A β-cyclodextrin Modified Graphitic Carbon Nitride with Au Co-Catalyst for Efficient Photocatalytic Hydrogen Peroxide Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Photocatalysts
2.1.1. Preparation of g-C3N4
2.1.2. Preparation of β-Cyclodextrin–Carbon Nitride (β-CD-CN) Composite
2.1.3. Preparation of Metal Co-Catalyst/β-CD-CN Nanocomposite
2.2. Material Characterization
2.3. Photocatalytic Reactivity Test and H2O2 Decomposition
2.4. Electron Spin Resonance (ESR) Test
3. Results and Discussion
3.1. Material Characterization
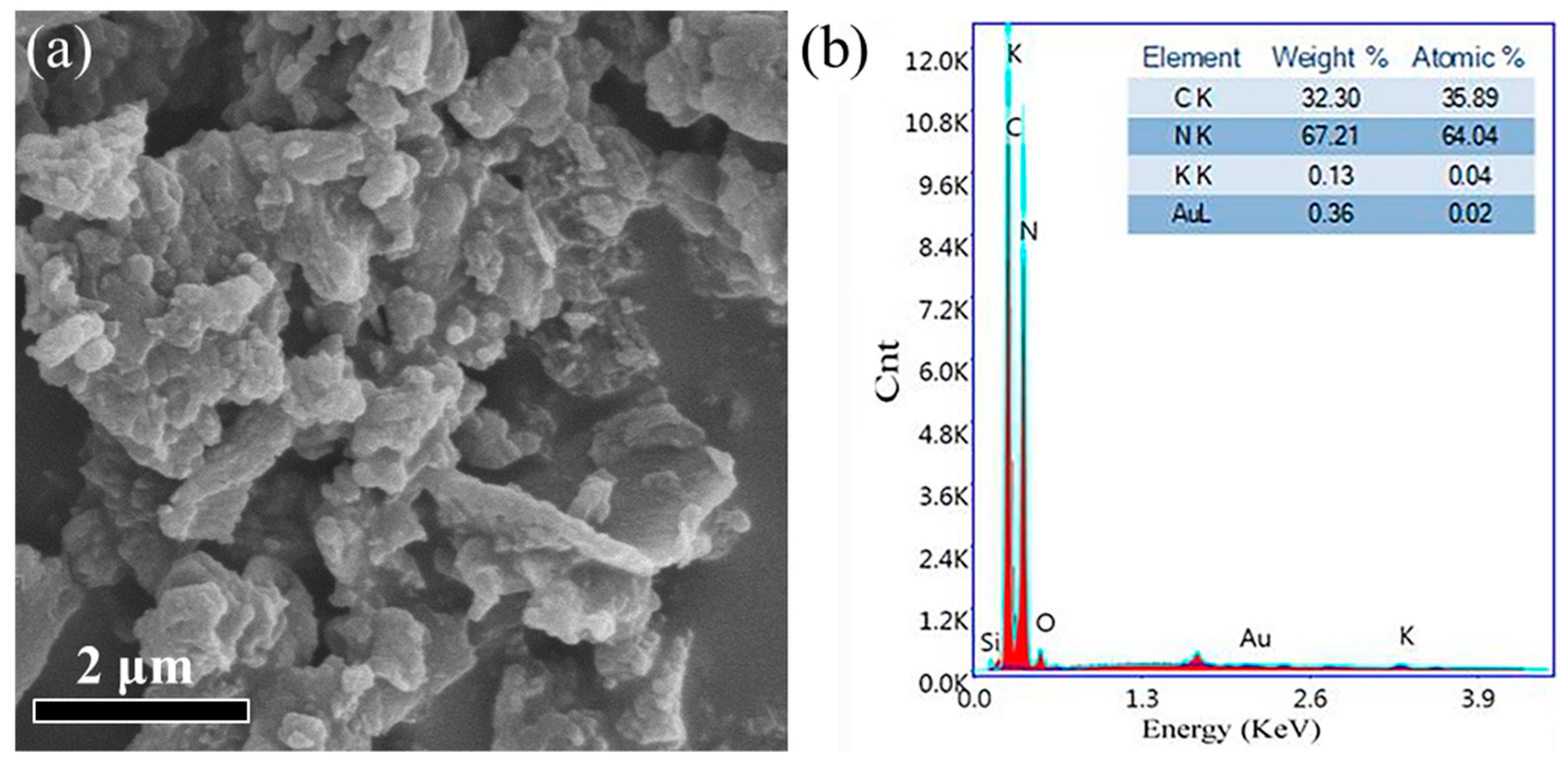
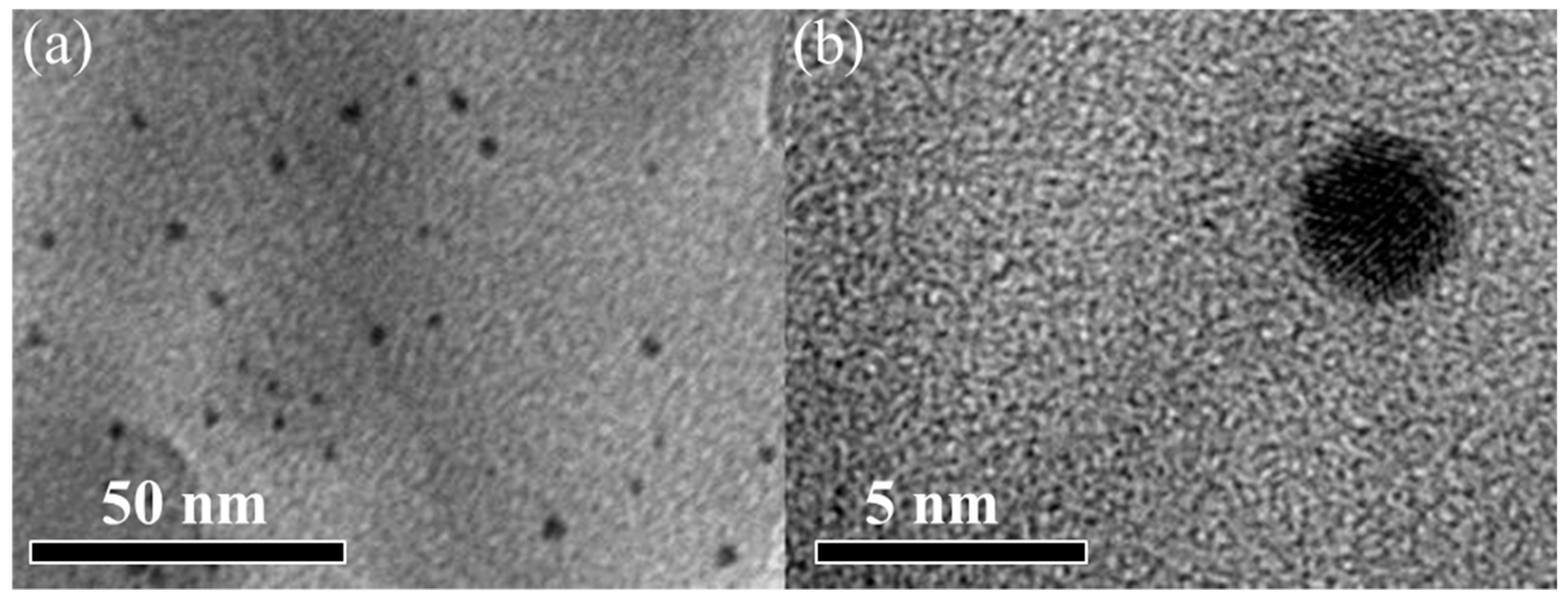
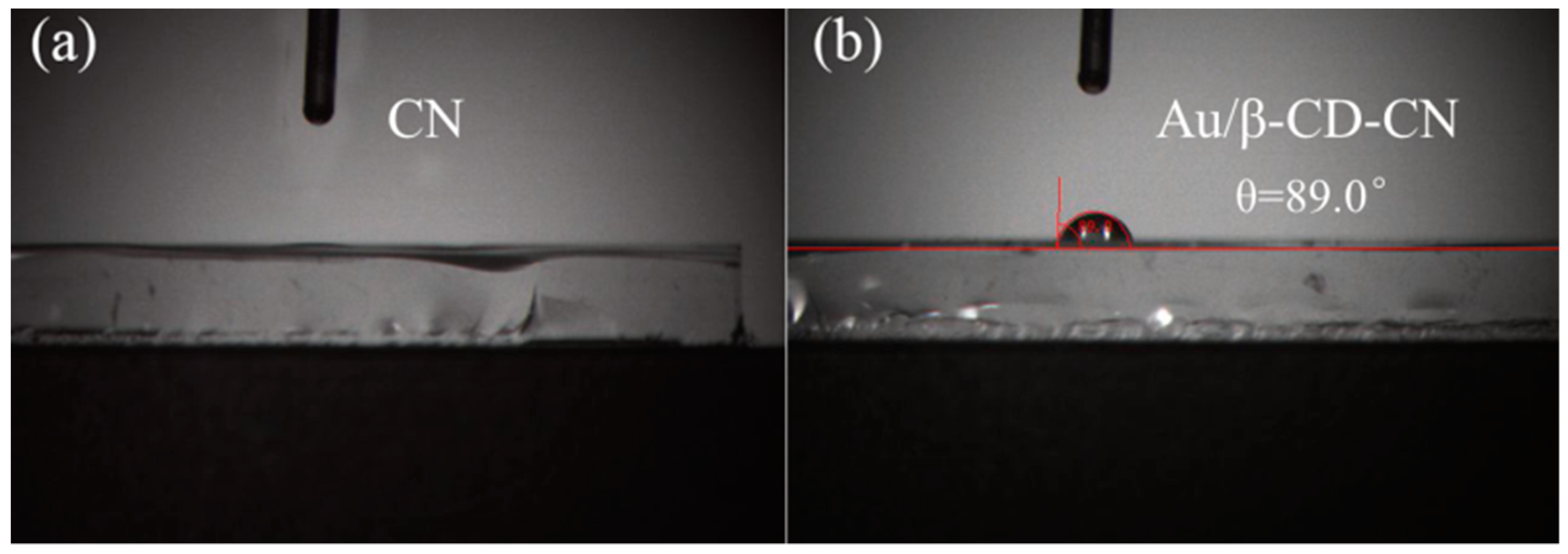
3.1.1. Surface Morphology Characterization
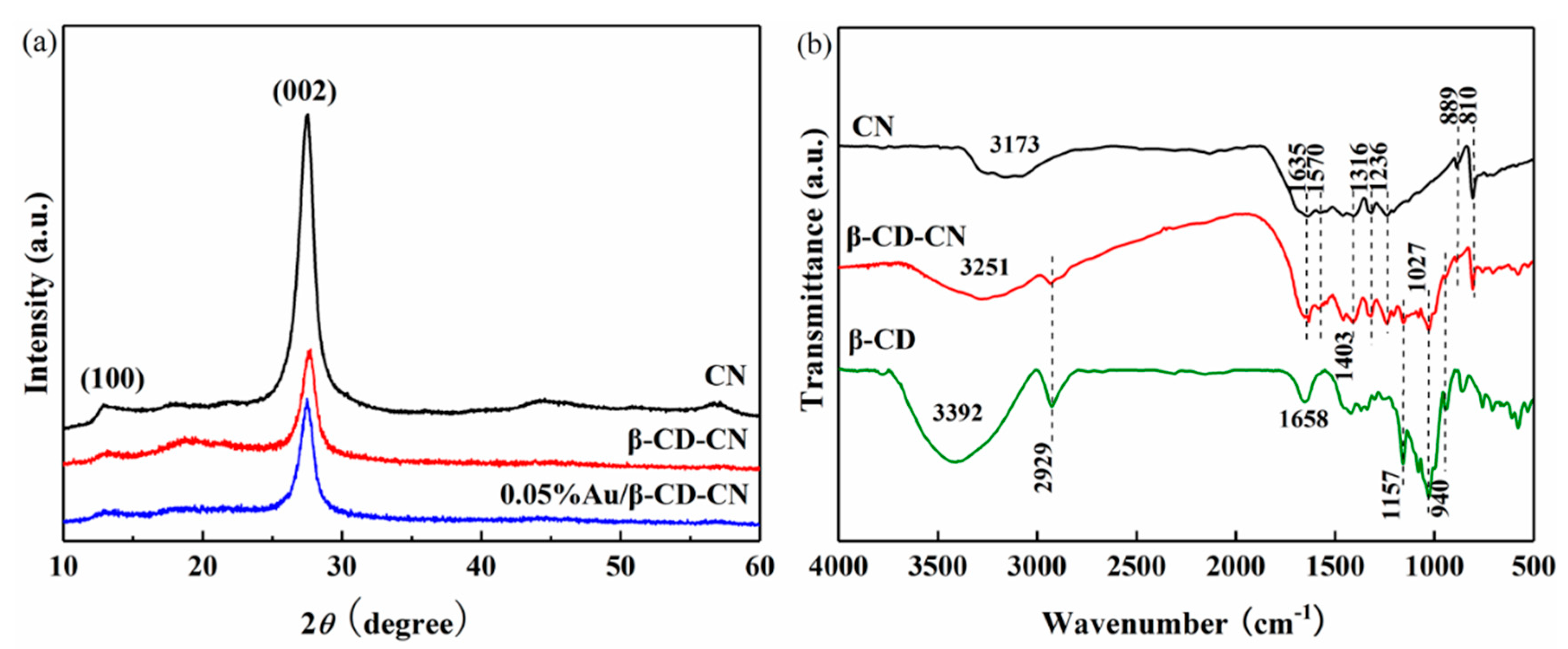
3.1.2. X-Ray Diffraction (XRD) and Fourier Transform Infrared (FT-IR) Characterizations
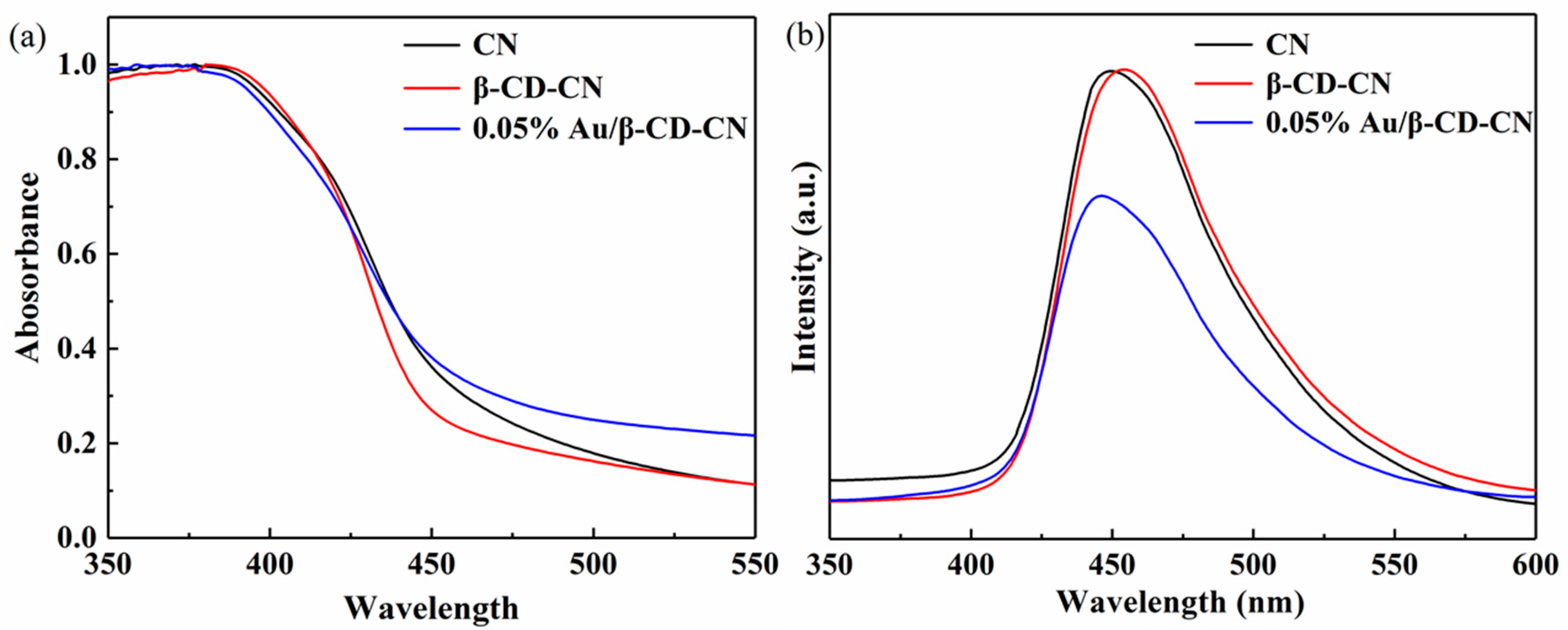
3.1.3. The Optical Absorption, Photoluminescence and Surface Properties
3.2. Photocatalytic Performance
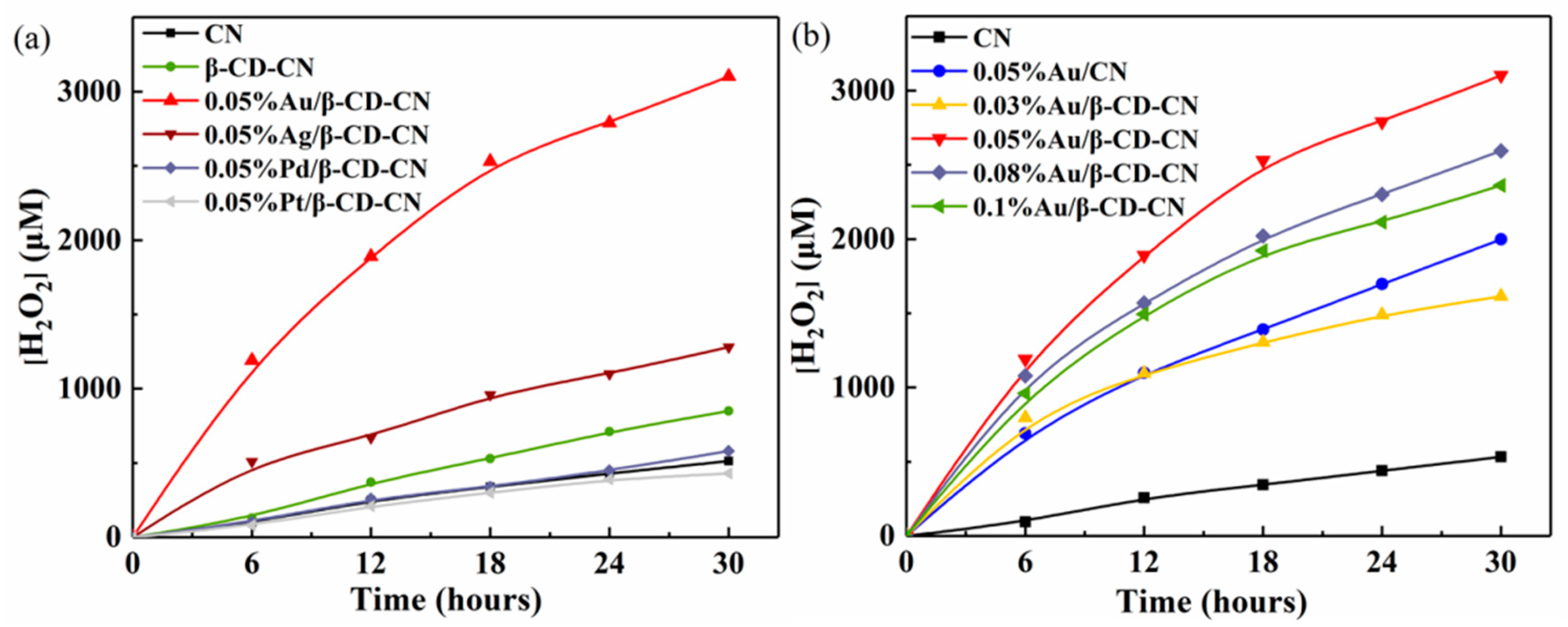
3.2.1. Photocatalytic H2O2 Production Activity
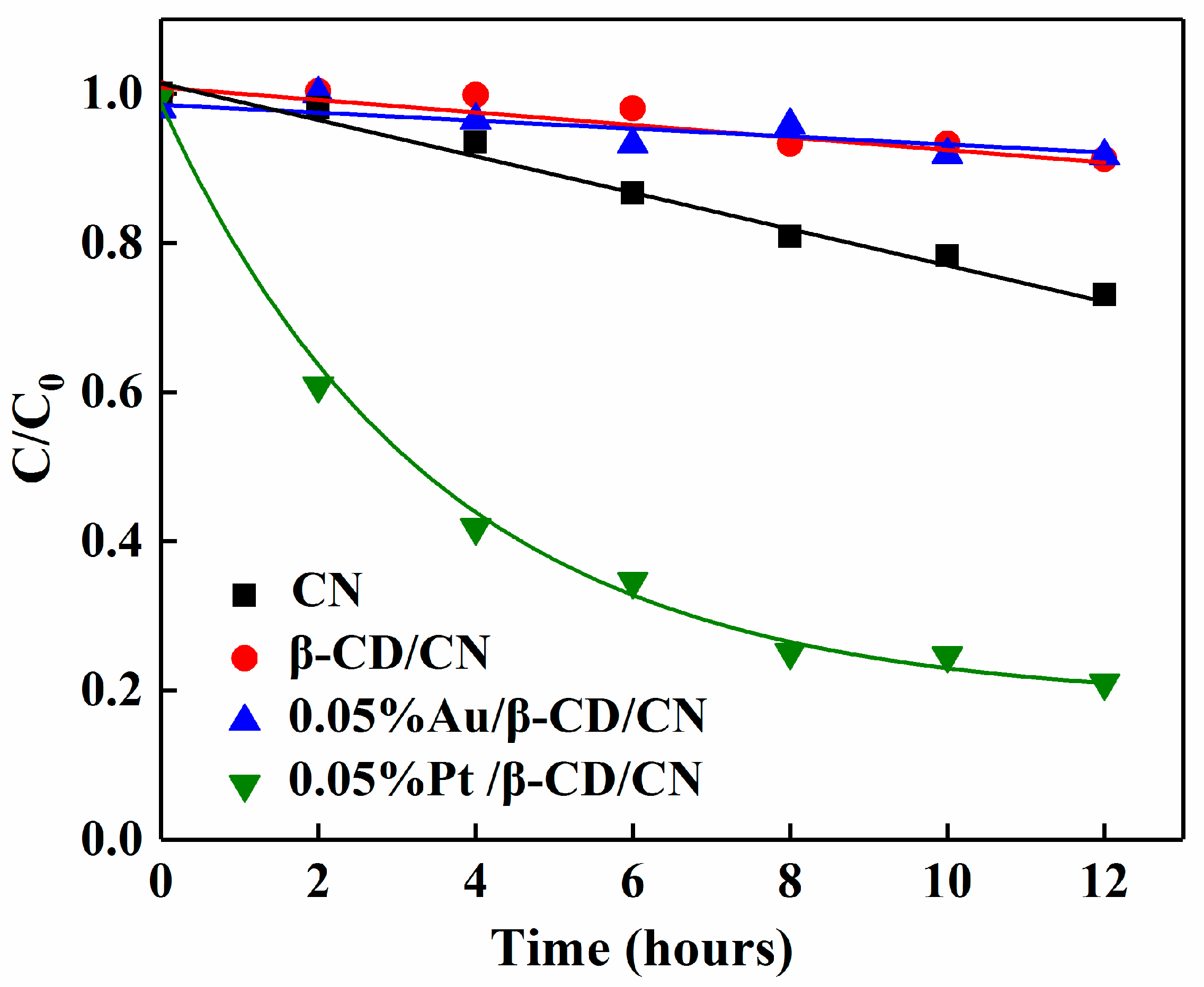
3.2.2. Photocatalytic H2O2 Decomposition Activity
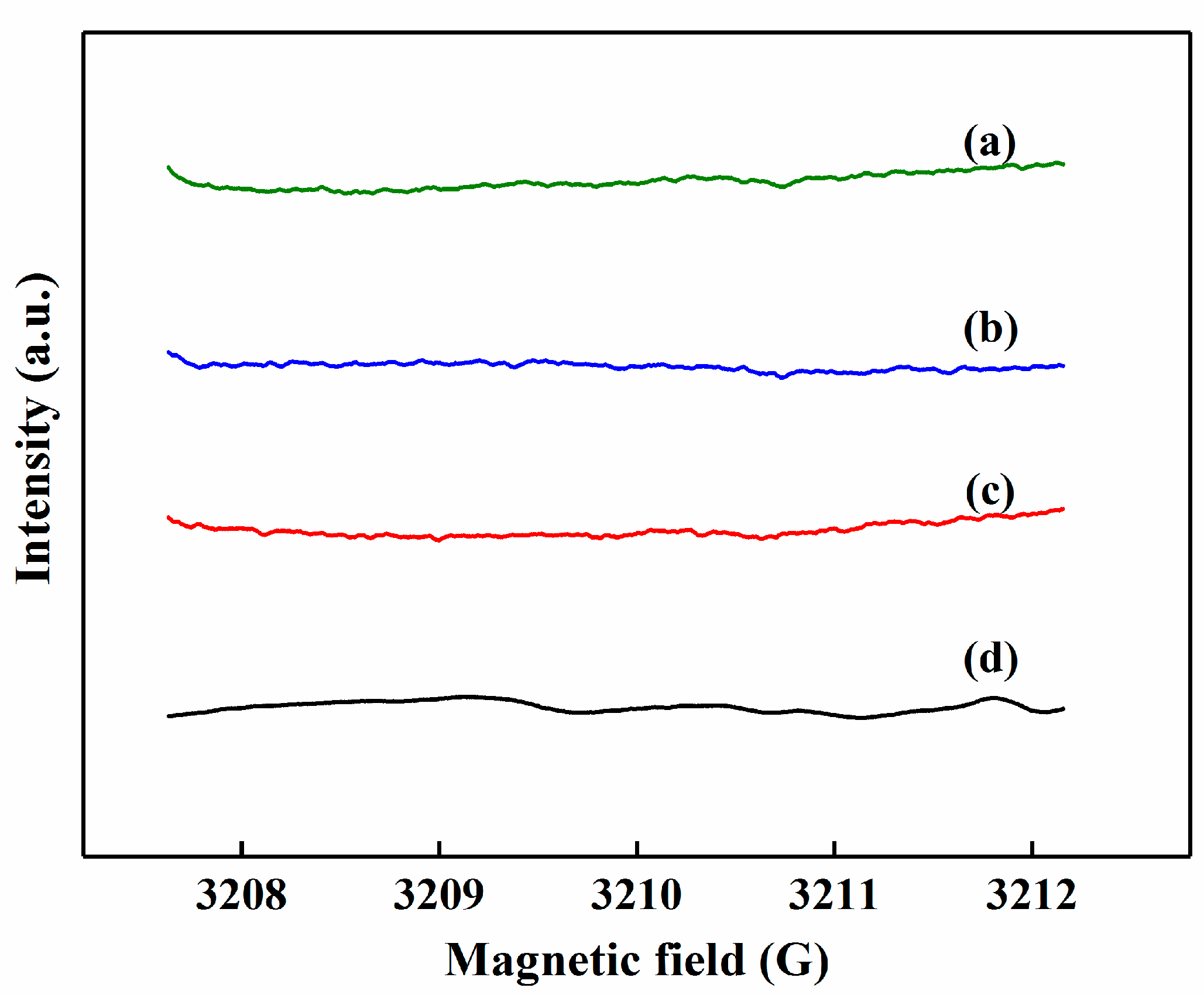
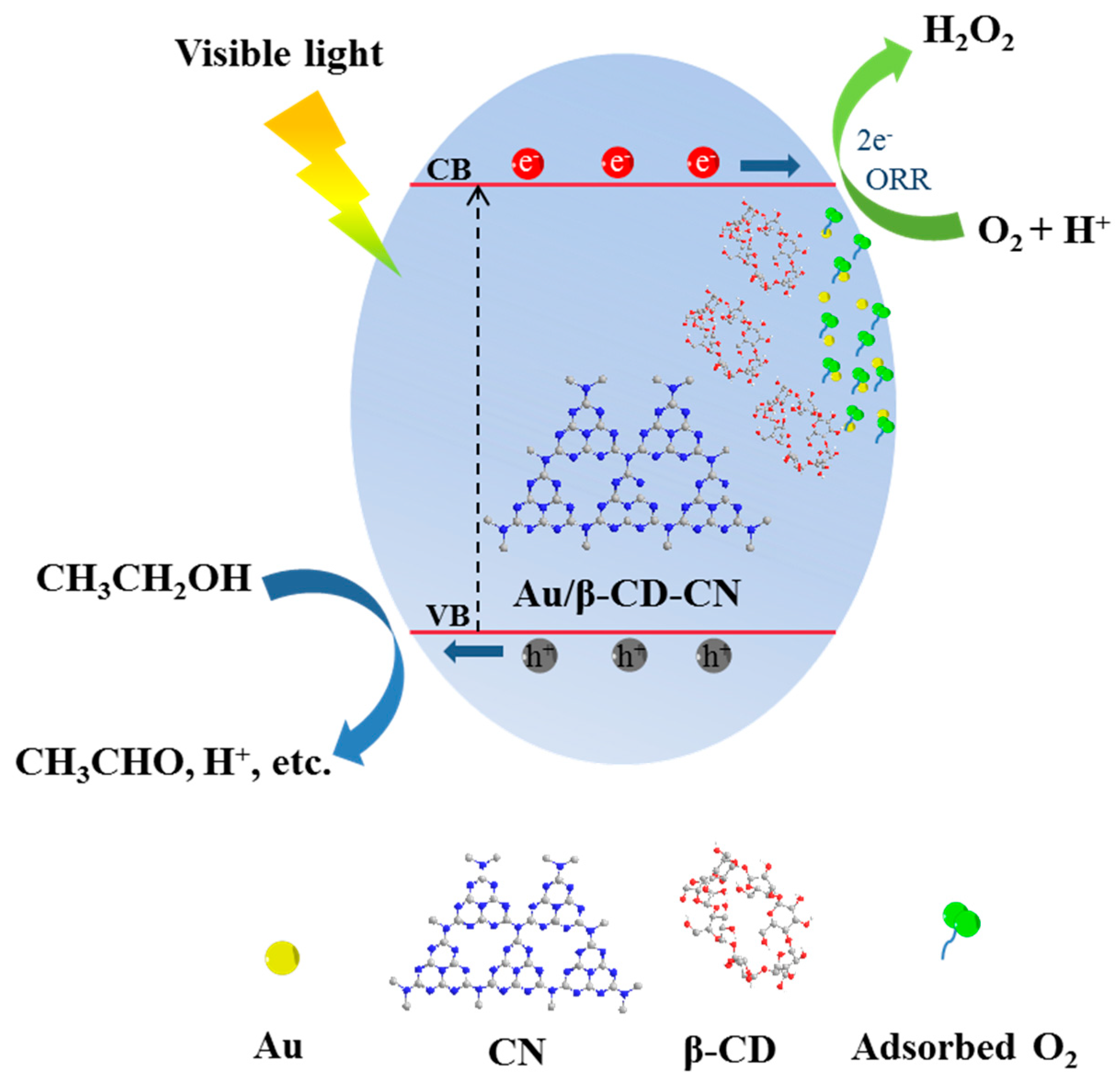
3.3. Plausible Mechanism of Photocatalysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yusuke, Y.; Akifumi, N.; Takamitsu, M.; Kei, O.; Shunichi, F. Acetate induced enhancement of photocatalytic hydrogen peroxide production from oxalic acid and dioxygen. J. Phys. Chem. A 2013, 117, 3751–3760. [Google Scholar]
- Lee, K.P.; Gopalan, A.I.; Komathi, S. Direct electrochemistry of cytochrome c and biosensing for hydrogen peroxide on polyaniline grafted multi-walled carbon nanotube electrode. Sens. Actuators B Chem. 2009, 141, 518–525. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Komathi, S.; Anand, G.S.; Lee, K.P. Nanodiamond based sponges with entrapped enzyme: A novel electrochemical probe for hydrogen peroxide. Biosens. Bioelectron. 2013, 46, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Komathi, S.; Gopalan, A.I.; Kim, S.K.; Anand, G.S.; Lee, K.P. Fabrication of horseradish peroxidase immobilized poly(N-[3-(trimethoxy silyl)propyl]aniline) gold nanorods film modified electrode and electrochemical hydrogen peroxide sensing. Electrochim. Acta 2013, 92, 71–78. [Google Scholar] [CrossRef]
- Bui, Q.T.; Yu, I.K.; Gopalan, A.L.; Saianand, G.; Kim, W.; Choi, S.H. Facile Fabrication of Metal Oxide Based Catalytic Electrodes by AC Plasma Deposition and Electrochemical Detection of Hydrogen Peroxide. Catalysts 2019, 9, 888. [Google Scholar] [CrossRef] [Green Version]
- Moon, G.H.; Kim, W.; Bokare, A.D.; Sung, N.E.; Choi, W. Solar production of H2O2 on reduced graphene oxide-TiO2 hybrid photocatalysts consisting of earth-abundant elements only. Energy Environ. Sci. 2014, 7, 4023–4028. [Google Scholar] [CrossRef]
- Zuo, G.F.; Li, B.D.; Guo, Z.L.; Wang, L.; Yang, F.; Hou, W.S.; Zhang, S.T.; Zong, P.X.; Liu, S.S.; Meng, X.G.; et al. Efficient Photocatalytic Hydrogen Peroxide Production over TiO2 Passivated by SnO2. Catalysts 2019, 9, 623. [Google Scholar] [CrossRef] [Green Version]
- Miwako, T.; Shin-Ichi, N.; Hiroaki, T. In situ liquid phase synthesis of hydrogen peroxide from molecular oxygen using gold nanoparticle-loaded titanium(IV) dioxide photocatalyst. J. Am. Chem. Soc. 2010, 132, 7850–7851. [Google Scholar]
- Meng, X.G.; Zong, P.X.; Wang, L.; Yang, F.; Hou, W.S.; Zhang, S.T.; Li, B.D.; Guo, Z.L.; Liu, S.S.; Zuo, G.F.; et al. Au-nanoparticle-supported ZnO as highly efficient photocatalyst for H2O2 production. Catal. Commun. 2020, 134, 105860. [Google Scholar] [CrossRef]
- Domènech, X.; Ayllón, J.A.; Peral, J. H2O2 Formation from photocatalytic processes at the ZnO/water interface. Environ. Sci. Pollut. R. 2001, 8, 285–287. [Google Scholar] [CrossRef]
- Chen, S.H.; Jiang, Y.S.; Lin, H.Y. Easy Synthesis of BiVO4 for Photocatalytic Overall Water Splitting. ACS Omega 2020, 5, 8927–8933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirakawa, H.; Shiota, S.; Shiraishi, Y.; Sakamoto, H.; Hirai, T. Au Nanoparticles Supported on BiVO4: Effective Inorganic Photocatalysts for H2O2 Production from Water and O2 under Visible Light. ACS Catal. 2016, 6, 4976–4982. [Google Scholar] [CrossRef]
- Yang, L.; Dong, G.; Jacobs, D.L.; Wang, Y.; Zang, L.; Wang, C. Two-channel photocatalytic production of H2O2 over g-C3N4 nanosheets modified with perylene imides. J. Catal. 2017, 352, 274–281. [Google Scholar] [CrossRef]
- Peng, Y.L.; Wang, L.Z.; Liu, Y.D.; Chen, H.J.; Lei, J.Y.; Zhang, J.L. Visible-light-driven photocatalytic H2O2 production on g-C3N4 loaded with CoP as a noble metal free cocatalyst. Eur. J. Inorg. Chem. 2017, 2017, 4797–4802. [Google Scholar] [CrossRef]
- Xiong, W.; Huang, F.; Zhang, R.Q. Recent developments on carbon nitride based films for photoelectrochemical water splitting. Sustain. Energy Fuels 2020, 4, 485–503. [Google Scholar] [CrossRef]
- Wu, C.B.; Yu, G.H.; Yin, Y.; Wang, Y.Z.; Chen, L.; Han, Q.; Tang, J.W.; Wang, B. Mesoporous Polymeric Cyanamide-Triazole-Heptazine Photocatalysts for Highly-Efficient Water Splitting. Small 2020, 16, 2003162. [Google Scholar] [CrossRef]
- Chi, H.L.; Liu, J.X.; Zhang, X.Y.; Xue, X.G.; Zhang, D.D.; Lin, X.C.; Huang, P.R.; Sun, L.X.; Xiong, J.; Cai, P.; et al. Synergetic defects boost charge separation in CN for enhanced photocatalytic water splitting. J. Mater. Chem. C 2020, 8, 9366–9372. [Google Scholar] [CrossRef]
- Maeda, K.; Wang, X.; Nishihara, Y. Photocatalytic Activities of Graphitic Carbon Nitride Powder for Water Reduction and Oxidation under Visible Light. J. Phys. Chem. C 2009, 113, 4940–4947. [Google Scholar] [CrossRef]
- Meng, X.G.; Liu, L.Q.; Ouyang, S.X.; Xu, H.; Wang, D.F.; Zhao, N.Q.; Ye, J.H. Nanometals for Solar-to-Chemical Energy Conversion: From Semiconductor-Based Photocatalysis to Plasmon-Mediated Photocatalysis and Photo-Thermocatalysis. Adv. Mater. 2016, 28, 6781–6803. [Google Scholar] [CrossRef]
- Samanta, S.; Martha, S.; Parida, K. Facile Synthesis of Au/g-C3N4 Nanocomposites: An Inorganic/Organic Hybrid Plasmonic Photocatalyst with Enhanced Hydrogen Gas Evolution Under Visible-Light Irradiation. ChemCatChem 2014, 6, 1453–1462. [Google Scholar]
- Zuo, G.F.; Liu, S.S.; Wang, L.; Song, H.; Zong, P.X.; Hou, W.S.; Li, B.D.; Guo, Z.L.; Meng, X.G.; Du, Y.; et al. Finely dispersed Au nanoparticles on graphitic carbon nitride as highly active photocatalyst for hydrogen peroxide production. Catal. Commun. 2019, 123, 69–72. [Google Scholar] [CrossRef]
- Liu, G.G.; Zhao, G.X.; Wei, Z.; Liu, Y.Y.; Hong, P.; Zhang, H.B.; Dong, H.; Meng, X.G.; Peng, L.; Kako, T.; et al. In Situ Bond Modulation of Graphitic Carbon Nitride to Construct p–n Homojunctions for Enhanced Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2016, 26, 6822–6829. [Google Scholar] [CrossRef]
- Kosaka, K.; Yamada, H.; Matsui, S.; Echigo, S.; Shishida, K. Comparison among the Methods for Hydrogen Peroxide Measurements To Evaluate Advanced Oxidation Processes: Application of a Spectrophotometric Method Using Copper(II) Ion and 2,9-Dimethyl-1,10-phenanthroline. Environ. Sci. Technol. 1998, 32, 3821–3824. [Google Scholar] [CrossRef]
- Jirkovsky, J.S.; Itai, P.; Elisabet, A.; Matej, H.; Simon, R.; Schiffrin, D.J. Single atom hot-spots at Au-Pd nanoalloys for electrocatalytic H2O2 production. J. Am. Chem. Soc. 2011, 133, 19432. [Google Scholar] [CrossRef]
- Samira, S.; Arnau, V.C.; Mohammadreza, K.; Davide, D.; Paolo, M.; Björn, W.; María, E.E.; Paoli, E.A.; Rasmus, F.; Hansen, T.W. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar]
- Liu, S.S.; Dong, W.; Zeng, X.F.; Guo, Z.L.; Zong, P.X.; Li, B.D.; Meng, X.G.; Zuo, G.F. β-cyclodextrin modified g-C3N4 nanosheet: A fluorescent drug carrier with ultrahigh drug loading capacity and pH-responsive release. J. Chem. Technol. Biot. 2019, 94, 628–633. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. Analysis Method: FTIR studies of β -phase crystal formation in stretched PVDF films. Polym. Test. 2003, 22, 699–704. [Google Scholar] [CrossRef]
- Song, W.C.; Hu, J.; Zhao, Y.; Shao, D.D.; Li, J.X. Efficient removal of cobalt from aqueous solution using β-cyclodextrin modified graphene oxide. RSC Adv. 2013, 3, 9514–9521. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Chen, D.H. Cyclodextrin conjugated magnetic colloidal nanoparticles as a nanocarrier for targeted anticancer drug delivery. Nanotechnology 2008, 19, 265602. [Google Scholar] [CrossRef]
- Xu, X.Y.; Shang, H.; Zhang, T.Y.; Shu, P.J.; Liu, Y.P.; Xie, J.; Zhang, D.Y.; Tan, H.; Li, J.S. A stimuli-responsive insulin delivery system based on reversible phenylboronate modified cyclodextrin with glucose triggered host-guest interaction. Int. J. Pharm. 2018, 548, 649–658. [Google Scholar] [CrossRef]
- Ge, L. Synthesis and photocatalytic performance of novel metal-free g-C3N4 photocatalysts. Mater. Lett. 2011, 65, 2652–2654. [Google Scholar] [CrossRef]
- Gopalan, S.A.; Gopalan, A.I.; Vinu, A.; Lee, K.P.; Kang, S.W. A new optical-electrical integrated buffer layer design based on gold nanoparticles tethered thiol containing sulfonated polyaniline towards enhancement of solar cell performance. Sol. Energy Mat. Sol. Cells 2018, 174, 112–123. [Google Scholar] [CrossRef]
- Anand, G.S.; Gopalan, A.I.; Kang, S.W.; Lee, K.P. Development of a surface plasmon assisted label-free calorimetric method for sensitive detection of mercury based on functionalized gold nanorods. J. Anal. Atom. Spectrom. 2013, 28, 488–498. [Google Scholar] [CrossRef]
- Lin, L.; Ou, H.H.; Zhang, Y.; Wang, X. Tri-s-triazine-based Crystalline Graphitic Carbon Nitrides for Highly Efficient Hydrogen Evolution Photocatalysis. ACS Catal. 2016, 6, 3921–3931. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Photocatalytic reduction of carbon dioxide with water vapors over montmorillonite modified TiO2 nanocomposites. Appl. Catal. B-Environ. 2013, 142, 512–522. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, W.; Pavaskar, P.; Aykol, M.; Cronin, S.B. Plasmon resonant enhancement of photocatalytic water splitting under visible illumination. Nano Lett. 2011, 11, 1111–1116. [Google Scholar] [CrossRef]
- Feng, D.X.; Nguyen, A.V.; Tong, X. Effect of contact angle and contact angle hysteresis on the floatability of spheres at the air-water interface. Adv. Colloid Interface Sci. 2017, 248, 69–84. [Google Scholar] [CrossRef]
- Kuriki, R.; Matsunaga, H.; Nakashima, T.; Wada, K.; Yamakata, A.; Ishitani, O.; Maeda, K. Nature-Inspired, Highly Durable CO2 Reduction System Consisting of a Binuclear Ruthenium(II) Complex and an Organic Semiconductor Using Visible Light. J. Am. Chem. Soc. 2016, 138, 5159–5170. [Google Scholar] [CrossRef]
- Balbuena, P.B.; Calvo, S.R.; Lamas, E.J.; Salazar, P.F.; Seminario, J.M. Adsorption and Dissociation of H2O2 on Pt and Pt-Alloy Clusters and Surfaces. J. Phys. Chem. B 2006, 110, 17452–17459. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kanazawa, S.; Sugano, Y.; Tsukamoto, D.; Sakamoto, H.; Ichikawa, S.; Hirai, T. Highly Selective Production of Hydrogen Peroxide on Graphitic Carbon Nitride (g-C3N4) Photocatalyst Activated by Visible Light. ACS Catal. 2014, 4, 774–780. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, G.; Zhang, Y.; Liu, S.; Guo, Z.; Zhao, Q.; Saianand, G.; Feng, L.; Li, L.; Li, W.; Zhang, N.; et al. A β-cyclodextrin Modified Graphitic Carbon Nitride with Au Co-Catalyst for Efficient Photocatalytic Hydrogen Peroxide Production. Nanomaterials 2020, 10, 1969. https://doi.org/10.3390/nano10101969
Zuo G, Zhang Y, Liu S, Guo Z, Zhao Q, Saianand G, Feng L, Li L, Li W, Zhang N, et al. A β-cyclodextrin Modified Graphitic Carbon Nitride with Au Co-Catalyst for Efficient Photocatalytic Hydrogen Peroxide Production. Nanomaterials. 2020; 10(10):1969. https://doi.org/10.3390/nano10101969
Chicago/Turabian StyleZuo, Guifu, Yuqian Zhang, Shanshan Liu, Zhaoliang Guo, Qiannan Zhao, Gopalan Saianand, Liwei Feng, Lijuan Li, Wangze Li, Ning Zhang, and et al. 2020. "A β-cyclodextrin Modified Graphitic Carbon Nitride with Au Co-Catalyst for Efficient Photocatalytic Hydrogen Peroxide Production" Nanomaterials 10, no. 10: 1969. https://doi.org/10.3390/nano10101969






