Morphology Related Defectiveness in ZnO Luminescence: From Bulk to Nano-Size
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Structural and Morphological Characterization
2.3. Spectroscopic Characterization
3. Results
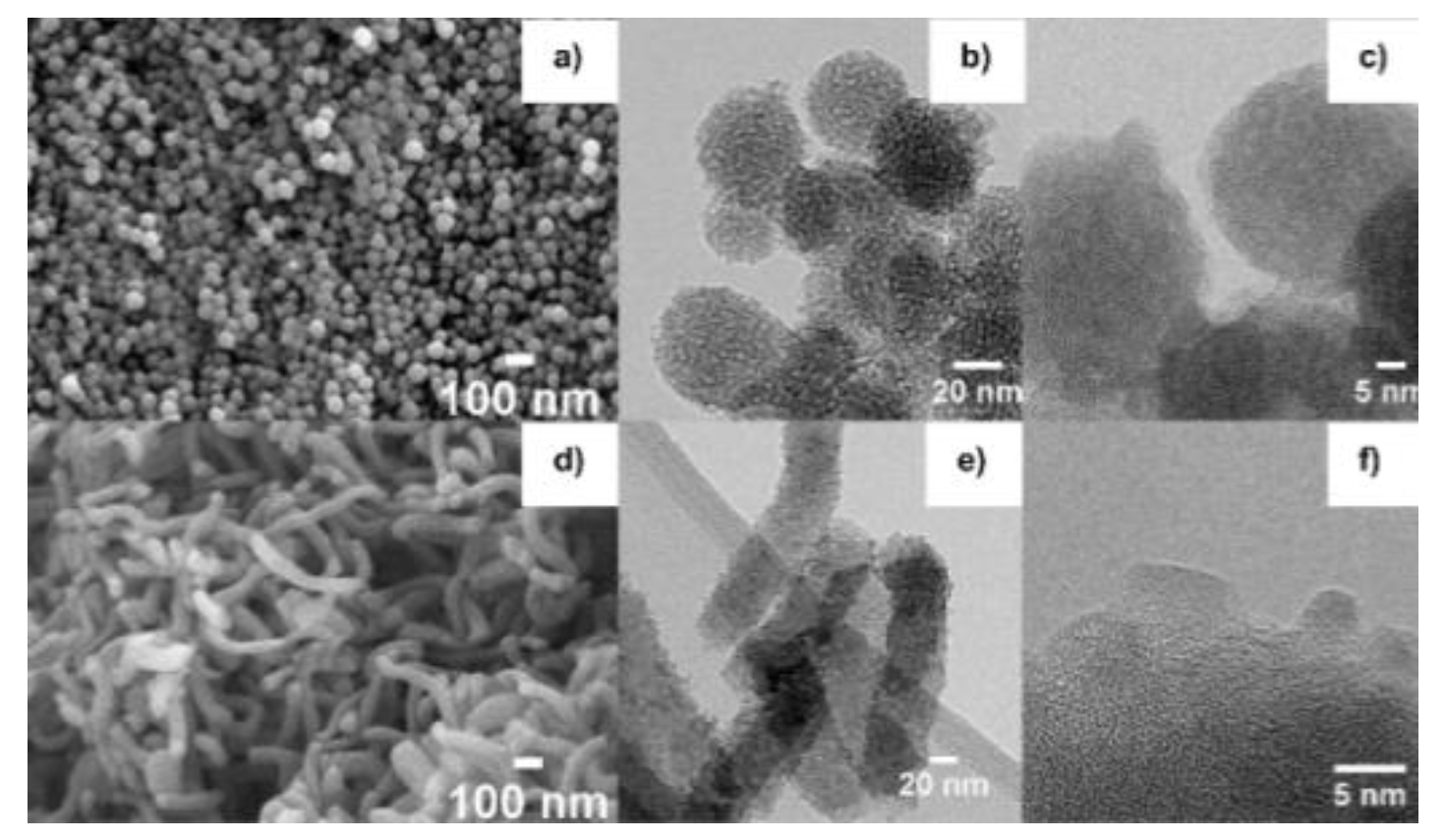
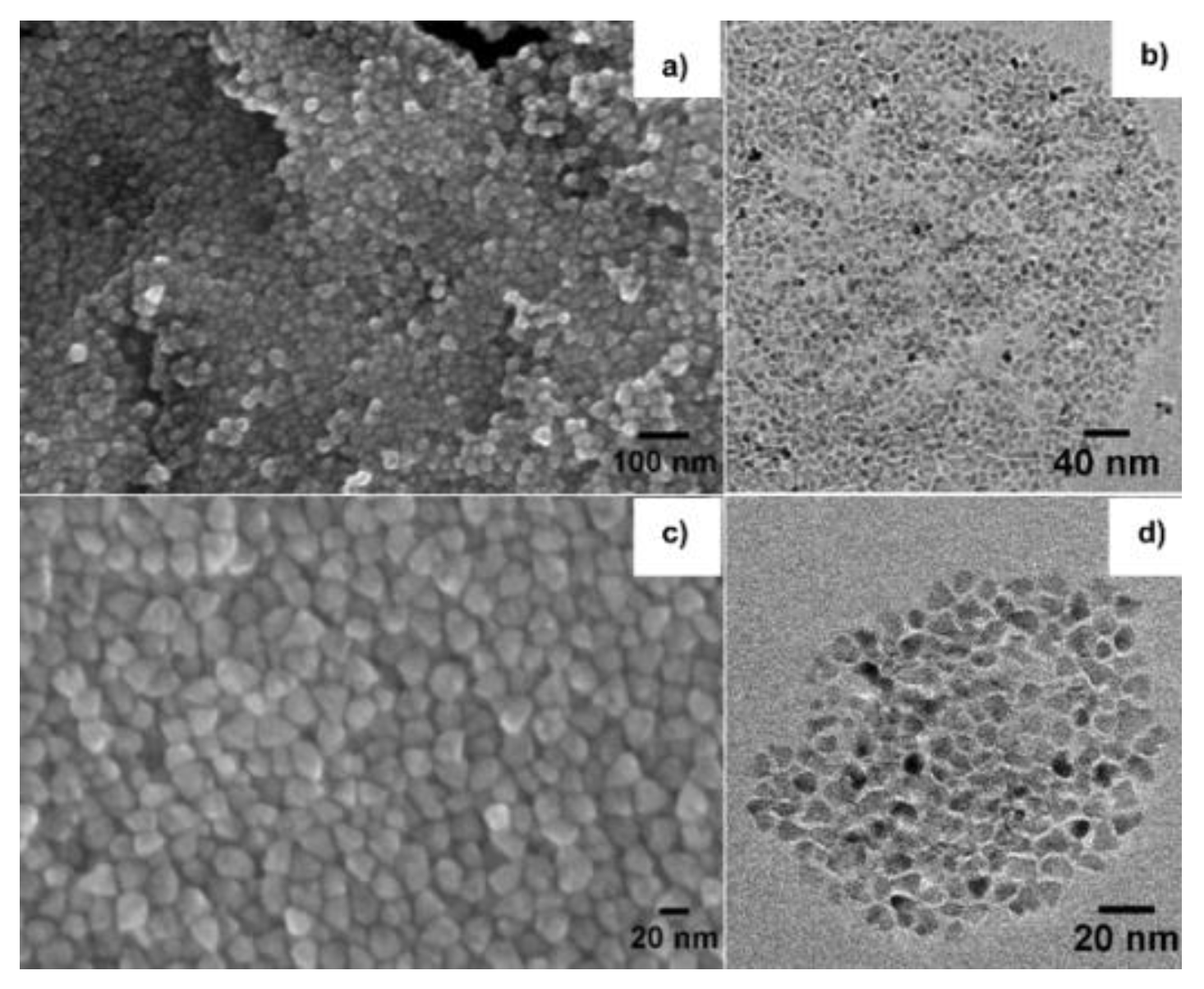
3.1. Structural and Morphological Properties
3.2. Radio- and Photo-Luminescence
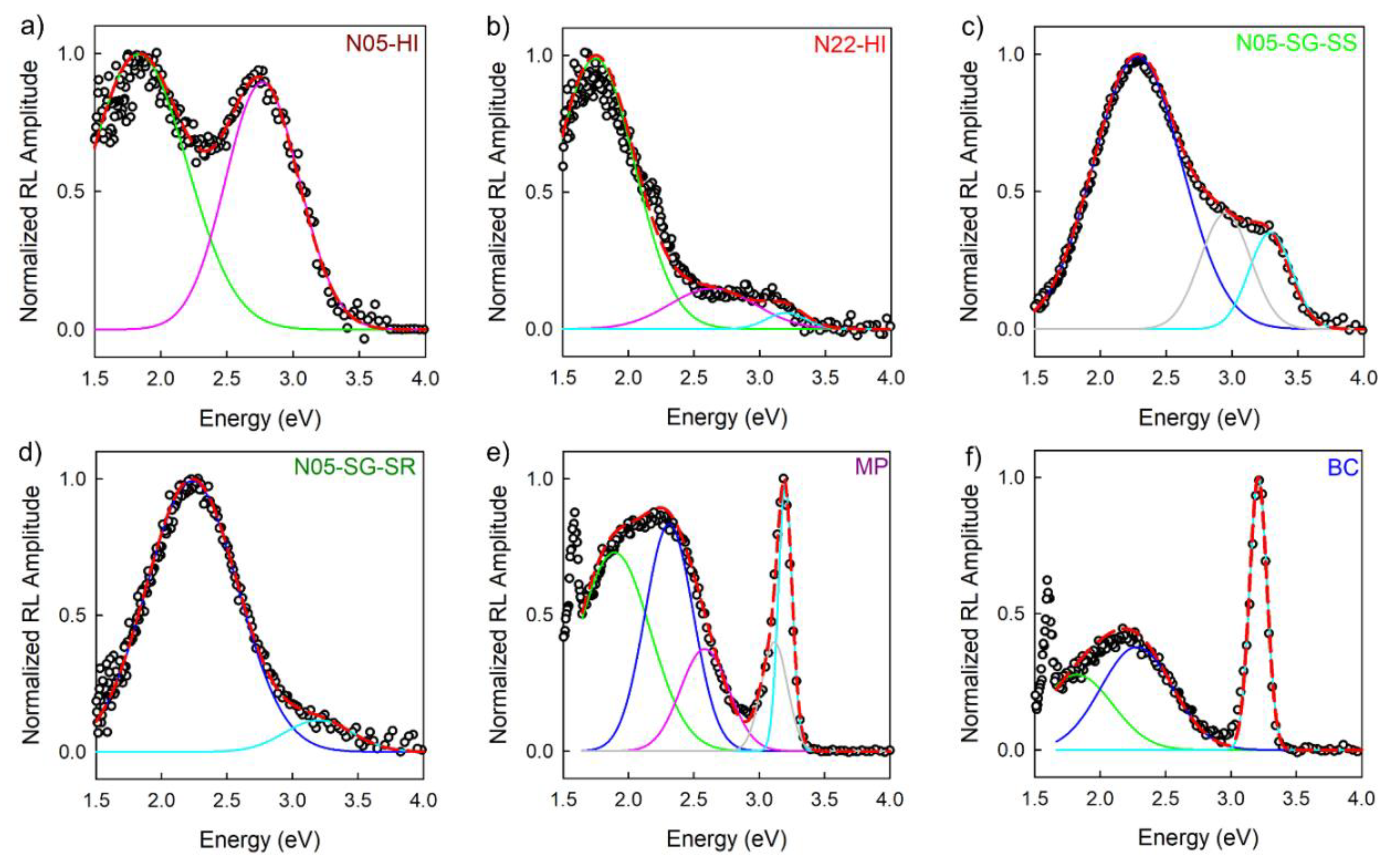
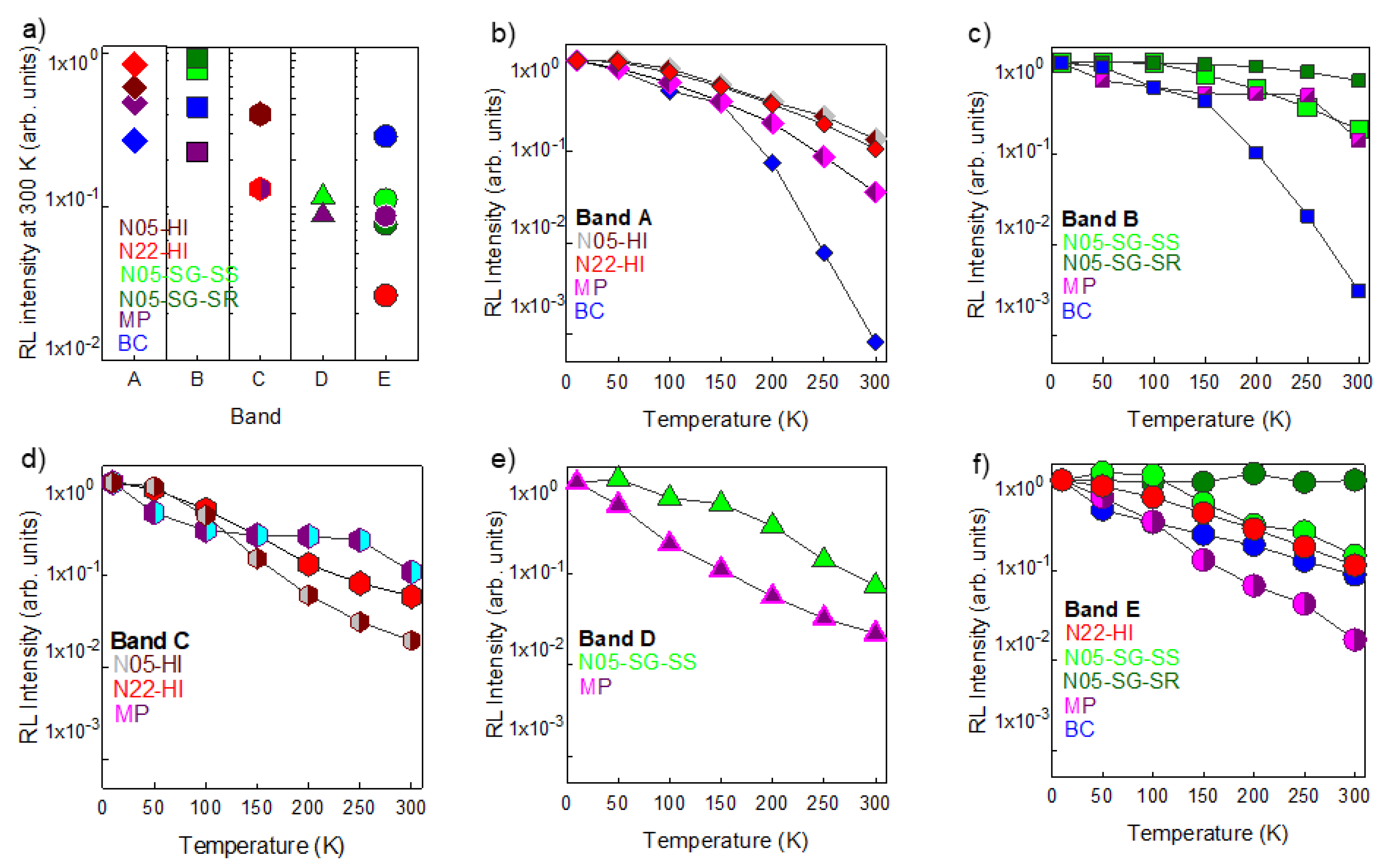
3.3. Analysis of Spectral Components
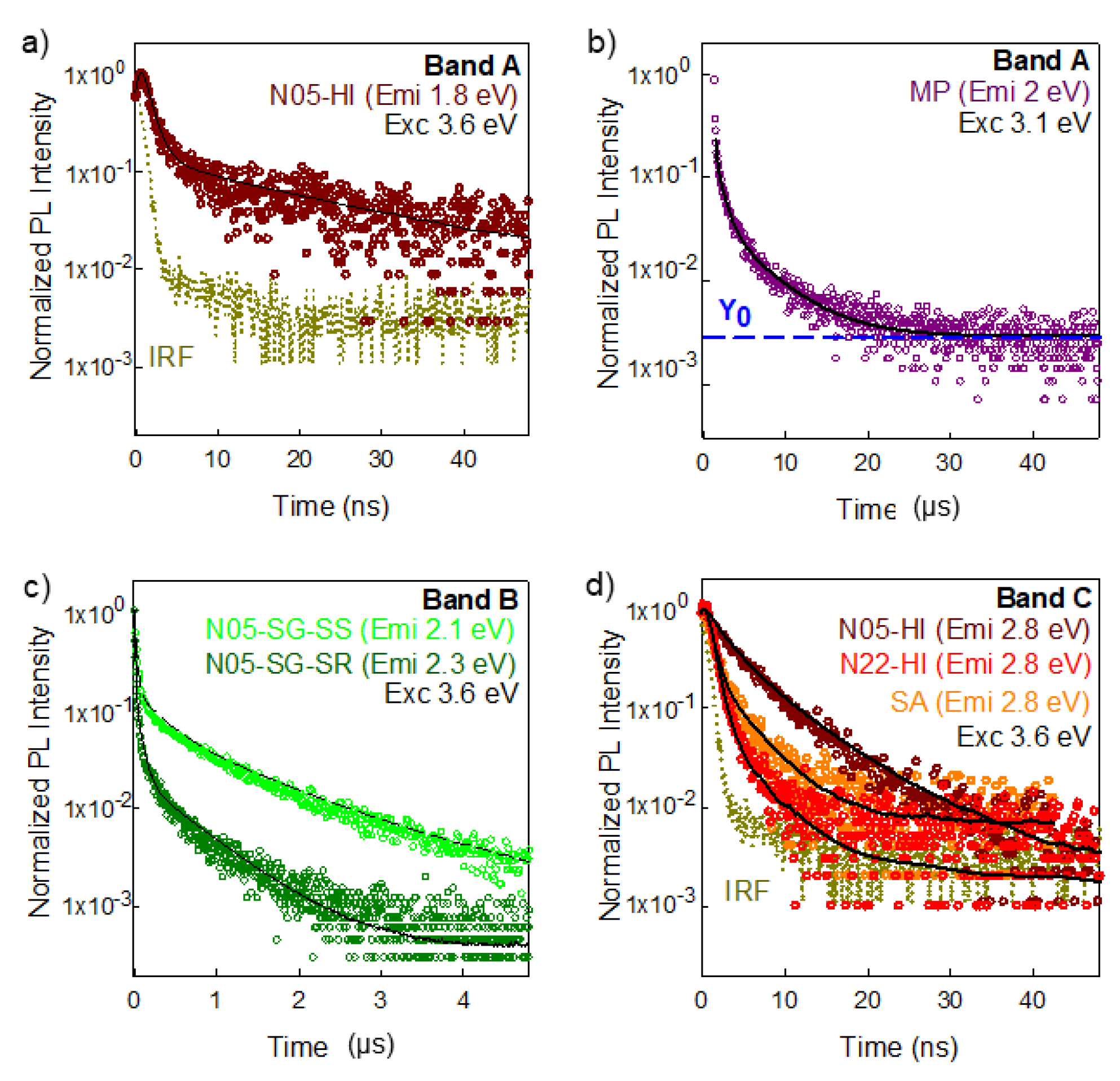
3.4. Time Resolved Luminescence
4. Discussion
4.1. Role of Excitation Energy
4.2. Role of Dimensionality
4.3. Role of Growth Conditions and Interfaces
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doǧan, S.; Avrutin, V.; Cho, S.J.; Morko̧, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 1–103. [Google Scholar] [CrossRef] [Green Version]
- Janotti, A.; Van De Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72. [Google Scholar] [CrossRef] [Green Version]
- Klingshirn, C.F. ZnO: Material, physics and applications. ChemPhysChem 2007, 8, 782–803. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, H.M.S.; Khan, F.; Nam, K.; Kim, H.Y.; Kim, S.D. Ultraviolet Photodetection Based on High-Performance Co-Plus-Ni Doped ZnO Nanorods Grown by Hydrothermal Method on Transparent Plastic Substrate. Nanomaterials 2020, 10, 1225. [Google Scholar] [CrossRef] [PubMed]
- Willander, M.; Nur, O.; Sadaf, J.R.; Qadir, M.I.; Zaman, S.; Zainelabdin, A.; Bano, N.; Hussain, I. Luminescence from Zinc Oxide Nanostructures and Polymers and their Hybrid Devices. Materials 2010, 3, 2643–2667. [Google Scholar] [CrossRef]
- Guo-Bin, Z.; Chao-Shu, S.; Zheng-Fu, H.; Jun-Yan, S.; Zhu-Xi, F.; Kirm, M.; Zimmerer, G. Photoluminescent Properties of ZnO Films Deposited on Si Substrates. Chin. Phys. Lett. 2001, 18, 441–442. [Google Scholar] [CrossRef]
- Rodnyi, P.A.; Khodyuk, I.V. Optical and luminescence properties of zinc oxide (Review). Opt. Spectrosc. 2011, 111, 776–785. [Google Scholar] [CrossRef] [Green Version]
- Ambersley, M.D.; Pitt, C.W. Piezoelectric ZnO transducers produced by r.f. magnetron sputtering. Thin Solid Film 1981, 80, 183–195. [Google Scholar] [CrossRef]
- Engineering Materials. In Introduction to Nano; Sengupta, A.; Sarkar, C.K. (Eds.) Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-662-47313-9. [Google Scholar]
- Yin, Y.; Talapin, D. The chemistry of functional nanomaterials. Chem. Soc. Rev. 2013, 42, 2484. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H. Optical Properties of ZnO Nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Mostoni, S.; Milana, P.; Di Credico, B.; D’Arienzo, M.; Scotti, R. Zinc-Based Curing Activators: New Trends for Reducing Zinc Content in Rubber Vulcanization Process. Catalysts 2019, 9, 664. [Google Scholar] [CrossRef] [Green Version]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sust. Energ. Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Cerrato, E.; Paganini, M.C.; Giamello, E. Photoactivity under visible light of defective ZnO investigated by EPR spectroscopy and photoluminescence. J. Photochem. Photobiol. A Chem. 2020, 397, 112531. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc Oxide Nanostructures for NO2 Gas–Sensor Applications: A Review. Nano Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Djurišić, A.B.; Ng, A.M.C.; Chen, X.Y. ZnO nanostructures for optoelectronics: Material properties and device applications. Prog. Quantum Electron. 2010, 34, 191–259. [Google Scholar] [CrossRef]
- Zu, P.; Tang, Z.K.; Wong, G.K.L.; Kawasaki, M.; Ohtomo, A.; Koinuma, H.; Segawa, Y. Ultraviolet spontaneous and stimulated emissions from ZnO microcrystallite thin films at room temperature. Solid State Commun. 1997, 103, 459–463. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Z.; Luo, Y.; Wang, L. Giant enhanced infrared and orange emissions of ZnO nanoparticles induced by rich oxygen atmosphere. Solid State Commun. 2008, 147, 447–451. [Google Scholar] [CrossRef]
- Achour, A.; Islam, M.; Vizireanu, S.; Ahmad, I.; Akram, M.A.; Saeed, K.; Dinescu, G.; Pireaux, J.J. Orange/red photoluminescence enhancement upon sf6 plasma treatment of vertically aligned ZnO nanorods. Nanomaterials 2019, 9, 794. [Google Scholar] [CrossRef] [Green Version]
- Vanheusden, K.; Seager, C.H.; Warren, W.L.; Tallant, D.R.; Voigt, J.A. Correlation between photoluminescence and oxygen vacancies in ZnO phosphors. Appl. Phys. Lett. 1995, 403, 403. [Google Scholar] [CrossRef]
- Kasai, P.H. Electron Spin Resonance Studies of Donors and Acceptors in ZnO. Phys. Rev. 1963, 130, 989–995. [Google Scholar] [CrossRef]
- Polarz, S.; Roy, A.; Lehmann, M.; Driess, M.; Kruis, F.E.; Hoffmann, A.; Zimmer, P. Structure-Property-Function Relationships in Nanoscale Oxide Sensors: A Case Study Based on Zinc Oxide. Adv. Funct. Mater. 2007, 17, 1385–1391. [Google Scholar] [CrossRef] [Green Version]
- Buryi, M.; Babin, V.; Chang, Y.-Y.; Remeš, Z.; Mičová, J.; Šimek, D. Influence of precursor age on defect states in ZnO nanorods. Appl. Surf. Sci. 2020, 525, 146448. [Google Scholar] [CrossRef]
- Zhang, M.; Averseng, F.; Krafft, J.-M.; Borghetti, P.; Costentin, G.; Stankic, S. Controlled Formation of Native Defects in Ultrapure ZnO for the Assignment of Green Emissions to Oxygen Vacancies. J. Phys. Chem. C 2020, 124, 12696–12704. [Google Scholar] [CrossRef]
- Li, M.; Xing, G.; Xing, G.; Wu, B.; Wu, T.; Zhang, X.; Sum, T.C. Origin of green emission and charge trapping dynamics in ZnO nanowires. Phys. Rev. B 2013, 87. [Google Scholar] [CrossRef]
- Zhou, X.; Kuang, Q.; Jiang, Z.Y.; Xie, Z.X.; Xu, T.; Huang, R.-B.; Zheng, L.S. The origin of green emission of ZnO microcrystallites: Surface-dependent light emission studied by cathodoluminescence. J. Phys. Chem. C 2007, 111, 12091–12093. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Mostoni, S.; Crapanzano, R.; Cepek, C.; Di Credico, B.; Fasoli, M.; Polizzi, S.; Vedda, A.; Villa, I.; Scotti, R. Insight into the Influence of ZnO Defectivity on the Catalytic Generation of Environmentally Persistent Free Radicals in ZnO/SiO2 Systems. J. Phys. Chem. C 2019, 123, 21651–21661. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Miyashita, S.; Obara, K.; Shishido, T.; Sakagami, N. Hydrothermal growth of ZnO single crystals and their optical characterization. J. Cryst. Growth 2000, 214, 72–76. [Google Scholar] [CrossRef]
- Frodason, Y.K.; Johansen, K.M.; Galeckas, A.; Vines, L. Broad luminescence from donor-complexed LiZn and NaZn acceptors in ZnO. Phys. Rev. B 2019, 100, 184102. [Google Scholar] [CrossRef]
- Lyons, J.L.; Alkauskas, A.; Janotti, A.; Van de Walle, C.G. Deep donor state of the copper acceptor as a source of green luminescence in ZnO. Appl. Phys. Lett. 2017, 111, 042101. [Google Scholar] [CrossRef]
- Cao, B.; Cai, W.; Zeng, H. Temperature-dependent shifts of three emission bands for ZnO nanoneedle arrays. Appl. Phys. Lett. 2006, 88, 18–21. [Google Scholar] [CrossRef]
- Han, N.S.; Shim, H.S.; Seo, J.H.; Kim, S.Y.; Park, S.M.; Song, J.K. Defect states of ZnO nanoparticles: Discrimination by time-resolved photoluminescence spectroscopy. J. Appl. Phys. 2010, 107. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Duan, G.; Li, Y.; Yang, S.; Xu, X.; Cai, W. Blue luminescence of ZnO nanoparticles based on non-equilibrium processes: Defect origins and emission controls. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Vistovskyy, V.V.; Zhyshkovych, A.V.; Halyatkin, O.O.; Mitina, N.E.; Zaichenko, A.S.; Rodnyi, P.A.; Vasil’Ev, A.N.; Gektin, A.V.; Voloshinovskii, A.S. The luminescence of BaF2 nanoparticles upon high-energy excitation. J. Appl. Phys. 2014, 116. [Google Scholar] [CrossRef]
- Villa, I.; Lauria, A.; Moretti, F.; Fasoli, M.; Dujardin, C.; Niederberger, M.; Vedda, A. Radio-luminescence spectral features and fast emission in hafnium dioxide nanocrystals. Phys. Chem. Chem. Phys. 2018, 20, 15907–15915. [Google Scholar] [CrossRef] [PubMed]
- Cybinska, J.; Guzik, M.; Lorbeer, C.; Zych, E.; Guyot, Y.; Boulon, G.; Mudring, A.-V. Design of LaPO4:Nd3+ materials by using ionic liquids. Opt. Mater. 2017, 63, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Tissue, B.M.; Bihari, B. Lanthanide luminescence as a probe of nanocrystalline materials. J. Fluoresc. 1998, 8, 289–294. [Google Scholar] [CrossRef]
- Najib, S.; Bakan, F.; Abdullayeva, N.; Bahariqushchi, R.; Kasap, S.; Franzò, G.; Sankir, M.; Demirci Sankir, N.; Mirabella, S.; Erdem, E. Tailoring morphology to control defect structures in ZnO electrodes for high-performance supercapacitor devices. Nanoscale 2020, 12, 16162–16172. [Google Scholar] [CrossRef]
- Xiong, G.; Pal, U.; Serrano, J.G. Correlations among size, defects, and photoluminescence in ZnO nanoparticles. J. Appl. Phys. 2007, 101. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Li, C.; Chai, Z. Defect luminescence and its mediated physical properties in ZnO. J. Lumin. 2019, 208, 225–237. [Google Scholar] [CrossRef]
- Zhou, H.; Alves, H.; Hofmann, D.M.; Kriegseis, W.; Meyer, B.K.; Kaczmarczyk, G.; Hoffmann, A. Behind the weak excitonic emission of ZnO quantum dots: ZnO/Zn(OH)2 core-shell structure. Appl. Phys. Lett. 2002, 80, 210–212. [Google Scholar] [CrossRef]
- Gbur, T.; Čuba, V.; Múčka, V.; Nikl, M.; Knížek, K.; Pospíšil, M.; Jakubec, I. Photochemical preparation of ZnO nanoparticles. J. Nanoparticle Res. 2011, 13, 4529–4537. [Google Scholar] [CrossRef]
- Tadiello, L.; D’Arienzo, M.; Di Credico, B.; Hanel, T.; Matejka, L.; Mauri, M.; Morazzoni, F.; Simonutti, R.; Spirkova, M.; Scotti, R. The filler–rubber interface in styrene butadiene nanocomposites with anisotropic silica particles: Morphology and dynamic properties. Soft Matter 2015, 11, 4022–4033. [Google Scholar] [CrossRef]
- Scotti, R.; Conzatti, L.; D’Arienzo, M.; Di Credico, B.; Giannini, L.; Hanel, T.; Stagnaro, P.; Susanna, A.; Tadiello, L.; Morazzoni, F. Shape controlled spherical (0D) and rod-like (1D) silica nanoparticles in silica/styrene butadiene rubber nanocomposites: Role of the particle morphology on the filler reinforcing effect. Polymer 2014, 55, 1497–1506. [Google Scholar] [CrossRef]
- Susanna, A.; D’Arienzo, M.; Di Credico, B.; Giannini, L.; Hanel, T.; Grandori, R.; Morazzoni, F.; Mostoni, S.; Santambrogio, C.; Scotti, R. Catalytic effect of ZnO anchored silica nanoparticles on rubber vulcanization and cross-link formation. Eur. Polym. J. 2017, 93, 63–74. [Google Scholar] [CrossRef]
- Susanna, A.; Armelao, L.; Callone, E.; Dirè, S.; D’Arienzo, M.; Di Credico, B.; Giannini, L.; Hanel, T.; Morazzoni, F.; Scotti, R. ZnO nanoparticles anchored to silica filler. A curing accelerator for isoprene rubber composites. Chem. Eng. J. 2015, 275, 245–252. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Snoeck, E.; Garcia, M.A.; Giannini, C.; Guagliardi, A.; Cervellino, A.; Gozzo, F.; Hernando, A.; Achterhold, K.; Ciobanu, N.; et al. Colloidal Synthesis and Characterization of Tetrapod-Shaped Magnetic Nanocrystals. Nano Lett. 2006, 6, 1966–1972. [Google Scholar] [CrossRef]
- Bu, W.; Chen, Z.; Chen, F.; Shi, J. Oleic Acid/Oleylamine Cooperative-Controlled Crystallization Mechanism for Monodisperse Tetragonal Bipyramid NaLa(MoO4)2 Nanocrystals. J. Phys. Chem. C 2009, 113, 12176–12185. [Google Scholar] [CrossRef]
- Snyder, A.W.; Love, J.D. Optical Waveguide Theory; Springer: Boston, MA, USA, 1984; ISBN 978-0-412-24250-2. [Google Scholar]
- Williams, F.E.; Eyring, H. The mechanism of the luminescence of solids. J. Chem. Phys. 1947, 15, 289–304. [Google Scholar] [CrossRef]
- Kumar, N.; Kaur, R.; Mehra, R.M. Photoluminescence studies in sol-gel derived Zno films. J. Lumin. 2007, 126, 784–788. [Google Scholar] [CrossRef]
- Fonoberov, V.A.; Alim, K.A.; Balandin, A.A.; Xiu, F.; Liu, J. Photoluminescence investigation of the carrier recombination processes in ZnO quantum dots and nanocrystals. Phys. Rev. B 2006, 73, 1–9. [Google Scholar] [CrossRef] [Green Version]
- El Filali, B.; Torchynska, T.V.; Polupan, G.; Shcherbyna, L. Radiative defects, emission and structure of ZnO nanocrystals obtained by electrochemical method. Mater. Res. Bull. 2017, 85, 161–167. [Google Scholar] [CrossRef]
- Chen, H.I.; Hsiao, J.J.; Fang, C.H.; Jiang, J.A.; Wang, J.C.; Wu, Y.F.; Nee, T.E. Investigation of the thermal photocarrier interaction–recombination in ZnO nanostructures fabricated by the hydrothermal method. J. Lumin. 2017, 190, 136–140. [Google Scholar] [CrossRef]
- Nikl, M. Scintillation detectors for X-rays. Meas. Sci. Technol. 2006, 17. [Google Scholar] [CrossRef]
- Vedda, A.; Fasoli, M. Tunneling recombinations in scintillators, phosphors, and dosimeters. Radiat. Meas. 2018, 118, 86–97. [Google Scholar] [CrossRef]
- Luo, Z.; Moch, J.G.; Johnson, S.S.; Chen, C.C. A Review on X-ray Detection Using Nanomaterials. Curr. Nanosci. 2017, 13. [Google Scholar] [CrossRef]
- Pozina, G.; Yang, L.L.; Zhao, Q.X.; Hultman, L.; Lagoudakis, P.G. Size dependent carrier recombination in ZnO nanocrystals. Appl. Phys. Lett. 2010, 97, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Dujardin, C.; Amans, D.; Belsky, A.; Chaput, F.; Ledoux, G.; Pillonnet, A. Luminescence and scintillation properties at the nanoscale. IEEE Trans. Nucl. Sci. 2010, 57, 1348–1354. [Google Scholar] [CrossRef]
- Principles of Fluorescence Spectroscopy; Lakowicz, J.R. (Ed.) Springer: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Yanagida, T.; Fujimoto, Y.; Yamanoi, K.; Kano, M.; Wakamiya, A.; Kurosawa, S.; Sarukura, N. Optical and scintillation properties of bulk ZnO crystal. Phys. Status Solidi 2012, 9, 2284–2287. [Google Scholar] [CrossRef]
- Demidenko, V.A.; Gorokhova, E.I.; Khodyuk, I.V.; Khristich, O.A.; Mikhrin, S.B.; Rodnyi, P.A. Scintillation properties of ceramics based on zinc oxide. Radiat. Meas. 2007, 42, 549–552. [Google Scholar] [CrossRef] [Green Version]
- Santos, D.A.A.; Junior, E.A.S.; Macêdo, M.A. Radioluminescence in ZnO. Radiat. Phys. Chem. 2010, 79, 612–614. [Google Scholar] [CrossRef]
- Wilkinson, J.; Ucer, K.B.; Williams, R.T. Picosecond excitonic luminescence in ZnO and other wide-gap semiconductors. Radiat. Meas. 2004, 38, 501–505. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, Z.; Wang, Y.; Finch, A.; Townsend, P.D. Temperature dependence of bulk luminescence from ZnO. Luminescence 2018, 33, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Colosimo, A.M.; Anwand, W.; Boatner, L.A.; Wagner, A.; Stepanov, P.S.; Trinh, T.T.; Liedke, M.O.; Krause-Rehberg, R.; Cowan, T.E.; et al. ZnO Luminescence and scintillation studied via photoexcitation, X-ray excitation and gamma-induced positron spectroscopy. Sci. Rep. 2016, 6, 31238. [Google Scholar] [CrossRef] [Green Version]
- Dell’Orto, E.; Fasoli, M.; Ren, G.; Vedda, A. Defect-Driven Radioluminescence Sensitization in Scintillators: The Case of Lu2Si2O7:Pr. J. Phys. Chem. C 2013, 117, 20201–20208. [Google Scholar] [CrossRef]
- Derenzo, S.E.; Weber, M.J.; Klintenberg, M.K. Temperature dependence of the fast, near-band-edge scintillation from CuI, HgI2, PbI2, ZnO:Ga and CdS:In. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2002, 486, 214–219. [Google Scholar] [CrossRef]
- Van Dijken, A.; Meulenkamp, E.A.; Vanmaekelbergh, D.; Meijerink, A. The Kinetics of the Radiative and Nonradiative Processes in Nanocrystalline ZnO Particles upon Photoexcitation. J. Phys. Chem. B 2000, 104, 1715–1723. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crapanzano, R.; Villa, I.; Mostoni, S.; D’Arienzo, M.; Di Credico, B.; Fasoli, M.; Scotti, R.; Vedda, A. Morphology Related Defectiveness in ZnO Luminescence: From Bulk to Nano-Size. Nanomaterials 2020, 10, 1983. https://doi.org/10.3390/nano10101983
Crapanzano R, Villa I, Mostoni S, D’Arienzo M, Di Credico B, Fasoli M, Scotti R, Vedda A. Morphology Related Defectiveness in ZnO Luminescence: From Bulk to Nano-Size. Nanomaterials. 2020; 10(10):1983. https://doi.org/10.3390/nano10101983
Chicago/Turabian StyleCrapanzano, Roberta, Irene Villa, Silvia Mostoni, Massimiliano D’Arienzo, Barbara Di Credico, Mauro Fasoli, Roberto Scotti, and Anna Vedda. 2020. "Morphology Related Defectiveness in ZnO Luminescence: From Bulk to Nano-Size" Nanomaterials 10, no. 10: 1983. https://doi.org/10.3390/nano10101983
APA StyleCrapanzano, R., Villa, I., Mostoni, S., D’Arienzo, M., Di Credico, B., Fasoli, M., Scotti, R., & Vedda, A. (2020). Morphology Related Defectiveness in ZnO Luminescence: From Bulk to Nano-Size. Nanomaterials, 10(10), 1983. https://doi.org/10.3390/nano10101983







