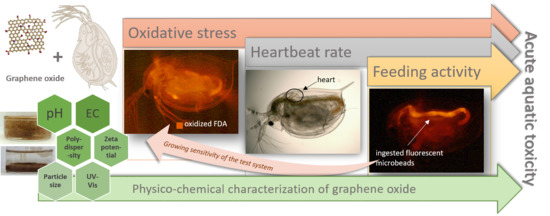
Ecotoxicity Assessment of Graphene Oxide by Daphnia magna through a Multimarker Approach from the Molecular to the Physiological Level including Behavioral Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the GO Nanoparticles
2.2. Physico-Chemical Characterization of the Assembled Ecotoxicity Test Systems
2.2.1. Electric Conductivity and pH Measurements
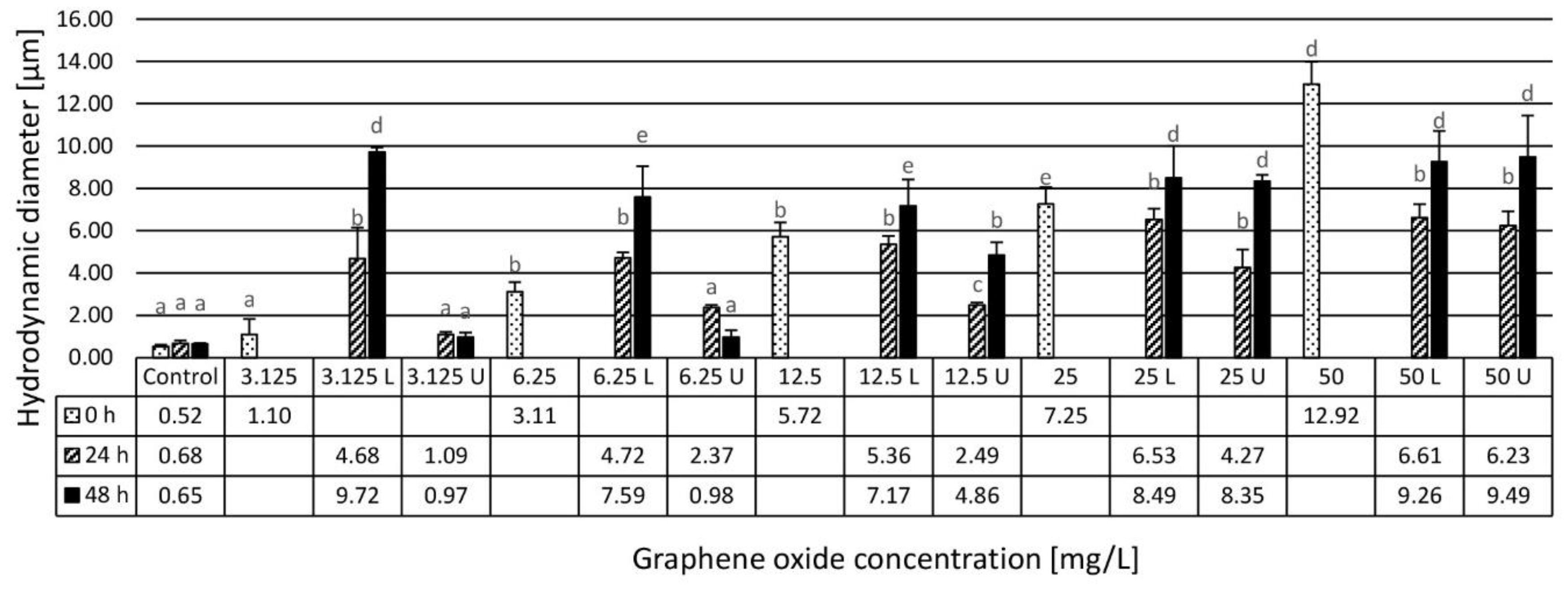
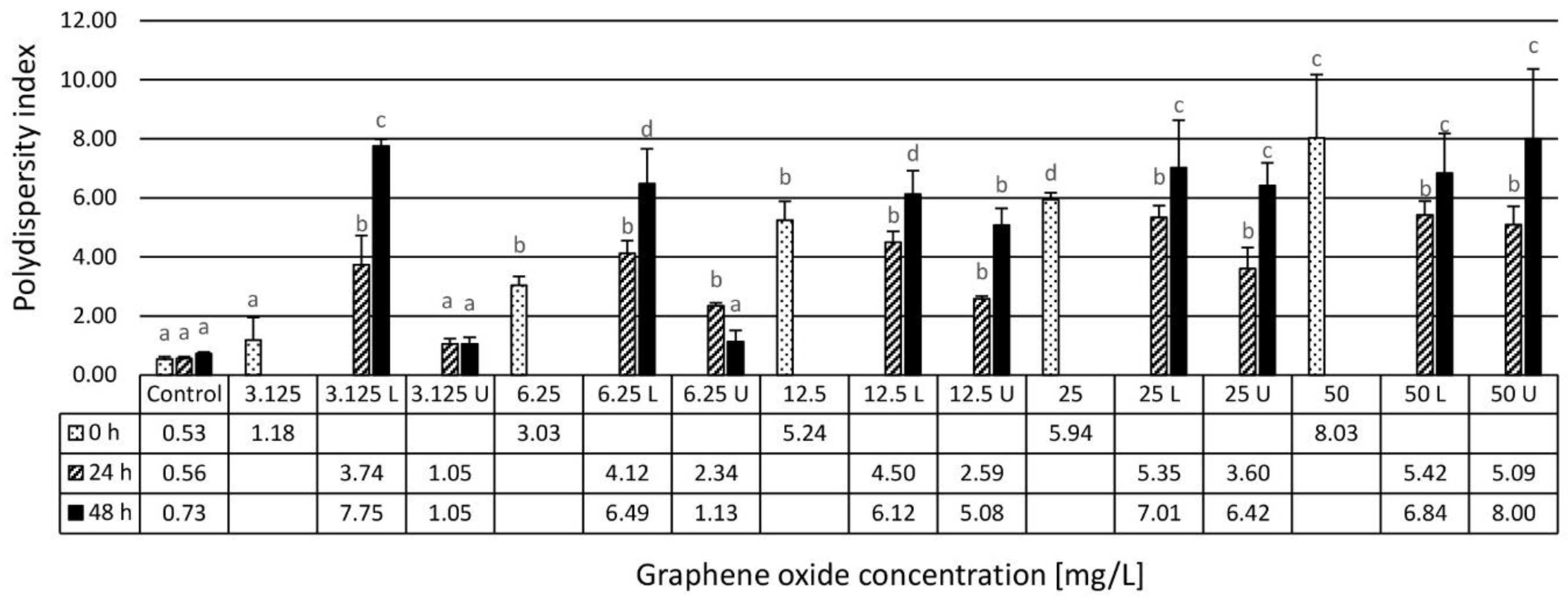
2.2.2. Dynamic Light Scattering Method Characterization and Zeta Potential Determination
2.2.3. UV-Vis Spectroscopy for GO Concentration Determination
2.3. Applied Ecotoxicity Test Methods
2.3.1. Daphnia magna Cultures
2.3.2. Daphnia magna Lethality and Immobilization Assay
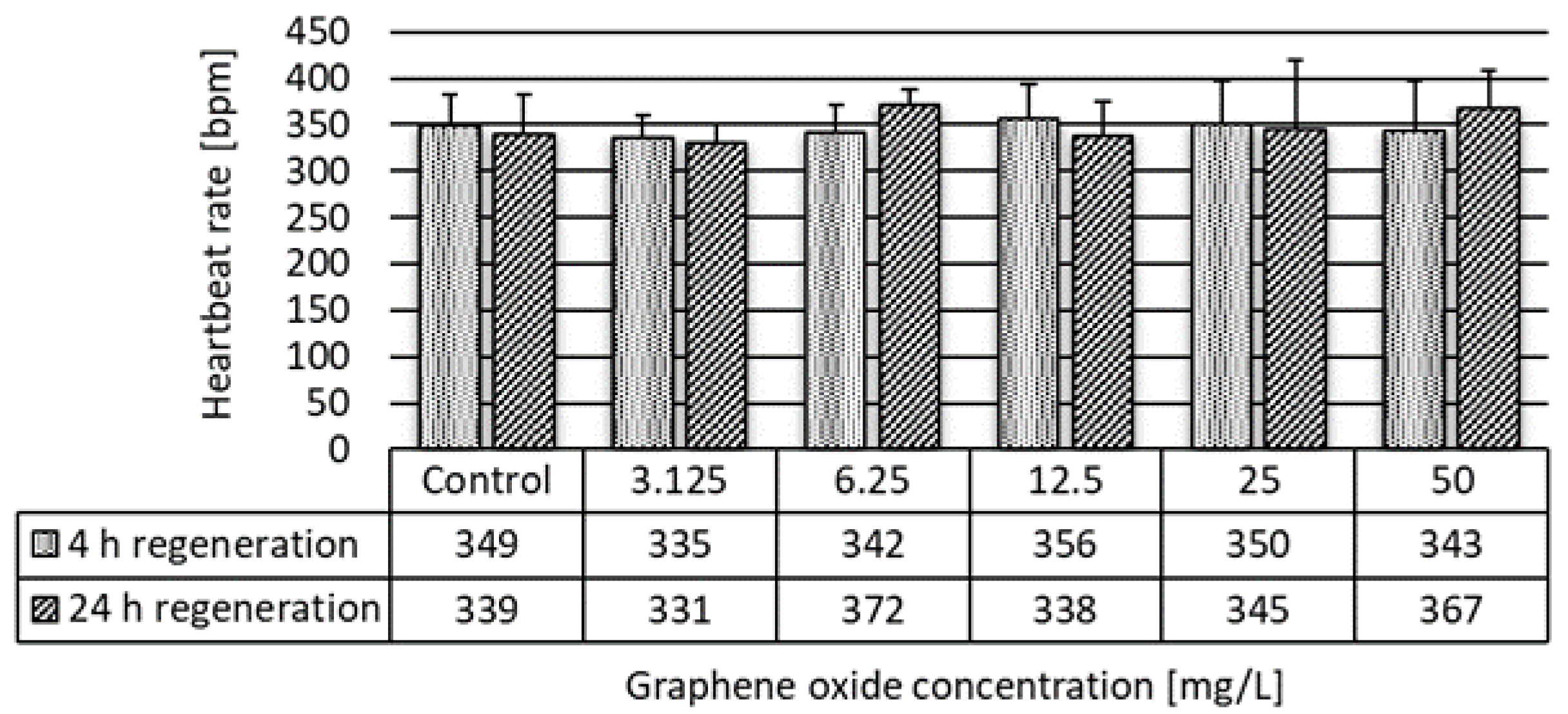
2.3.3. Daphnia magna Heartbeat Rate Assay
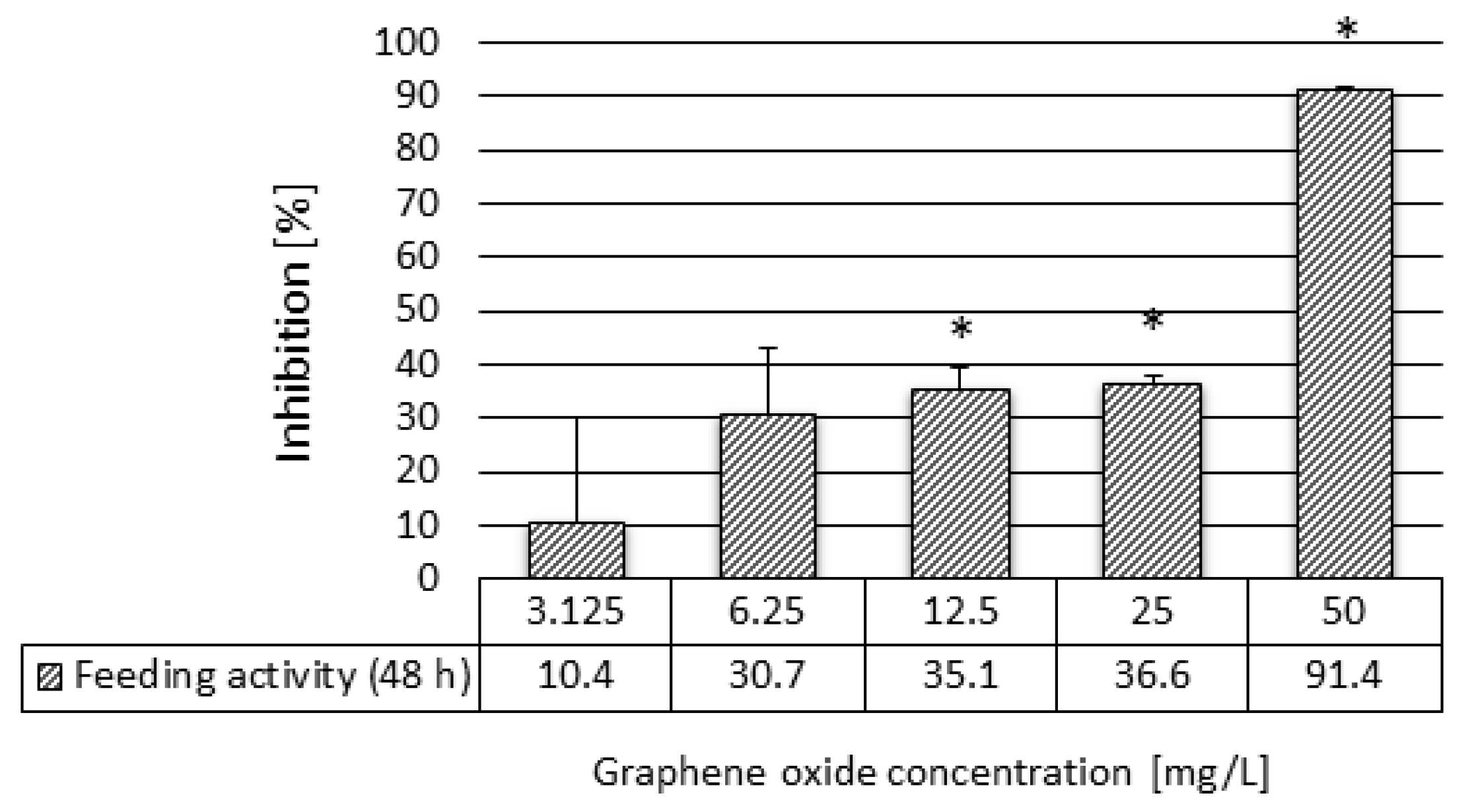
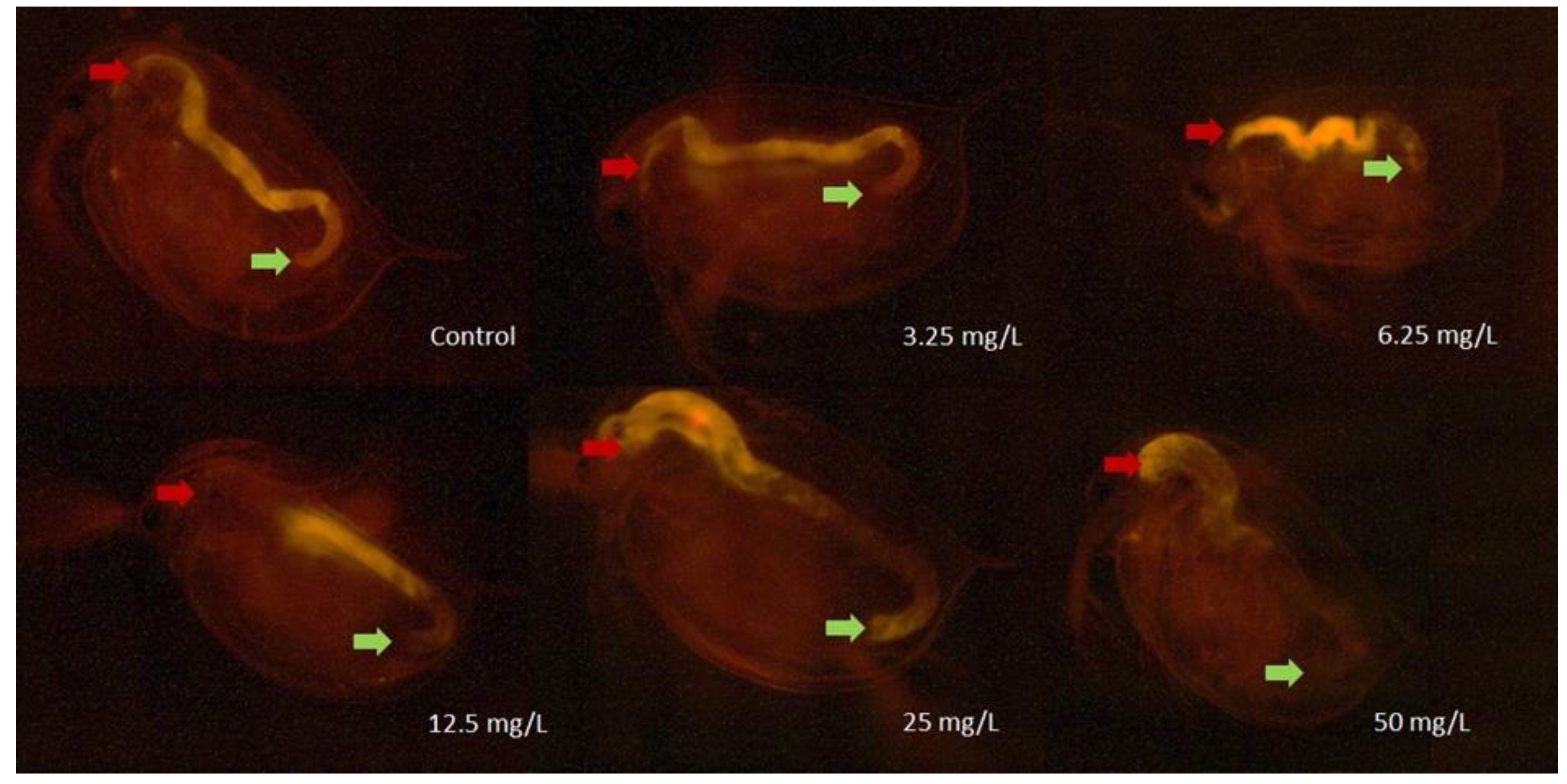
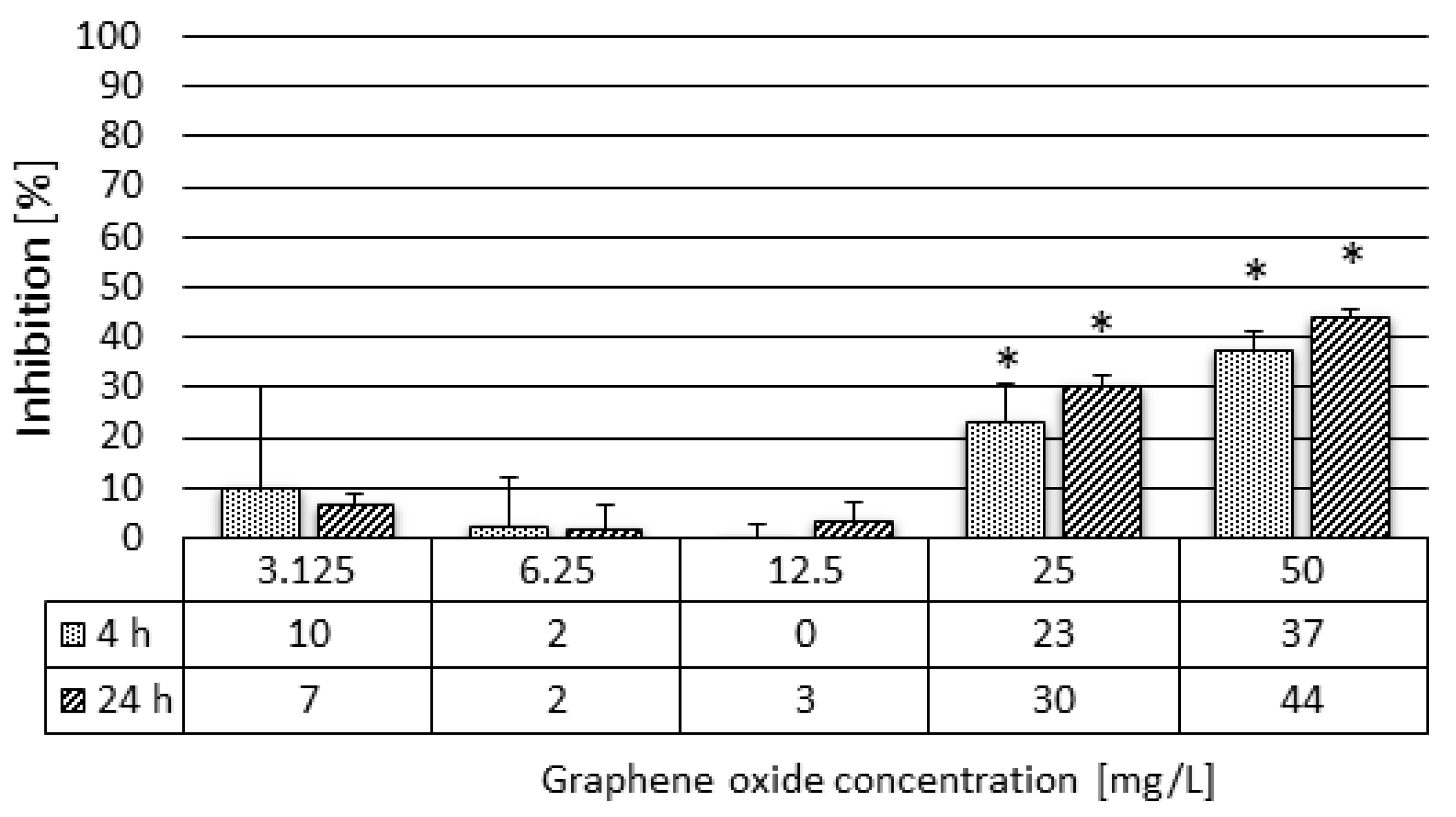
2.3.4. Daphnia magna Feeding Activity Assay
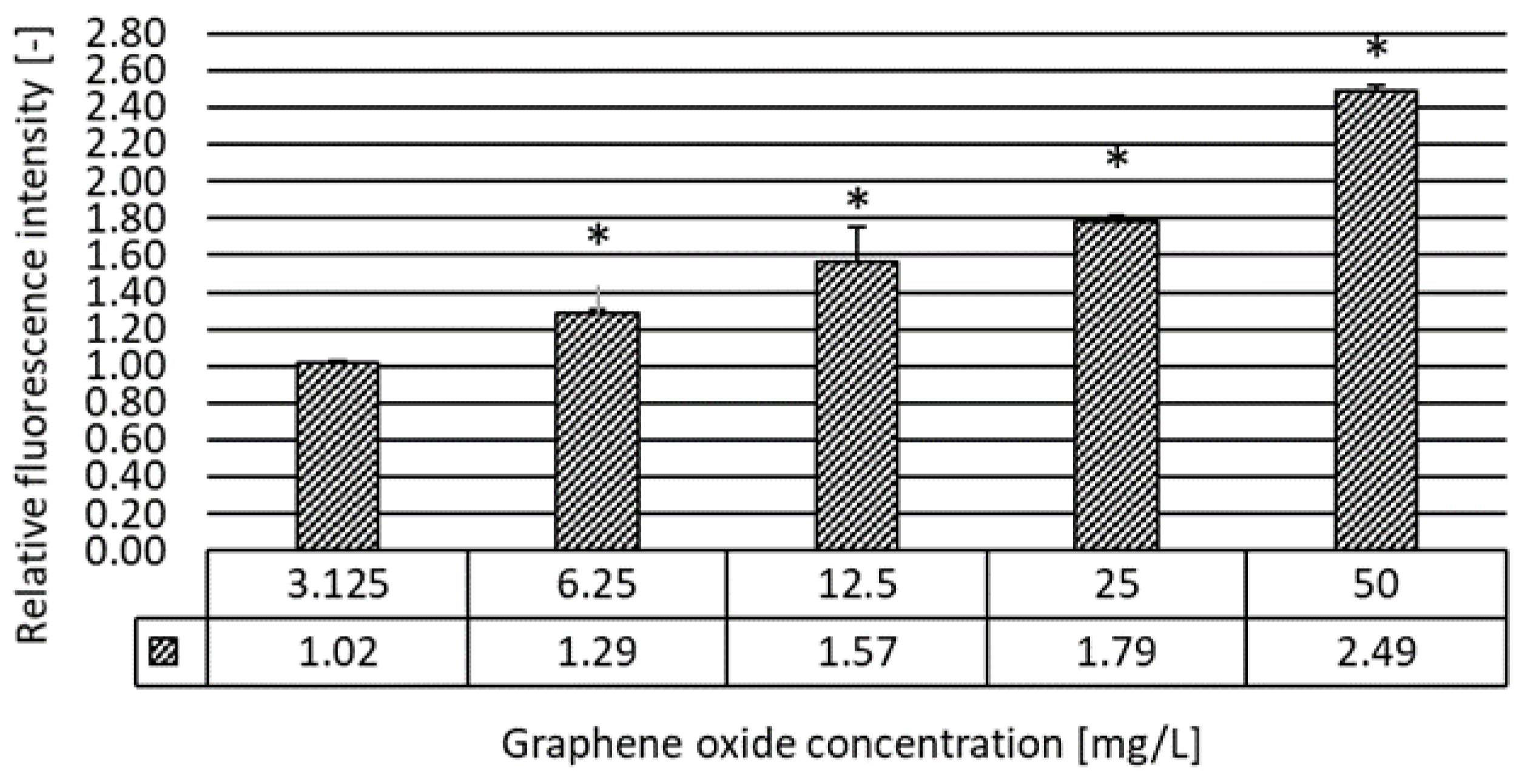
2.3.5. Oxidative Stress Assay
2.3.6. Recovery after GO Exposure
2.4. Data Evaluation and Statistical Analysis
3. Results
3.1. Results of the Physico-Chemical Characterization of the Assembled Ecotoxicity Test Systems
3.2. Results of the Ecotoxicity Experiments
4. Discussion
4.1. Physico-Chemical Characterization and Stability Evaluation of Graphene Oxide
4.2. Acute Ecotoxicity of Graphene Oxide to Daphnia Magna
4.3. Evaluation of the Sensitivity of the Applied Ecotoxicity Endpoints
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Fal, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Duch, M.C.; Mansukhani, N.D.; Hersam, M.C.; Bouchard, D. Deposition and release of graphene oxide nanomaterials using a quartz crystal microbalance. Environ. Sci. Technol. 2014, 48, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Duch, M.C.; Manuskhani, N.D.; Hersam, M.C.; Bouchard, D. Colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment. Environ. Sci. Technol. 2013, 47, 6288–6296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Z.; White, J.C.; Xing, B. Graphene in the aquatic environment: Adsorption, dispersion, toxicity and transformation. Environ. Sci. Technol. 2014, 48, 9995–10009. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.D.M.; Bartlett, N.J.; Lloyd, V.K. Daphnia as an emerging epigenetic model organism. Genet. Res. Int. 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Baun, A.; Hartmann, N.B.; Grieger, K.; Kusk, K.O. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: A brief review and recommendations for future toxicity testing. Ecotoxicology 2008, 17, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lin, Z.; Chen, Y.; Tian, S. Acute toxicities of six manufactured nanomaterial suspensions to Daphnia magna. J. Nanopart. Res. 2008, 11, 67–75. [Google Scholar] [CrossRef]
- Kim, K.T.; Klaine, S.J.; Lin, S.; Ke, P.C.; Kim, S.D. Acute toxicity of a mixture of copper and single-walled carbon nanotubes to Daphnia magna. Environ. Toxicol. Chem. 2010, 29, 122. [Google Scholar] [CrossRef]
- Li, S.; Pan, X.; Wallis, L.K.; Fan, Z.; Chen, Z.L.; Diamond, S.A. Comparison of TiO2 nanoparticle and graphene–TiO2 nanoparticle composite phototoxicity to Daphnia magna and Oryzias latipes. Chemosphere 2014, 112, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano, A.M.; Maul, J.D.; Saed, M.; Shah, S.A.; Green, M.J.; Canas-Carrell, J.E. Bioaccumulation, stress, and swimming impairment in Daphnia magna exposed to multi-wall carbon nanotubes, graphene, and graphene oxide. Environ. Toxicol. Chem. 2017, 36, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Huang, B.; Zhu, X.; Jiang, Y.; Chen, B.; Tao, Y.; Zhou, J.; Cai, Z. Mechanisms underlying the acute toxicity of fullerene to Daphnia magna: Energy acquisition restriction and oxidative stress. Water Res. 2017, 123, 696. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Farah, S.; Farkas, A.; Madarász, J.; László, K. Comparison of thermally and chemically reduced graphene oxides by thermal analysis and Raman spectroscopy. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- OECD 202. OECD Guideline for Testing Chemicals. Daphnia Magna Acute Immobilization Test; OECD Publisher: Paris, France, 2004. [Google Scholar]
- Fekete-Kertész, I.; Piszmán, D.; Molnár, M. Particle size and concentration dependent ecotoxicity of nano- and microscale TiO2−comparative study by different aquatic test organisms of different trophic levels. Water Air Soil Pollut. 2017, 228, 245. [Google Scholar] [CrossRef]
- Kamaya, M.; Sonamoto, M.; Nagashima, K.; Ginatullina, E.N. A simple bioassay using fluorescent microbeads and Daphnia magna. J. Environ. Sci. Eng. 2011, 5, 1613–1616. [Google Scholar]
- Ulm, L.; Krivohlavek, A.; Jurašin, D.; Ljubojević, M.; Šinko, G.; Crnković, T.; Žuntar, I.; Šikić, S.; Vrček, I.V. Response of biochemical biomarkers in the aquatic crustacean Daphnia magna exposed to silver, nanoparticles. Environ. Sci. Pollut. Res. 2015, 22, 19990–19999. [Google Scholar] [CrossRef]
- Zuin, S.; Micheletti, C.; Critto, A.; Pojana, G.; Johnston, H.; Stone, V.; Tran, L.; Marcomini, A. Weight of evidence approach for the relative hazard ranking of nanomaterials. Nanotoxicology 2011, 5, 445–458. [Google Scholar] [CrossRef]
- Lv, X.; Yang, Y.; Tao, Y.; Jiang, Y.; Chen, B.; Zhu, X.; Li, B. A mechanism study on toxicity of graphene oxide to Daphnia magna: Direct link between bioaccumulation and oxidative stress. Environ. Pollut. 2018, 234, 953–959. [Google Scholar] [CrossRef]
- Wu, L.; Liu, L.; Gao, B.; Muñoz-Carpena, R.; Zhang, M.; Chen, H.; Zhou, Z.; Wang, H. Aggregation kinetics of graphene oxides in aqueous solutions: Experiments, mechanisms, and modelling. Langmuir 2013, 29, 15174–15181. [Google Scholar] [CrossRef] [PubMed]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Balog, S.; Rodriguez-Lorenzo, L.; Monnier, C.A.; Obiols-Rabasa, M.; Rothen-Rutishauser, B.; Schurtenberger, P.; Petri-Fink, A. Characterizing nanoparticles in complex biological media and physiological fluids with depolarized dynamic light scattering. Nanoscale 2015, 7, 5991. [Google Scholar] [CrossRef] [Green Version]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nano EHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Li, Z.; Lu, Z.; Li, Y.; Yin, J.J.; Zhao, Y. Unraveling stress induced toxicity properties of graphene oxide and the underlying mechanism. Adv. Mater. 2012, 24, 5391–5397. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.K.; Dong, S.P.; Petersen, E.J.; Gao, S.X.; Huang, Q.G.; Mao, L. Biological uptake and depuration of radio-labeled graphene by Daphnia magna. Environ. Sci. Technol. 2013, 47, 12524–12531. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, W.; Xu, Z.; Peng, W.; Luo, S. Transgenerational effects of reduced graphene oxide modified by Au, Ag, Pd, Fe3O4, Co3O4 and SnO2 on two generations of Daphnia magna. Carbon 2017, 122, 669–679. [Google Scholar] [CrossRef]
- Liu, Y.; Han, W.; Xu, Z.; Fan, W.; Peng, W.; Luo, S. Comparative toxicity of pristine graphene oxide and its carboxyl, imidazole or polyethylene glycol functionalized products to Daphnia magna: A two generation study. Environ. Pollut. 2018, 237, 218–227. [Google Scholar] [CrossRef]
- Loureiro, S.; Gonçalves, S.F.; Gonçalves, G.; Hortiguela, M.J.; Rebelo, S.; Ferro, M.C.; Vila, M. Eco-friendly profile of pegylated nano-graphene oxide at different levels of an aquatic trophic chain. Ecotoxicol. Environ. Saf. 2018, 162, 192–200. [Google Scholar] [CrossRef]
- Lovern, S.B.; Strickler, J.R.; Klaper, R. Behavioral and physiological changes in Daphnia magna when exposed to nanoparticle suspensions (titanium dioxide, Nano-C60, and C60HxC70Hx). Environ. Sci. Technol. 2007, 41, 4465–4470. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.H.; Cui, M.M.; Shi, Z.W.; Tan, C.; Yang, X.P. Enhanced oxidative stress and physiological damage in Daphnia magna by copper in the presence of nano-TiO2. J. Nanomat. 2012, 398720. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Wang, Q.; Zhao, H.; Wang, L.; Guo, S.; Li, X. Ecotoxicological effects of graphene oxide on the protozoan Euglena gracilis. Chemosphere 2015, 128, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Gao, Y.; Hu, X.G. L-Cysteine: A biocompatible, breathable and beneficial coating for graphene oxide. Biomaterials 2015, 52, 301–311. [Google Scholar] [CrossRef]
- Bownik, A. Physiological endpoints in daphnid acute toxicity tests. Sci. Total Environ. 2020, 700, 134400. [Google Scholar] [CrossRef] [PubMed]

| Graphene Oxide Concentration [mg/L] | Phase | Zeta Potential [mV] | ||
|---|---|---|---|---|
| 0 h | 24 h | 48 h | ||
| Control | – | −14.9 +/− −9.2 | −16.7 +/− −3.6 | −14.9 +/− −5.8 |
| 3.125 | Upper Lower | −18.4 +/− −3.2 | −14.6 +/− −4.9 | −10.2 +/− −8.2 |
| −17.0 +/− −3.4 | −16.3 +/− −2.5 | |||
| 6.25 | Upper Lower | −17.9 +/− −2.4 | −14.8 +/− −3.4 | −15.8 +/− −3.1 |
| −16.4 +/− −3.4 | −14.0 +/− −3.1 | |||
| 12.5 | Upper Lower | −18.9 +/− −2.9 | −17.6 +/− −3.0 | −12.2 +/− −5.3 |
| −13.6 +/− −3.1 | −14.9 +/− −3.1 | |||
| 25 | Upper Lower | −18.0 +/− −4.8 | −15.1 +/− −2.9 | −11.5 +/− −5.0 |
| −13.6 +/− −3.4 | −13.9 +/− −3.0 | |||
| 50 | Upper Lower | −12.2 +/− −4.8 | −14.5 +/− −2.7 | −16.4 +/− −2.8 |
| −13.4 +/− −7.3 | −14.3 +/− −3.2 | |||
| Graphene Oxide Concentration [mg/L] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 3.125 | 6.25 | 12.5 | 25 | 50 | |||||||
| Vol. | Depth | Vol. | Depth | Vol. | Depth | Vol. | Depth | Vol. | Depth | Vol. | Depth | |
| Upper phase | – | – | 19.8 | 16 | 18.4 | 15 | 16.3 | 13 | 13.9 | 11 | 11.8 | 9 |
| Lower phase | – | – | 5.2 | 4 | 6.6 | 5 | 8.8 | 7 | 11.1 | 9 | 13.2 | 11 |
| Ecotoxicity Assay | Exposure Period | EC20 | EC50 | LOEC |
|---|---|---|---|---|
| Lethality | 24 h | >50 | >50 | >50 |
| 48 h | >50 | >50 | >50 | |
| Immobilization | 24 h | >50 | >50 | >50 |
| 48 h | 50 | >50 | 50 | |
| Heartbeat rate | 24 h | 41.84 | >50 | 50.00 |
| 48 h | 14.77 | >50 | 12.50 | |
| Feeding activity | 48 h | 8.10 | 29.18 | 12.50 |
| Oxidative stress | 48 h | 4.78 | 12.55 | 6.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fekete-Kertész, I.; László, K.; Terebesi, C.; Gyarmati, B.S.; Farah, S.; Márton, R.; Molnár, M. Ecotoxicity Assessment of Graphene Oxide by Daphnia magna through a Multimarker Approach from the Molecular to the Physiological Level including Behavioral Changes. Nanomaterials 2020, 10, 2048. https://doi.org/10.3390/nano10102048
Fekete-Kertész I, László K, Terebesi C, Gyarmati BS, Farah S, Márton R, Molnár M. Ecotoxicity Assessment of Graphene Oxide by Daphnia magna through a Multimarker Approach from the Molecular to the Physiological Level including Behavioral Changes. Nanomaterials. 2020; 10(10):2048. https://doi.org/10.3390/nano10102048
Chicago/Turabian StyleFekete-Kertész, Ildikó, Krisztina László, Csilla Terebesi, Benjámin Sándor Gyarmati, Shereen Farah, Rita Márton, and Mónika Molnár. 2020. "Ecotoxicity Assessment of Graphene Oxide by Daphnia magna through a Multimarker Approach from the Molecular to the Physiological Level including Behavioral Changes" Nanomaterials 10, no. 10: 2048. https://doi.org/10.3390/nano10102048
APA StyleFekete-Kertész, I., László, K., Terebesi, C., Gyarmati, B. S., Farah, S., Márton, R., & Molnár, M. (2020). Ecotoxicity Assessment of Graphene Oxide by Daphnia magna through a Multimarker Approach from the Molecular to the Physiological Level including Behavioral Changes. Nanomaterials, 10(10), 2048. https://doi.org/10.3390/nano10102048