Innovative Method Using Adhesive Force for Surface Micromachining of Carbon Nanowall
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparing the Substrates and Deposition of Adhesion-Increased Layer
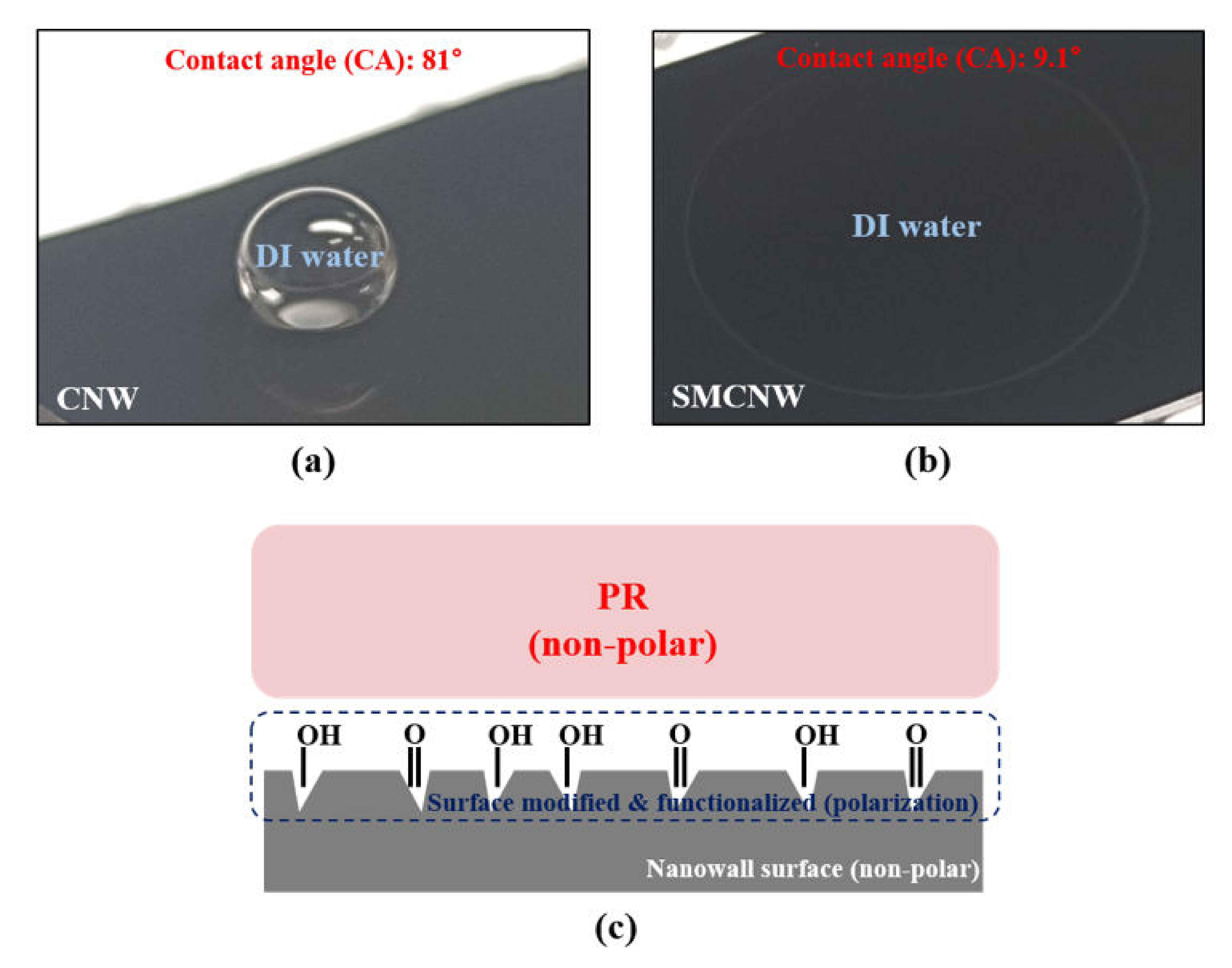
2.3. Growth of CNW and Its Surface Modification
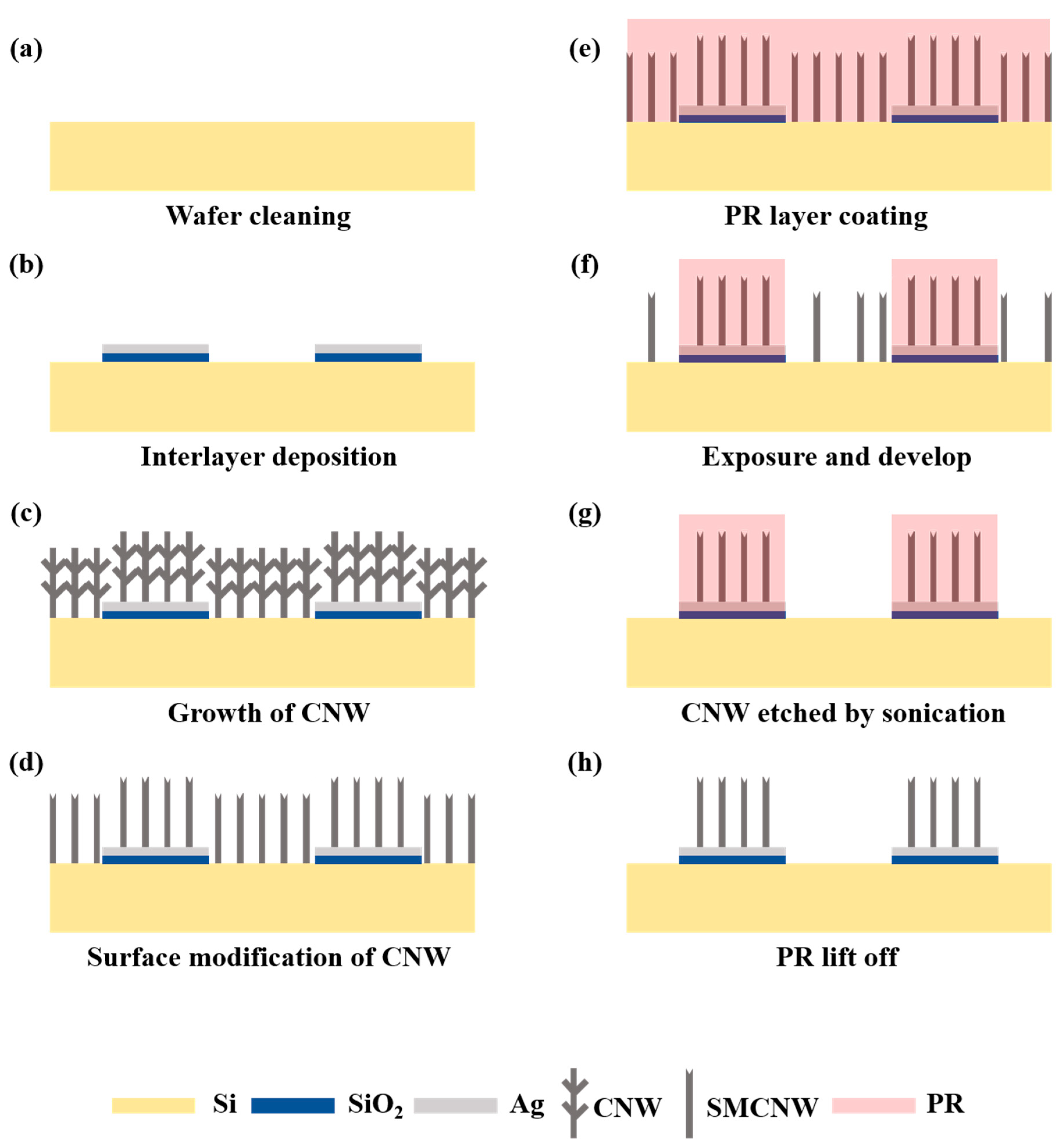
2.4. Innovative Method of Top-Down Surface Micromachining of CNW Based on Its Adhesive Properties
2.5. Characterization and Measurement of the Samples
3. Results and Discussion
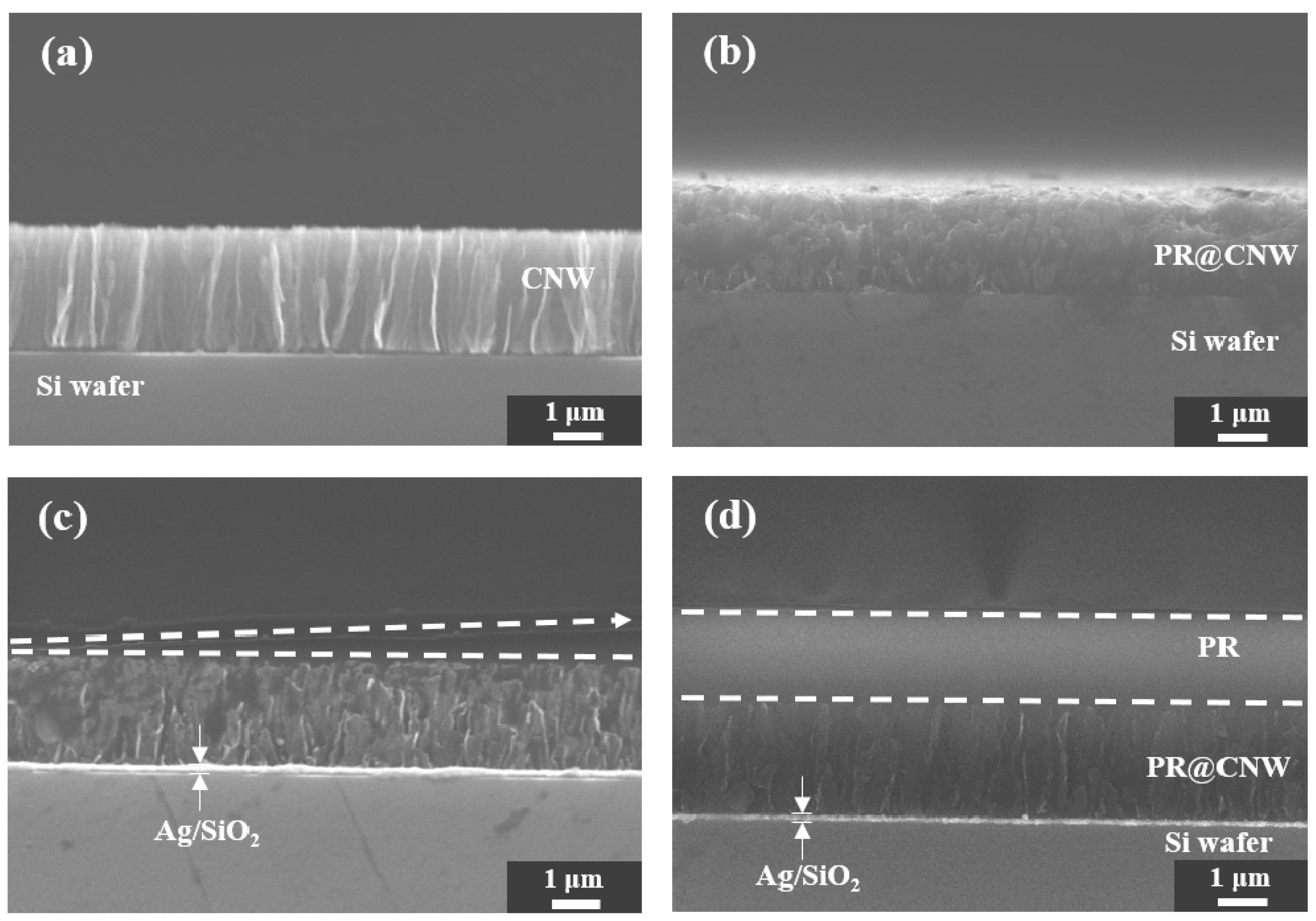
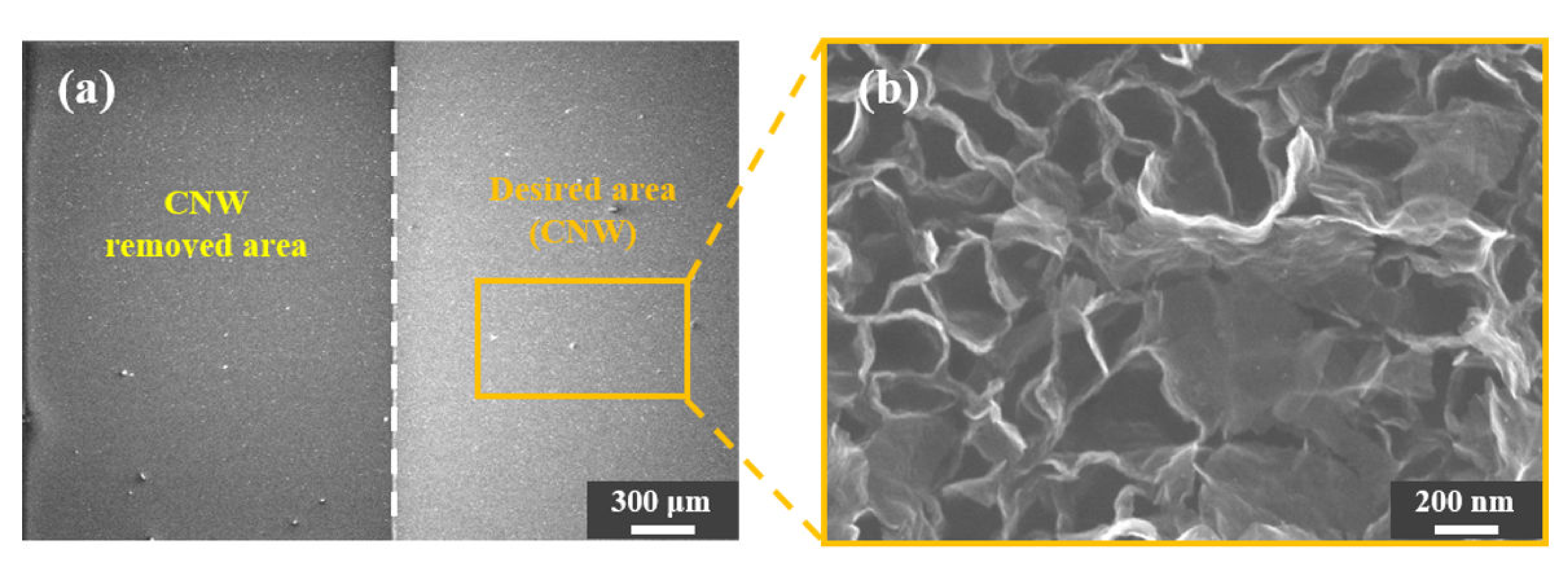
3.1. Application of the CNW Photolithography Process
3.2. Observation of the Variation in the SMCNW to which Surface Micromachining Technology Was Applied via Physical Force
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, T.-L.; Chou, C.-Y.; Wang, C.-P.; Teng, T.-C.; Han, H.-C. Picosecond laser-direct fabrication of graphene-based electrodes for a gas sensor module with wireless circuits. Microelectron. Eng. 2019, 210, 19–26. [Google Scholar] [CrossRef]
- Wang, C.; Tong, H.; Lu, J.; Liu, B.; Zheng, F.; Tao, W.; Zhang, W.; Chen, Q. Boosting Oxygen Evolution Reaction on Graphene through Engineering Electronic Structure. Carbon 2020, 170, 414–420. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, D.; Zhou, J.; Deng, J.; Yu, Y.; Deng, J.; Cai, Q.; Li, Z.; Lu, W.; Chen, X. Enhanced infrared photoresponse induced by symmetry breaking in a hybrid structure of graphene and plasmonic nanocavities. Carbon 2020, 170, 49–58. [Google Scholar] [CrossRef]
- Song, H.; Li, Q.; Zhang, Y. CNT-based sensor array for selective and steady detection of SO2 and NO. Mater. Res. Bull. 2020, 124, 110772. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Ramazani, A. Anticancer DOX delivery system based on CNTs: Functionalization, targeting and novel technologies. J. Control. Release 2020, 327, 198–224. [Google Scholar] [CrossRef]
- Liu, Y.; He, D.; Dubrunfaut, O.; Zhang, A.; Zhang, H.; Pichon, L.; Bai, J. GO-CNTs hybrids reinforced epoxy composites with porous structure as microwave absorbers. Compos. Sci. Technol. 2020, 200, 108450. [Google Scholar] [CrossRef]
- Dong, Y.; Fan, X.; Wei, H.; Hou, Z.; Li, M.; Qu, Q.; Yin, X.; Cheng, L.; Zhang, L. A lightweight CNWs-SiO2/3Al2O3·2SiO2 porous ceramic with excellent microwave absorption and thermal insulation properties. Ceram. Int. 2020, 46, 20395–20403. [Google Scholar] [CrossRef]
- Ren, F.; Xue, J.; Liu, X.; Cheng, L. In situ construction of CNWs/SiC-NWs hybrid network reinforced SiCN with excellent electromagnetic wave absorption properties in X band. Carbon 2020, 168, 278–289. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, H.J.; Song, Y.I.; Choi, Y.S.; Suh, S.-J. Morphological optimization and nitrogen functionalization of vertically oriented CNW for high performance electrical double layer capacitor electrode. Electrochim. Acta 2020, 348, 136210. [Google Scholar] [CrossRef]
- Masoudzadeh, F.; Jamshidi, M.; Fasihi, M. Preparation and application of cellulose nano whiskers (CNWs) in engineered cementitious composites. J. Build. Eng. 2019, 21, 213–221. [Google Scholar] [CrossRef]
- Makris, T.D.; Giorgi, L.; Giorgi, R.; Lisi, N.; Salernitano, E. CNT growth on alumina supported nickel catalyst by thermal CVD. Diam. Relat. Mater. 2005, 14, 815–819. [Google Scholar] [CrossRef]
- Gnanaprakasa, T.J.; Gu, Y.; Eddy, S.K.; Han, Z.; Beck, W.J.; Muralidharan, K.; Raghavan, S. The role of copper pretreatment on the morphology of graphene grown by chemical vapor deposition. Microelectron. Eng. 2015, 131, 1–7. [Google Scholar] [CrossRef]
- Naghdi, S.; Rhee, K.Y.; Park, S.-J. A catalytic, catalyst-free, and roll-to-roll production of graphene via chemical vapor deposition: Low temperature growth. Carbon 2018, 127, 1–12. [Google Scholar] [CrossRef]
- Cushing, G.W.; Johánek, V.; Navin, J.K.; Harrison, I. Graphene Growth on Pt(111) by Ethylene Chemical Vapor Deposition at Surface Temperatures near 1000 K. J. Phys. Chem. C 2015, 119, 4759–4768. [Google Scholar] [CrossRef]
- Vizireanu, S.; Stoica, S.D.; Luculescu, C.; Nistor, L.C.; Mitu, B.; Dinescu, G. Plasma techniques for nanostructured carbon materials synthesis. A case study: Carbon nanowall growth by low pressure expanding RF plasma. Plasma Sources Sci. Technol. 2010, 19, 34016. [Google Scholar] [CrossRef]
- Lisi, N.; Giorgi, R.; Re, M.; Dikonimos, T.; Giorgi, L.; Salernitano, E.; Gagliardi, S.; Tatti, F. Carbon nanowall growth on carbon paper by hot filament chemical vapour deposition and its microstructure. Carbon 2011, 49, 2134–2140. [Google Scholar] [CrossRef]
- Dikonimos, T.; Giorgi, L.; Giorgi, R.; Lisi, N.; Salernitano, E.; Rossi, R. DC plasma enhanced growth of oriented carbon nanowall films by HFCVD. Diam. Relat. Mater. 2007, 16, 1240–1243. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Shiji, K.; Amano, H.; Hori, M. Fabrication of vertically aligned carbon nanowalls using capacitively coupled plasma-enhanced chemical vapor deposition assisted by hydrogen radical injection. Appl. Phys. Lett. 2004, 84, 4708–4710. [Google Scholar] [CrossRef]
- Shih, W.-C.; Jeng, J.-M.; Huang, C.-T.; Lo, J.-T. Fabrication of carbon nanoflakes by RF sputtering for field emission applications. Vacuum 2010, 84, 1452–1456. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Holloway, B.C.; Outlaw, R.; Zhao, X.; Hou, K.; Shutthanandan, V.; Manos, D.M. A mechanism for carbon nanosheet formation. Carbon 2007, 45, 2229–2234. [Google Scholar] [CrossRef]
- Zhao, J.; Shaygan, M.; Eckert, J.; Meyyappan, M.; Rümmeli, M.H. A Growth Mechanism for Free-Standing Vertical Graphene. Nano Lett. 2014, 14, 3064–3071. [Google Scholar] [CrossRef] [PubMed]
- Mironovich, K.V.; Itkis, D.M.; Semenenko, D.A.; Dagesian, S.A.; Yashina, L.V.; Kataev, E.Y.; Mankelevich, Y.A.; Suetin, N.V.; Krivchenko, V.A. Tailoring of the carbon nanowall microstructure by sharp variation of plasma radical composition. Phys. Chem. Chem. Phys. 2014, 16, 25621–25627. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Kondo, H.; Ishikawa, K.; Sekine, M.; Hiramatsu, M.; Hori, M. Density control of carbon nanowalls grown by CH4/H2 plasma and their electrical properties. Carbon 2014, 68, 380–388. [Google Scholar] [CrossRef]
- Zang, X.; Zhou, Q.; Chang, J.; Liu, Y.; Lin, L. Graphene and carbon nanotube (CNT) in MEMS/NEMS applications. Microelectron. Eng. 2015, 132, 192–206. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetič, M.; Panjan, P.; Hauptman, N.; Klanjsek-Gunde, M.; Balat-Pichelin, M. Etching of carbon–tungsten composite with oxygen plasma. Surf. Coat. Technol. 2010, 204, 1503–1508. [Google Scholar] [CrossRef]
- Pears, K.A.; Stolze, J. Carbon etching with a high density plasma etcher. Microelectron. Eng. 2005, 81, 7–14. [Google Scholar] [CrossRef]
- Choi, H.; Kwon, S.H.; Kang, H.; Kim, J.H.; Choi, W. Analysis of plasma-grown carbon oxide and reduced-carbon-oxide nanowalls. RSC Adv. 2020, 10, 9761–9767. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Z.; Qi, J.; Shi, J.; Hao, T.; Feng, J. Understanding the growth mechanism of vertically aligned graphene and control of its wettability. Carbon 2016, 103, 339–345. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, H.; Lee, S.; Kim, Y.; Choi, W.; Kang, H. Solubility of modified catalyst-free carbon nanowall with organic solvents. Appl. Surf. Sci. 2020, 529, 147161. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially Resolved Raman Spectroscopy of Single- and Few-Layer Graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Soin, N.; Roy, S.S.; Ray, S.C.; McLaughlin, J. Excitation energy dependence of Raman bands in multiwalled carbon nanotubes. J. Raman Spectrosc. 2010, 41, 1227–1233. [Google Scholar] [CrossRef]




| Parameter | SiO2 Target | Ag Target |
|---|---|---|
| Substrate temperature | Room temperature | Room temperature |
| Injection gas | Ar: 34 sccm O2: 6 sccm | Ar: 40 sccm |
| RF power | 150 W | 150 W |
| Deposition time | 60 min | 3 min |
| Working pressure | 1.5 × 10−2 Torr | 1.5 × 10−2 Torr |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Kwon, S.; Lee, S.; Kim, Y.; Kang, H.; Kim, J.H.; Choi, W. Innovative Method Using Adhesive Force for Surface Micromachining of Carbon Nanowall. Nanomaterials 2020, 10, 1978. https://doi.org/10.3390/nano10101978
Choi H, Kwon S, Lee S, Kim Y, Kang H, Kim JH, Choi W. Innovative Method Using Adhesive Force for Surface Micromachining of Carbon Nanowall. Nanomaterials. 2020; 10(10):1978. https://doi.org/10.3390/nano10101978
Chicago/Turabian StyleChoi, Hyeokjoo, Seokhun Kwon, Seokwon Lee, Yonghyeon Kim, Hyunil Kang, Jung Hyun Kim, and Wonseok Choi. 2020. "Innovative Method Using Adhesive Force for Surface Micromachining of Carbon Nanowall" Nanomaterials 10, no. 10: 1978. https://doi.org/10.3390/nano10101978
APA StyleChoi, H., Kwon, S., Lee, S., Kim, Y., Kang, H., Kim, J. H., & Choi, W. (2020). Innovative Method Using Adhesive Force for Surface Micromachining of Carbon Nanowall. Nanomaterials, 10(10), 1978. https://doi.org/10.3390/nano10101978






