Effects of Zn-Doped Mesoporous Bioactive Glass Nanoparticles in Etch-and-Rinse Adhesive on the Microtensile Bond Strength
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MBN and MBN-Zn
2.2. Characterisation of MBN and MBN-Zn
2.3. Fabrication of Experimental Samples
2.3.1. Preparation of DA Mixed with MBN (DA-MBN) and DA Mixed with Zn-Doped MBN (DA-MBN-Zn)
2.3.2. Resin Disk Preparation
2.3.3. Resin/Dentin Beam Preparation
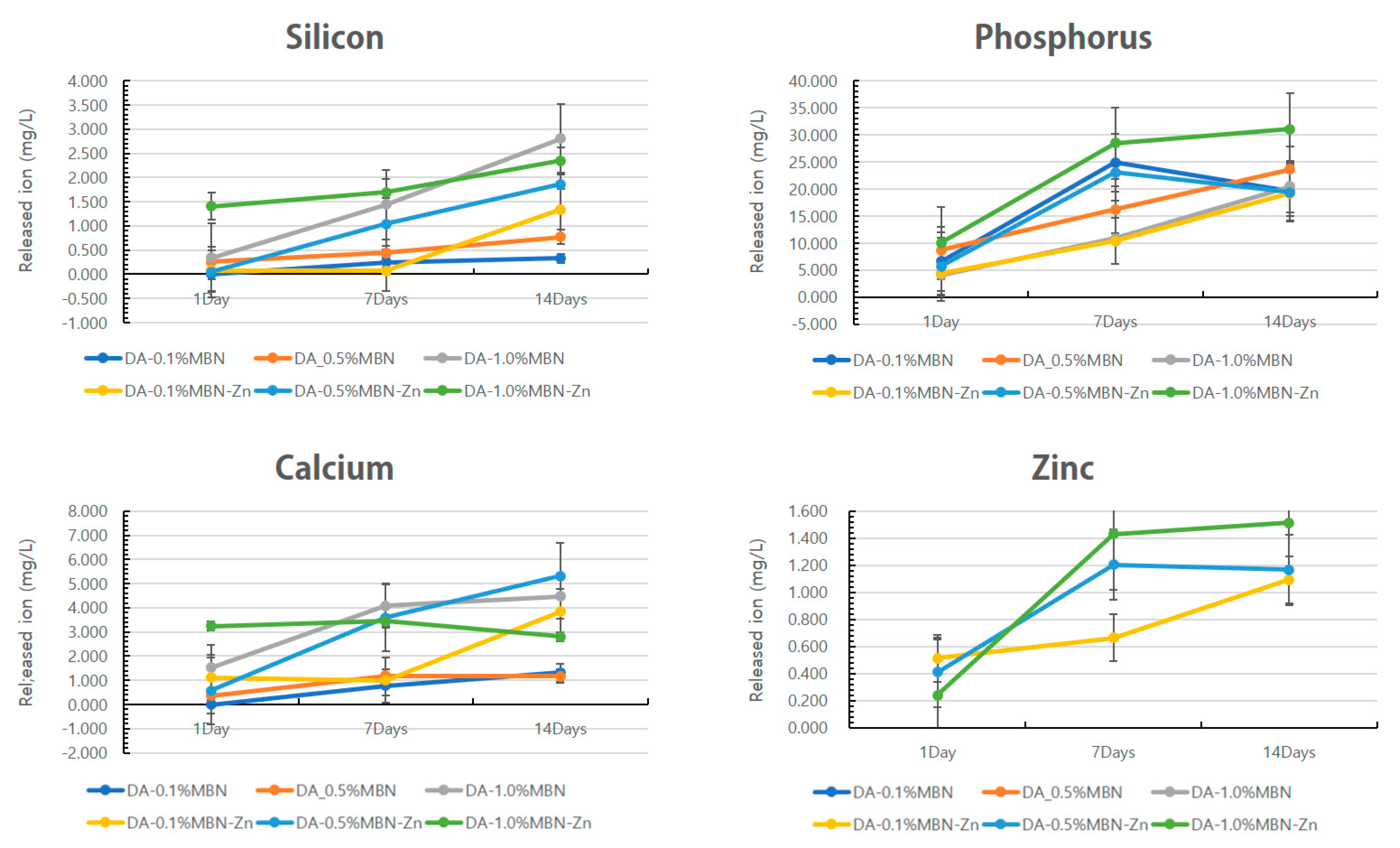
2.4. Dissolution Test
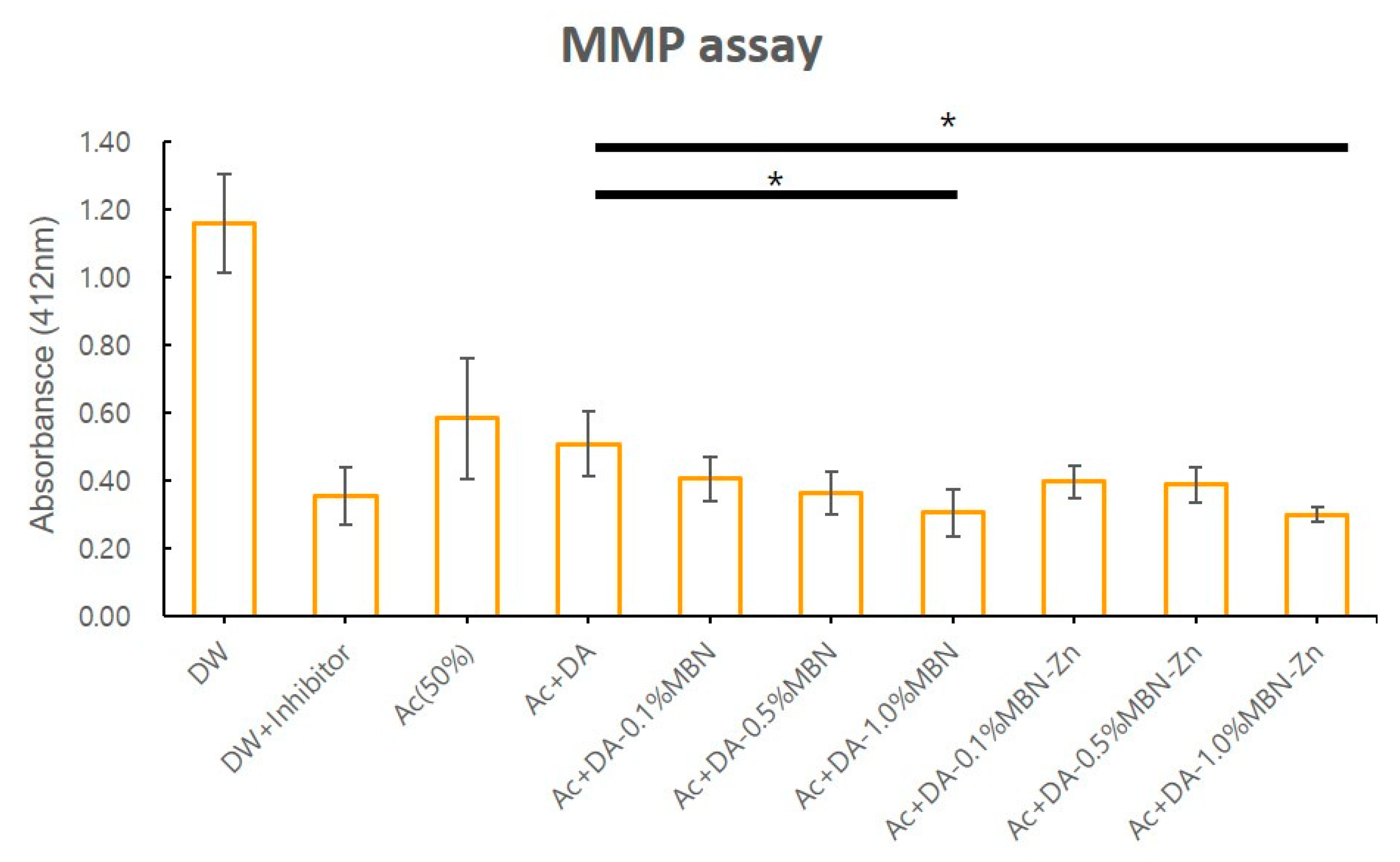
2.5. Matrix Metalloproteinases (MMPs) Inhibition Test
2.6. Mechanical Properties
2.6.1. Microtensile Bond Strength (MTBS) Test
2.6.2. Thermocycling Test
2.7. Biological Properties
2.7.1. Cell Culture and MTT Assay
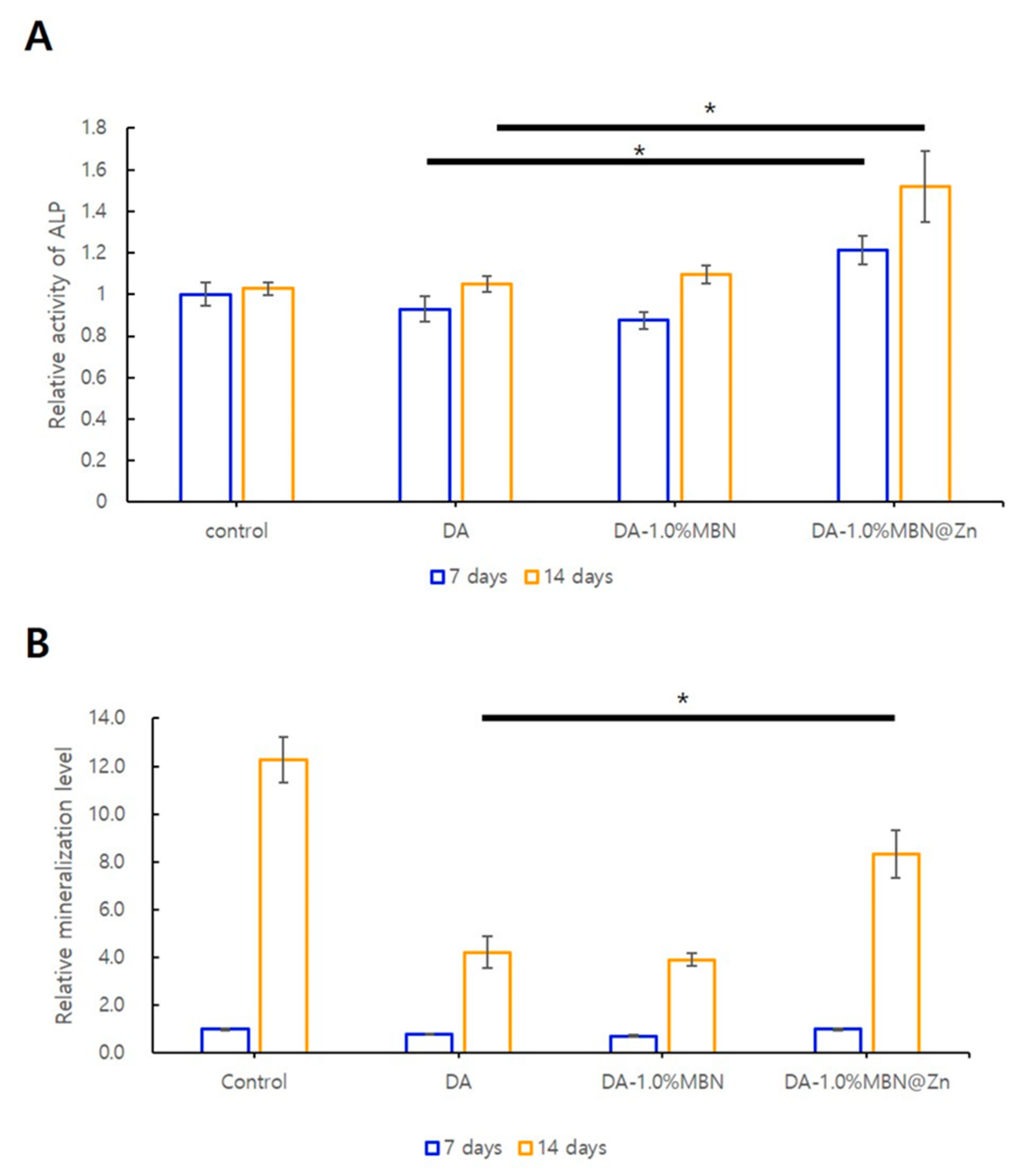
2.7.2. Quantitative Assay of Alkaline Phosphatase (ALP) Activity
2.7.3. Alizarin Red S (ARS) Staining and Quantitative Detection
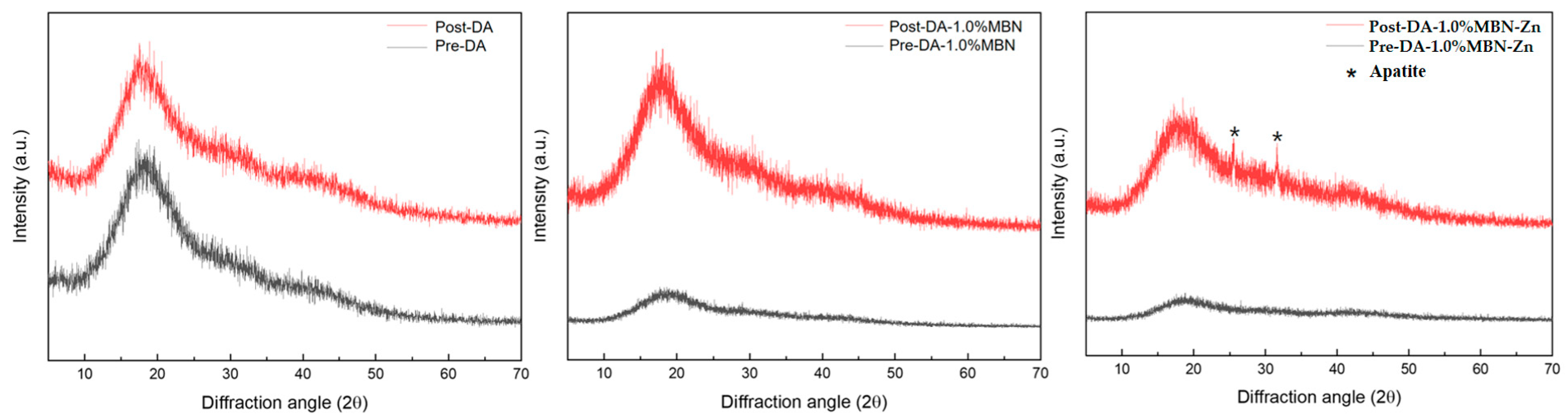
2.8. Non-Cellular Bioactivity of DA-MBN and DA-MBN-Zn
2.9. Statistical Analysis
3. Results
3.1. Characterisation of MBN and MBN-Zn
3.2. Characterisation of DA-MBN and DA-MBN-Zn
In-Vitro Dissolution Test
3.3. MMP Inhibition Test
3.4. Mechanical Properties
3.4.1. MTBS Test
3.4.2. MTBS Test after Thermocycling
3.5. Biological Properties
3.5.1. MTT Assay
3.5.2. ALP Activity in hDPSCs
3.5.3. ARS Staining
3.6. Non-Cellular Bioactivity of DA-MBN and DA-MBN-Zn
3.6.1. SEM after SBF Soaking
3.6.2. XRD after SBF Soaking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Perdigao, J.; Reis, A.; Loguercio, A.D. Dentin adhesion and MMPs: A comprehensive review. J. Esthet. Restor. Dent. 2013, 25, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, N.; Kojima, K.; Masuhara, E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J. Biomed. Mater. Res. 1982, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Inokoshi, S.; Braem, M.; Lambrechts, P.; Vanherle, G. Morphological aspects of the resin-dentin interdiffusion zone with different dentin adhesive systems. J. Dent. Res. 1992, 71, 1530–1540. [Google Scholar] [CrossRef]
- Hashimoto, M.; Ohno, H.; Kaga, M.; Endo, K.; Sano, H.; Oguchi, H. In vivo degradation of resin-dentin bonds in humans over 1 to 3 years. J. Dent. Res. 2000, 79, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spencer, P. Hybridization efficiency of the adhesive/dentin interface with wet bonding. J. Dent. Res. 2003, 82, 141–145. [Google Scholar] [CrossRef]
- Hashimoto, M.; Ohno, H.; Kaga, M.; Sano, H.; Tay, F.R.; Oguchi, H.; Araki, Y.; Kubota, M. Over-etching effects on micro-tensile bond strength and failure patterns for two dentin bonding systems. J. Dent. 2002, 30, 99–105. [Google Scholar] [CrossRef]
- Spencer, P.; Jonggu Park, Q.Y.; Misra, A.; Bohaty, B.S.; Singh, V.; Parthasarathy, R.; Sene, F.; de Paiva Goncalves, S.E.; Laurence, J. Durable bonds at the adhesive/dentin interface: An impossible mission or simply a moving target? Braz. Dent. Sci. 2012, 15, 4–18. [Google Scholar] [CrossRef] [Green Version]
- Barcellos, D.C.; Batista, G.R.; Silva, M.A.; Pleffken, P.R.; Rangel, P.M.; Fernandes, V.V., Jr.; Di Nicolo, R.; Torres, C.R. Two-year clinical performance of self-etching adhesive systems in composite restorations of anterior teeth. Oper. Dent. 2013, 38, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Barcellos, D.C.; Fonseca, B.M.; Pucci, C.R.; Cavalcanti, B.; Persici Ede, S.; Goncalves, S.E. Zn-doped etch-and-rinse model dentin adhesives: Dentin bond integrity, biocompatibility, and properties. Dent. Mater. 2016, 32, 940–950. [Google Scholar] [CrossRef] [Green Version]
- Toledano, M.; Sauro, S.; Cabello, I.; Watson, T.; Osorio, R. A Zn-doped etch-and-rinse adhesive may improve the mechanical properties and the integrity at the bonded-dentin interface. Dent. Mater. 2013, 29, e142–e152. [Google Scholar] [CrossRef]
- Carrilho, M.R.; Tay, F.R.; Donnelly, A.M.; Agee, K.A.; Tjaderhane, L.; Mazzoni, A.; Breschi, L.; Foulger, S.; Pashley, D.H. Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. J. Biomed. Mater. Res. B 2009, 90, 373–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledano, M.; Yamauti, M.; Ruiz-Requena, M.E.; Osorio, R. A ZnO-doped adhesive reduced collagen degradation favouring dentine remineralization. J. Dent. 2012, 40, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Woessner, J.F., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio, R.; Yamauti, M.; Osorio, E.; Ruiz-Requena, M.E.; Pashley, D.; Tay, F.; Toledano, M. Effect of dentin etching and chlorhexidine application on metalloproteinase-mediated collagen degradation. Eur. J. Oral Sci. 2011, 119, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Brinckerhoff, C.E.; Matrisian, L.M. Matrix metalloproteinases: A tail of a frog that became a prince. Nat. Rev. Mol. Cell Biol. 2002, 3, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [Green Version]
- Loffek, S.; Schilling, O.; Franzke, C.W. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.; Jung, H.S.; Kim, H.J.; Jang, J.H.; Kim, D.S.; Choi, K.K.; Kim, S.Y. Effect of zinc on the collagen degradation in acid-etched dentin. J. Dent. Sci. 2018, 13, 97–102. [Google Scholar] [CrossRef]
- Femiano, F.; Femiano, R.; Femiano, L.; Jamilian, A.; Rullo, R.; Perillo, L. Dentin caries progression and the role of metalloproteinases: An update. Eur. J. Paediatr. Dent. 2016, 17, 243–247. [Google Scholar]
- Bourd-Boittin, K.; Fridman, R.; Fanchon, S.; Septier, D.; Goldberg, M.; Menashi, S. Matrix metalloproteinase inhibition impairs the processing, formation and mineralization of dental tissues during mouse molar development. Exp. Cell Res. 2005, 304, 493–505. [Google Scholar] [CrossRef]
- Mazzoni, A.; Mannello, F.; Tay, F.R.; Tonti, G.A.; Papa, S.; Mazzotti, G.; Di Lenarda, R.; Pashley, D.H.; Breschi, L. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J. Dent. Res. 2007, 86, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Sulkala, M.; Tervahartiala, T.; Sorsa, T.; Larmas, M.; Salo, T.; Tjaderhane, L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch. Oral Biol 2007, 52, 121–127. [Google Scholar] [CrossRef]
- Osorio, R.; Yamauti, M.; Osorio, E.; Ruiz-Requena, M.E.; Pashley, D.H.; Tay, F.R.; Toledano, M. Zinc reduces collagen degradation in demineralized human dentin explants. J. Dent. 2011, 39, 148–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaussain-Miller, C.; Fioretti, F.; Goldberg, M.; Menashi, S. The role of matrix metalloproteinases (MMPs) in human caries. J. Dent. Res. 2006, 85, 22–32. [Google Scholar] [CrossRef]
- Steinsvoll, S. Periodontal disease, matrix metalloproteinases and chemically modified tetracyclines. Microb. Ecol. Health Dis. 2004, 16, 1–7. [Google Scholar]
- Toledano, M.; Yamauti, M.; Osorio, E.; Osorio, R. Zinc-inhibited MMP-mediated collagen degradation after different dentine demineralization procedures. Caries Res. 2012, 46, 201–207. [Google Scholar] [CrossRef]
- Kim, J.; Uchiyama, T.; Carrilho, M.; Agee, K.A.; Mazzoni, A.; Breschi, L.; Carvalho, R.M.; Tjaderhane, L.; Looney, S.; Wimmer, C.; et al. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent. Mater. 2010, 26, 771–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breschi, L.; Martin, P.; Mazzoni, A.; Nato, F.; Carrilho, M.; Tjaderhane, L.; Visintini, E.; Cadenaro, M.; Tay, F.R.; De Stefano Dorigo, E.; et al. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent. Mater. 2010, 26, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonocore, M.G. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J. Dent. Res. 1955, 34, 849–853. [Google Scholar] [CrossRef]
- Kaya, S.; Cresswell, M.; Boccaccini, A.R. Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mat. Sci. Eng. C-Mater. 2018, 83, 99–107. [Google Scholar] [CrossRef]
- Kwon, S.; Singh, R.K.; Perez, R.A.; Abou Neel, E.A.; Kim, H.W.; Chrzanowski, W. Silica-based mesoporous nanoparticles for controlled drug delivery. J. Tissue Eng. 2013, 4, 2041731413503357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Santhiya, D. Mesoporous Bioactive Glass and its Applications. In Bioactive Glasses; Elsevier: Amsterdam, The Netherlands, 2018; pp. 63–85. [Google Scholar]
- Xia, W.; Chang, J. Preparation and characterization of nano-bioactive-glasses (NBG) by a quick alkali-mediated sol–gel method. Mater. Lett. 2007, 61, 3251–3253. [Google Scholar] [CrossRef]
- Lusvardi, G.; Malavasi, G.; Menabue, L.; Aina, V.; Morterra, C. Fluoride-containing bioactive glasses: Surface reactivity in simulated body fluids solutions. Acta Biomater. 2009, 5, 3548–3562. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; ISO 10993-12; ISO: Geneva, Swtizerland, 2012. [Google Scholar]
- Sabatini, C.; Scheffel, D.L.; Scheffel, R.H.; Agee, K.A.; Rouch, K.; Takahashi, M.; Breschi, L.; Mazzoni, A.; Tjaderhane, L.; Tay, F.R.; et al. Inhibition of endogenous human dentin MMPs by Gluma. Dent. Mater. 2014, 30, 752–758. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, V.V.B., Jr.; Rodrigues, J.R.; da Silva, J.M.F.; Pagani, C.; Malaquias, H.; Balducci, I. Microtensile bond strength of two self-adhesive cements to enamel and dentin: Bonding efficiency and thermocycling effect. Braz. Dent. Sci. 2012, 15, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Han, Y.; Zhao, X.Y.; Wang, Z.Y.; He, H.M. Microtensile bond strength of one- and two-step self-etching adhesives on sclerotic dentin: The effects of thermocycling. Oper. Dent. 2010, 35, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Toledano, M.; Aguilera, F.S.; Osorio, E.; Cabello, I.; Osorio, R. Microanalysis of thermal-induced changes at the resin-dentin interface. Microsc. Microanal. 2014, 20, 1218–1233. [Google Scholar] [CrossRef]
- Jun, S.K.; Yang, S.A.; Kim, Y.J.; El-Fiqi, A.; Mandakhbayar, N.; Kim, D.S.; Roh, J.; Sauro, S.; Kim, H.W.; Lee, J.H.; et al. Multi-functional nano-adhesive releasing therapeutic ions for MMP-deactivation and remineralization. Sci. Rep. 2018, 8, 5663. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; El-Fiqi, A.; Mandakhbayar, N.; Lee, H.H.; Kim, H.W. Drug/ion co-delivery multi-functional nanocarrier to regenerate infected tissue defect. Biomaterials 2017, 142, 62–76. [Google Scholar] [CrossRef]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar]
- Carrilho, E.; Cardoso, M.; Marques Ferreira, M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP Based Dental Adhesives: Adhesive Interface Characterization and Adhesive Stability-A Systematic Review. Materials 2019, 12, 790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz, M.A.; Luque-Martinez, I.; Malaquias, P.; Hass, V.; Reis, A.; Campanha, N.H.; Loguercio, A.D. In vitro longevity of bonding properties of universal adhesives to dentin. Oper. Dent. 2015, 40, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Neščáková, Z.; Zheng, K.; Liverani, L.; Nawaz, Q.; Galusková, D.; Kaňková, H.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Multifunctional zinc ion doped sol—Gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019, 4, 312–321. [Google Scholar] [CrossRef]
- Braga, R.R.; Fronza, B.M. The use of bioactive particles and biomimetic analogues for increasing the longevity of resin-dentin interfaces: A literature review. Dent. Mater. J. 2020, 39, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makowski, G.S.; Ramsby, M.L. Differential effect of calcium phosphate and calcium pyrophosphate on binding of matrix metalloproteinases to fibrin: Comparison to a fibrin-binding protease from inflammatory joint fluids. Clin. Exp. Immunol. 2004, 136, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Kremer, E.A.; Chen, Y.; Suzuki, K.; Nagase, H.; Gorski, J.P. Hydroxyapatite induces autolytic degradation and inactivation of matrix metalloproteinase-1 and -3. J. Bone Miner. Res. 1998, 13, 1890–1902. [Google Scholar] [CrossRef]
- Tjaderhane, L.; Larjava, H.; Sorsa, T.; Uitto, V.J.; Larmas, M.; Salo, T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J. Dent. Res. 1998, 77, 1622–1629. [Google Scholar] [CrossRef]
- Tezvergil-Mutluay, A.; Seseogullari-Dirihan, R.; Feitosa, V.P.; Cama, G.; Brauer, D.S.; Sauro, S. Effects of Composites Containing Bioactive Glasses on Demineralized Dentin. J. Dent. Res. 2017, 96, 999–1005. [Google Scholar] [CrossRef]
- Bauer, J.; Silva, E.S.A.; Carvalho, E.M.; Ferreira, P.V.C.; Carvalho, C.N.; Manso, A.P.; Carvalho, R.M. Dentin pretreatment with 45S5 and niobophosphate bioactive glass: Effects on pH, antibacterial, mechanical properties of the interface and microtensile bond strength. J. Mech. Behave. Biomed. 2019, 90, 374–380. [Google Scholar] [CrossRef]
- Saboia, V.P.; Silva, F.C.; Nato, F.; Mazzoni, A.; Cadenaro, M.; Mazzotti, G.; Giannini, M.; Breschi, L. Analysis of differential artificial ageing of the adhesive interface produced by a two-step etch-and-rinse adhesive. Eur. J. Oral Sci. 2009, 117, 618–624. [Google Scholar] [CrossRef]
- Tjaderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.; Carvalho, R.M.; Tay, F.R.; et al. Strategies to prevent hydrolytic degradation of the hybrid layer-A review. Dent. Mater. 2013, 29, 999–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, F.R.; Pashley, D.H. Dental adhesives of the future. J. Adhes. Dent. 2002, 4, 91–103. [Google Scholar] [PubMed]
- Kavrik, F.; Kucukyilmaz, E. The effect of different ratios of nano-sized hydroxyapatite fillers on the micro-tensile bond strength of an adhesive resin. Microsc. Res. Tech. 2019, 82, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Reichl, F.X.; Shi, J.; He, X.; Hickel, R.; Högg, C. Cytotoxicity and DNA double-strand breaks in human gingival fibroblasts exposed to eluates of dental composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 34, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Tauböck, T.T.; Marovic, D.; Zeljezic, D.; Steingruber, A.D.; Attin, T.; Tarle, Z. Genotoxic potential of dental bulk-fill resin composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2017, 33, 788–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helvatjoglu-Antoniades, M.; Koliniotou-Kubia, E.; Dionyssopoulos, P. The effect of thermal cycling on the bovine dentine shear bond strength of current adhesive systems. J. Oral Rehabil. 2004, 31, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.L.; Colucci, V.; Palma-Dibb, R.G.; Corona, S.A. Assessment of in vitro methods used to promote adhesive interface degradation: A critical review. J. Esthet. Restor. Dent. 2007, 19, 340–353. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.P.; Gerlach, R.F.; Line, S.R. Inhibition of human gingival gelatinases (MMP-2 and MMP-9) by metal salts. Dent. Mater. 2000, 16, 103–108. [Google Scholar] [CrossRef]
- Tezvergil-Mutluay, A.; Agee, K.A.; Hoshika, T.; Carrilho, M.; Breschi, L.; Tjaderhane, L.; Nishitani, Y.; Carvalho, R.M.; Looney, S.; Tay, F.R.; et al. The requirement of zinc and calcium ions for functional MMP activity in demineralizaed dentin matrices. Dent. Mater. 2010, 26, 1059–1067. [Google Scholar] [CrossRef] [Green Version]



| Composition (mol%) | ||||
|---|---|---|---|---|
| SiO2 | CaO | P2O5 | ZnO | |
| MBN | 65 | 31 | 4 | 0 |
| MBN-Zn | 60 | 31 | 4 | 5 |
| Group | n | MTBS | MTBS after Thermocycling |
|---|---|---|---|
| DA | 15 | 36.59 ± 10.66 | 28.77 ± 4.16 |
| DA-0.1% MBN | 15 | 36.83 ± 10.18 | 35.66 ± 4.35 |
| DA-0.5% MBN | 15 | 37.59 ± 11.31 | 36.98 ± 5.30 |
| DA-1.0% MBN | 15 | 37.64 ± 8.11 | 37.57 ± 10.29 * |
| DA-0.1% MBN-Zn | 15 | 36.25 ± 7.50 | 35.59 ± 10.02 |
| DA-0.5% MBN-Zn | 15 | 37.02 ± 8.89 | 35.81 ± 11.35 |
| DA-1.0% MBN-Zn | 15 | 38.39 ± 8.09 | 37.85 ± 7.12 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Sun, W.; Kim, Y.; Kim, I.-R.; Gong, M.-K.; Yoon, S.-Y.; Bae, M.-K.; Park, B.-S.; Park, S.-B.; Kim, Y.-I. Effects of Zn-Doped Mesoporous Bioactive Glass Nanoparticles in Etch-and-Rinse Adhesive on the Microtensile Bond Strength. Nanomaterials 2020, 10, 1943. https://doi.org/10.3390/nano10101943
Choi Y, Sun W, Kim Y, Kim I-R, Gong M-K, Yoon S-Y, Bae M-K, Park B-S, Park S-B, Kim Y-I. Effects of Zn-Doped Mesoporous Bioactive Glass Nanoparticles in Etch-and-Rinse Adhesive on the Microtensile Bond Strength. Nanomaterials. 2020; 10(10):1943. https://doi.org/10.3390/nano10101943
Chicago/Turabian StyleChoi, Yeonju, Woogyeong Sun, Yeon Kim, In-Ryoung Kim, Mi-Kyung Gong, Seog-Young Yoon, Moon-Kyoung Bae, Bong-Soo Park, Soo-Byung Park, and Yong-Il Kim. 2020. "Effects of Zn-Doped Mesoporous Bioactive Glass Nanoparticles in Etch-and-Rinse Adhesive on the Microtensile Bond Strength" Nanomaterials 10, no. 10: 1943. https://doi.org/10.3390/nano10101943





