Y.; Hierro, I.D.; Fajardo, M. Copper, Copper Oxide Nanoparticles and Copper Complexes Supported on Mesoporous SBA-15 as Catalysts in the Selective Oxidation of Benzyl Alcohol in Aqueous Phase. Microporous Mesoporous Mater. 2016, 220, 136–147. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Hussain, I.; Li, C.; Li, J.; Sun, X.; Shen, J.; Han, H.; Wang, L. Iron-Copper Bimetallic Nanoparticles Supported on Hollow Mesoporous Silica Spheres: The Effect of Fe/Cu Ratio on Heterogeneous Fenton Degradation of a Dye. RSC Adv. 2016, 6, 54623–54635. [Google Scholar] [CrossRef]
- Mohan, A.; Rout, L.; Thomas, A.M.; Nagappan, S.; Parambadath, S.; Park, S.S.; Ha, C.S. Silver Nanoparticles Impregnated pH-Responsive Nanohyhybrid System for the Catalytic Reduction of Dyes. Microporous Mesoporous Mater. 2020, 303, 110260–110269. [Google Scholar] [CrossRef]
- Liu, F.; Urban, M.W. Dual pH and Temperature Responsiveness of poly(2-(N, N-dimethylamino)ethyl methacrylate-co-n-butyl acrylate)] Colloidal Dispersions and Their Films. Macromolecules 2008, 41, 6531–6539. [Google Scholar] [CrossRef]
- Yuan, W.; Zhao, Z.; Yuan, J.; Gu, S.; Zhang, F.; Xie, X.; Ren, J. Synthesis of pH and Temperature-Responsive Chitosan-graft-poly(2-(N,N-dimethylamino)ethyl methacrylate) Copolymer and Gold Nanoparticle Stabilization by its Micelles. Polym. Int. 2011, 60, 194–201. [Google Scholar] [CrossRef]
- Ganesamoorthy, S.; Jerome, P.; Shanmugasundaram, K.; Karvembu, R. Highly Efficient Homogeneous and Hetrogenized Ruthenium Catalyst for Transfer Hydrogenation of Carbonyl Compounds. RSC Adv. 2014, 4, 27955–27962. [Google Scholar] [CrossRef]
- Babu, S.G.; Priyadarsini, P.A.; Karvembu, R. Copper on Boehmite: A Simple, Selective, Efficient and Reusable Hetrogeneous Catalyst for Oxidation of alcohols with Periodic Acid in Water at Room Temperature. Appl. Catal. A Gen. 2011, 392, 1218–1224. [Google Scholar] [CrossRef]
- Rozner, S.D.; Alsters, P.L.; Neumann, R. Water Soluble and Self-Assembled Polyoxometalate as a Recyclable Catalyst for Oxidation of Alcohols in Water with Hydrogen Peroxide. J. Am. Chem. Soc. 2003, 125, 5280–5281. [Google Scholar] [CrossRef] [PubMed]
- Frija, L.M.T.; Alegria, E.C.B.A.; Sutradhar, M.; Cristiano, M.L.S.; Ismael, A.; Kopylovich, M.N.; Pombeiro, A.J.L. Copper(II) and Cobalt(II) Tetrazole-Saccharinate Complexes as Effective Catalysts for Oxidation of Secondary Alcohols. J. Mol. Catal. A Chem. 2016, 425, 283–290. [Google Scholar] [CrossRef]
- Ferraz, C.P.; Garcia, M.A.S.; Neto, E.T.; Rossi, L.M. Oxidation of Benzyl Alcohol Catalysed by Gold Catalyzed by Gold Nanoparticles under Alkaline Conditions: Weak vs. Strong Baes. RSC Adv. 2016, 6, 25279–25285. [Google Scholar] [CrossRef]
- Xu, S.; Wu, J.; Huang, P.; Lao, C.; Lai, H.; Wang, Y.; Wang, Z.; Zhong, G.; Fu, X.; Peng, F. Selective Catalytic Oxidation of Benzyl Alcohol to Benzaldehyde by Nitrates. Front. Chem. 2020, 8, 151. [Google Scholar] [CrossRef]
- Chen, G.J.; Wang, J.S.; Jin, F.Z.; Liu, M.Y.; Zhao, C.W.; Li, Y.A.; Dong, Y.B. Pd@Cu(II)-MOF-Catalysed Aerobic Oxidation of Benzylic Alcohols in Air with High Conversion and Selectivity. Inorg. Chem. 2016, 55, 3058–3064. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.U.; Raiisanen, M.T.; Leskela, M.; Repo, T. Copper Catalyzed Oxidation of Benzylic Alcohols in Water with H2O2. Appl. Catal. A Gen. 2012, 411, 180–187. [Google Scholar] [CrossRef]
- Meng, C.; Yang, K.; Fu, X.; Yuan, R. Photocatalytic Oxidation of Benzyl Alcohol by Homogeneous CuCl2/Solvent: A Model System to Explore the Role if Molecular Oxygen. ACS Catal. 2015, 5, 3760–3766. [Google Scholar] [CrossRef]
- Ali, R.; Adil, A.F.; Warthan, A.A.; Siddiqui, M.R.H. Identification of Active Phase for Selective Oxidation of Benzyl Alcohol with Moleular Oxygen Catalyzed Copper-Manganese Oxide Nanoparticles. J. Chem. 2012, 2013, 1–9. [Google Scholar]
- Lokhande, P.D.; Waghmare, S.R.; Gaikward, H.; Hankare, P.P. Copper Catalyzed Oxidation of Benzyl Alcohol to Benzaldehyde. J. Korean Chem. Soc. 2012, 56, 539–541. [Google Scholar] [CrossRef] [Green Version]
- Neves, P.; Valente, A.A.; Lin, Z. Mild Liquid Phase Oxidation of Benzyl Alcohol in the Presence of Microporous Framework Copper Silicates. Chem. Eur. J. 2020, 2020, 1172–1176. [Google Scholar] [CrossRef]
- Pina, C.D.; Falletta, E.; Rossi, M. Highly Selective Oxidation of Benzyl Alcohol to Benzaldehyde Catalysed by Bimetallic Gold-Copper Catalyst. J. Catal. 2008, 260, 384–386. [Google Scholar] [CrossRef]
- Liu, K.; Quin, T.; Sun, Y.; Hou, C.; Cao, X.; Jiang, S. Synergistic Effect Between Ag and Mn3O4 in the Gas Phase Oxidation of Alcohols. Catal. Commun. 2018, 113, 15–18. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.H.; Qi, M.Y.; Li, J.Y.; Tang, Z.R.; Anpo, M.; Yamada, Y.M.A.; Xu, Y.J. Photoredox Dual Reaction for Selective Alcohol Oxidation and Hydrogen Evolution Over Nickel Surface-Modified ZnIn2S4. Appl. Catal. B 2020, 271, 118946–118953. [Google Scholar] [CrossRef]
- Luo, N.; Wang, M.; Li, H.; Zhang, J.; Liu, H.; Wang, F. Photocatalytic Oxidation-Hydrogenolysis of Lignin β-O-4 Models Via a Dual Light Wavelength Switching Strategy. ACS Catal. 2016, 6, 7716–7721. [Google Scholar] [CrossRef]

| Entry | Substrate | Product | Time (min) | Conversion b (Selectivity c) (%) | Isolated Yield d (%) | TON d | TOF d |
|---|---|---|---|---|---|---|---|
| 1 | 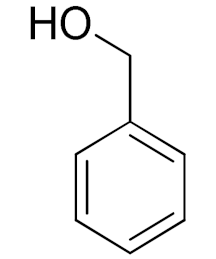 | 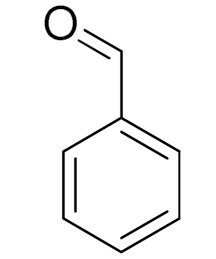 | 45 | 99 (99) | 90 | 31 | 42 |
| 2 |  | 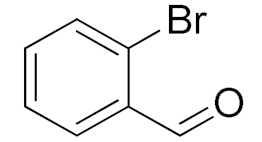 | 180 | 82 (98) | 71 | 26 | 9 |
| 3 | 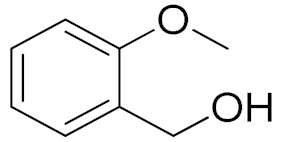 | 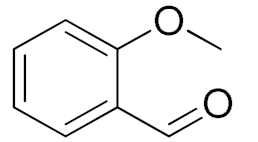 | 180 | 80 (94) | 74 | 25 | 9 |
| 4 | 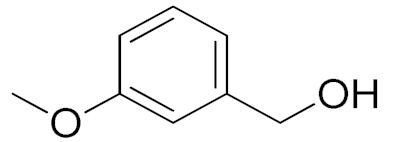 | 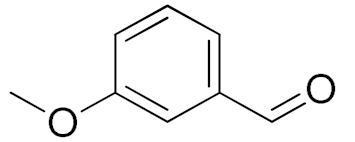 | 240 | 88 (98) | 78 | 28 | 7 |
| 5 | 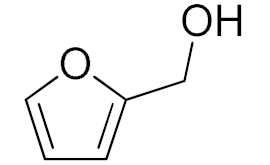 | 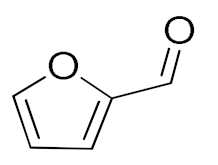 | 120 | 94 (99) | 87 | 30 | 15 |
| 6 | 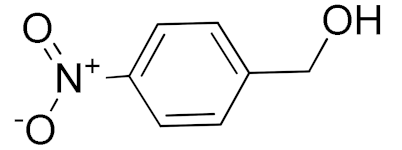 | 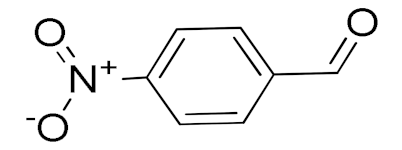 | 300 | 87 (95) | 79 | 28 | 6 |
| 7 | 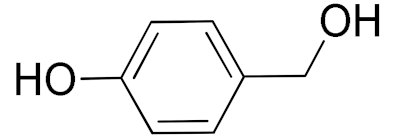 | 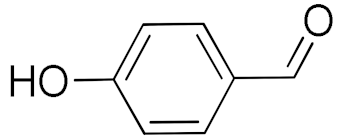 | 480 | 89 (99) | 80 | 28 | 4 |
| 8 |  | 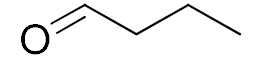 | 1440 | 76 (97) | 62 | 24 | 1 |
| 9 | 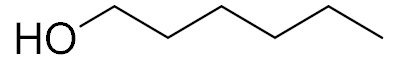 | 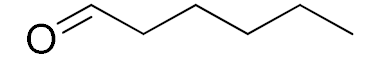 | 1440 | 75 (89) | 88 | 24 | 1 |
| 10 |  |  | 720 | 88 (97) | 81 | 28 | 3 |
| 11 |  | 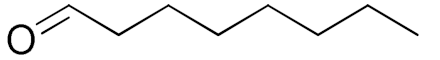 | 720 | 89 (88) | 70 | 28 | 3 |
| 12 | 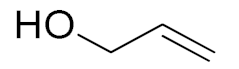 | 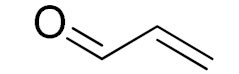 | 180 | 68 (88) | 32 | 22 | 7 |
| 13 |  |  | 300 | 60 (95) | 52 | 19 | 4 |
| 14 | 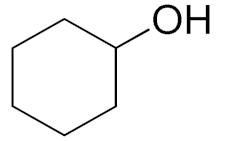 |  | 300 | 62 (91) | 54 | 20 | 4 |
| 15 |  | 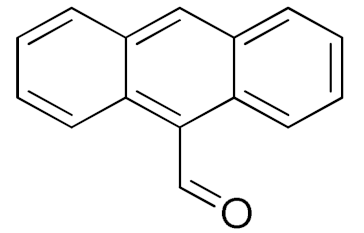 | 1440 | 91 (99) | 83 | 29 | 2 |
| Entry | Catalyst | Oxidant | Catalyst Amount (mg) | Time (h) | Temp. (°C) | Yield (%) | TON | TOF | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cu(NO3)2 | H2O2 (1.2 mL) | 10 | 1.5 | 80 | 52 | 78 | 52 | [40] |
| 2 | Pd@Cu(II)-MOF | O2 | 23 | 25 | 130 | 99 | 50 | 2 | [41] |
| 3 | CuSO4 | H2O2 (1 mL) | 10 | 1.5 | 80 | 62 | 186 | 124 | [42] |
| 4 | CuCl2/CH3COCH3 | O2 | 25 | 1 | 25 | 95 | 190 | 190 | [43] |
| 5 | CuMn2 oxide | O2 | 200 | 0.6 | 102 | 99 | - | - | [44] |
| 6 | CuCl2/THF | O2 | 20 | 2 | 80 | 85 | 43 | 22 | [45] |
| 7 | CuSH/1NaK | t-BuOOH (1.6 mL) | 20 | 24 | 70 | 72 | 165 | 7 | [46] |
| 8 | Au-Cu/SiO2 | O2 | 200 | 10 | 319 | 92 | 702 | 280 | [47] |
| 9 | AgCu/SiC | O2 | 280 | 2.5 | 280 | 99 | 1151 | 460 | [48] |
| 10 | CuNPs/p(DMAEMA-co-TBA)/TSBA | H2O2 (1 mL) | 5.0 | 1 | 25 | 99 | 31 | 42 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, A.M.; Peter, J.; Nagappan, S.; Mohan, A.; Ha, C.-S. Dual Stimuli-Responsive Copper Nanoparticles Decorated SBA-15: A Highly Efficient Catalyst for the Oxidation of Alcohols in Water. Nanomaterials 2020, 10, 2051. https://doi.org/10.3390/nano10102051
Thomas AM, Peter J, Nagappan S, Mohan A, Ha C-S. Dual Stimuli-Responsive Copper Nanoparticles Decorated SBA-15: A Highly Efficient Catalyst for the Oxidation of Alcohols in Water. Nanomaterials. 2020; 10(10):2051. https://doi.org/10.3390/nano10102051
Chicago/Turabian StyleThomas, Anju Maria, Jerome Peter, Saravanan Nagappan, Anandhu Mohan, and Chang-Sik Ha. 2020. "Dual Stimuli-Responsive Copper Nanoparticles Decorated SBA-15: A Highly Efficient Catalyst for the Oxidation of Alcohols in Water" Nanomaterials 10, no. 10: 2051. https://doi.org/10.3390/nano10102051