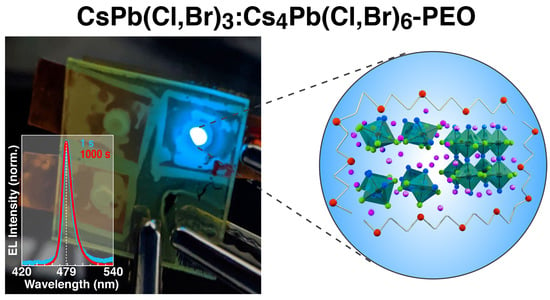
Suppression of Electric Field-Induced Segregation in Sky-Blue Perovskite Light-Emitting Electrochemical Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solvent Engineering
2.2. Thin Films Morphology and Structure Description
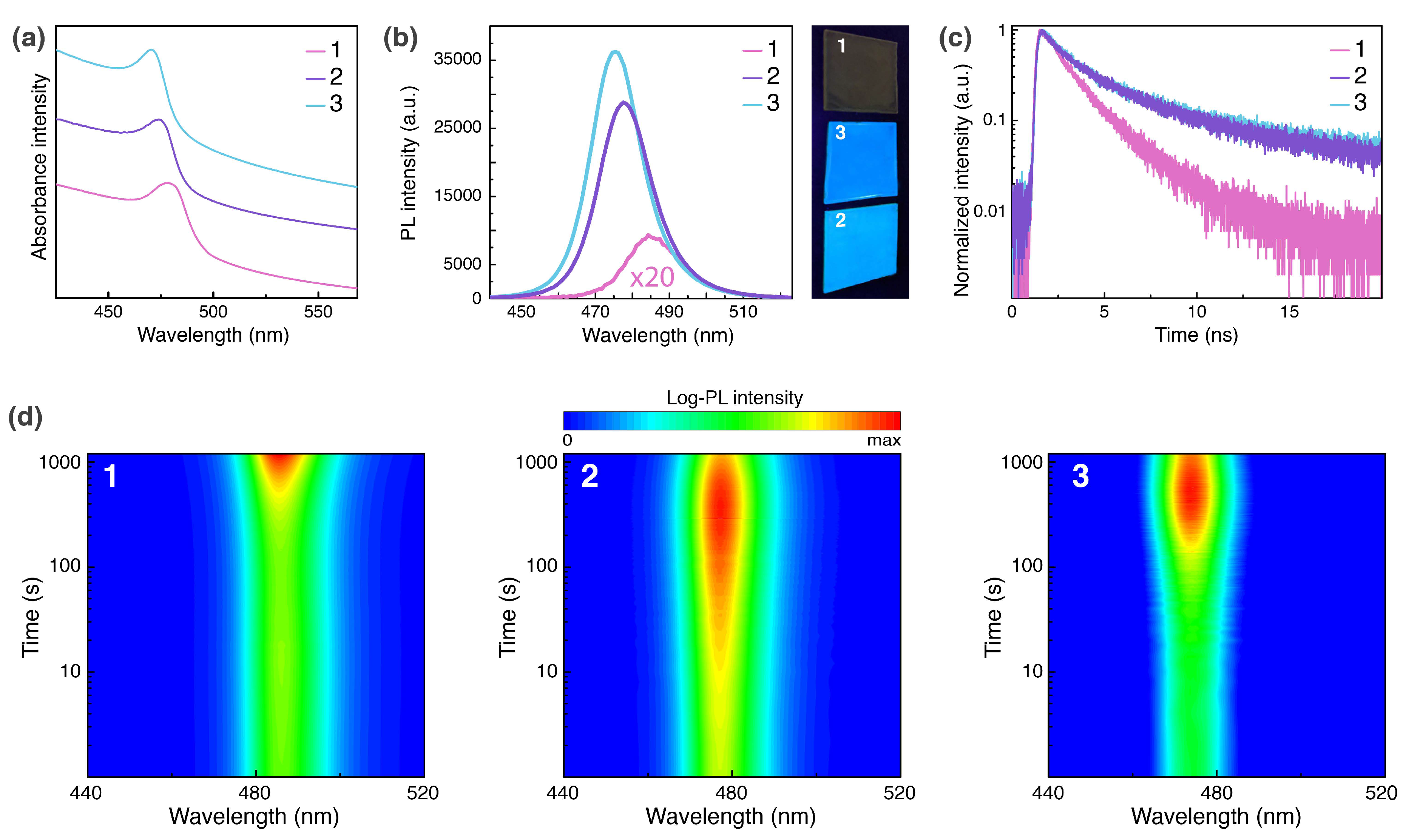
2.3. Photophysical Properties
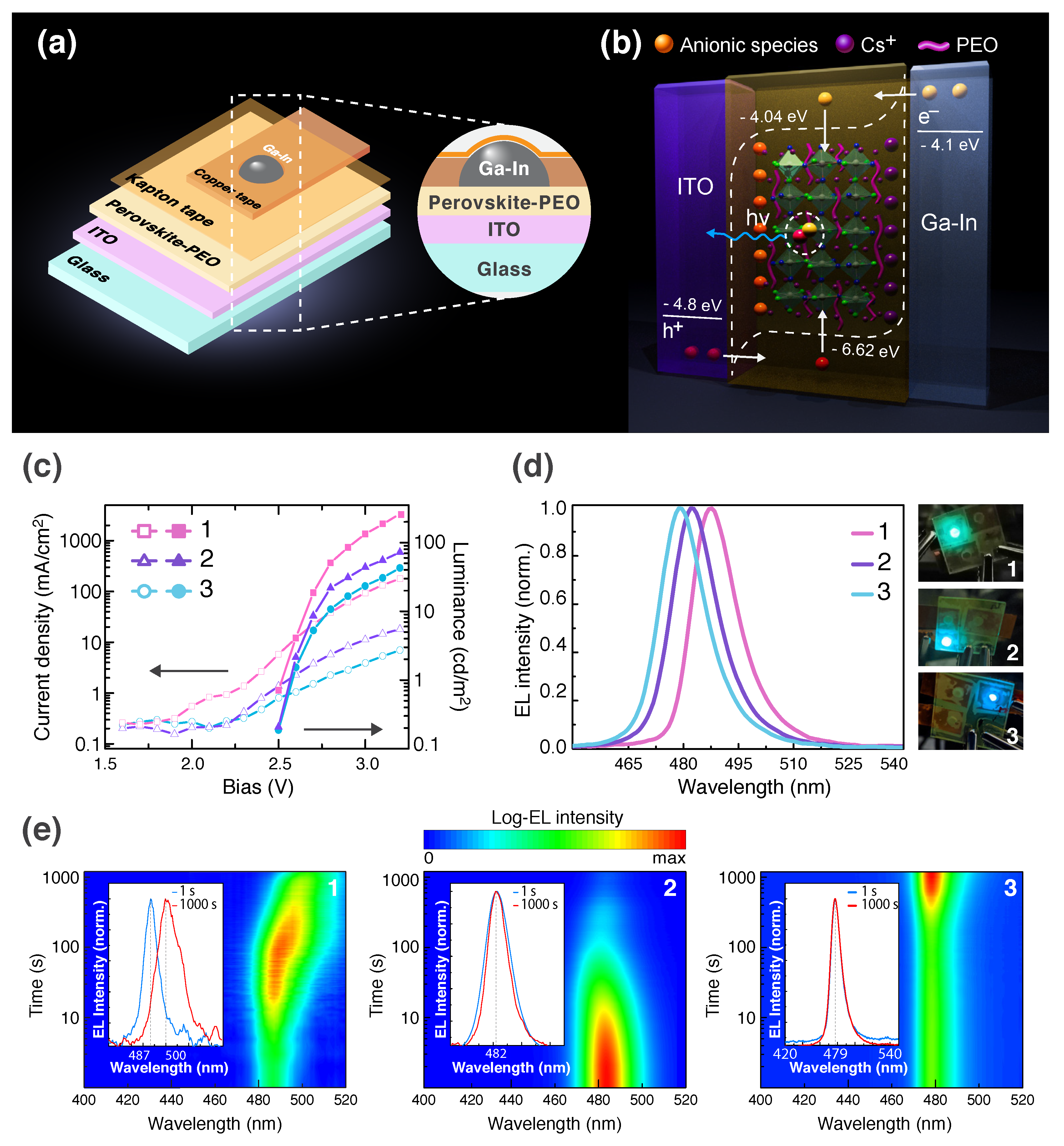
2.4. PeLECs Fabrication and Characterization
3. Experimental Section
3.1. Materials
3.2. Preparation of Composite Solutions
3.3. Thin Films Deposition
3.4. Characterization of Thin Films
3.5. Device Fabrication and Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EL | Electroluminescence |
| EQE | External quantum efficiency |
| PeLECs | Perovskite light-emitting electrochemical cells |
| PeLEDs | Perovskite light-emitting devices |
| PEA | Phenylethylammonium |
| PEO | Poly(ethylene oxide) |
| PL | Photoluminescence |
| SHV | Surface halide vacancies |
References
- Huang, H.; Bodnarchuk, M.I.; Kershaw, S.V.; Kovalenko, M.V.; Rogach, A.L. Lead halide perovskite nanocrystals in the research spotlight: Stability and defect tolerance. ACS Energy Lett. 2017, 2, 2071–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, W.; Tress, W. Review on Recent Progress of All-Inorganic Metal Halide Perovskites and Solar Cells. Adv. Mater. 2019, 31, 1902851. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bao, Q.; Shen, L.; Ding, L. Potential applications for perovskite solar cells in space. Nano Energy 2020, 76, 105019. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Zhang, H.; Ma, W.; Wang, L.; Zong, X. Organic-Inorganic Hybrid Perovskites: Game-Changing Candidates for Solar Fuel Production. Nano Energy 2020, 71, 104647. [Google Scholar] [CrossRef]
- Becker, M.A.; Vaxenburg, R.; Nedelcu, G.; Sercel, P.C.; Shabaev, A.; Mehl, M.J.; Michopoulos, J.G.; Lambrakos, S.G.; Bernstein, N.; Lyons, J.L.; et al. Bright triplet excitons in caesium lead halide perovskites. Nature 2018, 553, 189–193. [Google Scholar] [CrossRef]
- Herz, L.M. Charge-carrier mobilities in metal halide perovskites: Fundamental mechanisms and limits. ACS Energy Lett. 2017, 2, 1539–1548. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef]
- Liashenko, T.G.; Cherotchenko, E.D.; Pushkarev, A.P.; Pakštas, V.; Naujokaitis, A.; Khubezhov, S.A.; Polozkov, R.G.; Agapev, K.B.; Zakhidov, A.A.; Shelykh, I.A.; et al. Electronic structure of CsPbBr3−xClx perovskites: Synthesis, experimental characterization, and DFT simulations. Phys. Chem. Chem. Phys. 2019, 21, 18930–18938. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.; Wang, S.; Guo, J.; Yu, W.W.; Rogach, A.L. Metal halide perovskite light-emitting devices: Promising technology for next-generation displays. Adv. Funct. Mater. 2019, 29, 1902008. [Google Scholar] [CrossRef]
- Mak, C.H.; Huang, X.; Liu, R.; Tang, Y.; Han, X.; Ji, L.; Zou, X.; Zou, G.; Hsu, H.Y. Recent progress in surface modification and interfacial engineering for high-performance perovskite light-emitting diodes. Nano Energy 2020, 73, 104752. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Wu, Q.; Cao, F.; Yang, D.; Shang, Y.; Ning, Z.; Zhang, W.; Zheng, W.; Yan, Y.; et al. Trifluoroacetate induced small-grained CsPbBr 3 perovskite films result in efficient and stable light-emitting devices. Nat. Commun. 2019, 10, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Zhao, J.; Yang, G.; Xu, X.; Pan, G. Ammonium acetate passivated CsPbI3 perovskite nanocrystals for efficient red light-emitting diodes. Nanoscale 2020, 12, 7712–7719. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, Y.; Kershaw, S.V.; Li, T.; Wang, C.; Zhang, X.; Wang, W.; Li, D.; Wang, Y.; Lu, M.; et al. Zn-alloyed CsPbI3 nanocrystals for highly efficient perovskite light-emitting devices. Nano Lett. 2019, 19, 1552–1559. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, X.; Bai, X.; Wu, H.; Shen, X.; Zhang, Y.; Zhang, W.; Zheng, W.; Song, H.; Yu, W.W.; et al. Spontaneous silver doping and surface passivation of CsPbI3 perovskite active layer enable light-emitting devices with an external quantum efficiency of 11.2%. ACS Energy Lett. 2018, 3, 1571–1577. [Google Scholar] [CrossRef]
- Li, G.; Rivarola, F.W.R.; Davis, N.J.; Bai, S.; Jellicoe, T.C.; de la Peña, F.; Hou, S.; Ducati, C.; Gao, F.; Friend, R.H.; et al. Highly efficient perovskite nanocrystal light-emitting diodes enabled by a universal crosslinking method. Adv. Mater. 2016, 28, 3528–3534. [Google Scholar] [CrossRef]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J.; et al. Highly efficient perovskite-quantum-dot light-emitting diodes by surface engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef]
- Gangishetty, M.K.; Hou, S.; Quan, Q.; Congreve, D.N. Reducing Architecture Limitations for Efficient Blue Perovskite Light-Emitting Diodes. Adv. Mater. 2018, 30, 1706226. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Vashishtha, P.; Ng, M.; Shivarudraiah, S.B.; Halpert, J.E. High efficiency blue and green light-emitting diodes using Ruddlesden—Popper inorganic mixed halide perovskites with butylammonium interlayers. Chem. Mater. 2018, 31, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Hoke, E.T.; Slotcavage, D.J.; Dohner, E.R.; Bowring, A.R.; Karunadasa, H.I.; McGehee, M.D. Reversible photo-induced trap formation in mixed-halide hybrid perovskites for photovoltaics. Chem. Sci. 2015, 6, 613–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.J.; Kuno, M.; Kamat, P.V. Shift happens. How halide ion defects influence photoinduced segregation in mixed halide perovskites. ACS Energy Lett. 2017, 2, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Brennan, M.C.; Draguta, S.; Kamat, P.V.; Kuno, M. Light-induced anion phase segregation in mixed halide perovskites. ACS Energy Lett. 2017, 3, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Bischak, C.G.; Hetherington, C.L.; Wu, H.; Aloni, S.; Ogletree, D.F.; Limmer, D.T.; Ginsberg, N.S. Origin of reversible photoinduced phase separation in hybrid perovskites. Nano Lett. 2017, 17, 1028–1033. [Google Scholar] [CrossRef] [Green Version]
- Bischak, C.G.; Wong, A.B.; Lin, E.; Limmer, D.T.; Yang, P.; Ginsberg, N.S. Tunable polaron distortions control the extent of halide demixing in lead halide perovskites. J. Phys. Chem. Lett. 2018, 9, 3998–4005. [Google Scholar] [CrossRef] [Green Version]
- Barker, A.J.; Sadhanala, A.; Deschler, F.; Gandini, M.; Senanayak, S.P.; Pearce, P.M.; Mosconi, E.; Pearson, A.J.; Wu, Y.; Srimath Kandada, A.R.; et al. Defect-assisted photoinduced halide segregation in mixed-halide perovskite thin films. ACS Energy Lett. 2017, 2, 1416–1424. [Google Scholar] [CrossRef]
- Draguta, S.; Sharia, O.; Yoon, S.J.; Brennan, M.C.; Morozov, Y.V.; Manser, J.S.; Kamat, P.V.; Schneider, W.F.; Kuno, M. Rationalizing the light-induced phase separation of mixed halide organic—Inorganic perovskites. Nat. Commun. 2017, 8, 200. [Google Scholar] [CrossRef] [Green Version]
- Vashishtha, P.; Halpert, J.E. Field-driven ion migration and color instability in red-emitting mixed halide perovskite nanocrystal light-emitting diodes. Chem. Mater. 2017, 29, 5965–5973. [Google Scholar] [CrossRef]
- Li, J.; Shan, X.; Bade, S.G.R.; Geske, T.; Jiang, Q.; Yang, X.; Yu, Z. Single-layer halide perovskite light-emitting diodes with sub-band gap turn-on voltage and high brightness. J. Phys. Chem. Lett. 2016, 7, 4059–4066. [Google Scholar] [CrossRef]
- Shan, X.; Li, J.; Chen, M.; Geske, T.; Bade, S.G.R.; Yu, Z. Junction Propagation in Organometal Halide Perovskite–Polymer Composite Thin Films. J. Phys. Chem. Lett. 2017, 8, 2412–2419. [Google Scholar] [CrossRef]
- Jiang, Y.; Qin, C.; Cui, M.; He, T.; Liu, K.; Huang, Y.; Luo, M.; Zhang, L.; Xu, H.; Li, S.; et al. Spectra stable blue perovskite light-emitting diodes. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, J.; Zhao, Y.; Askerka, M.; Quan, L.N.; Gong, X.; Zhao, W.; Zhao, J.; Tan, H.; Long, G.; Gao, L.; et al. Color-stable highly luminescent sky-blue perovskite light-emitting diodes. Nat. Commun. 2018, 9, 3541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumawat, N.K.; Liu, X.K.; Kabra, D.; Gao, F. Blue perovskite light-emitting diodes: Progress, challenges and future directions. Nanoscale 2019, 11, 2109–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.w.; Kedem, N.; Kulbak, M.; Yang, W.S.; Jeon, N.J.; Seo, J.; Kim, G.; Kim, K.J.; Shin, T.J.; Hodes, G.; et al. Understanding how excess lead iodide precursor improves halide perovskite solar cell performance. Nat. Commun. 2018, 9, 3301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Hou, Y.; Sun, H.T.; Mohammed, O.F.; Sargent, E.H.; Bakr, O.M. Reducing Defects in Halide Perovskite Nanocrystals for Light-Emitting Applications. J. Phys. Chem. Lett. 2019, 10, 2629–2640. [Google Scholar] [CrossRef] [Green Version]
- Davies, C.L.; Filip, M.R.; Patel, J.B.; Crothers, T.W.; Verdi, C.; Wright, A.D.; Milot, R.L.; Giustino, F.; Johnston, M.B.; Herz, L.M. Bimolecular recombination in methylammonium lead triiodide perovskite is an inverse absorption process. Nat. Commun. 2018, 9, 293. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.; Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019, 48, 3842–3867. [Google Scholar] [CrossRef]
- Pushkarev, A.; Korolev, V.; Anoshkin, S.; Komissarenko, F.; Makarov, S.; Zakhidov, A. Cesium lead mixed-halide perovskites in polymer matrix. J. Phys. Conf. Ser. Iop Publ. 2018, 1092, 012122. [Google Scholar] [CrossRef]
- Yun, J.S.; Seidel, J.; Kim, J.; Soufiani, A.M.; Huang, S.; Lau, J.; Jeon, N.J.; Seok, S.I.; Green, M.A.; Ho-Baillie, A. Critical role of grain boundaries for ion migration in formamidinium and methylammonium lead halide perovskite solar cells. Adv. Energy Mater. 2016, 6, 1600330. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Cacovich, S.; Stavrakas, C.; Philippe, B.; Richter, J.M.; Alsari, M.; Booker, E.P.; Hutter, E.M.; Pearson, A.J.; et al. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 2018, 555, 497–501. [Google Scholar] [CrossRef]
- Lee, J.W.; Bae, S.H.; Hsieh, Y.T.; De Marco, N.; Wang, M.; Sun, P.; Yang, Y. A bifunctional lewis base additive for microscopic homogeneity in perovskite solar cells. Chem 2017, 3, 290–302. [Google Scholar] [CrossRef]
- Lin, H.S.; Okawa, S.; Ma, Y.; Yotsumoto, S.; Lee, C.; Tan, S.; Manzhos, S.; Yoshizawa, M.; Chiashi, S.; Lee, H.M.; et al. Polyaromatic Nano-tweezers on Semiconducting Carbon Nanotubes for the Growth and Interfacing of Lead-Halide Perovskite Crystal Grains in Solar Cells. Chem. Mater. 2020, 32, 5125–5133. [Google Scholar] [CrossRef]
- Hou, Y.; Aydin, E.; De Bastiani, M.; Xiao, C.; Isikgor, F.H.; Xue, D.J.; Chen, B.; Chen, H.; Bahrami, B.; Chowdhury, A.H.; et al. Efficient tandem solar cells with solution-processed perovskite on textured crystalline silicon. Science 2020, 367, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Shi, Y.; Shi, T.; Chu, S.; Chen, W.; Ighodalo, K.O.; Zhao, J.; Li, X.; Xiao, Z. Suppression and Reversion of Light-Induced Phase Separation in Mixed-Halide Perovskites by Oxygen Passivation. ACS Energy Lett. 2019, 4, 2052–2058. [Google Scholar] [CrossRef]
- Wang, X.; Ling, Y.; Lian, X.; Xin, Y.; Dhungana, K.B.; Perez-Orive, F.; Knox, J.; Chen, Z.; Zhou, Y.; Beery, D.; et al. Suppressed phase separation of mixed-halide perovskites confined in endotaxial matrices. Nat. Commun. 2019, 10, 695. [Google Scholar] [CrossRef]
- Thote, A.; Jeon, I.; Lee, J.W.; Seo, S.; Lin, H.S.; Yang, Y.; Daiguji, H.; Maruyama, S.; Matsuo, Y. Stable and reproducible 2D/3D formamidinium–lead–iodide perovskite solar cells. ACS Appl. Energy Mater. 2019, 2, 2486–2493. [Google Scholar] [CrossRef]
- Han, T.H.; Lee, J.W.; Choi, Y.J.; Choi, C.; Tan, S.; Lee, S.J.; Zhao, Y.; Huang, Y.; Kim, D.; Yang, Y. Surface-2D/Bulk-3D Heterophased Perovskite Nanograins for Long-Term-Stable Light-Emitting Diodes. Adv. Mater. 2020, 32, 1905674. [Google Scholar] [CrossRef]
- Li, J.; Bade, S.G.R.; Shan, X.; Yu, Z. Single-layer light-emitting diodes using organometal halide perovskite/poly (ethylene oxide) composite thin films. Adv. Mater. 2015, 27, 5196–5202. [Google Scholar] [CrossRef]
- Liang, D.; Peng, Y.; Fu, Y.; Shearer, M.J.; Zhang, J.; Zhai, J.; Zhang, Y.; Hamers, R.J.; Andrew, T.L.; Jin, S. Color-pure violet-light-emitting diodes based on layered lead halide perovskite nanoplates. ACS Nano 2016, 10, 6897–6904. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Yang, Z.; Zhou, N.; Deng, Y.; Zhao, J.; Xiao, X.; Rudd, P.; Moran, A.; Yan, Y.; et al. Efficient sky-blue perovskite light-emitting diodes via photoluminescence enhancement. Nat. Commun. 2019, 10, 5633. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.P.; Kim, J.; Kim, B.S.; Kim, H.M.; Kim, J.; Yusoff, A.R.b.M.; Jang, J.; Nazeeruddin, M.K. High-Efficiency, Blue, Green, and Near-Infrared Light-Emitting Diodes Based on Triple Cation Perovskite. Adv. Opt. Mater. 2017, 5, 1600920. [Google Scholar] [CrossRef]
- Braly, I.L.; Stoddard, R.J.; Rajagopal, A.; Uhl, A.R.; Katahara, J.K.; Jen, A.K.Y.; Hillhouse, H.W. Current-induced phase segregation in mixed halide hybrid perovskites and its impact on two-terminal tandem solar cell design. ACS Energy Lett. 2017, 2, 1841–1847. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhao, L.; Tran, N.L.; Lin, Y.L.; Silver, S.H.; Kerner, R.A.; Yao, N.; Kahn, A.; Scholes, G.D.; Rand, B.P. Mixed-halide perovskites with stabilized bandgaps. Nano Lett. 2017, 17, 6863–6869. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Sun, W.; Wang, Z.; Bai, Y.; Hayat, T.; Alsaedi, A.; Tan, Z. High Performance Quasi-2D Perovskite Sky-Blue Light-Emitting Diodes Using a Dual-Ligand Strategy. Small 2020, 16, 2002940. [Google Scholar] [CrossRef]
- Nitsch, K.; Dušek, M.; Nikl, M.; Polak, K.; Rodova, M. Ternary alkali lead chlorides: Crystal growth, crystal structure, absorption and emission properties. Prog. Cryst. Growth Charact. Mater. 1995, 30, 1–22. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, Z.; Fu, Q.; Chen, Z.; He, J.; Zhang, S.; Yan, L.; Hu, Y.; Luo, W. Growth and characterization of all-inorganic lead halide perovskite semiconductor CsPbBr3 single crystals. CrystEngComm 2017, 19, 6797–6803. [Google Scholar] [CrossRef]
- Palazon, F.; Urso, C.; De Trizio, L.; Akkerman, Q.; Marras, S.; Locardi, F.; Nelli, I.; Ferretti, M.; Prato, M.; Manna, L. Postsynthesis transformation of insulating Cs4PbBr6 nanocrystals into bright perovskite CsPbBr3 through physical and chemical extraction of CsBr. ACS Energy Lett. 2017, 2, 2445–2448. [Google Scholar] [CrossRef]
- Dao, V.D.; Vu, N.H.; Yun, S. Recent advances and challenges for solar-driven water evaporation system toward applications. Nano Energy 2020, 68, 104324. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Zhang, L.; Yin, L. PbCl2-tuned inorganic cubic CsPbBr3 (Cl) perovskite solar cells with enhanced electron lifetime, diffusion length and photovoltaic performance. J. Power Sources 2017, 360, 11–20. [Google Scholar] [CrossRef]
- Jella, V.; Ippili, S.; Yoon, S.G. Halide (Cl/Br)-Incorporated Organic–Inorganic Metal Trihalide Perovskite Films: Study and Investigation of Dielectric Properties and Mechanical Energy Harvesting Performance. ACS Appl. Electron. Mater. 2020, 2, 2579–2590. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liashenko, T.G.; Pushkarev, A.P.; Naujokaitis, A.; Pakštas, V.; Franckevičius, M.; Zakhidov, A.A.; Makarov, S.V. Suppression of Electric Field-Induced Segregation in Sky-Blue Perovskite Light-Emitting Electrochemical Cells. Nanomaterials 2020, 10, 1937. https://doi.org/10.3390/nano10101937
Liashenko TG, Pushkarev AP, Naujokaitis A, Pakštas V, Franckevičius M, Zakhidov AA, Makarov SV. Suppression of Electric Field-Induced Segregation in Sky-Blue Perovskite Light-Emitting Electrochemical Cells. Nanomaterials. 2020; 10(10):1937. https://doi.org/10.3390/nano10101937
Chicago/Turabian StyleLiashenko, Tatiana G., Anatoly P. Pushkarev, Arnas Naujokaitis, Vidas Pakštas, Marius Franckevičius, Anvar A. Zakhidov, and Sergey V. Makarov. 2020. "Suppression of Electric Field-Induced Segregation in Sky-Blue Perovskite Light-Emitting Electrochemical Cells" Nanomaterials 10, no. 10: 1937. https://doi.org/10.3390/nano10101937