A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications
Abstract
:1. Introduction
2. Biodegradable and Non-Biodegradable Polymer Nanomaterials (PNM): General Properties
2.1. Biodegradable Polymer Properties
- (1)
- In situ administration of formulations
- (2)
- On-demand delivery of the molecular targeted agent
- (3)
- The fact that a targeted agent can be combined with radiotherapy and immunotherapy
- (4)
- The use of FDA approved biodegradable polymers [35]
2.2. Non-Biodegradable Polymer Properties
3. Chitosan-Based Nanoparticles
4. Alginate-Based Nanoparticles
5. Albumin-Based Nanoparticles
6. Hydroxyapatite-Based Nanoparticles
7. Hyaluronic Acid-Based Nanoparticles
8. Conclusions
Funding
Conflicts of Interest
References
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano Struct. Nano Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Domg, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. Reactive and Functional Polymers. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Tiyaboonchai, W. Chitosan Nanoparticles: A Promising System for Drug Delivery. Naresuan Univ. J. 2003, 11, 51–66. [Google Scholar]
- Jeong, B.; Choi, Y.K.; Bae, Y.H.; Zentner, G.; Kim, S.W. New biodegradable polymers for injectable drug delivery systems. J. Control. Release 1999, 62, 109–114. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Hassan, S.; Zhang, X. Droplet-Based Microgels: Attractive Materials for Drug Delivery Systems. Res. Dev. Mater. Sci. 2019, 11, 1183–1185. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Pugazhendhi, A.; Gopalakrishnan, K.; Sivagurunathan, P.; Saratale, G.D.; Dung, T.N.B.; Kannapiran, E. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol. Rep. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Chen, M.; Yin, M. Design and development of fluorescent nanostruc-tures for bioimaging. Prog. Polym. Sci. 2014, 39, 365–395. [Google Scholar] [CrossRef]
- Baba, M.; Matsumoto, Y.; Kashio, A.; Cabral, H.; Nishiyama, N.; Kataoka, K.; Yamasoba, T. Micellization of cisplatin (NC-6004) reduces its oto-toxicity in guinea pigs. J. Control. Release 2012, 157, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Cramer, N.B.; Standsburry, J.W.; Bowman, C.N. Recent advances and developments in composite dental restorative materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Vinugopal, J.R.; Ravichandran, R.; Ramalingam, M.; Raghunath, M.; Ramakrishna, S. Nanofiber technology for controllingstem cell functions and tissue engineering. Micro Nanotechnol. Eng. Stem Cells Tissues 2013, 2, 27–51. [Google Scholar]
- Cheng, Z.; Zaki, A.A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional nanoparticles: Cost versus benefit of adding targeting andimaging capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenera-tive medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Pugazhendhi, A.; Prabakar, D.; Jacob, J.M.; Karuppusamy, I.; Saratale, R.G. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb. Pathog. 2018, 114, 41–45. [Google Scholar] [CrossRef]
- Nanotechnology for Health: Vision Paper and Basis for a Strategic Agenda for Nanomedicine. Available online: https://op.europa.eu/en/publication-detail/-/publication/60816548-e372-4216-a253c4442b527a21/language-en (accessed on 30 September 2020).
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Nejati-Koshki, K.; Mesgari, M.; Ebrahimi, E.; Abbasalizadeh, F.; Aval, S.F.; Khandaghi, A.A.; Abasi, M.; Akbarzadeh, A. Synthesis and in vitro study of cisplatin-loaded Fe3O4 nanoparticles modified with PLGA-PEG6000 copolymers in treatment of lung cancer. J. Microencapsul. 2014, 31, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Larguinno, M.; Cordeiro, A.; Raposo, L.R.; Costa, P.M.; Santos, S.; Diniz, M.S.; Fernandes, A.R.; Baptista, P.V. Gold-nanobeacons for gene therapy: Evaluation of genotoxicity, cell toxicity, and proteome profiling analysis. Nanotoxicology 2013, 8, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2012, 56, 1649–1659. [Google Scholar] [CrossRef]
- Feng, S.S. Nanoparticles of biodegradable polymers for new-concept chemotherapy. Expert Rev. Med. Devices 2014, 1, 115–125. [Google Scholar] [CrossRef]
- Yeo, W.W.Y.; Hosseinkhani, H.; Abdul Rahman, S.; Rosli, R.; Domb, A.J.; Abdullah, S. Safety Profile of Dextran-Spermine Gene Delivery Vector in Mouse Lungs. J. Nanosci. Nanotechnol. 2014, 14, 3328–3336. [Google Scholar] [CrossRef]
- Alibolandi, M.; Abnous, K.; Ramezani, M.; Hosseinkhani, H.; Hadizadeh, F. Synthesis of AS1411-aptamer-conjugated CdTe quantum dots with high fluorescence strength for probe labeling tumor cells. J. Fluoresc. 2014, 24, 1519–1529. [Google Scholar] [CrossRef]
- Dragan, E.S.; Bucataria, F. Design and characterization of anionic hydrogels confined in Daisogel silica composites microspheres and their application in sustained release of proteins. Colloid. Surf. A 2016, 489, 46–56. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Ou, K.L.; Mao, C.; Hosseinkhani, H. Importance of dual delivery systems for bone tissue engineering. J. Control. Release 2016, 225, 152–169. [Google Scholar] [CrossRef]
- Qi, X.; Wei, W.; Li, J.; Liu, Y.; Hu, X.; Zhang, J.; Bi, L.; Dong, W. Fabrication and Characterization of a Novel Anticancer Drug Delivery System: Salecan/Poly(methacrylic acid) Semi-interpenetrating Polymer Network Hydrogel. ACS Biomater. Sci. Eng. 2015, 1, 1287–1299. [Google Scholar] [CrossRef]
- Dinu, M.V.; Dragan, E.S. Evaluation of Cu2+, Co2+ and Ni2+ ions removal from aqueous solution using a novel chitosan/clinoptilolite composite: Kinetics and isotherms. Chem. Eng. J. 2010, 160, 157–163. [Google Scholar] [CrossRef]
- Qi, X.; Wei, W.; Li, J.; Zuo, G.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Salecan-Based pH-Sensitive Hydrogels for Insulin Delivery. Mol. Pharmaceut. 2017, 14, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Pande, V. Studies on the characteristics of zaltoprofen loaded gelatin nanoparticles by nanoprecipitation. Inventi Rapid NDDS 2015, 3, 1–7. [Google Scholar]
- Prajapati, S.K.; Jain, A.; Jain, A. Biodegradable polymers and constructs: A novel approach in drug delivery. Eur. Polym. J. 2019, 120, 109191. [Google Scholar] [CrossRef]
- Ossipov, D.A. Nanostructured hyaluronic acid-based materials for active delivery to cancer. Expert Opin. Drug Deliv. 2010, 7, 681–703. [Google Scholar] [CrossRef] [PubMed]
- Kluin, O.S.; van der Mei, H.C.; Busscher, H.J.; Neut, D. Biodegradable vs. non-biodegradable antibiotic delivery devices in the treatment of osteomyelitis. Expert Opin. Drug Deliv. 2013, 10, 341–351. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Yousefi, B.; Asemi, Z.; Nikfar, B.; Mansournia, M.A.; Hallajzadeh, J. Chitosan: A compound for drug delivery system in gastric cancer—A review. Carbohydr. Polym. 2020, 242, 116403. [Google Scholar] [CrossRef]
- LeHoux, J.G.; Grondin, F. Some effects of chitosan on liver function in the rat. Endocrinology 1993, 132, 1078–1084. [Google Scholar] [CrossRef]
- Peniston, Q.P.; Johnson, E.L. Process for the Manufacturer of Chitosan. U.S. Patent US4195175A, 25 March 1980. [Google Scholar]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Huang, G.; Liu, Y.; Chen, L. Chitosan and its derivatives as vehicles for drug delivery. Drug Deliv. 2017, 24, 108–113. [Google Scholar] [CrossRef]
- Prabaharan, M.; Mano, J.F. Chitosan-Based Particles as Controlled Drug Delivery Systems. Drug Deliv. 2005, 12, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Maitra, A.; Kumar, P.G.; De, T.; Sahoo, S.K. Process for the Preparation of Highly Monodispersed Hydrophilicpolymeric Nanoparticles of Size Less than 100 nm. U.S. Patent US5874111A, 23 February 1999. [Google Scholar]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Chitosan and chitosan/ethylene oxide propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm. Res. 1997, 14, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.A.; Fresneau, M.P.; Marazuela, A.; Fabra, A.; Alonso, M.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control. Release 2001, 73, 255–267. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.J.; Zhao, H.Y.; Zheng, J.M.; Xu, H.; Wei, G.; Hao, J.S.; Cui, F.D. Bioadhesive polysaccharide in protein delivery system: Chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int. J. Pharm. 2002, 249, 139–147. [Google Scholar] [CrossRef]
- Giacone, D.V.; Dartora, V.F.; de Matos, J.K.; Passos, J.S.; Miranda, D.A.; de Oliveira, E.A.; Silveira, E.R.; Costa-Lotufo, L.V.; Maria-Engler, S.S.; Lopes, L.B. Effect of nanoemulsion modification with chitosan and sodium alginate on the topical delivery and efficacy of the cytotoxic agent piplartine in 2D and 3D skin cancer models. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- El-Shabouri, M.H. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. J. Pharm. 2002, 249, 101–108. [Google Scholar] [CrossRef]
- Bodmeier, R.; Chen, H.G.; Paeratakul, O. A novel approach to the oral delivery of micro- or nanoparticles. Pharm. Res. 1989, 6, 413–417. [Google Scholar] [CrossRef]
- Afshar, M.; Dai, Y.-N.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. Preparation and characterization of sodium alginate/polyvinyl alcohol hydrogel containing drug-loaded chitosan nanoparticles as a drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 56, 101530. [Google Scholar] [CrossRef]
- Gomathi, T.; Sudha, P.N.; Florence, J.A.K.; Venkatesan, J.; Anil, S. Fabrication of letrozole formulation using chitosan nanoparticles through ionic gelation method. Int. J. Biol. Macromol. 2017, 104, 1820–1832. [Google Scholar] [CrossRef]
- Wang, J.; Tan, J.; Luo, J.; Huang, P.; Zhou, W.; Chen, L.; Long, L.; Zhang, L.M.; Zhu, B.; Yang, L.; et al. Enhancement of scutellarin oral delivery efficacy by vitamin B12-modified amphiphilic chitosan derivatives to treat type II diabetes induced-retinopathy. J. Nanobiotechnol. 2017, 15, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lv, T.; Zhang, H.; Xie, X.; Li, Z.; Chen, H. Folate and heptamethine cyanine modified chitosan-based nanotheranostics for tumor targeted near-infrared fluorescence imaging and photodynamic therapy. Biomacromolecules 2017, 18, 2146–2160. [Google Scholar] [CrossRef] [PubMed]
- Kamel, K.; Khalil, I.A.; Rateb, M.E.; Elgendy, H.; Elhawary, S. Chitosan-coated cinnamon/oregano-loaded solid lipid nanoparticles to augment 5-fluorouracil cytotoxicity for colorectal cancer: Extracts standardization, nanoparticles optimization and cytotoxicity evaluation. J. Agric. Food Chem. 2017, 65, 7966–7981. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T.; Liu, Z.K.; Zhu, Q.L.; Rong, X.H.; Liang, C.L.; Wang, J. Redox-responsive nano-carriers for drug and gene co-delivery based on chitosan derivatives modified mesoporous silica nanoparticles. Colloids Surf. B Biointerfaces 2017, 155, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Bayati, K.; Ho, E.A. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv. Transl. Res. 2017, 7, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kwon, S.; Lee, M.; Chung, H.; Kim, J.H.; Kim, Y.S.; Park, R.W.; Kim, I.S.; Seo, S.B.; Kwon, I.C.; et al. Self-assembled nanoparticles based on glycol chitosan bearing hydrophobic moieties as carriers for doxorubicin: In vivo biodistribution and anti-tumor activity. Biomaterials 2006, 27, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Belbekhouche, S.; Mansour, O.; Carbonnier, B. Promising sub-100 nm tailor made hollow chitosan/poly(acrylic acid) nanocapsules for antibiotic therapy. J. Colloid Interface Sci. 2018, 522, 183–190. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan, C.; Jato, J.L.V.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym.Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Shu, X.Z.; Zhu, K.J. The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur. J. Pharm. Biopharm. 2002, 54, 235–243. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, J.J.; Liu, Y.M.; Wu, X.L. MicroRNA-34A inhibits the growth, invasion and metastasis of gastric cancer by targeting PDGFR and MET expression. Biosci. Rep. 2014, 43, e00112. [Google Scholar] [CrossRef]
- Chi, J.; Jiang, Z.; Qiao, J.; Zhang, W.; Peng, Y.; Liu, W.; Han, B. Antitumor evaluation of carboxymethyl chitosan based norcantharidin conjugates against gastric cancer as novel polymer therapeutics. Int. J. Biol. Macromol. 2019, 136, 1–12. [Google Scholar] [CrossRef]
- Gong, Y.; Tao, L.; Wang, F.; Liu, W.; Jing, L.; Liu, D.; Zhou, N. Chitosan as an adjuvant for a Helicobacter pylori therapeutic vaccine. Mol. Med. Rep. 2015, 12, 4123–4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, S.; Aggarwal, P.; Pathak, A.; Bhandari, R.; Duffoo, F.; Gulati, S.C. Molecular genetics of head and neck cancer (review). Mol. Med. Rep. 2012, 6, 19–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Øilo, M.; Bakken, V. Biofilm and dental biomaterials. Materials 2015, 8, 2887–2900. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Schiroky, L.V.; Garcia, I.M.; Ogliari, F.A.; Samuel, S.M.W.; Collares, F.M. Triazine compound as copolymerized antibacterial agent in adhesive resins. Braz. Dent. J. 2017, 28, 196–200. [Google Scholar] [CrossRef]
- Degrazia, F.W.; Leitune, V.C.B.; Gracia, I.M.; Arthur, R.A.; Samuel, S.M.W.; Collares, F.M. Effect of silver nanoparticles on the physicochemical and antimicrobial properties of an orthodontic adhesive. J. Appl. Oral. Sci. 2016, 24, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Wassel, M.O.; Khattab, M.A. Antibacterial activity against Streptococcus mutans and inhibition of bacterial induced enamel demineralization of propolis, miswak, and chitosan nanoparticles based dental varnishes. J. Adv. Res. 2017, 8, 387–392. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, C.; Melo, M.A.; Bai, Y.; Cheng, L.; Xu, H.H. A novel protein-repellent dental composite containing 2-methacryloyloxyethyl phosphorylcholine. Int. J. Oral. Sci. 2015, 7, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Fornell, A.C.; Skold-Larsoon, K.; Hallgren, A.; Bergstand, F.; Twetman, S. Effect of a hydrophobic tooth coating on gingival health, mutans streptococci, and enamel demineralization in adolescents with fixed orthodontic appliances. Acta Odontol. Scand. 2002, 60, 37–41. [Google Scholar] [CrossRef]
- Nguyen, S.; Hiorth, M.; Rykke, M.; Smistad, G. Polymer coated liposomes for dental drug delivery—Interactions with parotid saliva and dental enamel. Eur. J. Pharm. Sci. 2013, 50, 78–85. [Google Scholar] [CrossRef]
- Tonnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Ciofani, G.; Raffa, V.; Pizzorusso, T.; Menciassi, A.; Dario, P. Characterization of an alginate-based drug delivery system for neurological applications. Med. Eng. Phys. 2008, 30, 848–855. [Google Scholar] [CrossRef]
- Lencina, M.S.; Ciolino, A.E.; Andreucetti, N.A.; Villar, M.A. Thermoresponsive hydrogels based on alginate-g-poly (N-isopropylacrylamide) copolymers obtained by low doses of gamma radiation. Eur. Polym. J. 2015, 68, 641–649. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Z.; Liu, B.; Yang, J.; Yang, X.; Yu, Y. A smart drug delivery system responsive to pH/enzyme stimuli based on hydrophobic modified sodium alginate. Eur. Polym. J. 2020, 133, 109779. [Google Scholar] [CrossRef]
- Binauld, S.; Stenzel, M. Acid-degradable polymers for drug delivery: A decade of innovation. Chem. Commun. 2013, 49, 2082–2102. [Google Scholar] [CrossRef]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.-L. Injectable thermoresponsive hydrogel formed by alginate-g-poly (N-isopropylacrylamide) that releases doxorubicin-encapsulated micelles as a smart drug delivery system. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef]
- Luo, R.C.; Lim, Z.H.; Li, W.; Shi, P.; Chen, C.-H. Near-infrared light triggerable deformation-free polysaccharide double network hydrogels. Chem. Commun. 2014, 50, 7052–7055. [Google Scholar] [CrossRef]
- Dong, L.; Xia, S.; Wu, K.; Huang, Z.; Chen, H.; Chen, J.; Zhang, J. A pH/enzyme-responsive tumor-specific delivery system for doxorubicin. Biomaterials 2010, 31, 6309–6316. [Google Scholar] [CrossRef]
- Wu, J.L.; Wang, C.Q.; Zhuo, R.X.; Cheng, S.X. Multi-drug delivery system based on alginate/calcium carbonate hybrid nanoparticles for combination chemotherapy. Colloids Surf. B Biointerfaces 2014, 123, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yan, Y.; Shen, Q.; Ma, D.; Huang, L.; Cai, X.; Tan, S. A colon targeted drug delivery system based on alginate modificated graphene oxide for colorectal liver metastasis. Mater. Sci. Eng. C 2017, 79, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, Z.; Nourbakhsh, M.S.; Kiani, S.; Daemi, H.; Ashtiani, M.K.; Baharv, H. Effect of chemical composition and sulfated modification of alginate in the development of delivery systems based on electrostatic interactions for small molecule drugs. Mater. Lett. 2020, 263, 127235. [Google Scholar] [CrossRef]
- Hügl, S.; Scheper, V.; Gepp, M.M.; Lenarz, T.; Rau, T.S.; Schwieger, J. Coating stability and insertion forces of an alginate-cell-based drug delivery implant system for the inner ear. J. Mech. Behav. Biomed. Mater. 2019, 97, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Jahanshahi, M.; Najafpour, G.D. Production of biological nanoparticles from bovine serum albumin for drug delivery. Afr. J. Biotechnol. 2006, 5, 1918–1923. [Google Scholar]
- Joshi, M.; Nagarsenkar, M.; Prabhakar, B. Albumin nano-carriers for pulmonary drug delivery: An attractive approach. J. Drug Deliv. Sci. Technol. 2020, 56, 101529. [Google Scholar] [CrossRef]
- Patil, G.V. Biopolymer albumin for diagnosis and in drug delivery. Drug Dev. Res. 2003, 58, 219–247. [Google Scholar] [CrossRef]
- Irache, J.M.; Merodio, M.; Arnedo, A.; Camapanero, M.A.; Mirshahi, M.; Espuelas, S. Albumin nanoparticles for the intravitreal delivery of anticytomegaloviral drugs. Mini Rev. Med. Chem. 2005, 5, 293–305. [Google Scholar] [CrossRef]
- Arshady, R. Preparation of microspheres and microcapsules by interfacial polycondensation techniques. J. Microcapsul. 1989, 6, 13–28. [Google Scholar] [CrossRef]
- Oakenfull, D.; Pearce, J.; Burley, R.W.; Damodaran, S.; Paraf, A. (Eds.) Food Proteins and Their Applications; Marcel Dekker: New York, NY, USA, 1997; Volume 487, p. 681. [Google Scholar]
- Hu, Y.J.; Liu, Y.; Sun, T.Q.; Bai, A.M.; Lü, J.Q.; Pi, Z.B. Binding of anti-inflammatory drug cromolyn sodiumto bovine serumalbumin. Int. J. Biol. Macromol. 2006, 39, 280–285. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hematara, T.; Herbert, E.; Kreuter, J. Transferrin- and transferrinreceptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Kim, T.H.; Jiang, H.H.; Youn, Y.S.; Park, C.W.; Tak, K.K.; Lee, S.; Kim, H.; Jon, S.; Chen, X.; Lee, K.C. Preparation and characterization of water-soluble albuminbound curcumin nanoparticles with improved antitumor activity. Int. J. Pharm. 2011, 403, 285–291. [Google Scholar] [CrossRef]
- Dreis, S.; Rothweiler, F.; Michaelis, M.; Cinatl, J., Jr.; Kreuter, J.; Langer, K. Preparation, characterization and maintenance of drug efficacy of doxorubicin-loaded human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2007, 341, 207–214. [Google Scholar] [CrossRef]
- Iwao, Y.; Tomiguchi, I.; Domura, A.; Mantaira, Y.; Minami, A.; Suzuki, T.; Ikawa, T.; Kimura, S.I.; Itai, S. Inflamed site-specific drug delivery system based on the interaction of human serum albumin nanoparticles with myeloperoxidase in a murine model of experimental colitis. Eur. J. Pharm. Biopharm. 2018, 125, 141–147. [Google Scholar] [CrossRef]
- Siri, M.; Grasselli, M.; Alonso, S.d.V. Correlation between assembly structure of a gamma irradiated albumin nanoparticle and its function as a drug delivery system. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125176. [Google Scholar] [CrossRef]
- Stein, N.; Mulac, D.; Fabian, J.; Herrmann, F.C.; Langer, K. Nanoparticle albumin-bound mTHPC for photodynamic therapy: Preparation and comprehensive characterization of a promising drug delivery system. Int. J. Pharm. 2020, 582, 119347. [Google Scholar] [CrossRef]
- Gomes, D.S.; Santos, A.M.C.; Neves, G.A.; Menezes, R.R. A brief review on hydroxyapatite production and use in biomedicine. Cerâmica 2019, 65, 282–302. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Espanol, M.; Guillem-Marti, J.; Kempf, D.; Diez-Escudero, A.; Ginebra, M.-P. Ion-doping as a strategy to modulate hydroxyapatite nanopartilce internalization. Nanoscale 2016, 8, 1595–1607. [Google Scholar] [CrossRef] [Green Version]
- Cellet, T.S.P.; Pereira, G.M.; Muniz, E.C.; Silva, R.; Rubira, A.F. Hydroxyapatite nanowhiskers embedded in chondroitin sulfate microsphere as colon targeted drug delivery system. J. Mater. Chem. B 2015, 3, 6837–6846. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozlowsa, J. Characterization of collagen/hydroxyapatite composite sponges as a potential bone substitute. Int. J. Biol. Macromol. 2010, 47, 483–487. [Google Scholar] [CrossRef]
- Li, D.; Huang, X.; Wu, Y.; Li, J.; Cheng, W.; He, J.; Tian, H.; Huang, Y. Preparation of pH-responsive mesoporous hydroxyapatite nanoparticles for intracellular controlled release of an anticancer drug. Biomater. Sci. 2016, 4, 272–280. [Google Scholar] [CrossRef]
- Venkatasubbu, G.D.; Ramasamy, S.; Avadhani, G.S.; Ramakrishnan, V.; Kumar, J. Surface modification and paclitaxel drug delivery of folic acid modified polyethylene glycol functionalized hydroxyapatite nanoparticles. Powder Technol. 2013, 235, 437–442. [Google Scholar] [CrossRef]
- Ghafarinazari, A.; Tahari, A.; Moztarzadeh, F.; Mozafari, M.; Bahroloom, M.E. Ion exchange behaviour of silver-doped apatite micro and nanoparticles as antibacterial biomaterial. Micro Nano Lett. 2011, 6, 713–717. [Google Scholar] [CrossRef]
- Mizuno, S.; Glowacki, J. Three dimensional composite of demineralized bone powder and collagen for in vitro analysis of chondroinduction of human dermal fibroblasts. Biomaterials 1996, 17, 1819–1825. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Wang, M.; Joseph, R.; Bonfield, W. Hydroxyapatite-poly- ethylene composites for bone substitution: Effects of ceramic particle size and morphology. Biomaterials 1998, 19, 2357–2366. [Google Scholar] [CrossRef]
- Bonfield, W.; Wang, M.; Tanner, K.E. Interfaces in analogue biomaterials. Acta Mater. 1998, 46, 2509–2518. [Google Scholar] [CrossRef]
- Di Silvio, L.; Dalby, M.J.; Bonfield, W. Osteoblast behaviour on HA/PE composite surfaces with different HA volumes. Biomaterials 2002, 23, 101–107. [Google Scholar] [CrossRef]
- Wang, M.; Bonfield, W. Chemically coupled hydroxyapatite-polyethylene composites: Processing and characterization. Mater. Lett. 2000, 44, 119–124. [Google Scholar] [CrossRef]
- Kikuchi, M.; Itoh, S.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Self-organization mechanism in a bone-like hy-droxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials 2001, 22, 1705–1711. [Google Scholar] [CrossRef]
- Li, J.; Fartash, B.; Hermannson, L. Hydroxyapatite-alu-mina composites and bone-bonding. Biomaterials 1995, 16, 417–422. [Google Scholar] [CrossRef]
- Lopes, M.A.; Silva, R.F.; Monteiro, F.J.; Santos, J.D. Microstructural dependence of Young’s moduli of P2O5 glass reinforced hydroxyapatite for biomedical applications. Biomaterials 2000, 21, 749–754. [Google Scholar] [CrossRef]
- Liu, Q.; de Wijn, J.R.; van Blitterswijk, C.A. Composite biomaterials with chemical bonding between hy-droxyapatite filler particles and PEG/PBT copoly-mer matrix. J. Biomed. Mater. Res. 1998, 40, 490–497. [Google Scholar] [CrossRef]
- Ghiasi, B.; Sefidbakht, Y.; Rezaei, M. Hydroxyapatite for Biomedicine and Drug Delivery. Nanomater. Adv. Biol. Appl. 2019, 104, 85–120. [Google Scholar]
- Choi, K.Y.; Min, K.H.; Na, J.H.; Choi, K.; Kim, K.; Park, J.H.; Kwon, I.C.; Jeong, S.Y. Self-assembled hyaluronic acid nanoparticles as a potential drug carrier for cancer therapy: Synthesis, characterization, and in vivo biodistribution. J. Mater. Chem. 2009, 19, 4102–4107. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Cheng, D.; Han, W.; Yang, K.; Song, Y.; Jiang, M.; Song, E. One-step facile synthesis of hyaluronic acid functionalized fluorescent gold nanoprobes sensitive to hyaluronidase in urine specimen from bladder cancer patients. Talanta 2014, 130, 408–414. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Z.Q.; Wang, S.J.; Liu, P.; Qin, Y.H.; Ma, Y.; Li, X.C.; Huo, Z.-J. Hyaluronic acidtagged silica nanoparticles in colon cancer therapy: Therapeutic efficacy evaluation. Int. J. Nanomed. 2015, 10, 6445–6454. [Google Scholar]
- Wang, H.; Sun, G.; Zhang, Z.; Ou, Y. Transcription activator, hyaluronic acid and tocopheryl succinate multi-functionalized novel lipid carriers encapsulating etoposide for lymphoma therapy. Biomed. Pharmacother. 2017, 91, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, H. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J. Control. Release 2018, 278, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Pang, L.; Feng, H.; Dong, H.; Wang, S.; Cong, H.; Shen, Y.; Bing, Y. Recent advantage of hyaluronic acid for anticancer application: A review of “3S” transition approach. Carbohydr. Polym. 2020, 238, 116204. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Fu, J.; Li, R.; Zhang, F.; Ling, G.; Zhang, P. A potential carrier for anti-tumor targeted delivery-hyaluronic acid nanoparticles. Carbohydr. Polym. 2019, 208, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Choi, K.; Jeong, S.Y. PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials 2011, 32, 1880–1889. [Google Scholar] [CrossRef]
- Na, K.; Lee, C.-S. Photochemically Triggered Cytosolic Drug Delivery Using pHResponsive Hyaluronic Acid Nanoparticles for Light-Induced Cancer Therapy. Biomaterials 2014, 15, 4228–4238. [Google Scholar]
- Yoon, H.Y.; Koo, H.; Ki Choi, Y.; Kwon, I.C.; Choi, K.; Jae HyungPark, J.H.; Kim, K. Photo-crosslinked hyaluronic acid nanoparticles with improved stability for in vivo tumor-targeted drug delivery. Biomaterials 2013, 34, 5273–5280. [Google Scholar] [CrossRef]
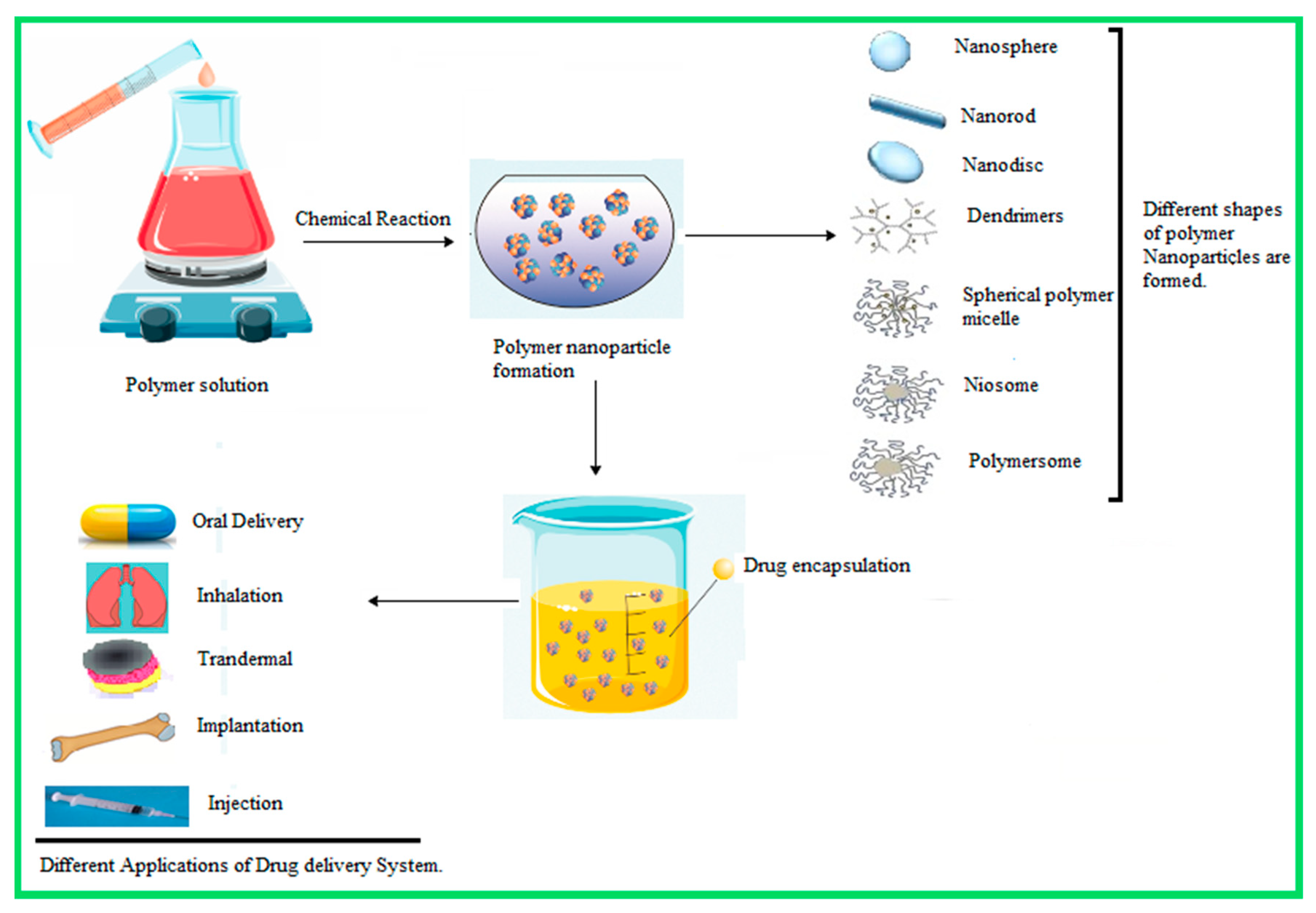
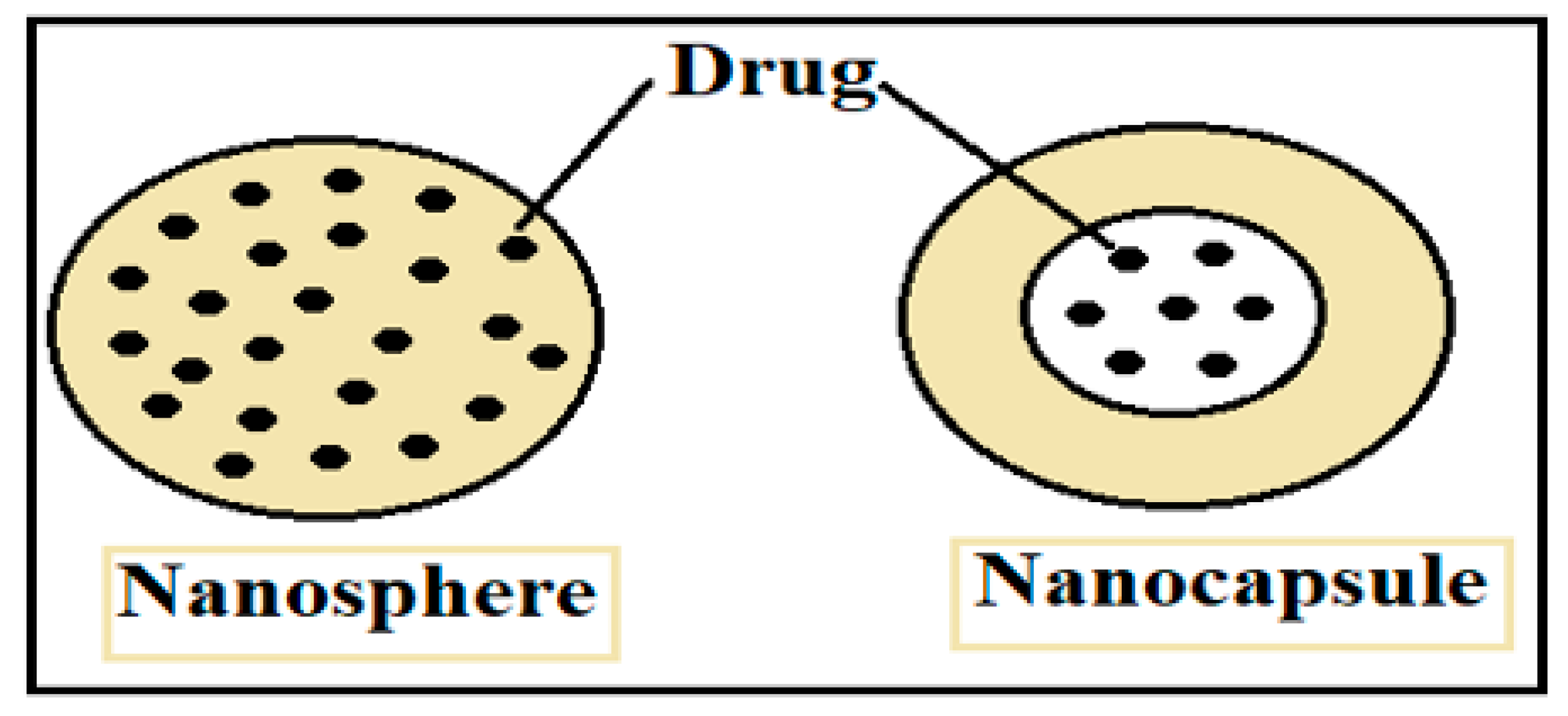


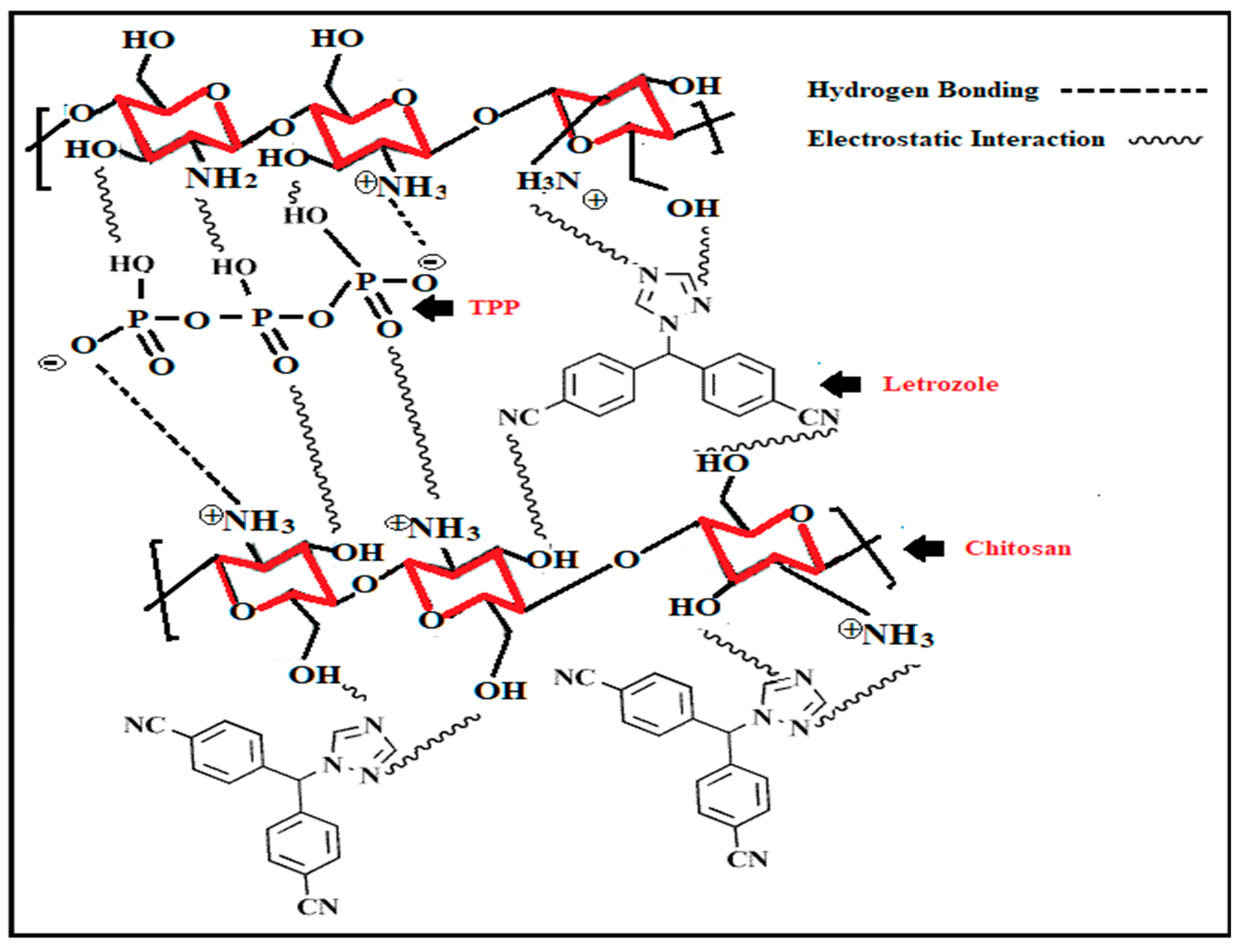


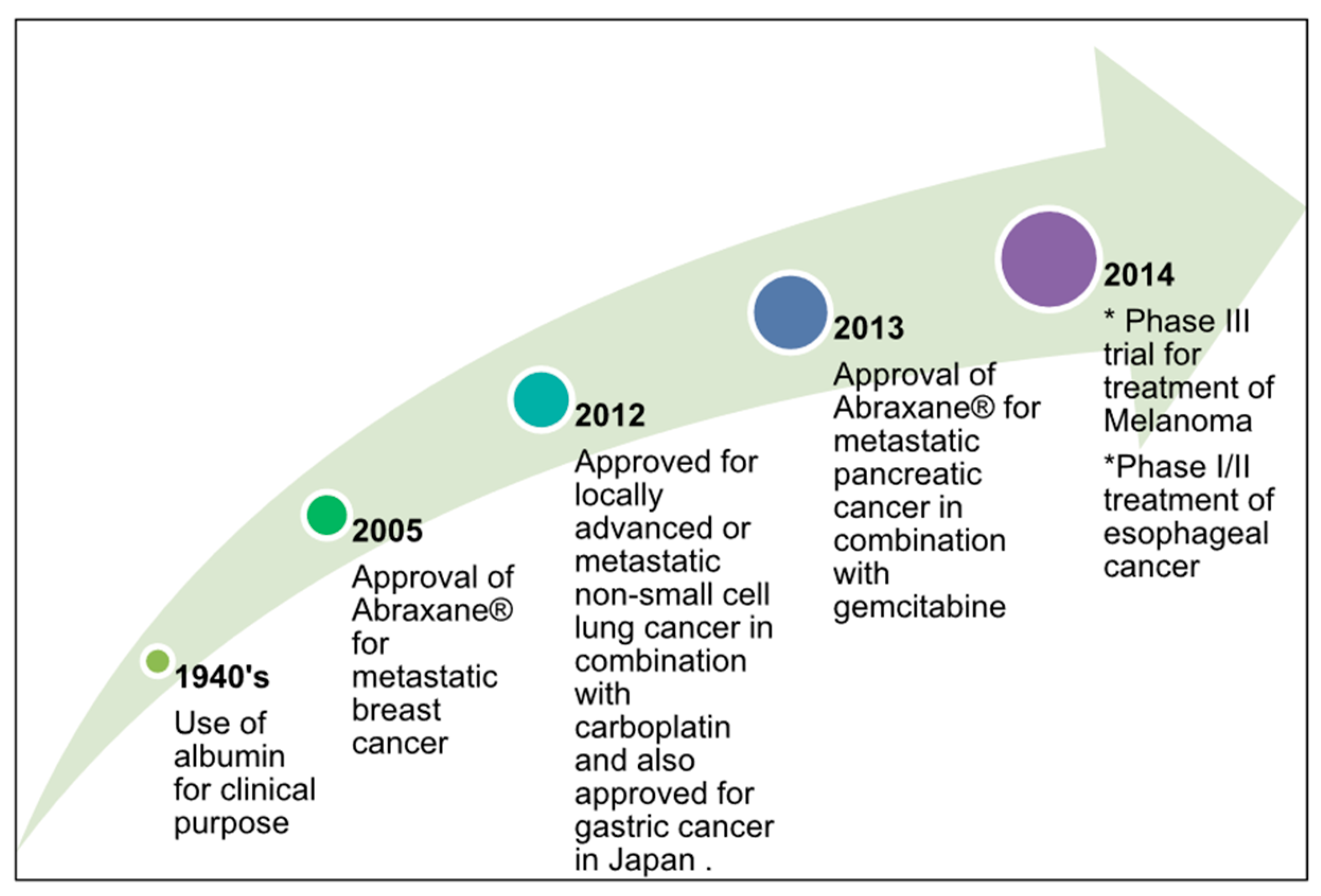

| Nanoparticle Drug Delivery System |
|---|
|
|
|
|
|
|
| Polymeric carriers |
|
|
|
|
|
|
| Polymer/Modified Polymer | Preparation Method | Model Drug |
|---|---|---|
| Chitosan–folic acid | Ionic gelation | 5-flurouracil |
| Chitosan/alginate and solid lipid NPs | Ionic gelation | Ciprofloxacin |
| PVA/chitosan-gelation | Electrospinning method | Erythromycin |
| Alginate calcium carbonate | Co-precipitation | Paclitaxel |
| PEGylated albumin | RAFT polymerization | Lysozyme |
| Bovine serum albumin | Multistep process | Temozolomide |
| Egg albumin | Desolvation method | Curcumin |
| PEG-modified human serum albumin | Solid dispersion technology | Paclitaxel |
| Advantages | Disadvantages | Biomedical Applications | Method of Preparation |
|---|---|---|---|
| Bioactive (hydration shell) | Strong hydration shell, ionic surface, fragile | Drug delivery system, tissue engineering’ implants | Wet method, dry method, high-temperature process |
| Ease of modification and surface functionalization | Precipitation and turbid solution | - | - |
| Good attachment to polymers | Surface corona formation, aggregation | - | - |
| Ease of composite formation | Dispersity in chemical composition, size and shape polymorphism | - | - |
| Biodegradable, biocompatibility | High pH sensitivity and solubility | - | - |
| Self-assembly | Low stability | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.-u. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. https://doi.org/10.3390/nano10101970
Idrees H, Zaidi SZJ, Sabir A, Khan RU, Zhang X, Hassan S-u. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials. 2020; 10(10):1970. https://doi.org/10.3390/nano10101970
Chicago/Turabian StyleIdrees, Humaira, Syed Zohaib Javaid Zaidi, Aneela Sabir, Rafi Ullah Khan, Xunli Zhang, and Sammer-ul Hassan. 2020. "A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications" Nanomaterials 10, no. 10: 1970. https://doi.org/10.3390/nano10101970





