Well-Adhered Copper Nanocubes on Electrospun Polymeric Fibers
Abstract
1. Introduction
2. Materials and Methods
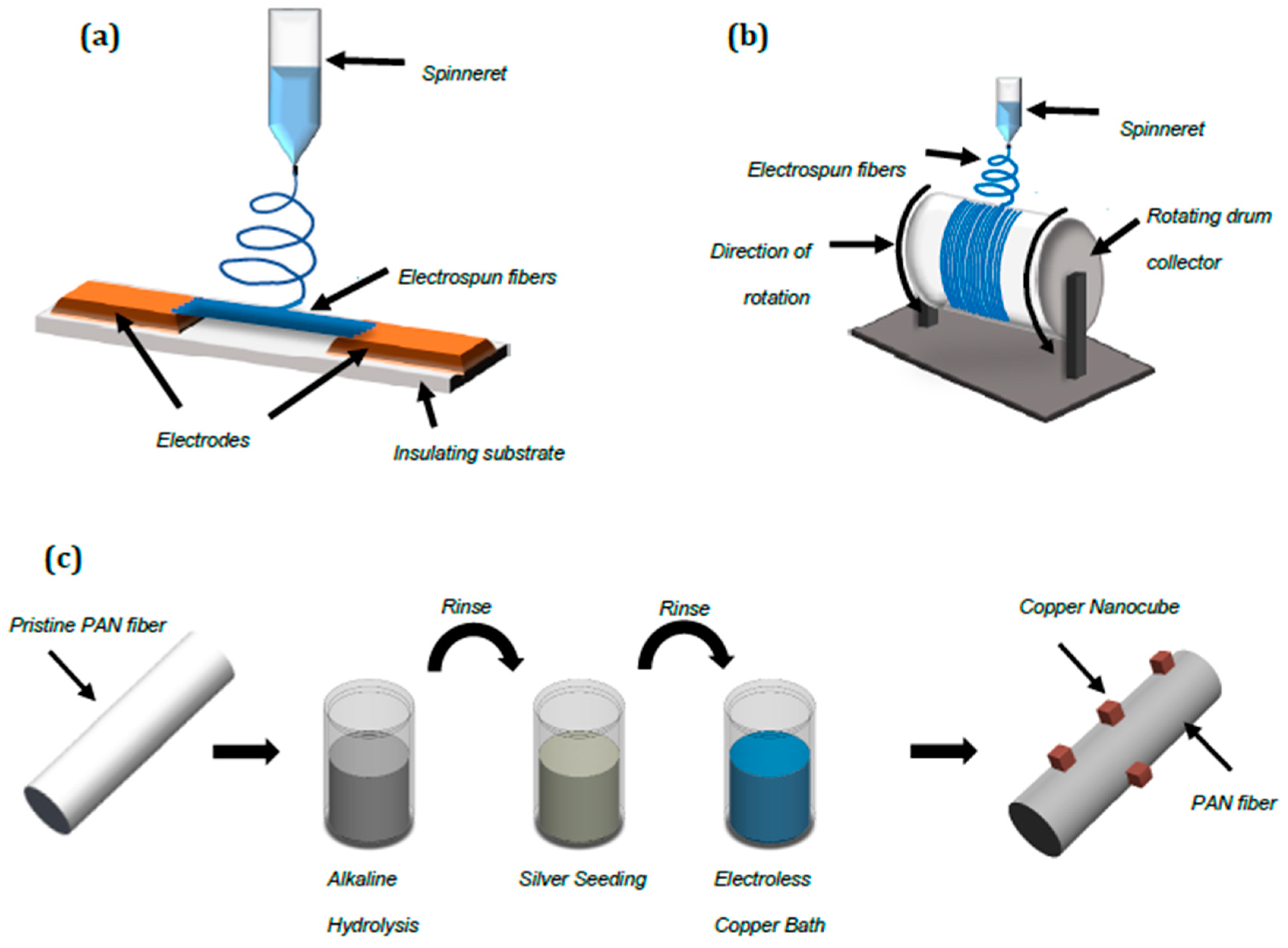
2.1. Nanocube Growth on Aligned Fiber Mats
2.2. Electroless Copper Deposition
2.3. Tensile Testing
2.4. Characterization
3. Results and Discussion
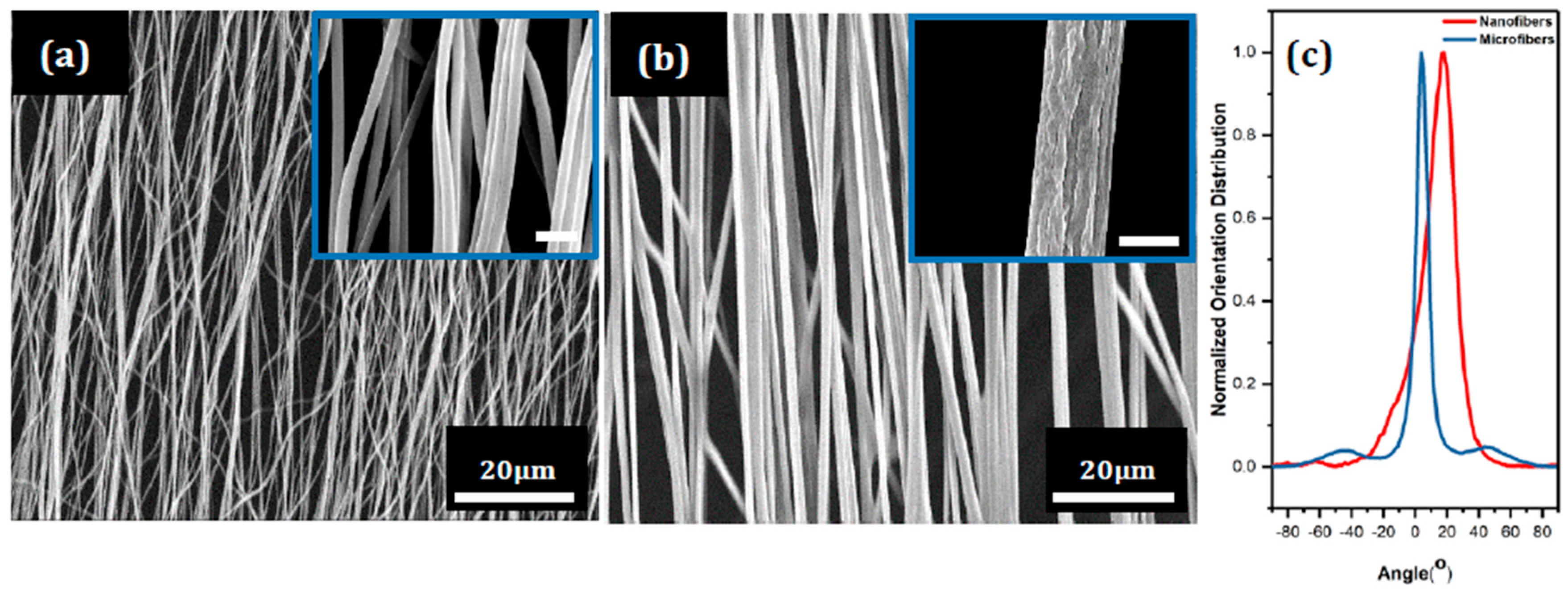
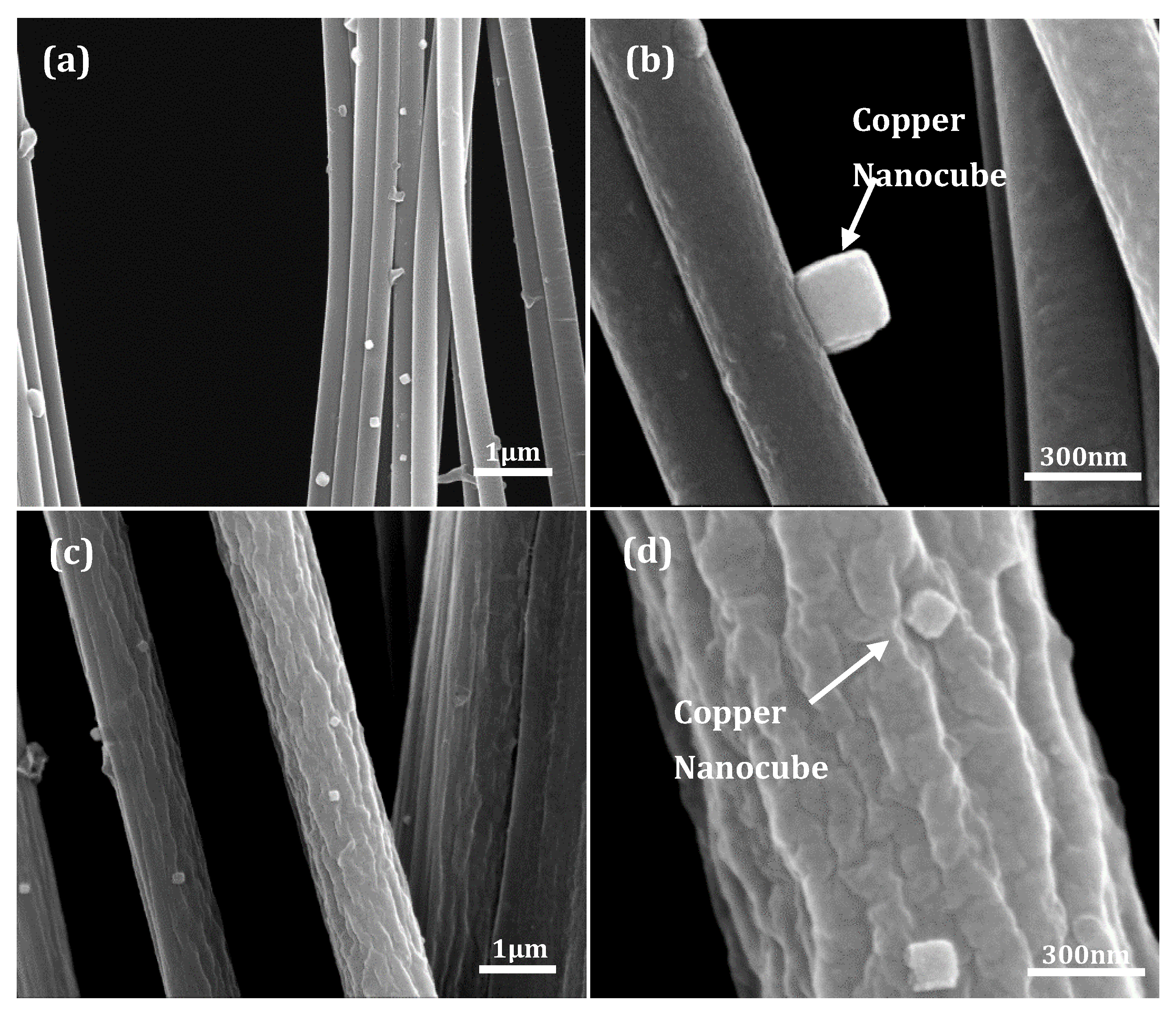
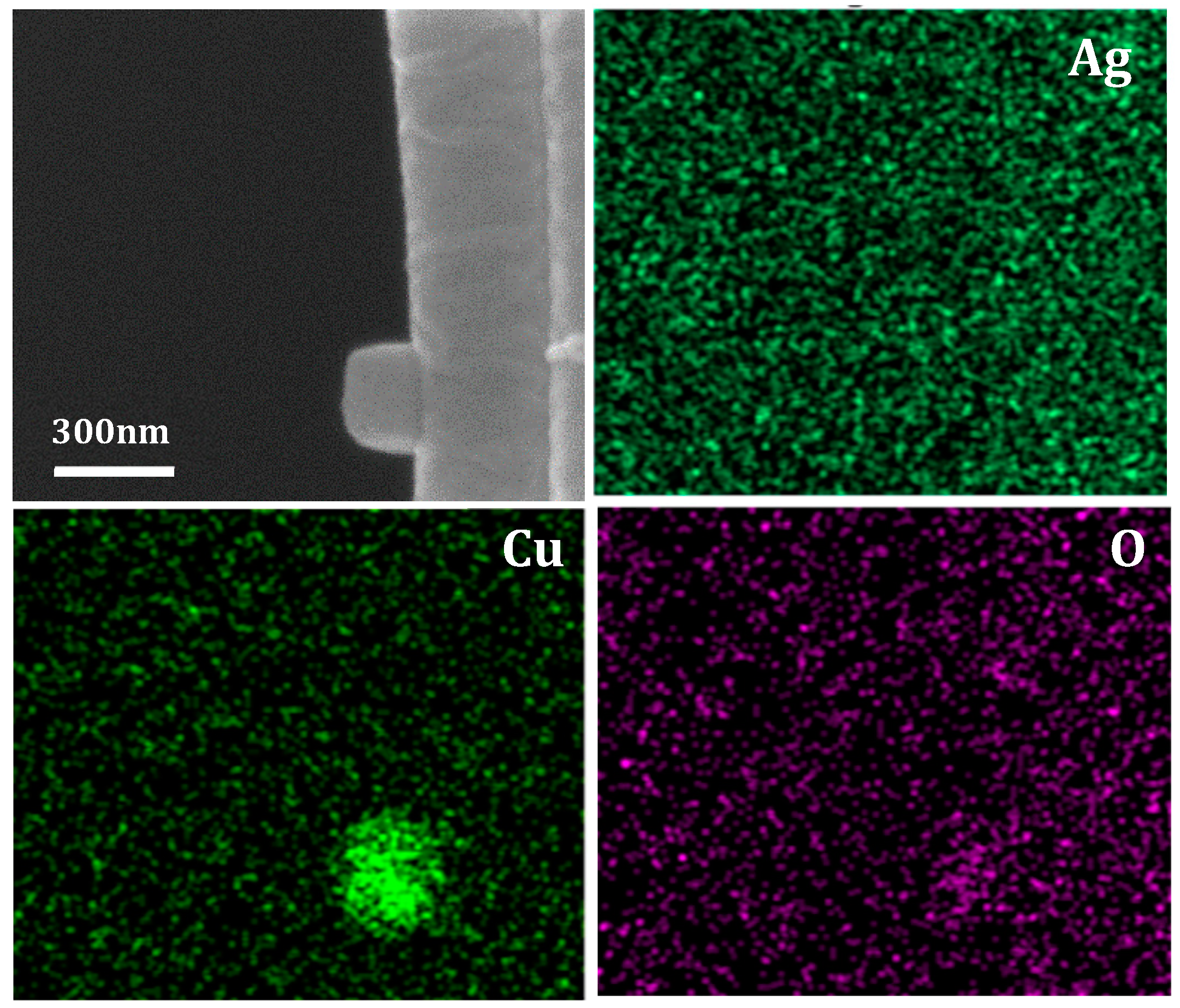
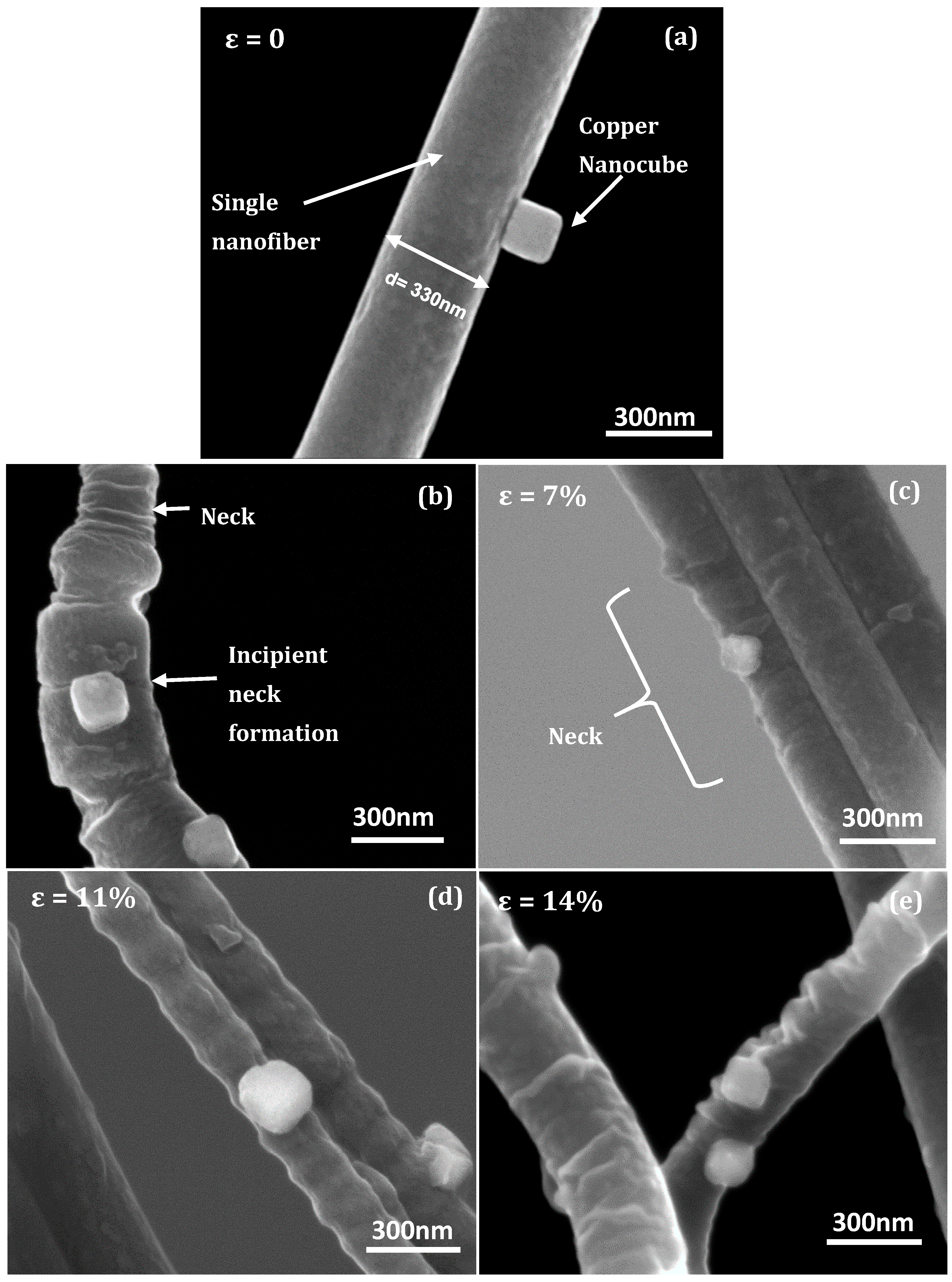
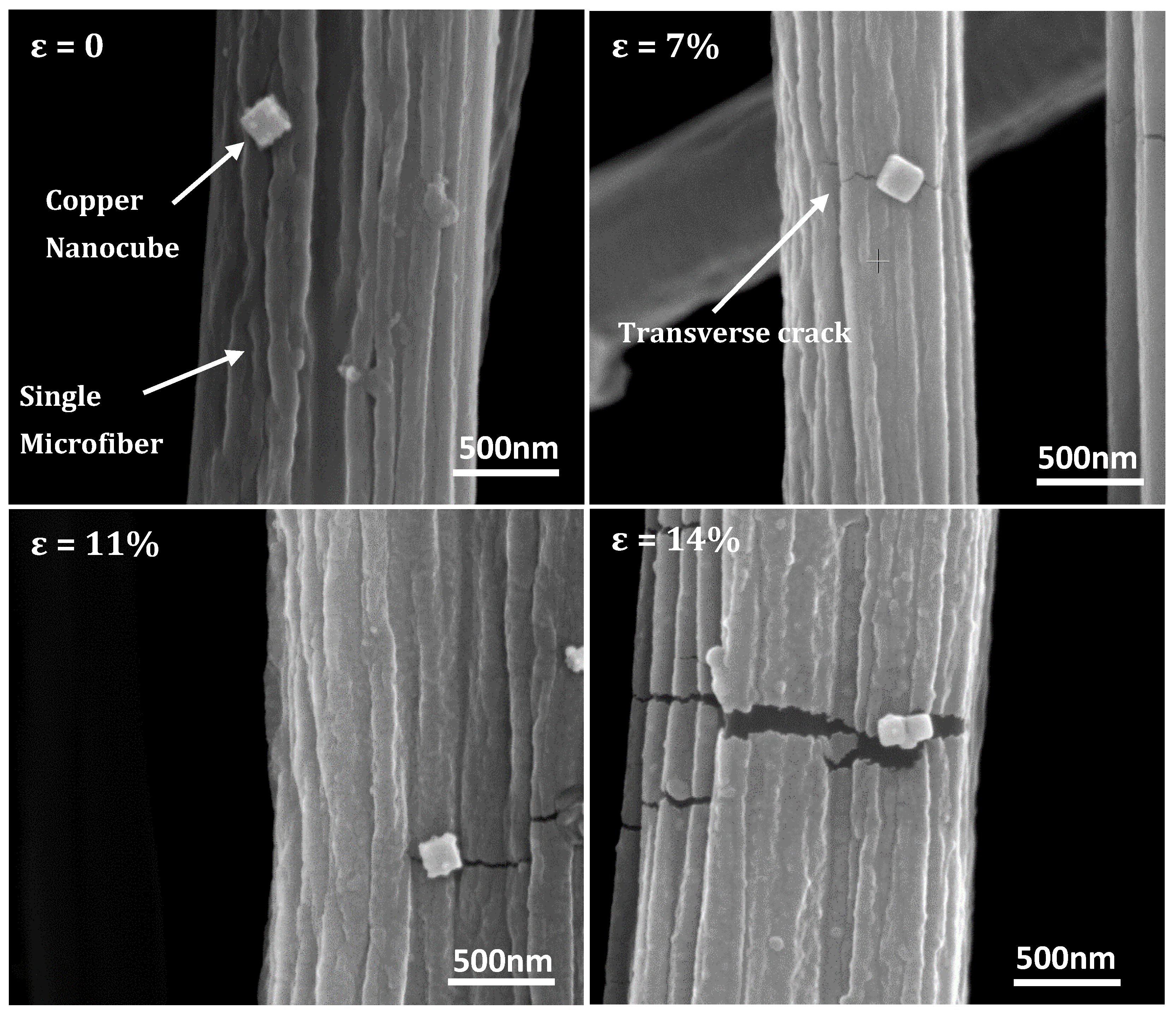
3.1. Fiber Deformation and Copper Nanocube Adhesion
3.2. Raman Spectroscopy
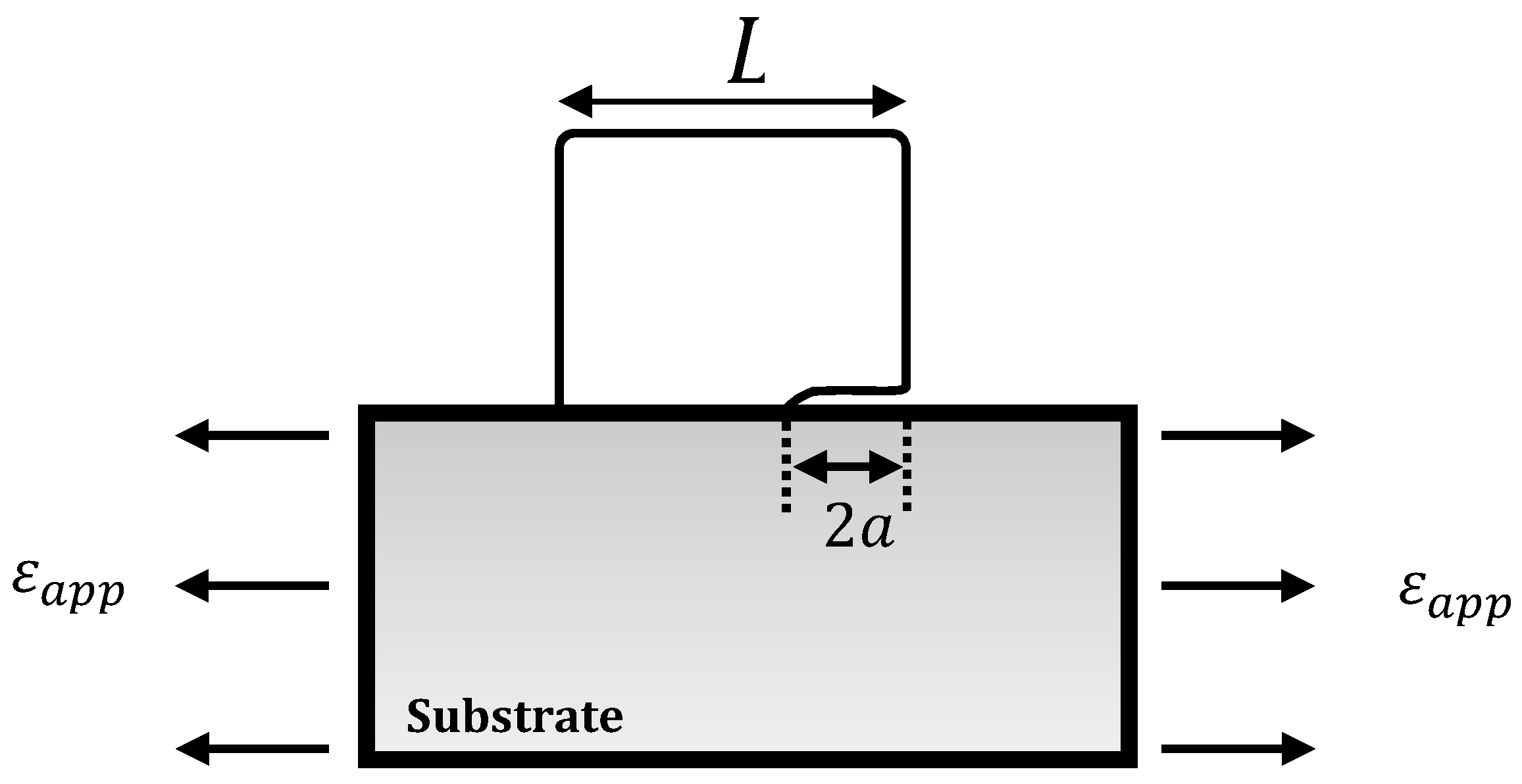
3.3. Interfacial Adhesion Energy
3.3.1. Strain Energy Release Model
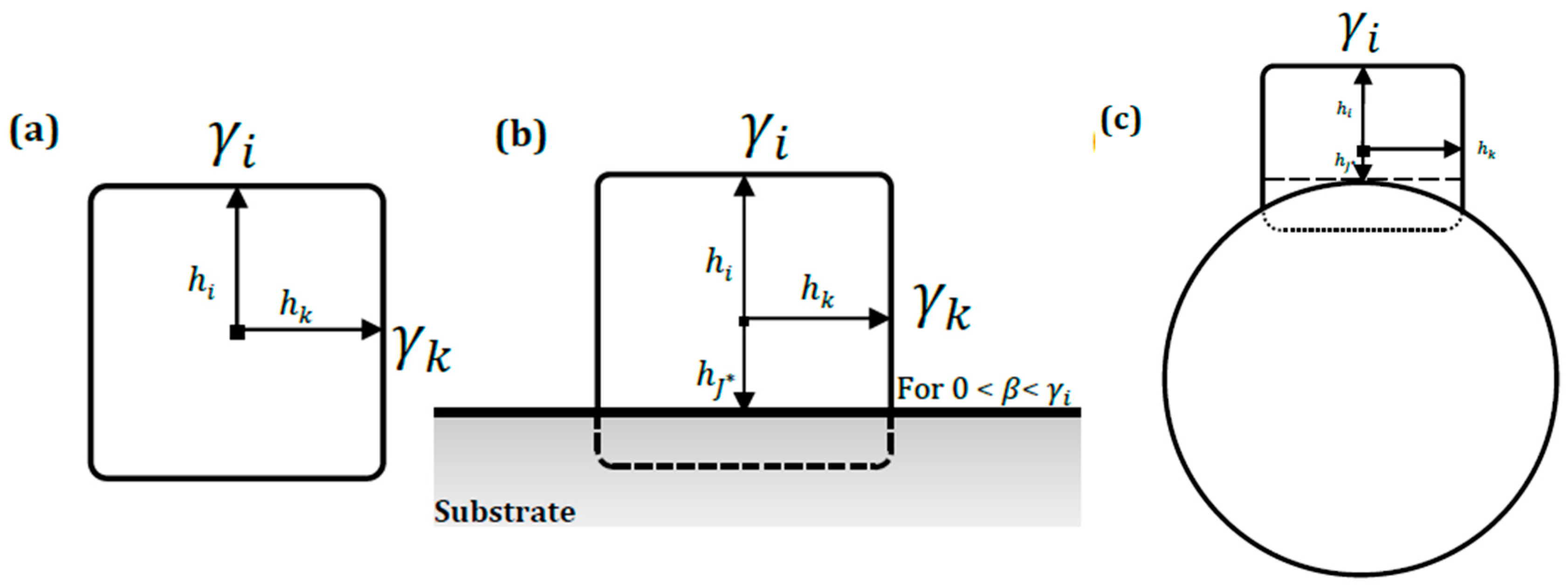
3.3.2. Gibbs–Wulff–Kaischew Shape Theory for Quantifying Adhesion
4. Adhesion Energy Qualification and Contextualization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Gao, Y.; Bach Truong, Y.; Zhu, Y.; Louis Kyratzis, I. Electrospun antibacterial nanofibers: Production, activity, and in vivo applications. J. Appl. Polymer Sci. 2014, 131, 40797. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Wang, C.; Cheng, C.; Wang, X. Facile Immobilization of Ag Nanocluster on Nanofibrous Membrane for Oil/Water Separation. ACS Appl. Mater. Interfaces 2014, 6, 15272–15282. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Barhate, R.S.; Sundarrajan, S.; Pliszka, D.; Ramakrishna, S. Fine chemical processing: The potential of nanofibres in filtration. Filtr. Sep. 2008, 45, 32–35. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, R.; Li, Y.; Sun, B.; Li, Y.; Zhang, N.; Qiu, J.; Li, X.; Wang, C. Robust and durable superhydrophobic electrospun nanofibrous mats via a simple Cu nanocluster immobilization for oil-water contamination. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 173–183. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Skupov, K.M.; Zhigalina, O.M.; Naumkin, A.; Modestov, A.D.; Basu, V.G.; Sufiyanova, A.E.; Razorenov, D.Y. New Carbon Nanofiber Composite Materials Containing Lanthanides and Transition Metals Based on Electrospun Polyacrylonitrile for High Temperature Polymer Electrolyte Membrane Fuel Cell Cathodes. Polymers 2020, 12, 1340. [Google Scholar] [CrossRef]
- Schaefer, H.-E. Nanoscience. In The Science of the Small in Physics, Engineering, Chemistry, Biology and Medicine; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Sarkar, S.; Guibal, E.; Quignard, F.; Sengupta, A.K. Polymer-supported metals and metal oxide nanoparticles: Synthesis, characterization, and applications. J. Nanopart. Res. 2012, 14, 1–24. [Google Scholar] [CrossRef]
- An, S.; Jo, H.S.; Kim, D.; Lee, H.J.; Ju, B.-K.; Al-Deyab, S.S.; Ahn, J.-H.; Qin, Y.; Swihart, M.T.; Yarin, A.; et al. Self-Junctioned Copper Nanofiber Transparent Flexible Conducting Film via Electrospinning and Electroplating. Adv. Mater. 2016, 28, 7149–7154. [Google Scholar] [CrossRef]
- Morinaga, A.; Tsutsumi, H.; Katayama, Y. Electrospun Cu-Deposited Flexible Fibers as an Efficient Oxygen Evolution Reaction Electrocatalyst. Chem. Phys. Chem. 2019, 20, 2899. [Google Scholar] [CrossRef]
- Faupel, F.; Zaporojtchenko, V.; Strunskus, T.; Erichsen, J.; Dolgner, K.; Thran, A.; Kiene, M. Fundamental aspects of polymer metallization. In Metallization of Polymers 2; Springer: Berlin/Heidelberg, Germany, 2002; pp. 73–96. [Google Scholar]
- Wei, Q.; Gao, W.; Hou, D.; Wang, X. Surface modification of polymer nanofibres by plasma treatment. Appl. Surf. Sci. 2005, 245, 16–20. [Google Scholar] [CrossRef]
- Dong, H.; Fey, E.; Gandelman, A.; Jones, W.E. Synthesis and Assembly of Metal Nanoparticles on Electrospun Poly (4-vinylpyridine) Fibers and Poly (4-vinylpyridine) Composite Fibers. Chem. Mater. 2006, 18, 2008–2011. [Google Scholar] [CrossRef]
- Chen, L.; Si, L.; Wu, F.; Chan, S.Y.; Yu, P.; Xin, J.H. Electrical and mechanical self-healing membrane using gold nanoparticles as localized “nano-heaters”. J. Mater. Chem. C 2016, 4, 10018–10025. [Google Scholar] [CrossRef]
- Ghavami Nejad, A.; Unnithan, A.R.; Sasikala, A.R.K.; Samarikhalaj, M.; Thomas, R.G.; Jeong, Y.Y.; Nasseri, S.; Murugesan, P.; Wu, D.; Park, C.H.; et al. Mussel-Inspired Electrospun Nanofibers Functionalized with Size-Controlled Silver Nanoparticles for Wound Dressing Application. ACS Appl. Mater. Interfaces 2015, 7, 12176–12183. [Google Scholar] [CrossRef] [PubMed]
- Naseeb, N.; Mohammed, A.A.; Laoui, T.; Khan, Z. A Novel PAN-GO-SiO₂ Hybrid Membrane for Separating Oil and Water from Emulsified Mixture. Materials 2019, 12, 212. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, J.; Qiang, R.; Nan, N.; You, X.; Zhou, Y.; Shao, W.; Liu, F.; Liu, R.-T. Electrospun Conductive Nanofiber Yarn for a Wearable Yarn Supercapacitor with High Volumetric Energy Density. Materials 2019, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Arslan, O.; Eren, H.; Biyikli, N.; Uyar, T. Reusable and Flexible Heterogeneous Catalyst for Reduction of TNT by Pd Nanocube Decorated ZnO Nanolayers onto Electrospun Polymeric Nanofibers. Chem. Select. 2017, 2, 8790–8798. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, Z.; Guo, J.; Bai, L.; Jia, L.; Song, P.; Mei, L.; Liu, Y. Palladium Nanocubes Assembled Electrospun Nanofiber Membrane: Facile Preparation and Catalytic Properties. Macromol. Mater. Eng. 2017, 302, 1600432. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, X.; Wang, Y.; Xie, S. Shape-controlled synthesis of metal nanocrystals. MRS Bull. 2013, 38, 335–344. [Google Scholar] [CrossRef]
- Yang, H.-J.; He, S.-Y.; Chen, H.-L.; Tuan, H.-Y. Monodisperse Copper Nanocubes: Synthesis, Self-Assembly, and Large-Area Dense-Packed Films. Chem. Mater. 2014, 26, 1785–1793. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, P.; Liu, M. Synthesis of well-defined copper nanocubes by a one-pot solution process. Nanotechnology 2006, 17, 6000–6006. [Google Scholar] [CrossRef]
- Jin, M.; He, G.; Zhang, H.; Zeng, J.; Xie, Z.; Xia, Y. Shape-Controlled Synthesis of Copper Nanocrystals in an Aqueous Solution with Glucose as a Reducing Agent and Hexadecylamine as a Capping Agent. Angew. Chem. Int. Ed. 2011, 50, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Susman, M.; Feldman, Y.; Vaskevich, A.; Rubinstein, I. Chemical Deposition of Cu sub(2)O Nanocrystals with Precise Morphology Control. ACS Nano 2014, 8, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Wang, X.; Suo, Z.; Vlassak, J. Metal films on polymer substrates stretched beyond 50%. Appl. Phys. Lett. 2007, 91, 221909. [Google Scholar] [CrossRef]
- Lu, N.; Yoon, J.; Suo, Z. Delamination of stiff islands patterned on stretchable substrates. Int. J. Mater. Res. 2007, 98, 717–722. [Google Scholar] [CrossRef]
- Asran, A.S.; Seydewitz, V.; Michler, G.H. Micromechanical properties and ductile behavior of electrospun polystyrene nanofibers. J. Appl. Polym. Sci. 2012, 125, 1663–1673. [Google Scholar] [CrossRef]
- Naraghi, M.; Chasiotis, I.; Kahn, H.; Wen, Y.; Dzenis, Y. Novel method for mechanical characterization of polymeric nanofibers. Rev. Sci. Instrum. 2007, 78, 85108. [Google Scholar] [CrossRef]
- Naraghi, M.; Chasiotis, I.; Kahn, H.; Wen, Y.; Dzenis, Y. Mechanical deformation and failure of electrospun polyacrylonitrile nanofibers as a function of strain rate. Appl. Phys. Lett. 2007, 91, 151901. [Google Scholar] [CrossRef]
- Richard, L.A.; Valter, S.; Ulf, W.G.; Peter, E.M.; Mikael, S.H.; Richard, T.O. Micromechanics of ultra-toughened electrospun PMMA/PEO fibres as revealed by in-situ tensile testing in an electron microscope. Sci. Rep. 2014, 4, 6335. [Google Scholar]
- Tan, E.; Ng, S.; Lim, C.T. Tensile testing of a single ultrafine polymeric fiber. Biomaterials 2005, 26, 1453–1456. [Google Scholar] [CrossRef]
- Pai, C.-L.; Boyce, M.C.; Rutledge, G.C. Mechanical properties of individual electrospun PA 6(3)T fibers and their variation with fiber diameter. Polymer 2011, 52, 2295–2301. [Google Scholar] [CrossRef]
- Papkov, D.; Zou, Y.; Andalib, M.N.; Goponenko, A.; Cheng, S.Z.D.; Dzenis, Y.A. Simultaneously Strong and Tough Ultrafine Continuous Nanofibers. ACS Nano 2013, 7, 3324–3331. [Google Scholar] [CrossRef] [PubMed]
- Michler, G.H.; Kausch, H.H.; Adhikari, R. Modeling of Thin Layer Yielding in Polymers. J. Macromol. Sci. Part B 2006, 45, 727–739. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Aminu, T.Q.; Bahr, D. Probing Adhesion of Metallic Nanoparticles on Polymeric Fibrous and Flat Architectures. MRS Adv. 2018, 3, 2749–2756. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Petitet, J.-P.; Froigneux, E.; Moreau, M.; Rouzaud, J.-N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2267–2276. [Google Scholar] [CrossRef]
- Yin, L.; Wang, J.; Lin, F.; Nuli, Y.; Yang, J. Polyacrylonitrile/graphene composite as a precursor to a sulfur-based cathode material for high-rate rechargeable Li–S batteries. Energy Environ. Sci. 2012, 5, 6966. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Lu, N.; Oh, K.-H.; Suo, Z.; Vlassak, J.J. Islands stretch test for measuring the interfacial fracture energy between a hard film and a soft substrate. J. Appl. Phys. 2013, 113, 223702. [Google Scholar] [CrossRef]
- Janssen, M. Fracture Mechanics, 2nd ed.; Spon Press: London, UK, 2004. [Google Scholar]
- He, M.; Evans, A.; Hutchinson, J. Convergent debonding of films and fibers. Acta Mater. 1997, 45, 3481–3489. [Google Scholar] [CrossRef]
- Naraghi, M.; Chasiotis, I. Mechanics of PAN nanofibers. In Major Accomplishments in Composite Materials and Sandwich Structures; Springer: Berlin/Heidelberg, Germany, 2009; pp. 757–778. [Google Scholar]
- Davis, J.R. Tensile Testing, 2nd ed.; ASM International: Materials Park, OH, USA, 2004. [Google Scholar]
- Budevski, E.B.; Staikov, G.T.; Lorenz, W.J. Electrochemical Phase Formation and Growth: An Introduction to the Initial Stages of Metal Deposition; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Li, R.; Zhang, X.; Dong, H.; Li, Q.; Shuai, Z.; Hu, W. Gibbs-Curie-Wulff Theorem in Organic Materials: A Case Study on the Relationship between Surface Energy and Crystal Growth. Adv. Mater. 2015, 28, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Dhanaraj, G.; Byrappa, K.; Prasad, V.; Dudley, M.; Schmetzer, K. Springer Handbook of Crystal Growth. Jpn. Mag. Mineral. Petrol. Sci. 2010, 39, 193. [Google Scholar]
- Winterbottom, W. Equilibrium shape of a small particle in contact with a foreign substrate. Acta Met. 1967, 15, 303–310. [Google Scholar] [CrossRef]
- Han, Y.; Lai, K.C.; Lii-Rosales, A.; Tringides, M.C.; Evans, J.W.; Thiel, P.A. Surface energies, adhesion energies, and exfoliation energies relevant to copper-graphene and copper-graphite systems. Surf. Sci. 2019, 685, 48–58. [Google Scholar] [CrossRef]
- Naraghi, M.; Kolluru, P.V.; Chasiotis, I. Time and strain rate dependent mechanical behavior of individual polymeric nanofibers. J. Mech. Phys. Solids 2014, 62, 257–275. [Google Scholar] [CrossRef]
- Naraghi, M.; Arshad, S.; Chasiotis, I. Molecular orientation and mechanical property size effects in electrospun polyacrylonitrile nanofibers. Polymer 2011, 52, 1612–1618. [Google Scholar] [CrossRef]
- Baji, A.; Mai, Y.-W.; Wong, S.-C.; Abtahi, M.; Chen, P. Electrospinning of polymer nanofibers: Effects on oriented morphology, structures and tensile properties. Compos. Sci. Technol. 2010, 70, 703–718. [Google Scholar] [CrossRef]
- Pai, C.-L.; Boyce, M.C.; Rutledge, G.C. Morphology of Porous and Wrinkled Fibers of Polystyrene Electrospun from Dimethylformamide. Macromolecules 2009, 42, 2102–2114. [Google Scholar] [CrossRef]
- Kisin, S.; Vukić, J.B.; Van Der Varst, P.G.T.; De With, G.; Koning, C.E. Estimating the Polymer−Metal Work of Adhesion from Molecular Dynamics Simulations. Chem. Mater. 2007, 19, 903–907. [Google Scholar] [CrossRef]
- Yeager, J.D.; Phillips, D.J.; Rector, D.M.; Bahr, D. Characterization of flexible ECoG electrode arrays for chronic recording in awake rats. J. Neurosci. Methods 2008, 173, 279–285. [Google Scholar] [CrossRef]
- Cordill, M.; Marx, V.; Kirchlechner, C. Ductile film delamination from compliant substrates using hard overlayers. Thin Solid Films 2014, 571, 302–307. [Google Scholar] [CrossRef] [PubMed]


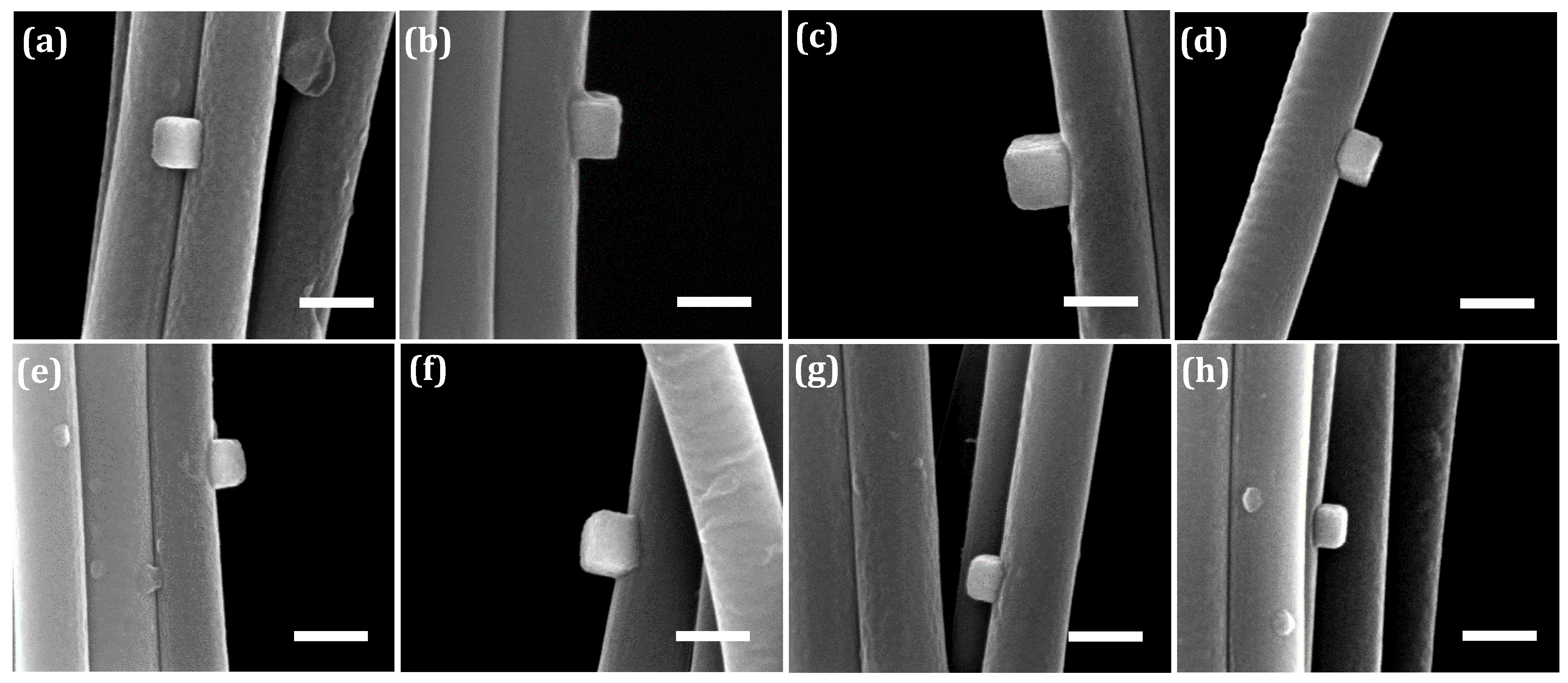
| Figure Reference | A (nm) | B (nm) | Adhesion Energy (J/m2) | Average Adhesion Energy (J/m2) |
|---|---|---|---|---|
| Figure 3b | 223 ± 5 | 208 ± 3 | 0.93 ± 0.05 | 1.08 ± 0.30 |
| Figure 5a | 157 ± 2 | 148 ± 2 | 0.66 ± 0.004 | |
| Figure 11a | 185 ± 4 | 133 ± 5 | 1.55 ± 0.03 | |
| Figure 11b | 216 ± 4 | 187 ± 8 | 1.14 ± 0.04 | |
| Figure 11c | 239 ± 3 | 229 ± 5 | 0.91 ± 0.02 | |
| Figure 11d | 149 ± 3 | 139 ± 2 | 0.66 ± 0.03 | |
| Figure 11e | 185 ± 5 | 144 ± 6 | 1.33 ± 0.02 | |
| Figure 11f | 190 ± 6 | 151 ± 16 | 1.30 ± 0.20 | |
| Figure 11g | 153 ± 5 | 128 ± 4 | 1.02 ± 0.02 | |
| Figure 11h | 137 ± 5 | 103 ± 2 | 1.27 ± 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminu, T.Q.; Brockway, M.C.; Skinner, J.L.; Bahr, D.F. Well-Adhered Copper Nanocubes on Electrospun Polymeric Fibers. Nanomaterials 2020, 10, 1982. https://doi.org/10.3390/nano10101982
Aminu TQ, Brockway MC, Skinner JL, Bahr DF. Well-Adhered Copper Nanocubes on Electrospun Polymeric Fibers. Nanomaterials. 2020; 10(10):1982. https://doi.org/10.3390/nano10101982
Chicago/Turabian StyleAminu, Temitope Q., Molly C. Brockway, Jack L. Skinner, and David F. Bahr. 2020. "Well-Adhered Copper Nanocubes on Electrospun Polymeric Fibers" Nanomaterials 10, no. 10: 1982. https://doi.org/10.3390/nano10101982
APA StyleAminu, T. Q., Brockway, M. C., Skinner, J. L., & Bahr, D. F. (2020). Well-Adhered Copper Nanocubes on Electrospun Polymeric Fibers. Nanomaterials, 10(10), 1982. https://doi.org/10.3390/nano10101982





