Role of Phase-Dependent Dielectric Properties of Alumina Nanoparticles in Electromagnetic-Assisted Enhanced Oil Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles
2.2. Fluids
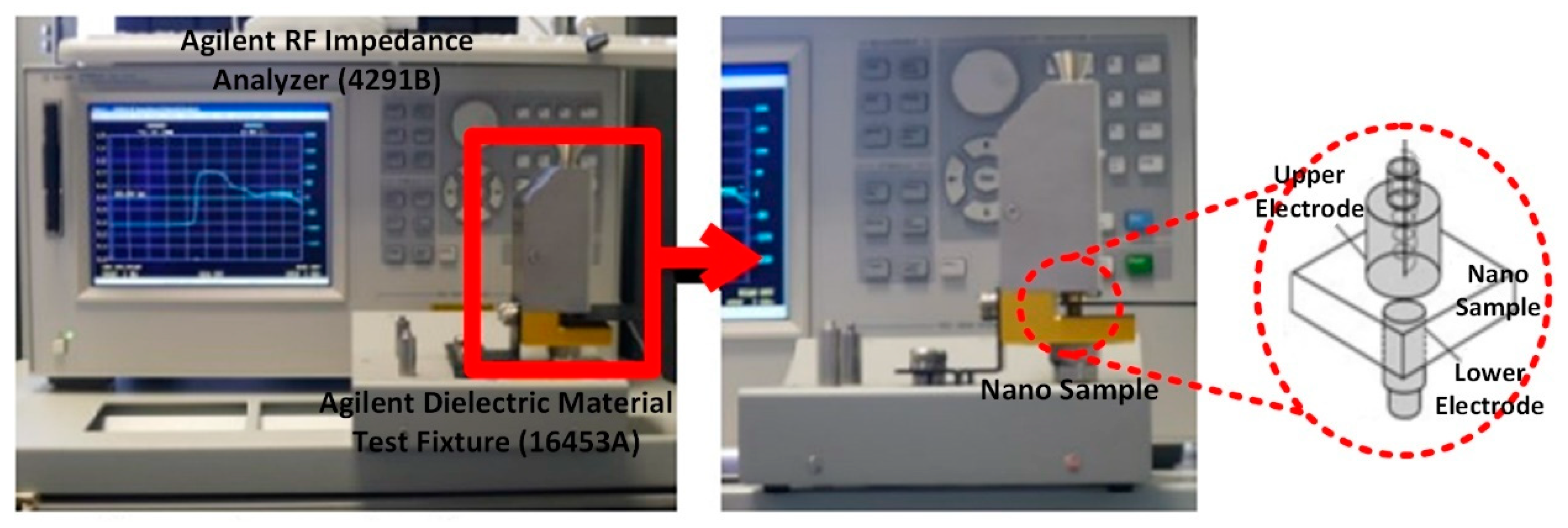
2.3. Dielectric Measurments
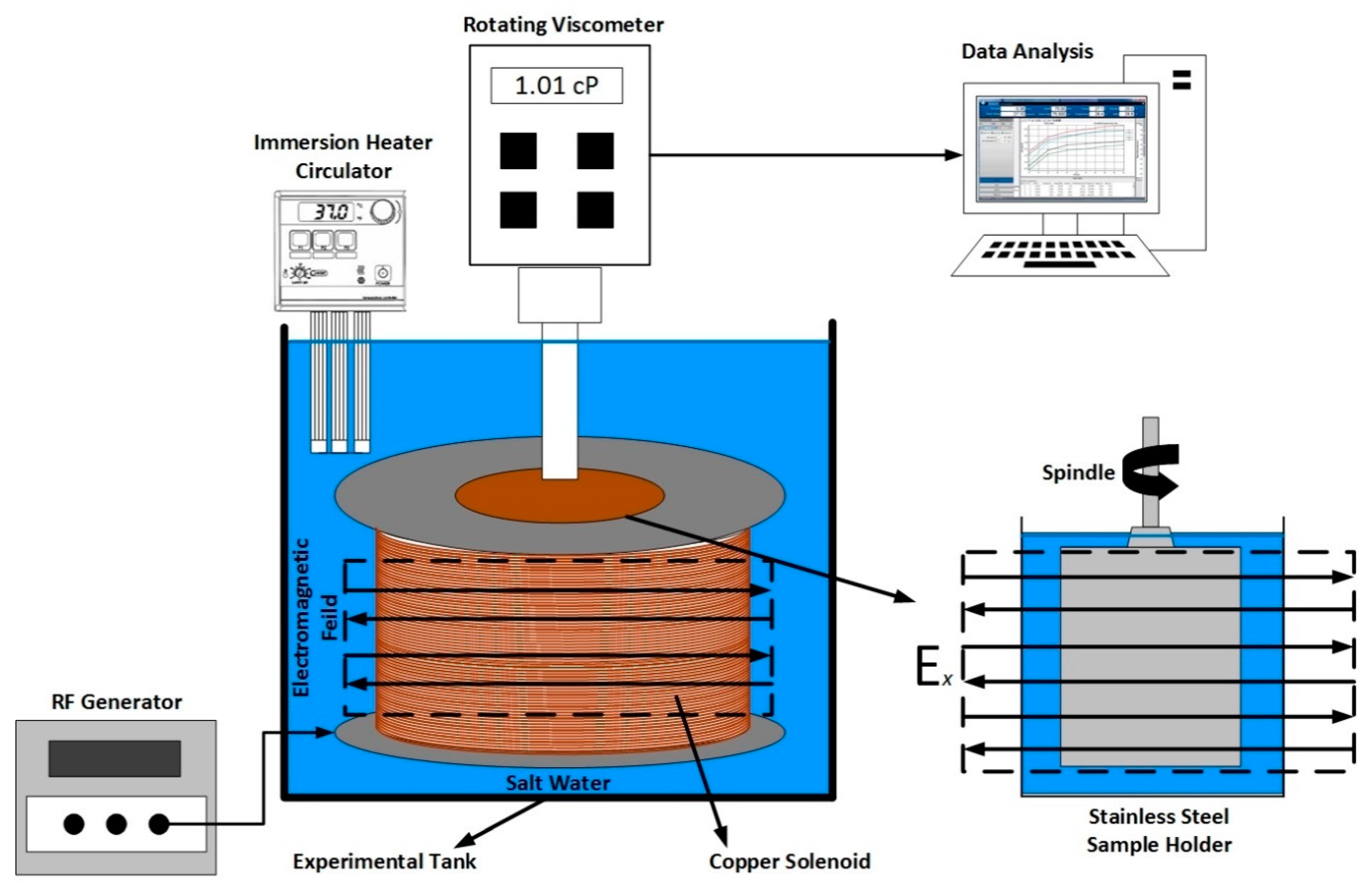
2.4. Viscosity Measurments
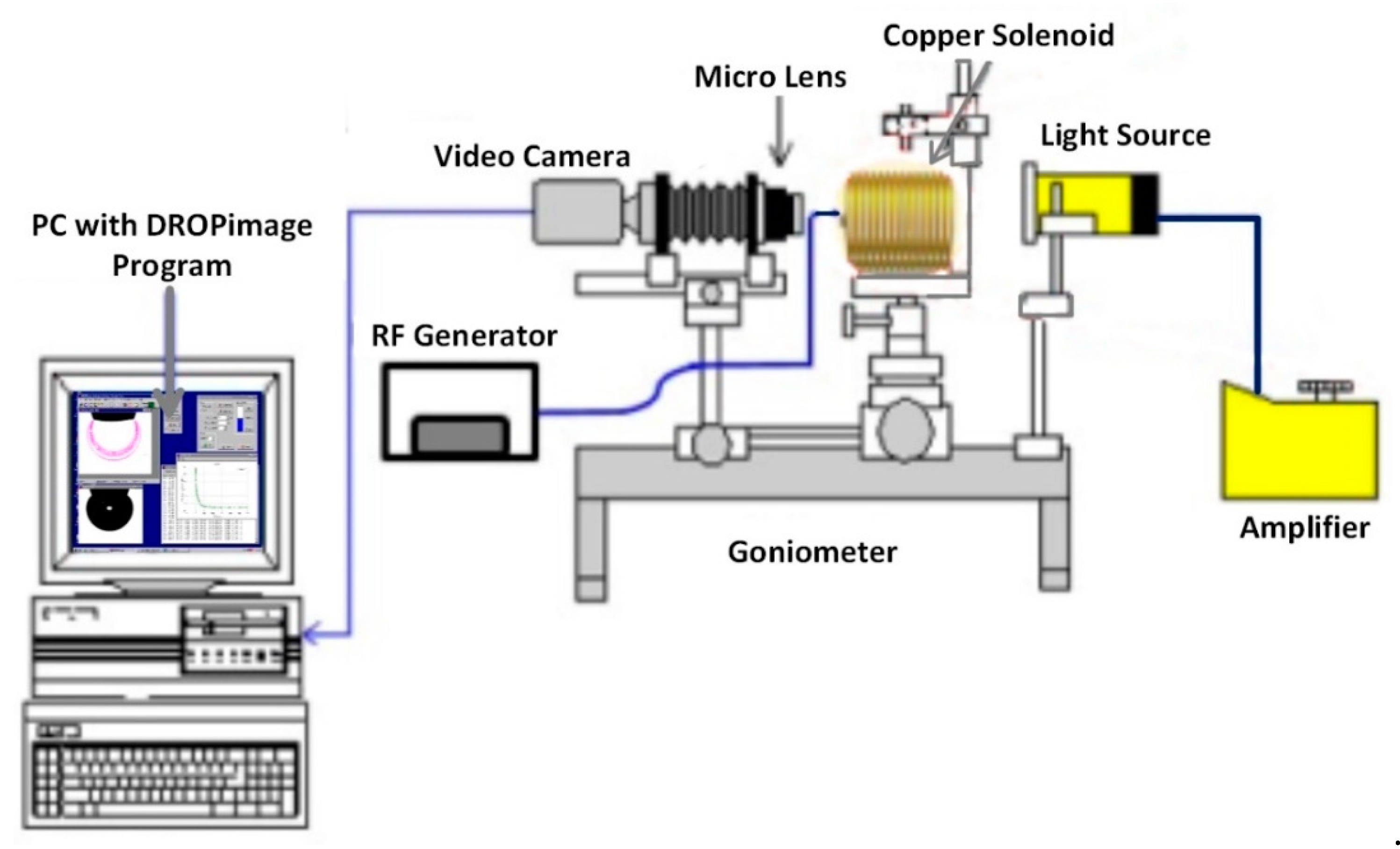
2.5. IFT and Wettability Measurments
2.6. Sandpack Flooding
3. Results and Discussion
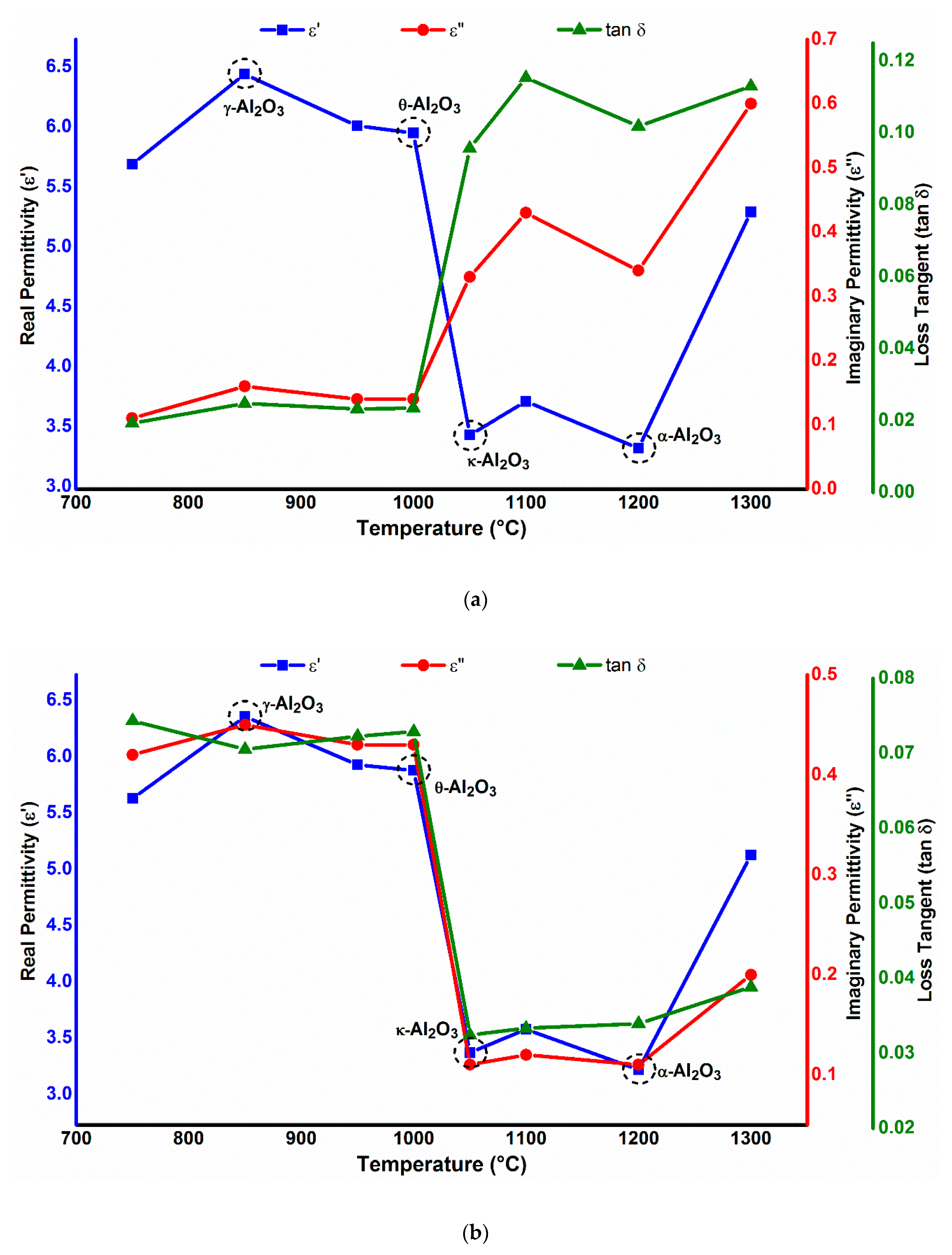
3.1. Phase-Dependent Dielectric Properties
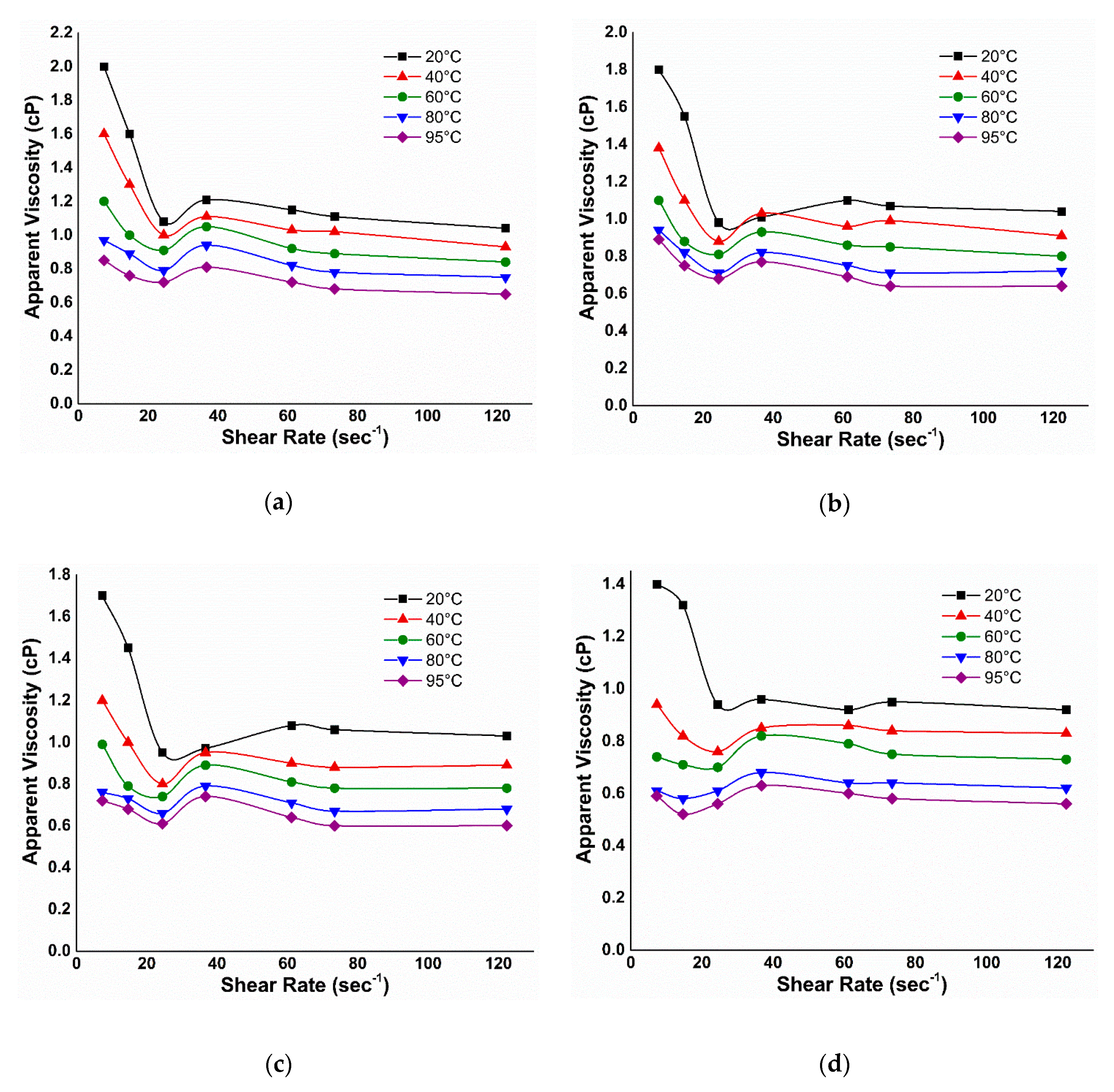
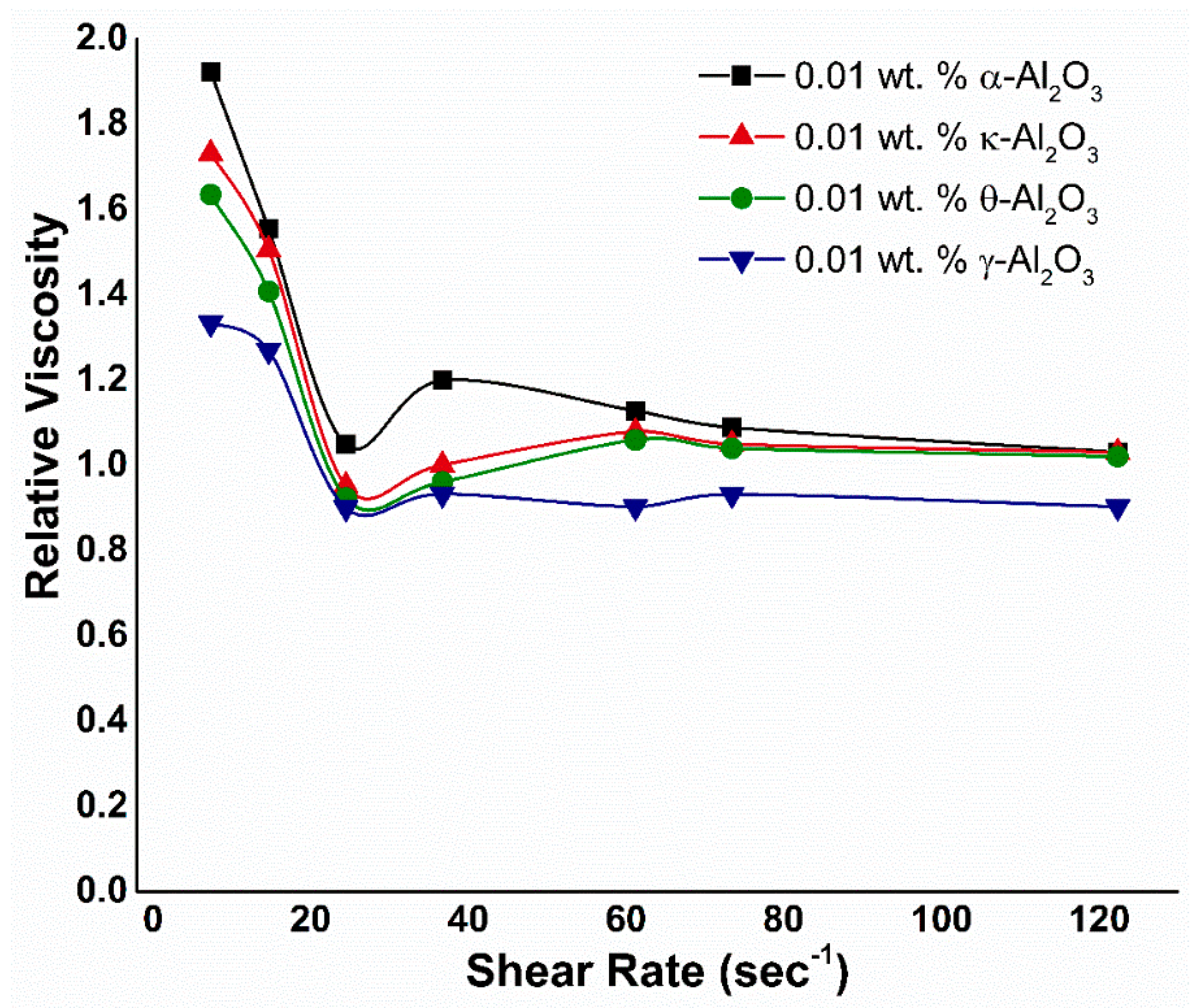
3.2. Viscosity
3.2.1. Effect of Temperature
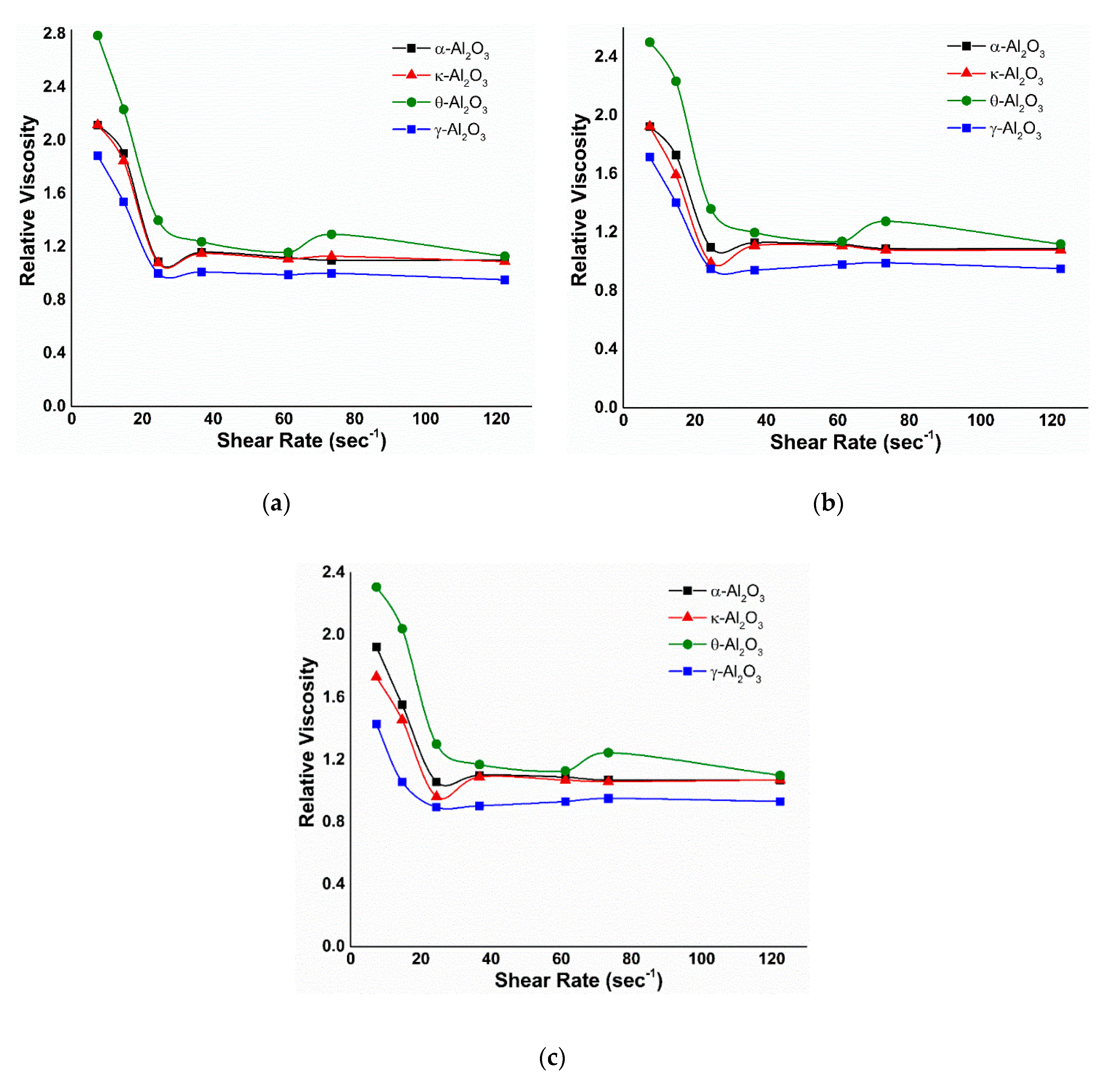
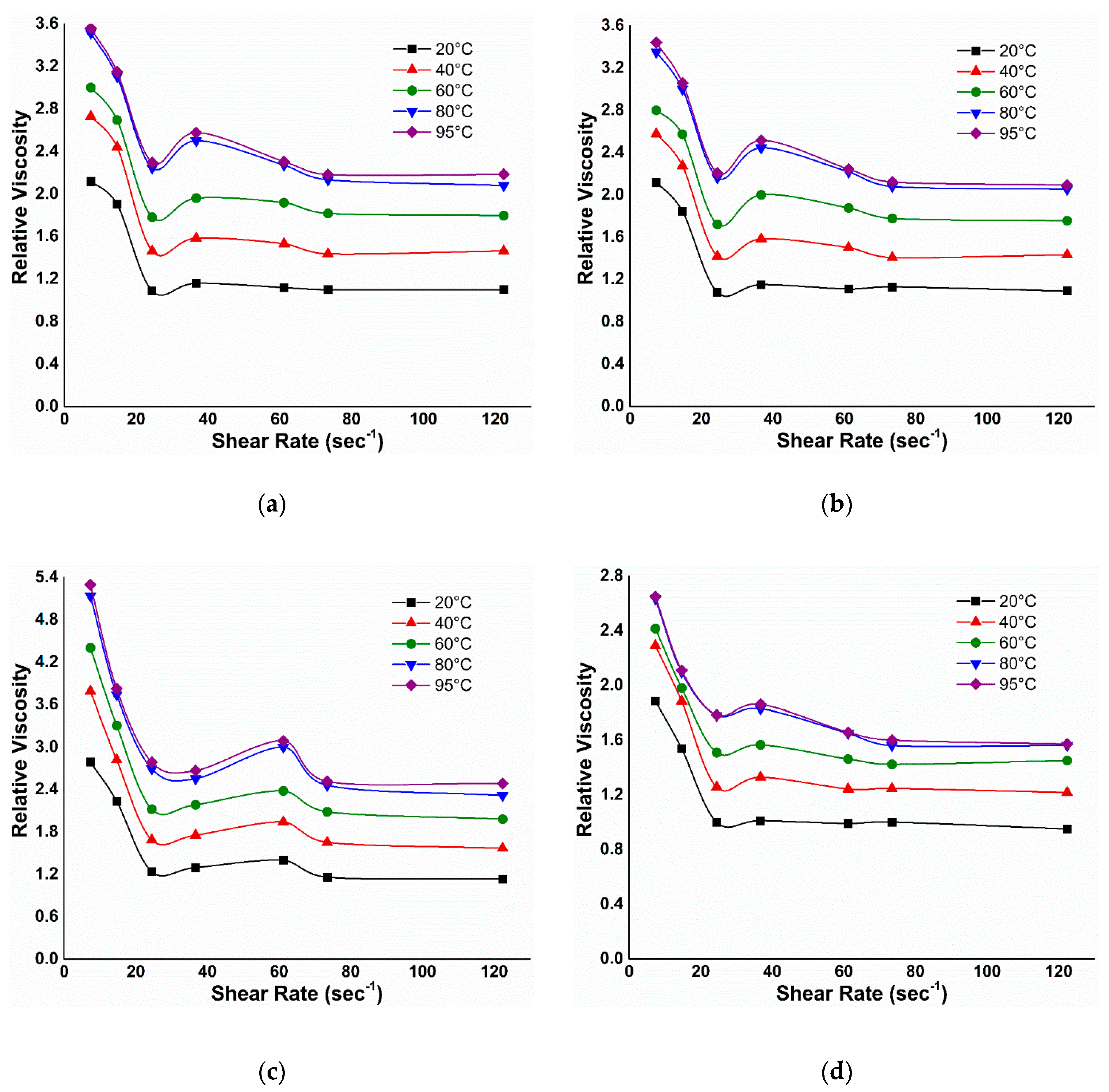
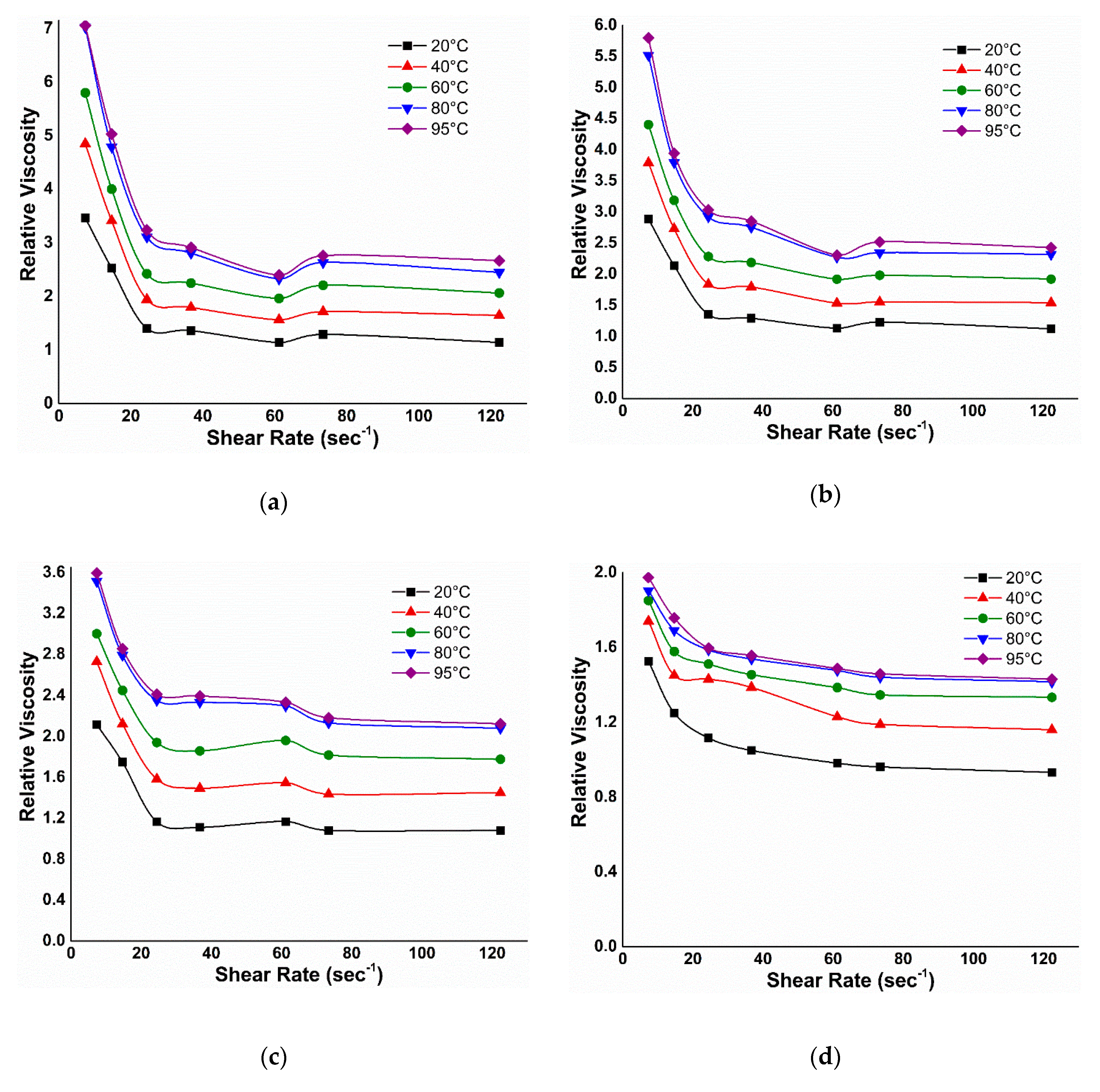
3.2.2. Viscosity under EM Field
3.2.3. Effect of Temperature
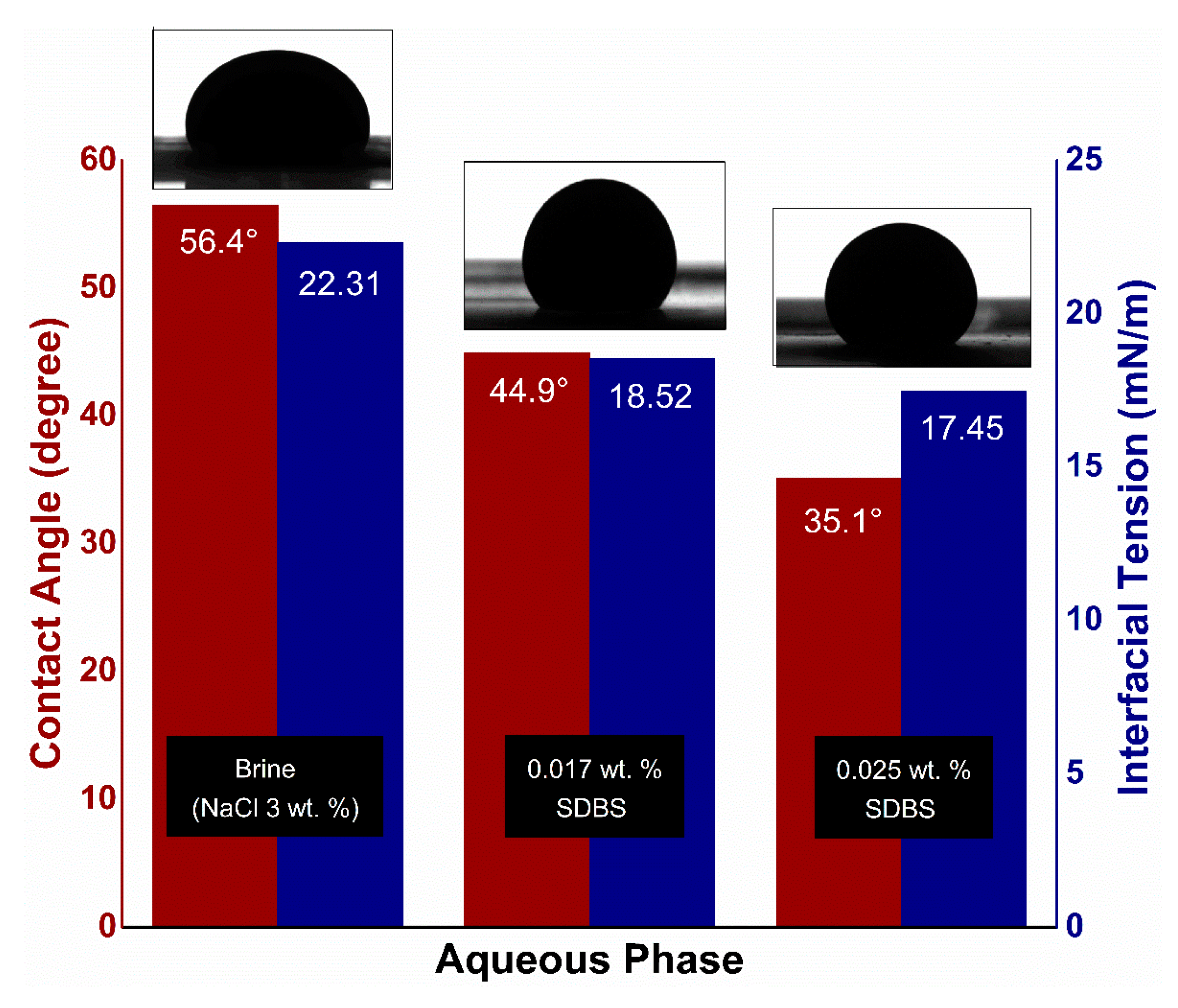
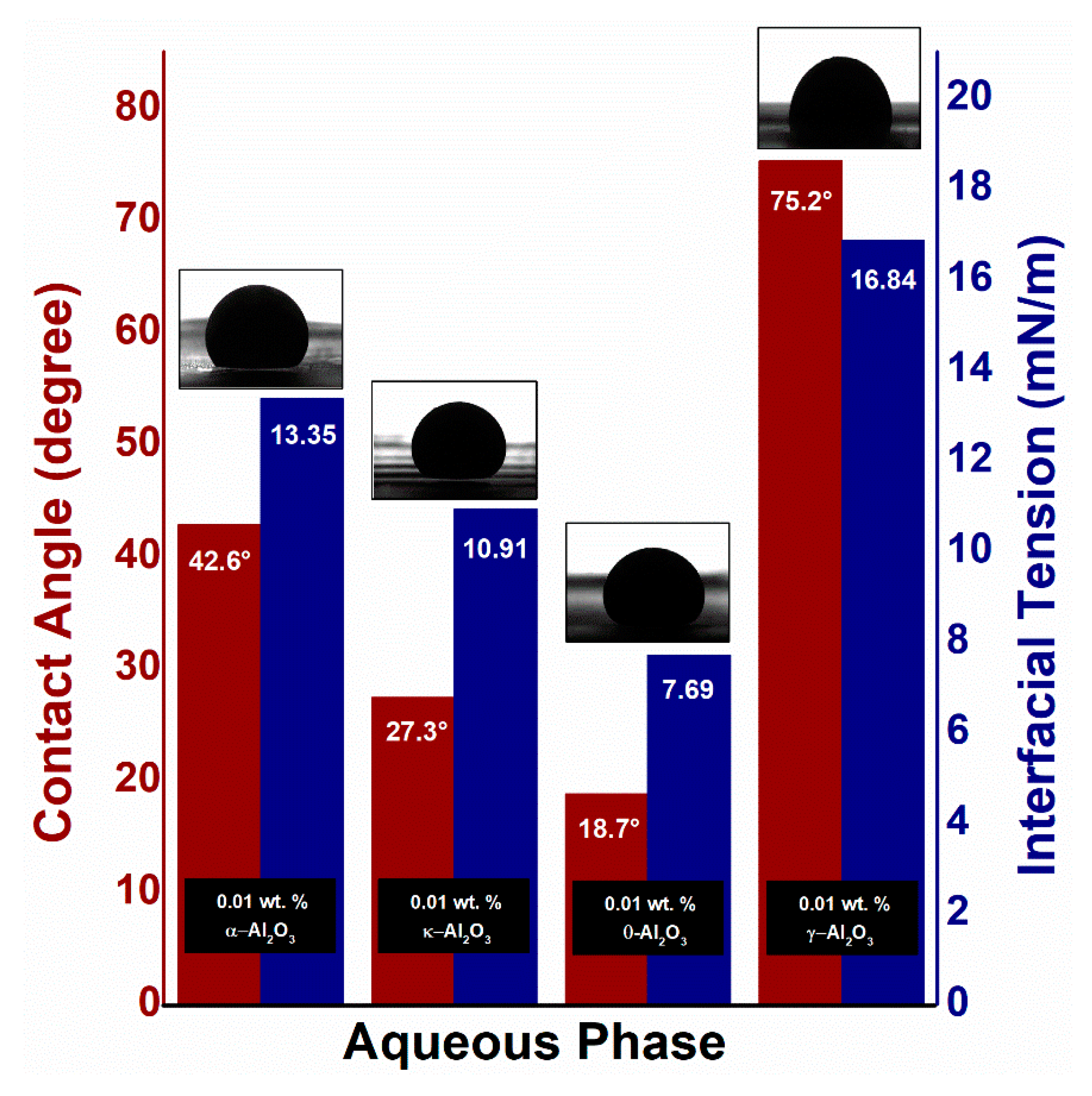
3.3. Interfacial Tension and Contact Angle
Impact of EM Field
3.4. Nanofluid Flooding
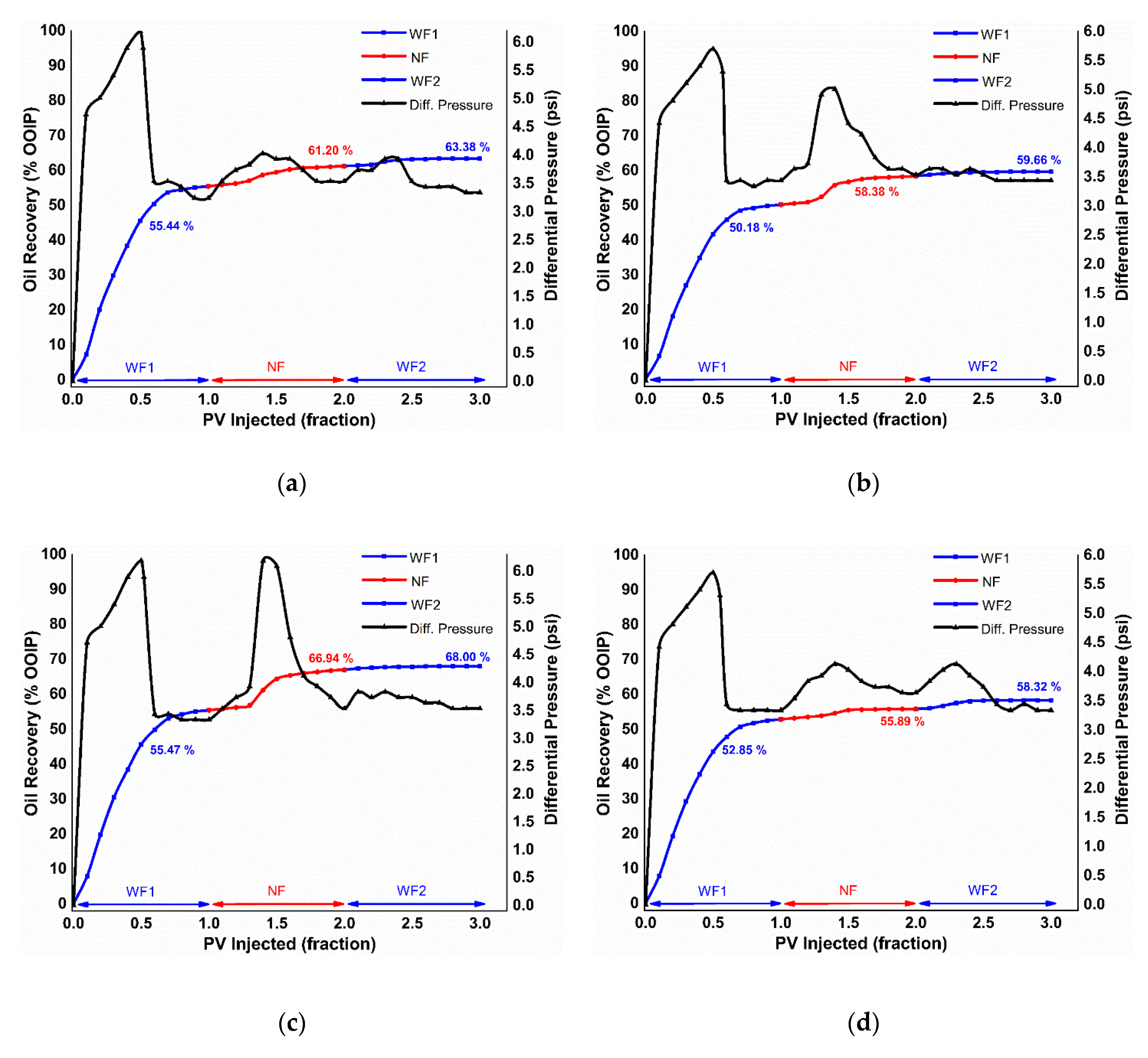
3.4.1. Conventional Nanofluid Flooding
Pressure Drop Profile
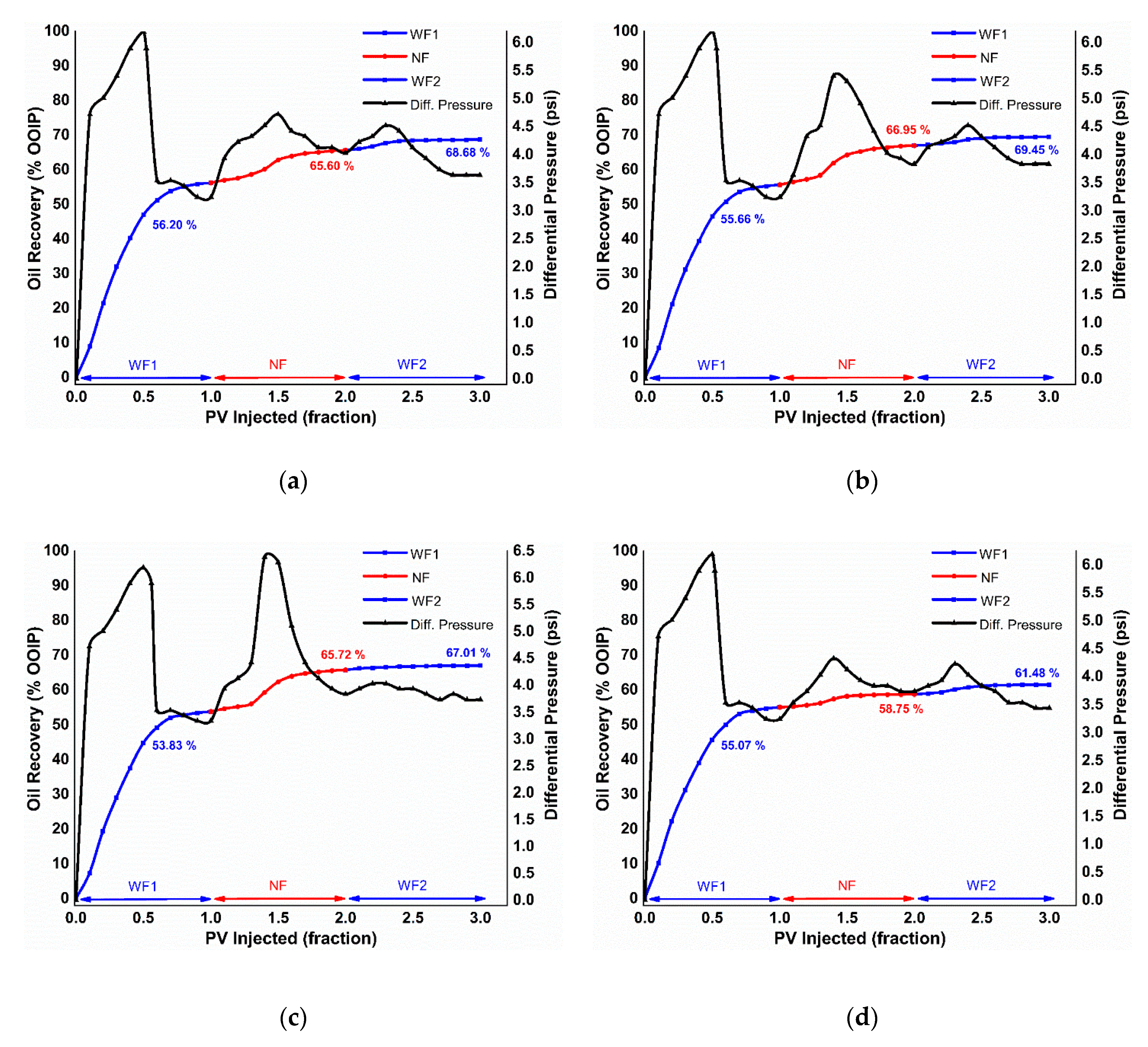
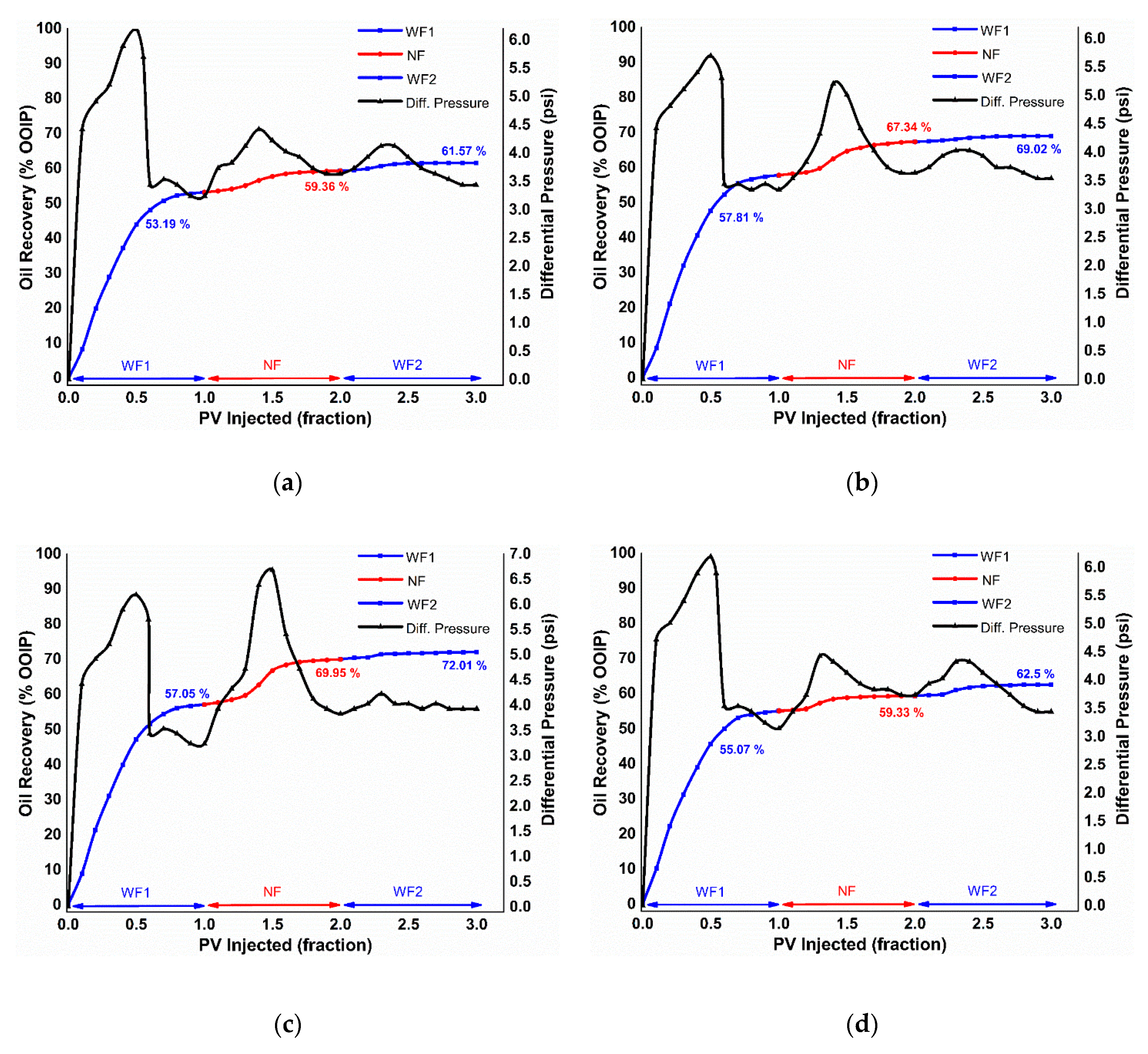
3.4.2. EM-Assisted Nanofluid Flooding
Pressure Drop Profile
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Ayatollahi, S.; Zerafat, M.M. Nanotechnology-assisted EOR techniques: New solutions to old challenges. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Skauge, T.; Spildo, K.; Skauge, A. Nano-sized particles for EOR. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Esfandyari Bayat, A.; Junin, R.; Samsuri, A.; Piroozian, A.; Hokmabadi, M. Impact of metal oxide nanoparticles on enhanced oil recovery from limestone media at several temperatures. Energy Fuels 2014, 28, 6255–6266. [Google Scholar] [CrossRef]
- Chen, H.; Ding, Y.; He, Y.; Tan, C. Rheological behaviour of ethylene glycol based titania nanofluids. Chem. Phys. Lett. 2007, 444, 333–337. [Google Scholar] [CrossRef]
- Bobbo, S.; Fedele, L.; Benetti, A.; Colla, L.; Fabrizio, M.; Pagura, C.; Barison, S. Viscosity of water based SWCNH and TiO2 nanofluids. Exp. Therm. Fluid Sci. 2012, 36, 65–71. [Google Scholar] [CrossRef]
- Roustaei, A.; Saffarzadeh, S.; Mohammadi, M. An evaluation of modified silica nanoparticles’ efficiency in enhancing oil recovery of light and intermediate oil reservoirs. Egypt. J. Pet. 2013, 22, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Hendraningrat, L.; Torsæter, O.; Ntnu, T. Improved oil recovery by hydrophilic silica nanoparticles suspension: 2-phase flow experimental studies. In Proceedings of the IPTC 2013: International Petroleum Technology Conference, Beijing, China, 26–28 March 2013; pp. 1–15. [Google Scholar]
- Suresh, S. Studies on the dielectric properties of CdS nanoparticles. Appl. Nanosci. 2014, 4, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Parvazdavani, M.; Masihi, M.; Ghazanfari, M.H. Monitoring the influence of dispersed nano-particles on oil–water relative permeability hysteresis. J. Pet. Sci. Eng. 2014, 124, 222–231. [Google Scholar] [CrossRef]
- Karimi, A.; Fakhroueian, Z.; Bahramian, A.; Pour Khiabani, N.; Darabad, J.B.; Azin, R.; Arya, S. Wettability alteration in carbonates using zirconium oxide nanofluids: EOR implications. Energy Fuels 2012, 26, 1028–1036. [Google Scholar] [CrossRef]
- Hwang, C.-C.; Wang, L.; Lu, W.; Ruan, G.; Kini, G.C.; Xiang, C.; Samuel, E.L.G.; Shi, W.; Kan, A.T.; Wong, M.S. Highly stable carbon nanoparticles designed for downhole hydrocarbon detection. Energy Environ. Sci. 2012, 5, 8304–8309. [Google Scholar] [CrossRef]
- Hwang, C.-C.; Ruan, G.; Wang, L.; Zheng, H.; Samuel, E.L.G.; Xiang, C.; Lu, W.; Kasper, W.; Huang, K.; Peng, Z. Carbon-based nanoreporters designed for subsurface hydrogen sulfide detection. ACS Appl. Mater. Interfaces 2014, 6, 7652–7658. [Google Scholar] [CrossRef]
- Fernández-García, M.; Rodriguez, J.A. Metal oxide nanoparticles. Encycl. Inorg. Bioinorg. Chem. 2011. [Google Scholar] [CrossRef] [Green Version]
- Keblinski, P.; Prasher, R.; Eapen, J. Thermal conductance of nanofluids: Is the controversy over? J. Nanoparticle Res. 2008, 10, 1089–1097. [Google Scholar] [CrossRef]
- Fedele, L.; Colla, L.; Bobbo, S.; Barison, S.; Agresti, F. Experimental stability analysis of different water-based nanofluids. Nanoscale Res. Lett. 2011, 6, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; France, D.M.; Routbort, J.L.; Choi, S.U.S. Review and comparison of nanofluid thermal conductivity and heat transfer enhancements. Heat Transf. Eng. 2008, 29, 432–460. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsæter, O. Metal oxide-based nanoparticles: Revealing their potential to enhance oil recovery in different wettability systems. Appl. Nanosci. 2015, 5, 181–199. [Google Scholar] [CrossRef] [Green Version]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Samin, A.M.; Risal, A.R. Experimental investigation of minimization in surfactant adsorption and improvement in surfactant-foam stability in presence of silicon dioxide and aluminum oxide nanoparticles. J. Pet. Sci. Eng. 2017, 159, 115–134. [Google Scholar] [CrossRef]
- Giraldo, J.; Benjumea, P.; Lopera, S.; Cortés, F.B.; Ruiz, M.A. Wettability alteration of sandstone cores by alumina-based nanofluids. Energy Fuels 2013, 27, 3659–3665. [Google Scholar] [CrossRef]
- Ogolo, N.; Olafuyi, O.; Onyekonwu, M. Enhanced oil recovery using nanoparticles. Saudi Arab. Sect. Tech. Symp. Exhib. 2012, 9. [Google Scholar] [CrossRef] [Green Version]
- Molnes, S.N.; Torrijos, I.P.; Strand, S.; Paso, K.G.; Syverud, K. Sandstone injectivity and salt stability of cellulose nanocrystals (CNC) dispersions—Premises for use of CNC in enhanced oil recovery. Ind. Crops Prod. 2016, 93, 152–160. [Google Scholar] [CrossRef]
- Wei, B.; Li, Q.; Jin, F.; Li, H.; Wang, C. The potential of a novel nanofluid in enhancing oil recovery. Energy Fuels 2016, 30, 2882–2891. [Google Scholar] [CrossRef]
- Zaid, H.M.; Yahya, N.; Latiff, N.R.A. The effect of nanoparticles crystallite size on the recovery efficiency in dielectric nanofluid flooding. J. Nano Res. 2013, 21, 103–108. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsæter, O.; Ntnu, T. Unlocking the potential of metal oxides nanoparticles to enhance the oil recovery. In Proceedings of the Offshore Technology Conference-Asia, Kuala Lumpur, Malaysia, 25 March 2014. [Google Scholar] [CrossRef]
- Seid Mohammadi, M.; Moghadasi, J.; Naseri, S. An experimental investigation of wettability alteration in carbonate reservoir using γ-Al2O3 nanoparticles. Iran. J. Oil Gas Sci. Technol. 2014, 3, 18–26. [Google Scholar]
- Wasan, D.T.; Nikolov, A.D. Spreading of nanofluids on solids. Nature 2003, 423, 156. [Google Scholar] [CrossRef] [PubMed]
- Kondiparty, K.; Nikolov, A.; Wu, S.; Wasan, D. Wetting and spreading of nanofluids on solid surfaces driven by the structural disjoining pressure: Statics analysis and experiments. Langmuir 2011, 27, 3324–3335. [Google Scholar] [CrossRef] [PubMed]
- Zaid, H.M.; Adil, M.; Chuan, L.K.; Latiff, N.R.A. Stability and electrorheology of ZnO nanofluids in the presence of anionic surfactants. AIP Conf. Proc. 2016, 1787. [Google Scholar] [CrossRef]
- Alnarabiji, M.S.; Yahya, N.; Nadeem, S.; Adil, M.; Baig, M.K.; Ghanem, O.B.; Azizi, K.; Ahmed, S.; Maulianda, B.; Klemeš, J.J. Nanofluid enhanced oil recovery using induced ZnO nanocrystals by electromagnetic energy: Viscosity increment. Fuel 2018, 233, 632–643. [Google Scholar] [CrossRef]
- Latiff, N.R.A.; Yahya, N.; Zaid, H.M.; Demiral, B. Novel enhanced oil recovery method using dielectric zinc oxide nanoparticles activated by electromagnetic waves. In Proceedings of the National Postgraduate Conference (NPC), Kuala Lumpur, Malaysia, 19–20 September 2011. [Google Scholar]
- Haroun, M.; Hassan, S.; Ansari, A.; Kindy, N.; Sayed, N.; Ali, B.; Sarma, H. Smart nano-EOR process for Abu Dhabi carbonate reservoirs. Abu Dhabi Int. Pet. Exhib. Conf. 2012, 1–13. [Google Scholar] [CrossRef]
- Zaid, H.M.; Adil, M.; Lee, K.C.; Latiff, N.R.A. Influence of frequency-dependent dielectric loss on electrorheology of surface modified ZnO nanofluids. In Proceedings of the International Conference on Nanomaterials and Biomaterials (ICNB 2017), Amsterdam, The Netherlands, 11–13 December 2017. [Google Scholar] [CrossRef]
- Adil, M.; Zaid, H.M.; Chuan, L.K.; Latiff, N.R.A. Effect of dispersion stability on electrorheology of water-based ZnO nanofluids. Energy Fuels 2016, 30, 6169–6177. [Google Scholar] [CrossRef]
- Adil, M.; Mohd Zaid, H.; Chuan, L.K.; Ahmad Latiff, N.R. Effect of EM propagation medium on electrorheological characteristics of dielectric nanofluids. J. Dispers. Sci. Technol. 2017, 38, 570–576. [Google Scholar] [CrossRef]
- Adil, M.; Lee, K.; Zaid, H.M.; Latiff, N.R.A.; Alnarabiji, M.S. Experimental study on electromagnetic-assisted ZnO nanofluid flooding for enhanced oil recovery (EOR). PLoS ONE 2018, 13, e0193518. [Google Scholar] [CrossRef]
- Adil, M.; Mohd Zaid, H.; Kean Chuan, L. Electromagnetically-induced change in interfacial tension and contact angle of oil droplet using dielectric nanofluids. Fuel 2020, 259, 116274. [Google Scholar] [CrossRef]
- Lee, K.; Adil, M.; Mohd Zaid, H.; Guan, B.H.; Soleimani, H.; Weis, M. Wettability, interfacial tension (IFT) and viscosity alteration of nanofluids under electromagnetic (EM) waves for enhanced oil recovery (IFT) applications. In Advanced Structured Materials; Springer: Cham, Switzerland, 2019; Volume 92, pp. 305–311. [Google Scholar]
- Zaid, H.M.; Adil, M.; Chuan, L.K. The effect of calcination temperature on dielectric properties of ZnO and Al2O3 nanoparticles at radio frequencies. Key Eng. Mater. 2016, 708, 9–13. [Google Scholar] [CrossRef]
- Adil, M.; Zaid, H.M.; Lee, K.C.; Latiff, N.R.A. Effect of annealing temperature on phase transition of nanoalumina synthesized by auto-combustion route. J. Nano Res. 2016, 41, 74–86. [Google Scholar] [CrossRef]
- Adil, M.; Zaid, H.M.; Chuan, L.K.; Latiff, N.R.A. Effect of CMC on the stability of ZnO nanofluid at high temperature and salinity. AIP Conf. Proc. 2016, 1787, 050018. [Google Scholar]
- Adil, M.; Zaid, H.M.; Chuan, L.K.; Latiff, N.R.A. Designing and Experimental Validation of an Electromagnetic Setup at MHz Frequency Under Different Propagation Media. In Theory and Applications of Applied Electromagnets; Springer International Publishing: Cham, Switzerland, 2016; ISBN 9783319172682. [Google Scholar]
- Behi, M.; Mirmohammadi, S.A. Investigation on Thermal Conductivity, Viscosity and Stability of Nanofluids. Master’s Thesis, Royal Institute of Technology (KTH), Stock, Sweden, 2012. [Google Scholar]
- Wu, Y. Experimental Study of Polymer Enhanced Alkaline Flooding for Western Canadian Heavy Oil Recovery. Master’s Thesis, University of Regina, Regina, Saskatchewan, 2009. [Google Scholar]
- Vila, R.; Gonzalez, M.; Molla, J.; Ibarra, A. Dielectric spectroscopy of alumina ceramics over a wide frequency range. J. Nucl. Mater. 1998, 253, 141–148. [Google Scholar] [CrossRef]
- Nick, T. Measurement of Fluid Rheology and Interpretation of Rheograms; Kaltec Scientific Inc.: Novi, MI, USA, 2000; pp. 1–38. [Google Scholar]
- Rashin, M.N.; Hemalatha, J. Synthesis and viscosity studies of novel ecofriendly ZnO–coconut oil nanofluid. Exp. Therm. Fluid Sci. 2013, 51, 312–318. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Desgranges, F.; Roy, G.; Galanis, N.; Maré, T.; Boucher, S.; Mintsa, H.A. Temperature and particle-size dependent viscosity data for water-based nanofluids–hysteresis phenomenon. Int. J. Heat Fluid Flow 2007, 28, 1492–1506. [Google Scholar] [CrossRef]
- Masoumi, N.; Sohrabi, N.; Behzadmehr, A. A new model for calculating the effective viscosity of nanofluids. J. Phys. D Appl. Phys. 2009, 42, 55501. [Google Scholar] [CrossRef] [Green Version]
- Jarahnejad, M.; Haghighi, E.B.; Saleemi, M.; Nikkam, N.; Khodabandeh, R.; Palm, B.; Toprak, M.S.; Muhammed, M. Experimental investigation on viscosity of water-based Al2O3 and TiO2 nanofluids. Rheol. Acta 2015, 54, 411–422. [Google Scholar] [CrossRef]
- López-López, M.T.; Gómez-Ramírez, A.; Rodríguez-Arco, L.; Durán, J.D.G.; Iskakova, L.; Zubarev, A. Colloids on the frontier of ferrofluids. Rheological properties. Langmuir 2012, 28, 6232–6245. [Google Scholar] [CrossRef]
- Ghasemi, A.; Abedi, A.; Ghasemi, F. Propagation Engineering in Wireless Communications; Springer: Cham, Switzerland, 2012; ISBN 1461410770. [Google Scholar]
- Klingenberg, D.J.; Van Swol, F.; Zukoski, C.F. The small shear rate response of electrorheological suspensions. I. Simulation in the point–dipole limit. J. Chem. Phys. 1991, 94, 6160–6169. [Google Scholar] [CrossRef]
- Von Hippel, A.R. Dielectrics and Waves; Wiley: New York, NY, USA, 1954. [Google Scholar]
- Morrow, N.R. Wettability and its effect on oil recovery. J. Pet. Technol. 1990, 42, 1–476. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants—Similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Vafaei, S.; Borca-Tasciuc, T.; Podowski, M.Z.; Purkayastha, A.; Ramanath, G.; Ajayan, P.M. Effect of nanoparticles on sessile droplet contact angle. Nanotechnology 2006, 17, 2523. [Google Scholar] [CrossRef] [PubMed]
- Sefiane, K.; Skilling, J.; MacGillivray, J. Contact line motion and dynamic wetting of nanofluid solutions. Adv. Colloid Interface Sci. 2008, 138, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Wasan, D.; Nikolov, A.; Kondiparty, K. The wetting and spreading of nanofluids on solids: Role of the structural disjoining pressure. Curr. Opin. Colloid Interface Sci. 2011, 16, 344–349. [Google Scholar] [CrossRef]
- Jones, T.B.; Wang, K.-L.; Yao, D.-J. Frequency-dependent electromechanics of aqueous liquids: Electrowetting and dielectrophoresis. Langmuir 2004, 20, 2813–2818. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B.; Fowler, J.D.; Chang, Y.S.; Kim, C.-J. Frequency-based relationship of electrowetting and dielectrophoretic liquid microactuation. Langmuir 2003, 19, 7646–7651. [Google Scholar] [CrossRef]
- Nwidee, L.N.; Barifcani, A.; Maxim, L.; Sarmadivaleh, M.; Iglauer, S. A realistic look at nanostructured material as an innovative approach for enhanced oil recovery process upgrading. Recent Insights Pet. Sci. Eng. InTechOpen 2018, 155–188. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Murphy, M.J.; Yu, H.; Bagaria, H.G.; Yoon, K.Y.; Nielson, B.M.; Bielawski, C.W.; Johnston, K.P.; Huh, C.; Bryant, S.L. Investigation of nanoparticle adsorption during transport in porous media. SPE J. 2015, 20, 667–677. [Google Scholar] [CrossRef]
- Ogolo, N.A.; Onyekonwu, M.O.; Akaranta, O. Trapping mechanism of nanofluids on migrating fines in sand. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 5–7 August 2013. [Google Scholar]
- Hendraningrat, L.; Engeset, B.; Suwarno, S.; Li, S.; Torsæter, O. Laboratory investigation of porosity and permeability impairment in Berea sandstones due to hydrophilic nanoparticle retention. In Proceedings of the International Symposium of the Society of Core Analysts, Napa Valley, CA, USA, 16–19 September 2013; pp. 16–19. [Google Scholar]
- Taber, J.J. Technical screening guides for the enhanced recovery of oil. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Francisco, CA, USA, 5–8 October 1983. [Google Scholar]




| Calcination Temperature (°C) | Crystallite Size (nm) ± 2 | Lattice Parameter (nm) ± 0.01 | Crystal Structure | Crystallinity (%) | Porosity (%) | Surface Area (m2.gm−1) | Denotation |
|---|---|---|---|---|---|---|---|
| 850 | 94.3 | a = 3.93 b = 3.93 c = 3.93 | Cubic | 72.2 | 32.9 | 34.5 | γ-Al2O3 |
| 1000 | 64.9 | a = 3.93 b = 3.93 c = 3.93 | Monoclinic | 96.2 | 41.0 | 43.3 | θ-Al2O3 |
| 1050 | 55.1 | a = 3.93 b = 3.93 c = 3.93 | Orthorhombic | 96.4 | 48.3 | 52.4 | κ-Al2O3 |
| 1200 | 25.0 | a = 3.93 b = 3.93 c = 3.93 | Hexagonal | 98.8 | 50.3 | 121 | α-Al2O3 |
| Nanofluid | pH | SDBS Concentration (wt. %) | Ultrasonication Period (min) |
|---|---|---|---|
| γ-Al2O3 | 8 | 0.025 | 90 |
| θ-Al2O3 | 10 | 0.017 | 90 |
| κ-Al2O3 | 10 | 0.017 | 90 |
| α-Al2O3 | 8 | 0.017 | 90 |
| Fluid | Concentration (wt. %) | Properties | ||
|---|---|---|---|---|
| Density (g.cm−3) | Viscosity at 100 rpm (cP) | pH | ||
| Crude oil | – | 0.8021 | 7.50 | – |
| Brine (NaCl) | 3 | 1.0197 | 1.01 | 6.76 |
| SDBS | 0.017 | 1.0190 | 1.01 | 5.25 |
| 0.025 | 1.0194 | 1.02 | 5.16 | |
| γ-Al2O3 | 0.01 | 1.0170 | 0.92 | 5.03 |
| θ-Al2O3 | 0.01 | 1.0115 | 1.03 | 4.62 |
| κ-Al2O3 | 0.01 | 1.0191 | 1.04 | 4.62 |
| α-Al2O3 | 0.01 | 1.0196 | 1.04 | 4.59 |
| Nanoparticles | Specific Surface Area (m2·g−1) | Particle Number per Gram | Particle Number per 0.01 wt. % |
|---|---|---|---|
| α-Al2O3 | 121.2 | 61756296 | 617562 |
| κ-Al2O3 | 52.40 | 5494017 | 54940 |
| θ-Al2O3 | 43.39 | 3279527 | 32795 |
| γ-Al2O3 | 34.56 | 1237326 | 12373 |
| Initial Water Saturation (Swi) (% Pore Volume (PV)) | Oil Recovery (% OOIP) | Displacement Efficiency (ED) (%) | Total Oil Recovery (% OOIP) | Flooding Case | ||
|---|---|---|---|---|---|---|
| WF1 | NF | WF2 | ||||
| 29.75 | 55.44 | 5.76 | 2.17 | 12.94 | 63.38 | α-Al2O3 |
| 28.62 | 50.18 | 8.19 | 1.27 | 16.45 | 59.66 | κ-Al2O3 |
| 30.55 | 55.47 | 11.46 | 1.06 | 25.75 | 68.00 | θ-Al2O3 |
| 26.79 | 52.85 | 3.03 | 2.42 | 6.43 | 58.32 | γ-Al2O3 |
| Flooding Case | Residual Retention Factor | |
|---|---|---|
| NF | WF2 | |
| α-Al2O3 | 1.090 | 0.944 |
| κ-Al2O3 | 1.028 | 0.972 |
| θ-Al2O3 | 1.058 | 1.00 |
| γ-Al2O3 | 1.088 | 0.918 |
| Applied EM Frequency (MHz) | Initial Water Saturation (Swi) (% PV) | Oil Recovery (% OOIP) | Displacement Efficiency (ED) (%) | Total Oil Recovery (% OOIP) | Flooding Case | ||
|---|---|---|---|---|---|---|---|
| WF1 | NF | WF2 | |||||
| 18.8 | 28.62 | 56.20 | 9.39 | 3.08 | 21.45 | 68.68 | α-Al2O3 |
| 29.44 | 55.66 | 11.28 | 2.50 | 25.46 | 69.45 | κ-Al2O3 | |
| 29.75 | 53.83 | 11.89 | 1.29 | 25.76 | 67.01 | θ-Al2O3 | |
| 29.44 | 55.07 | 3.67 | 2.73 | 8.17 | 61.48 | γ-Al2O3 | |
| 167 | 28.62 | 53.19 | 6.16 | 2.21 | 13.17 | 61.57 | α-Al2O3 |
| 29.44 | 57.81 | 9.53 | 1.67 | 22.59 | 69.02 | κ-Al2O3 | |
| 29.75 | 57.05 | 12.90 | 2.05 | 30.04 | 72.01 | θ-Al2O3 | |
| 29.15 | 57.57 | 4.12 | 3.06 | 9.73 | 64.77 | γ-Al2O3 | |
| Applied EM Frequency (MHz) | Residual Retention Factor | Flooding Case | |
|---|---|---|---|
| NF | WF2 | ||
| 18.8 | 1.090 | 0.944 | α-Al2O3 |
| 1.028 | 0.972 | κ-Al2O3 | |
| 1.058 | 1.00 | θ-Al2O3 | |
| 1.088 | 0.918 | γ-Al2O3 | |
| 167 | 1.212 | 0.945 | α-Al2O3 |
| 1.088 | 0.972 | κ-Al2O3 | |
| 1.181 | 1.025 | θ-Al2O3 | |
| 1.187 | 0.921 | γ-Al2O3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adil, M.; Lee, K.C.; Zaid, H.M.; Manaka, T. Role of Phase-Dependent Dielectric Properties of Alumina Nanoparticles in Electromagnetic-Assisted Enhanced Oil Recovery. Nanomaterials 2020, 10, 1975. https://doi.org/10.3390/nano10101975
Adil M, Lee KC, Zaid HM, Manaka T. Role of Phase-Dependent Dielectric Properties of Alumina Nanoparticles in Electromagnetic-Assisted Enhanced Oil Recovery. Nanomaterials. 2020; 10(10):1975. https://doi.org/10.3390/nano10101975
Chicago/Turabian StyleAdil, Muhammad, Kean Chuan Lee, Hasnah Mohd Zaid, and Takaaki Manaka. 2020. "Role of Phase-Dependent Dielectric Properties of Alumina Nanoparticles in Electromagnetic-Assisted Enhanced Oil Recovery" Nanomaterials 10, no. 10: 1975. https://doi.org/10.3390/nano10101975





