Hollow Silica Particles: Recent Progress and Future Perspectives
Abstract
1. Introduction
2. Synthesis
3. Characterization
4. Applications
4.1. Thermal Insulation
4.2. Drug Delivery
4.3. Energy Storage
4.4. Functional Coatings
4.5. Catalysis
5. Outlook and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, X.; He, D.; He, X.; Wang, K.; Tang, J.; Zou, Z.; He, X.; Xiong, J.; Li, L.; Shangguan, J. Synthesis of hollow mesoporous silica nanorods with controllable aspect ratios for intracellular triggered drug release in cancer cells. ACS Appl. Mater. Interfaces 2016, 8, 20558–20569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-Q.; Li, H.-J.; Qian, D.-J.; Chen, M. Kinetically controlled template-free synthesis of hollow silica micro-/nanostructures with unusual morphologies. Nanotechnology 2014, 25, 135608–135619. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Zhang, W.; Zhang, J.; Li, H.; Han, H.; Zhai, T. Design of gold hollow nanorods with controllable aspect ratio for multimodal imaging and combined chemo-photothermal therapy in the Second near-infrared window. ACS Appl. Mater. Interfaces 2018, 10, 36703–36710. [Google Scholar] [CrossRef] [PubMed]
- Jamil, S.; Janjua, M.R.S.A.; Ahmad, T.; Mehmood, T.; Jing, S.; Jing, X. Zinc oxide hollow micro spheres and nano rods: Synthesis and applications in gas sensor. Mater. Chem. Phys. 2014, 147, 225–231. [Google Scholar] [CrossRef]
- Cordoba, R.; Ibarra, A.; Mailly, D.; Teresa, J.M.D. Vertical growth of superconducting crystalline hollow nanowires by He+ focused ion beam induced deposition. Nano Lett. 2018, 18, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, Z.; Luo, Y.; Xia, M.; Cao, C. Silicon hollow sphere anode with enhanced cycling stability by a template-free method. Nanotechnology 2017, 28, 165404. [Google Scholar] [CrossRef]
- Neshchimenko, V.; Li, C.; Mikhailov, M.; Lv, J. Optical radiation stability of ZnO hollow particles. Nanoscale 2018, 10, 22335–22347. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Zhu, X.; Kong, X.Z. Preparation of hollow TiO2 nanoparticles through TiO2 deposition on polystyrene latex particles and characterizations of their structure and photocatalytic activity. Nanoscale Res. Lett. 2012, 7, 646. [Google Scholar] [CrossRef]
- Nomura, T.; Tanii, S.; Ishikawa, M.; Tokumoto, H.; Konish, Y. Synthesis of hollow zirconia particles using wet bacterial templates. Adv. Powder Technol. 2013, 24, 1013–1016. [Google Scholar] [CrossRef]
- Gyger, F.; Hubner, M.; Feldmann, C.; Barsan, N.; Weimar, U. Nanoscale SnO2 hollow spheres and their application as a gas-sensing material. Chem. Mater. 2010, 22, 4821–4827. [Google Scholar] [CrossRef]
- Ramli, R.A. Hollow polymer particles: A review. RSC Adv. 2017, 7, 52632–52650. [Google Scholar] [CrossRef]
- Meng, Q.; Xiang, S.; Zhang, K.; Wang, M.; Bu, X.; Xue, P.; Liu, L.; Sun, H.; Yang, B. A facile two-step etching method to fabricate porous hollow silica particles. J. Colloid Interface Sci. 2012, 384, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Akane, Y.; Ogi, T.; OKuyama, K. Mesopore-free hollow silica particles with controllable diameter and shell thickness via additive-free synthesis. Langmuir 2012, 28, 8616–8624. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pasc, A.; Fierro, V.; Celzard, A. Hollow carbon spheres, synthesis and applications—A review. J. Mater. Chem. A 2016, 4, 12686–12713. [Google Scholar] [CrossRef]
- Mei, X.; Chen, D.; Li, N.; Xu, Q.; Ge, J.; Li, H.; Lu, J. Hollow mesoporous silica nanoparticles conjugated with pH-sensitive amphiphilic diblock polymer for controlled drug release. Microporous Mesoporous Mater. 2012, 152, 16–24. [Google Scholar] [CrossRef]
- Zou, X.; Tao, C.; Yang, K.; Yang, F.; Lv, H.; Yan, L.; Yan, H.; Li, Y.; Xi, Y.; Yuan, X.; et al. Rational design and fabrication of highly transparent, flexible, and thermally stable superhydrophobic coatings from raspberry-like hollow silica nanoparticles. Appl. Surface Sci. 2018, 440, 700–711. [Google Scholar] [CrossRef]
- Cao, X.; Chuan, X.; Masse, R.C.; Huang, D.; Li, S.; Cao, G. A three-layer design with mesoporous silica encapsulated by a carbon core and shell for high energy lithium ion battery anodes. J. Mater. Chem. A 2015, 3, 22739–22749. [Google Scholar] [CrossRef]
- Qiu, J.; Huo, D.; Xue, J.; Zhu, G.; Liu, H.; Xia, Y. Encapsulation of a Phase-Change Material in Nanocapsules with a Well-Defined Hole in the Wall for the Controlled Release of Drugs. Angew. Chem. Int. Ed. 2019, 58, 10606–10611. [Google Scholar] [CrossRef]
- Han, Y.; Liu, X.; Lu, Z. Systematic Investigation of Prelithiated SiO2 particles for high-performance anodes in lithium-ion battery. Appl. Sci. 2018, 8, 1245. [Google Scholar] [CrossRef]
- Ishii, H.; Sato, K.; Nagao, D.; Konno, M. Anionic liposome template synthesis of raspberry-like hollow silica particle under ambient conditions with basic catalyst. Colloids Surf. Biointerfaces 2012, 92, 372–376. [Google Scholar] [CrossRef]
- Shiomi, T.; Tsunoda, T.; Kawai, A.; Chiku, H.; Mizukami, F.; Sakaguchi, K. Formation of cage-like hollow spherical silica via a mesoporous structure by calcination of lysozyme–silica hybrid particles. Chem. Commun. 2007, 4404–4406. [Google Scholar] [CrossRef] [PubMed]
- Moghal, J.; Kobler, J.; Sauer, J.; Best, J.; Gardener, M.; Watt, A.A.R.; Wakefield, G. High-performance, single-layer antireflective optical coatings comprising mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; He, J. Surface hydrophobic co-modification of hollow silica nanoparticles toward large-area transparent superhydrophobic coatings. J. Colloid Interface Sci. 2013, 396, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Fuji, M.; Han, Y.S.; Takai, C. Synthesis and Applications of Hollow Particles. KONA Powder Part. J. 2013, 30, 47–68. [Google Scholar] [CrossRef]
- Bao, Y.; Shi, C.; Wang, T.; Li, X.; Ma, J. Recent progress in hollow silica: Template synthesis, morphologies and applications. Microporous Mesoporous Mater. 2016, 227, 121–136. [Google Scholar] [CrossRef]
- Sasidharan, M.; Liu, D.; Gunawardhana, N.; Yoshio, M.; Nakashima, K. Synthesis, characterization and application for lithium-ion rechargeable batteries of hollow silica nanospheres. J. Mater. Chem. 2011, 21, 13881–13888. [Google Scholar] [CrossRef]
- Meyer, E.E.; Rosenberg, K.J.; Israelachvili, J. Recent progress in understanding hydrophobic interactions. Proc. Natl. Acad. Sci. USA 2006, 103, 15739–15746. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Jafelicci, M.; Davolos, M.R.; Santos, F.J.D.; Andrade, S.J.D. Hollow silica particles from microemulsion. J. Non-Crystal. Solids 1999, 247, 98–102. [Google Scholar] [CrossRef]
- Hah, H.J.; Kim, J.S.; Jeon, B.J.; Koo, S.M.; Lee, Y.E. Simple preparation of monodisperse hollow silica particles without using template. Chem. Commun. 2003, 1712–1713. [Google Scholar] [CrossRef]
- Song, L.; Ge, X.; Zhang, Z. Interfacial fabrication of silica hollow particles in a reverse emulsion system. Chem. Lett. 2005, 34, 1314–1315. [Google Scholar] [CrossRef]
- Horikoshi, S.; Akao, Y.; Ogura, T.; Sakai, H.; Abe, M.; Serpone, N. On the stability of surfactant-free water-in-oil emulsions and synthesis of hollow SiO2 nanospheres. Colloids Surfaces Physicochem. Eng. Asp. 2010, 372, 55–60. [Google Scholar] [CrossRef]
- Hu, W.; Gu, H.; Wang, J.; Li, Y.; Wang, Z. One-step synthesis of silica hollow particles in a W/O inverse emulsion. Colloid Polym. Sci. 2013, 291, 2697–2704. [Google Scholar] [CrossRef]
- Okada, T.; Koide, T. Uniform-sized silica nanocapsules produced by addition of salts to a water-in-oil emulsion template. Langmuir 2018, 34, 9500–9506. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Huang, Z.; Pan, Y.; Xue, G. A facile and environmentally friendly method for the synthesis of hollow silica particles in a self-stable dispersion. J. Mater. Chem. 2010, 20, 5516–5520. [Google Scholar] [CrossRef]
- Nomura, T.; Morimoto, Y.; Ishikawa, M.; Tokumoto, H.; Konishi, Y. Synthesis of hollow silica microparticles from bacterial templates. Adv. Powder. Technol. 2010, 21, 8–12. [Google Scholar] [CrossRef]
- Wibowo, F.R.; Saputra, O.A.; Lestari, W.W.; Koketsu, M.; Mukti, R.R.; Martien, R. pH-Triggered Drug Release Controlled by Poly(Styrene Sulfonate) Growth Hollow Mesoporous Silica Nanoparticles. ACS Omega 2020, 5, 4261–4269. [Google Scholar] [CrossRef]
- Nomura, T.; Morimoto, Y.; Tokumoto, H.; Konishi, Y. Fabrication of silica hollow particles using Escherichia coli as a template. Mater. Lett. 2008, 62, 3727–3729. [Google Scholar] [CrossRef]
- Le, Y.; Chen, J.-F.; Wang, J.-X.; Shao, L.; Wang, W.-C. A novel pathway for synthesis of silica hollow spheres with mesostructured walls. Mater. Lett. 2004, 58, 2105–2108. [Google Scholar] [CrossRef]
- Fuji, M.; Shin, T.; Watanabe, H.; Takei, T. Shape-controlled hollow silica nanoparticles synthesized by an inorganic particle template method. Adv. Powder Technol. 2012, 23, 562–565. [Google Scholar] [CrossRef]
- Nakashima, Y.; Takai, C.; Khosroshahi, H.R.; Suthabanditpong, W.; Fuji, M. Synthesis of ultra-small hollow silica nanoparticles using the prepared amorphous calcium carbonate in one-pot process. Adv. Powder Technol. 2018, 29, 904–908. [Google Scholar] [CrossRef]
- Williamson, P.A.; Blower, P.J.; Green, M.A. Synthesis of porous hollow silica nanostructures using hydroxyapatite nanoparticle templates. Chem. Commun. 2011, 47, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, H.; Wu, M.; Zhong, Y.; Wang, D.; Jiao, Z. A soft–hard template approach towards hollow mesoporous silica nanoparticles with rough surfaces for controlled drug delivery and protein adsorption. J. Mater. Chem. B 2015, 3, 6480–6489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Y.; Li, Y.; Bandosz, T.J.; Akins, D.L. Synthesis of hollow ellipsoidal silica nanostructures using a wet-chemical etching approach. J. Colloid Interface Sci. 2012, 375, 106–111. [Google Scholar] [CrossRef]
- Li, W.; Tian, T.; Zhao, C.; Zhang, B.; Zhang, H.; Zhang, Q.; Geng, W. Investigation of selective etching mechanism and its dependency on the particle size in preparation of hollow silica spheres. J. Nanopart. Res. 2015, 17, 480–491. [Google Scholar] [CrossRef]
- Cheow, W.S.; Li, S.; Hadinoto, K. Spray drying formulation of hollow spherical aggregates of silica nanoparticles by experimental design. Chem. Eng. Res. Des. 2010, 88, 673–685. [Google Scholar] [CrossRef]
- Cho, Y.-S. Fabrication of Hollow or Macroporous Silica Particles by Spray Drying of Colloidal Dispersion. J. Dispers. Sci. Technol. 2016, 37, 23–33. [Google Scholar] [CrossRef]
- Kim, K.D.; Choia, K.Y.; Yang, J.W. Formation of spherical hollow silica particles from sodium silicate solution by ultrasonic spray pyrolysis method. Colloids Surfaces Physicochem. Eng. Asp. 2005, 254, 193–198. [Google Scholar] [CrossRef]
- Chung, Y.S.; Lim, J.S.; Park, S.B.; Okuyama, K. Templated Synthesis of Silica Hollow Particles by Using Spray Pyrolysis. J. Chem. Eng. Jpn. 2004, 37, 1099–1104. [Google Scholar] [CrossRef]
- Freudensprung, I.; Joe, D.; Nietzel, S.; Vollmer, D.; Klapper, M.; Müllen, K. Spherical Polyolefin Particles from Olefin Polymerization in the Confined Geometry of Porous Hollow Silica Particles. Macromol. Rapid Commun. 2016, 37, 1651–1656. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Ye, X.; Yu, H.; Li, J.; Zeng, F. Selective basic etching of bifunctional core–shell composite particles for the fabrication of organic functionalized hollow mesoporous silica nanospheres. New J. Chem. 2016, 40, 825–831. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, K.; Xu, X.; Tang, Y.; Han, Z.; Zhao, K.; Zhang, G.; Cui, J.; Ji, Y. Synthesis of Hollow Silica Particles Using Acid Dissolvable Resorcinol-Formaldehyde Resin Particles as Template. ChemistrySelect 2018, 3, 8919–8925. [Google Scholar] [CrossRef]
- Hwanga, H.S.; Baea, J.H.; Park, I.; Park, J.M.; Lim, K.T. Fabrication of hollow silica particles using copolymeric spheres prepared in supercritical carbon dioxide. J. Supercrit. Fluids 2009, 50, 292–296. [Google Scholar] [CrossRef]
- Park, I.; Ko, S.H.; An, Y.S.; Choi, K.H.; Chun, H.; Lee, S.; Kim, G. Monodisperse Polystyrene-Silica Core-Shell Particles and Silica Hollow Spheres Prepared by the Stober Method. J. Nanosci. Nanotechnol. 2009, 9, 7224–7228. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y. Synthesis and characterization of hollow mesoporous silica spheres using negative-charged polystyrene particles as templates. J. Inorg. Organomet. Polym. Mater. 2017, 27, 380–384. [Google Scholar] [CrossRef]
- Wang, Y.; Sunkara, B.; Zhan, J.; He, J.; Miao, L.; McPherson, G.L.; John, V.T.; Spinu, L. Synthesis of Submicrometer Hollow Particles with a Nanoscale Double-Layer Shell Structure. Langmuir 2012, 28, 13783–13787. [Google Scholar] [CrossRef]
- Toyama, N.; Ogawa, R.; Inoue, S.; Tansho, M.; Shimizu, T.; Umegaki, T.; Kojima, Y. Influence of morphology of hollow silica–alumina composite spheres on their activity for hydrolytic dehydrogenation of ammonia borane. J. Adv. Ceram. 2017, 6, 368–375. [Google Scholar] [CrossRef]
- Sharma, J.; Cullen, A.D.; Polizos, G.; Nawaz, K.; Wang, H.; Muralidharan, N.; Smith, B.D. Hybrid hollow silica particles: Synthesis and comparison of properties with pristine particles. RSC Adv. 2020, 10, 22331–22334. [Google Scholar] [CrossRef]
- Palanikumar, L.; Jeena, M.T.; Kim, K.; Oh, J.Y.; Kim, C.; Park, M.-H.; Ryu, J.-H. Spatiotemporally and Sequentially- Controlled Drug Release from Polymer Gatekeeper–Hollow Silica Nanoparticles. Sci. Rep. 2017, 7, 46540–46550. [Google Scholar] [CrossRef]
- Yu, S.-H.; Quan, B.; Jin, A.; Lee, K.-S.; Kang, S.H.; Kang, K.; Piao, Y.; Sung, Y.-E. Hollow nanostructured metal silicates with tunable properties for lithium ion battery anodes. ACS Appl. Mater. Interfaces 2015, 7, 25725–25732. [Google Scholar] [CrossRef]
- Liaoa, Y.; Wu, X.; Liu, H.; Chen, Y. Thermal conductivity of powder silica hollow spheres. Thermochim. Acta 2011, 526, 178–184. [Google Scholar] [CrossRef]
- Kwak, B.M.; Park, S.Y.; Chung, C.; Kwon, Y. Porous Silicone Polymeric Composites Filled with Hollow Silica Particles: Thermal Conductivity, Thermal Stability and Mechanical Properties. J. Nanosci. Nanotechnol. 2019, 19, 6321–6327. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.L.A.; Taniguchi, S.; Nguyen, T.T.; Arifa, A.F.; Iskandar, F.; Ogi, T. Precisely Tailored Synthesis of Hexagonal Hollow Silica Plate Particles and their Polymer Nanocomposite Films with Low Refractive Index. J. Colloid Interface Sci. 2020, 3, 64. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Luna, L.E.; Tan, W.S.; Rubner, M.F.; Cohen, R.E. Hollow Silica Nanoparticles in UV-Visible Antireflection Coatings for Poly(methyl methacrylate) Substrates. ACS Nano 2010, 4, 4308–4316. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Zou, X.; Du, K.; Zhang, L.; Yan, H.; Yuan, X. Ultralow-refractive-index optical thin films built from shape-tunable hollow silica nanomaterials. Opt. Lett. 2018, 43, 1802–1805. [Google Scholar] [CrossRef]
- Tao, C.; Yan, H.; Yuan, X.; Yin, Q.; Zhu, J.; Ni, W.; Yan, L.; Zhang, L. Sol-gel based antireflective coatings with superhydrophobicity and exceptionally low refractive indices built from trimethylsilanized hollow silica nanoparticles. Collloid Surf. A. Physicochem. Eng. Asp. 2016, 509, 307–313. [Google Scholar] [CrossRef]
- Jelle, B.P. Traditional, state-of-the-art and future thermal building insulation materials and solutions—Properties, requirements and possibilities. Energy Build. 2011, 43, 2549–2563. [Google Scholar] [CrossRef]
- Bergea, A.; Adl-Zarrabia, B.; Hagentof, C.-E. Assessing the thermal performance of district heating twin pipes with vacuum insulation panels. Energy Procedia 2015, 78, 382–387. [Google Scholar] [CrossRef]
- Engels, H.-W.; Pirkl, H.-G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile Materials and Sustainable Problem Solvers for Today’s Challenges. Angew. Chem. Int. Ed. 2013, 52, 9422–9441. [Google Scholar] [CrossRef]
- Zhang, J.; Fisher, T.S.; Ramachandran, P.V.; Gore, J.P.; Mudawar, I. A Review of Heat Transfer Issues in Hydrogen Storage Technologies. J. Heat Transfer. 2005, 127, 1391–1399. [Google Scholar] [CrossRef]
- Wicklen, B.; Kocjan, A.; Carosio, F.; Camino, G.; Antonitti, M.; Bergstrom, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 2015, 10, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-L.; Yang, N.; Apostolopoulou-Kalkavoura, V.; Qin, B.; Ma, Z.-Y.; Xing, W.-Y.; Qiao, C.; Bergstrçm, L.; Antonietti, M.; Yu, S.-H. Fire-Retardant and Thermally Insulating Phenolic-Silica Aerogels. Angew. Chem. Int. Ed. 2018, 57, 4538–4542. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nystrcm, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- Thaplial, P.C.; Singh, K. Aerogels as Promising Thermal Insulating Materials: An Overview. J. Mater. 2014, 2014, 10. [Google Scholar] [CrossRef]
- Gao, T.; Jelle, B.P.; Sandberg, L.I.C.; Gustavsen, A. Monodisperse Hollow Silica Nanospheres for Nano Insulation Materials: Synthesis, Characterization, and Life Cycle Assessment. ACS Appl. Mater. Interfaces 2013, 5, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, L.I.C.; Gao, T.; Jelle, B.P.; Gustavsen, A. Synthesis of Hollow Silica Nanospheres by Sacrificial Polystyrene Templates for Thermal Insulation Applications. Adv. Mater. Sci. Eng. 2013, 2013, 483651. [Google Scholar] [CrossRef]
- Wu, X.; Tian, Y.; Cui, Y.; Wei, L.; Wang, Q.; Chen, Y. Raspberry-like Silica Hollow Spheres: Hierarchical Structures by Dual Latex-Surfactant Templating Route. J. Phys. Chem. C 2007, 111, 9704–9708. [Google Scholar] [CrossRef]
- Ernawati, L.; Ogi, T.; Balgis, R.; Okuyama, K.; Stucki, M.; Hess, S.C.; Stark, W.J. Hollow Silica as an Optically Transparent and Thermally Insulating Polymer Additive. Langmuir 2016, 32, 338–345. [Google Scholar] [CrossRef]
- Takai, C.; Fuji, M.; Fujimoto, K. Thermal Insulation Film Achieved by Hollow Silica Nanoparticles. J. Soc. Powder Technol. Jpn. 2012, 49, 896–900. [Google Scholar] [CrossRef][Green Version]
- Fuji, M.; Takai, C. Nanoparticle Technology Handbook, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 493–497. [Google Scholar]
- Liao, Y.; Wu, X.; Wang, Z.; Yue, R.; Liu, G.; Chen, Y. Composite thin film of silica hollow spheres and waterborne polyurethane: Excellent thermal insulation and light transmission performances. Mater. Chem. Phys. 2012, 133, 642–648. [Google Scholar] [CrossRef]
- Hu, P.; Ai, D.; Jiang, X.; Zhang, X. Fabrication of hollow silica nanosphere and its application for thermal insulation coating. J. Thermoplast. Compos. Mater. 2020, 33, 198–213. [Google Scholar] [CrossRef]
- Hu, F.; Wu, S.; Sun, Y. Hollow-Structured Materials for Thermal Insulation. Adv. Mater. 2018, 31, 1801001. [Google Scholar] [CrossRef] [PubMed]
- Berge, A.; Johansson, P. Literature review of high-performance thermal insulation. In Report in Buildings Physics; Chalmers University of Technology: Gothenburg, Sweden, 2012. [Google Scholar]
- Peinado, A.; Alberto, G.; Marugán, P.; Pedro, F.; Márquez, G. A review of the application performances of concentrated solar power T systems. Appl. Energy 2019, 255, 113893. [Google Scholar] [CrossRef]
- Irshad, M.; Siraj, K.; Raza, R.; Ali, A.; Tiwari, P.; Zhu, B.; Rafique, A.; Ali, A.; Ullah, M.K.; Usman, A. A Brief Description of High Temperature Solid Oxide Fuel Cell’s Operation, Materials, Design, Fabrication Technologies and Performance. Appl. Sci. 2016, 6, 75. [Google Scholar] [CrossRef]
- Xiaowei, L.; jean-Charless, R.; Suyuan, Y. Effect of temperature on graphite oxidation behavior. Nucl. Eng. Des. 2004, 227, 273–280. [Google Scholar] [CrossRef]
- Barbe, C.; Bartlett, J.; Kong, L.; Finnie, K.; Lin, H.Q.; Larkin, M.; Callej, S.; Bush, A.; Calleja, G. Silica Particles: A Novel Drug-Delivery System. Adv. Mater. 2004, 16, 1959–1966. [Google Scholar] [CrossRef]
- Alexander, G.B.; Heston, W.M.; Iler, R.K. The Solubility of Amorphous Silica in Water. J. Phys. Chem. 1954, 58, 453–455. [Google Scholar] [CrossRef]
- Zhang, Y.; Ang, C.Y.; Li, M.; Tan, S.Y.; Qu, Q.; Luo, Z.; Zhao, Y. Polymer-Coated Hollow Mesoporous Silica Nanoparticles for Triple-Responsive Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 18179–18187. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Hou, X.; Su, X.; Chu, Q.; Fahruddin, N.; Zhao, J.X. Shape-Tunable Hollow Silica Nanomaterials Based on a Soft-Templating Method and Their Application as a Drug Carrier. ACS Appl. Mater. Interfaces 2014, 6, 21921–21930. [Google Scholar] [CrossRef]
- Teng, Y.; Du, Y.; Shi, J.; Pong, P.W.T. Magnetic iron oxide nanoparticle-hollow mesoporous silica Spheres: Fabrication and potential application in drug delivery. Curr. Appl. Phys. 2020, 20, 320–325. [Google Scholar] [CrossRef]
- Zhu, Y.; Meng, W.; Gao, H.; Hanagata, N. Hollow mesoporous silica/poly(l-lysine) particles for codelivery of drug and gene with enzyme-triggered release property. Phys. Chem. C 2011, 115, 13630–13636. [Google Scholar] [CrossRef]
- Zhu, Y.; Meng, W.; Hanagata, N. Cytosine-phosphodiester-guanine oligodeoxynucleotide (CpG ODN)-capped hollow mesoporous silica particles for enzyme-triggered drug delivery. Dalton Trans. 2011, 40, 10203–10208. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Quan, Z.; Zhang, H.; Qin, X.; Wang, R.; Yu, J. pH-triggered sustained drug release of multilayer encapsulation system with hollow mesoporous silica nanoparticles/chitosan/polyacrylic acid. Matter. Lett. 2020, 260, 126907–126911. [Google Scholar] [CrossRef]
- Bai, M.; Dong, H.; Su, X.; Yang, Y.; Guo, H. Hollow mesoporous silica nanoparticles as delivery vehicle of foot-and-mouth disease virus-like particles induce persistent immune responses in guinea pigs. J. Med. Virol. 2019, 91, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Liberman, A.; Wang, J.; Lu, N.; Viveros, R.D.; Allen, C.A.; Mattrey, R.F.; Blair, S.L.; Trogler, W.C.; Kim, M.J.; Kummel, A.C. Mechanically Tunable Hollow Silica Ultrathin Nanoshells for Ultrasound Contrast Agents. Adv. Funct. Mater. 2015, 8, 4049–4057. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Wang, W.; Kong, W.; Chen, Y. Organic-Inorganic Hybrid Hollow Mesoporous Organosilica Nanoparticles for Efficient Ultrasound-Based Imaging and Controlled Drug Release. J. Nanomater. 2014, 972475–972483. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, Y.; Sun, X.-G.; Li, Y.; Guo, B.; Chen, J.; Veith, G.M.; Hensley, D.K.; Paranthaman, M.P.; Goodenough, J.B.; et al. Superior Conductive Solid-like Electrolytes: Nanoconfining Liquids within the Hollow Structures. Nano Lett. 2015, 15, 3398–3402. [Google Scholar] [CrossRef]
- Cao, L.; Huang, J.; Lin, Z.; Yu, X.; Wu, X.; Zhang, B.; Zhan, Y.; Xie, F.; Zhang, W.; Chen, C.; et al. Amorphous SiO2/C composite as anode material for lithium-ion batteries. J. Mater. Res. 2018, 33, 1219–1225. [Google Scholar] [CrossRef]
- An, W.; Fu, J.; Su, J.; Wang, L.; Peng, X.; Wu, K.; Chen, Q.; Bi, Y.; Gao, B.; Zhang, X. Mesoporous hollow nanospheres consisting of carbon coated silica nanoparticles for robust lithium-ion battery anodes. J. Power Sources 2017, 345, 227–236. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Liu, H.; Liu, Z.-Q. SiO2@C hollow sphere anodes for lithium-ion batteries. J. Mater. Sci. Technol. 2017, 33, 239–245. [Google Scholar] [CrossRef]
- Cevik, E.; Gunday, S.T.; Akhtar, S.; Yamani, Z.H.; Bozkurt, A. Sulfonated Hollow Silica Spheres as Electrolyte Store/Release Agents: High-Performance Supercapacitor Applications. Energy Technol. 2019, 7, 1900511–1900520. [Google Scholar] [CrossRef]
- Shchukina, E.M.; Graham, M.; Zheng, Z.; Shchukin, D.G. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, Y.; Zhao, Z.; Thomas, H.; Zhu, X.; Moller, M. Inclusion of Phase-Change Materials in Submicron Silica Capsules Using a Surfactant-Free Emulsion Approach. Langmuir 2018, 34, 10397–10406. [Google Scholar] [CrossRef] [PubMed]
- Aftab, W.; Huang, X.; Wu, W.; Liang, Z.; Mahmood, A.; Zou, R. Nanoconfined phase change materials for thermal energy applications. Energy Environ. Sci. 2018, 11, 1392–1424. [Google Scholar] [CrossRef]
- Xu, L.; He, J. Fabrication of Highly Transparent Superhydrophobic Coatings from Hollow Silica Nanoparticles. Langmuir 2012, 28, 7512–7518. [Google Scholar] [CrossRef] [PubMed]
- Soled, S. Silica-supported catalysts get a new breath of life. Science 2015, 350, 1171–1172. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Kango, H.; Yamashita, H. Pd Nanoparticles and Aminopolymers Confined in Hollow Silica Spheres as Efficient and Reusable Heterogeneous Catalysts for Semihydrogenation of Alkynes. ACS Catal. 2019, 9, 1993–2006. [Google Scholar] [CrossRef]
- Li, L.; Ding, J.; Xue, J. Macroporous Silica Hollow Microspheres as Nanoparticle Collectors. Chem. Mater. 2009, 21, 3629–3637. [Google Scholar] [CrossRef]
- Jaricot, S.C.; Darbandi, M.; Nann, T. Au–silica nanoparticles by “reverse” synthesis of cores in hollow silica shells. Chem. Commun. 2007, 20, 2031–2033. [Google Scholar] [CrossRef]
- Park, J.C.; Bang, J.U.; Lee, J.; Ko, C.H.; Song, H. Ni@SiO2yolk-shell nanoreactor catalysts: High temperature stability and recyclability. J. Mater. Chem. 2010, 20, 1239–1246. [Google Scholar] [CrossRef]
- Demirors, A.F.; Blaaderen, A.V.; Imhof, A. Synthesis of Eccentric Titania−Silica Core−Shell and Composite Particles. Chem. Mater. 2009, 21, 979–984. [Google Scholar] [CrossRef]
- Ikeda, S.; Ikoma, Y.; Kobayashi, H.; Harada, T.; Torimoto, T.; Ohtani, B.; Matsumura, M. Encapsulation of titanium(iv) oxide particles in hollow silica for size-selective photocatalytic reactions. Chem. Commun. 2007, 3753–3755. [Google Scholar] [CrossRef] [PubMed]
- Xianliang, L.; Zousen, S.; Zhanchen, C.; Song, Z. Silver Chloride Loaded Hollow Mesoporous Silica Particles and Their Application in the Antibacterial Coatings on Denture Base. Chem. Res. Chin. Univ. 2018, 34, 495–499. [Google Scholar]
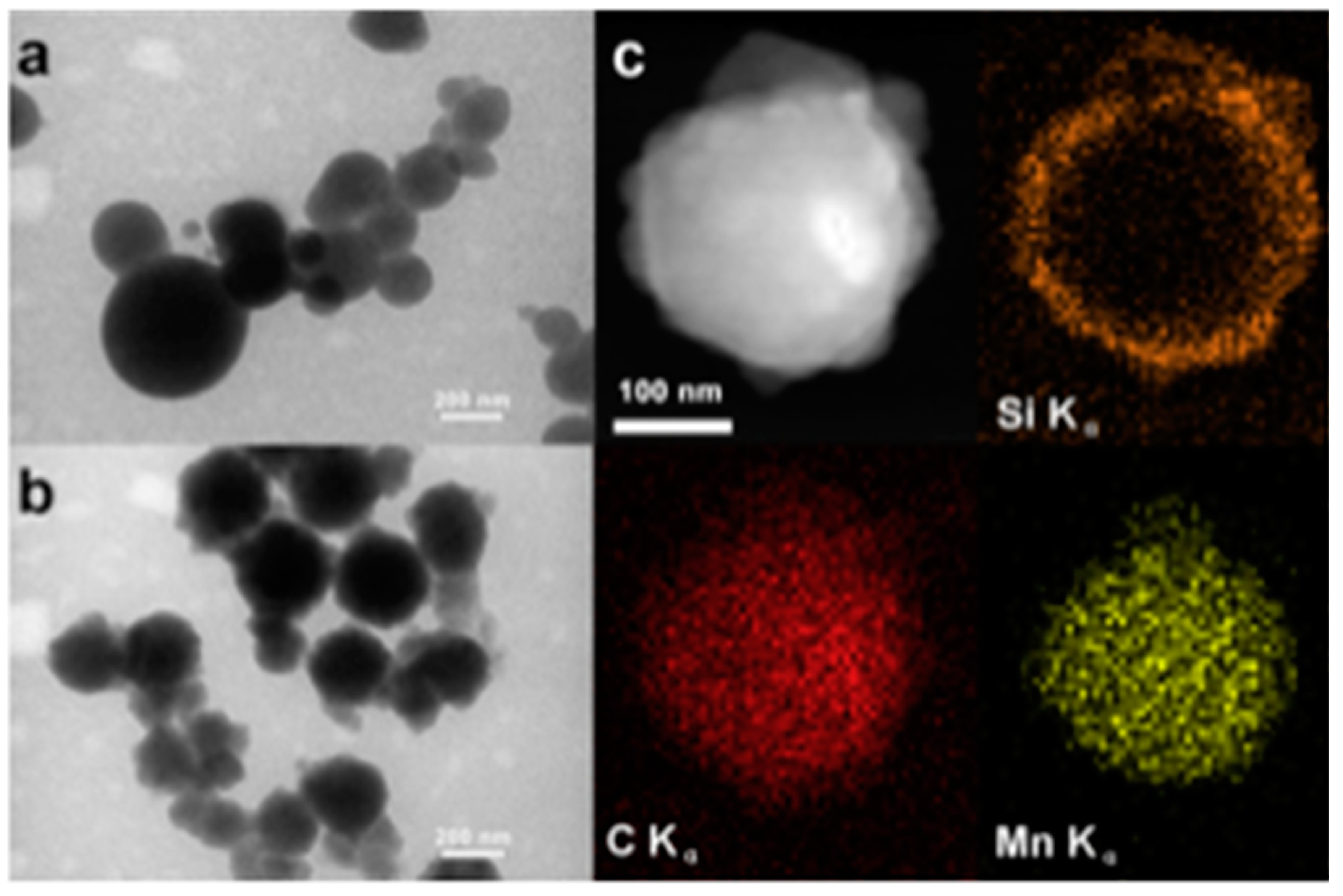
- Meng, Q.; Xiang, S.; Cheng, W.; Chen, Q.; Xue, P.; Zhang, K.; Sun, H.; Yang, B. Facile synthesis of manganese oxide loaded hollow silica particles and their application for methylene blue degradation. J. Colloid Interface Sci. 2013, 405, 28–34. [Google Scholar] [CrossRef]
- Han, Y.S.; Tarutani, Y.; Fuji, M.; Takahashi, M. Synthesis of hollow silica particle by combination of bubble templating method and sol-gel transformation. Adv. Mater. Res. 2006, 11, 673–676. [Google Scholar] [CrossRef]



| No. | Strategy | Advantages | Disadvantages | References |
|---|---|---|---|---|
| 1. | Polymer micelles/emulsions |
|
| [27,28,29,30,31] |
| Surfactant examples *: CTAB, PVP, and PTMS | ||||
| 2. | Inorganic particles as templates |
|
| [39,40,41,42] |
| Examples: carbon, calcium carbonate, and hydroxy apatite | ||||
| 3. | Polymer particles as templates |
|
| [50,51,52,53,54,55] |
| Examples: polystyrene and polyresorcinol | ||||
| 4. | Solid silica particle etching |
|
| [44,45] |
| 5. | Spray drying |
|
| [46,47] |
| 6. | Spray pyrolysis |
|
| [48,49] |
| 7. | Bacteria/virus templates |
|
| [36,37,38] |
| No. | Technique | Property Characterized | Application | References |
|---|---|---|---|---|
| 1. | SEM * | Size and surface features (e.g., roughness) | Composites | [13] |
| 2. | TEM * | Size, shell thickness, shell texture (e.g., solid or porous) | Composites | [13,23] |
| 3. | BET * and BJH * analysis | Shell pore size, pore volume, and surface area | Drug delivery and composites | [35,41,59] |
| 4. | XPS *, FTIR * | Surface chemistry | Composites, drug delivery, thermal insulation, battery anodes | [21,51,60] |
| 5. | Thermal conductivity measurement techniques, such as transient plane source | Thermal properties (e.g., thermal conductivity, thermal diffusivity, and heat capacity) | Thermal insulation materials (composites) | [61,62] |
| 6. | Nanoindentation | Mechanical properties (e.g., Young’s modulus, compressive strength) | Composites | [62] |
| 7. | AFM * | Size, surface characteristics, and mechanical properties (Young’s modulus) | Composites and functional coatings | [23] |
| 8. | UV-Vis * | Optical properties (e.g., reflectivity, visible transmittance, and opaqueness) | Functional coatings, e.g., reflective or antireflective coatings, superhydrophobic coatings | [63,64,65,66] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, J.; Polizos, G. Hollow Silica Particles: Recent Progress and Future Perspectives. Nanomaterials 2020, 10, 1599. https://doi.org/10.3390/nano10081599
Sharma J, Polizos G. Hollow Silica Particles: Recent Progress and Future Perspectives. Nanomaterials. 2020; 10(8):1599. https://doi.org/10.3390/nano10081599
Chicago/Turabian StyleSharma, Jaswinder, and Georgios Polizos. 2020. "Hollow Silica Particles: Recent Progress and Future Perspectives" Nanomaterials 10, no. 8: 1599. https://doi.org/10.3390/nano10081599
APA StyleSharma, J., & Polizos, G. (2020). Hollow Silica Particles: Recent Progress and Future Perspectives. Nanomaterials, 10(8), 1599. https://doi.org/10.3390/nano10081599