Comparative Study of Growth Morphologies of Ga2O3 Nanowires on Different Substrates
Abstract
:1. Introduction
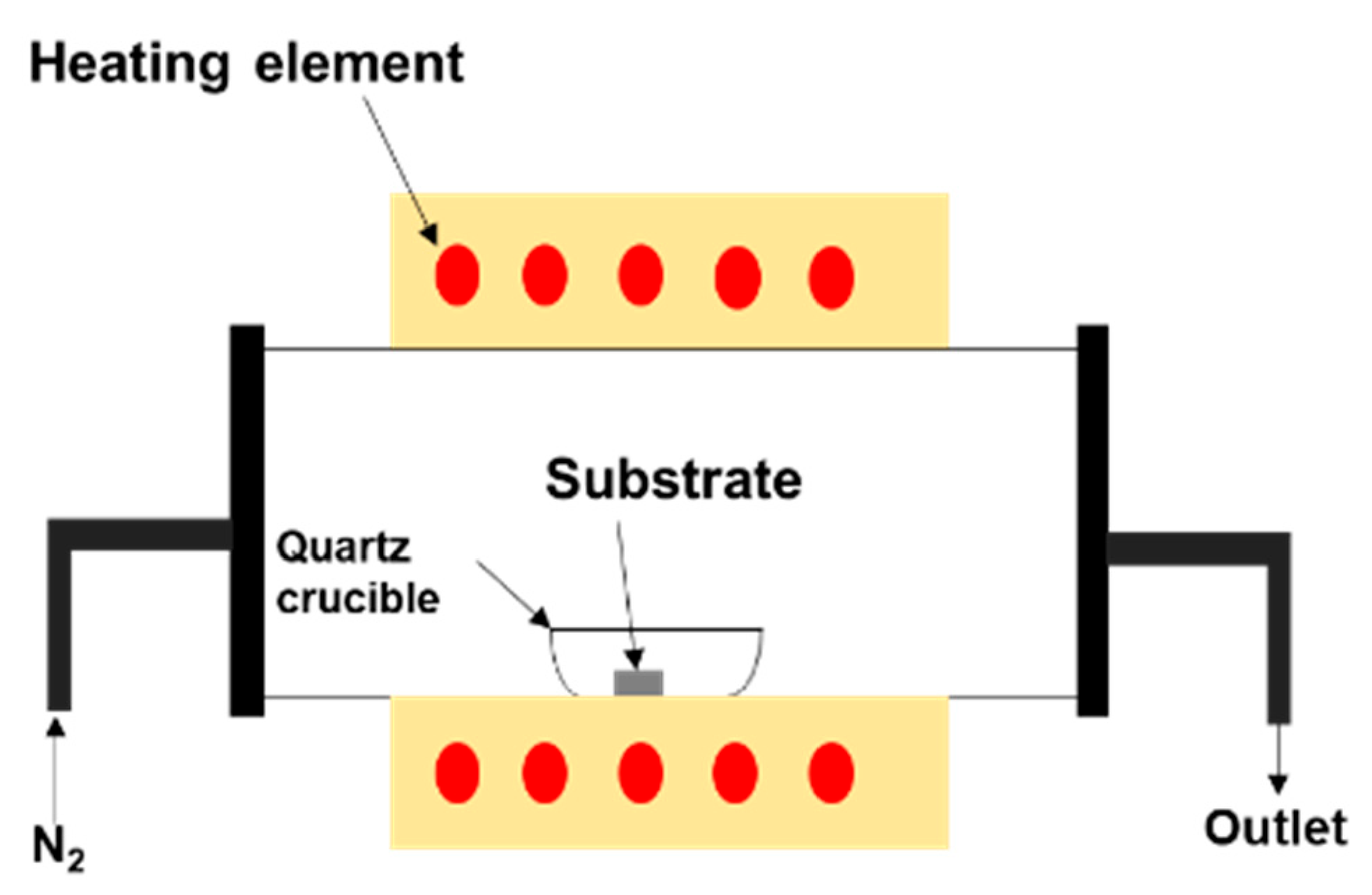
2. Materials and Methods
3. Results and Discussion
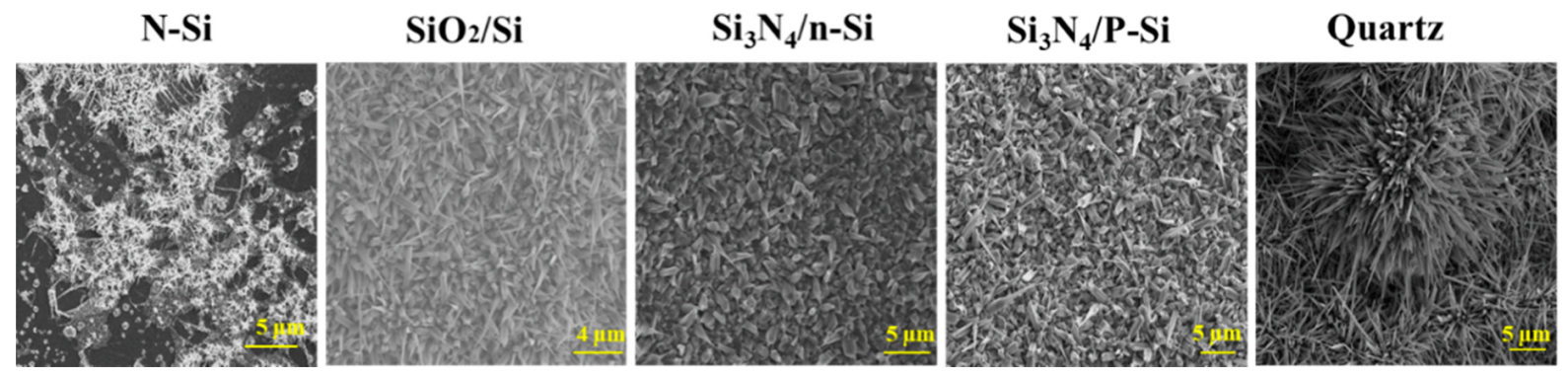
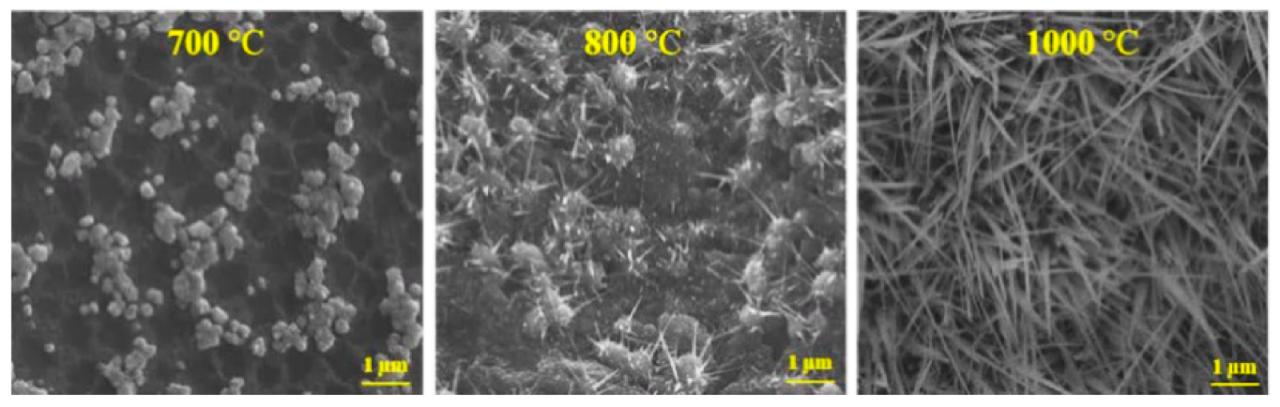
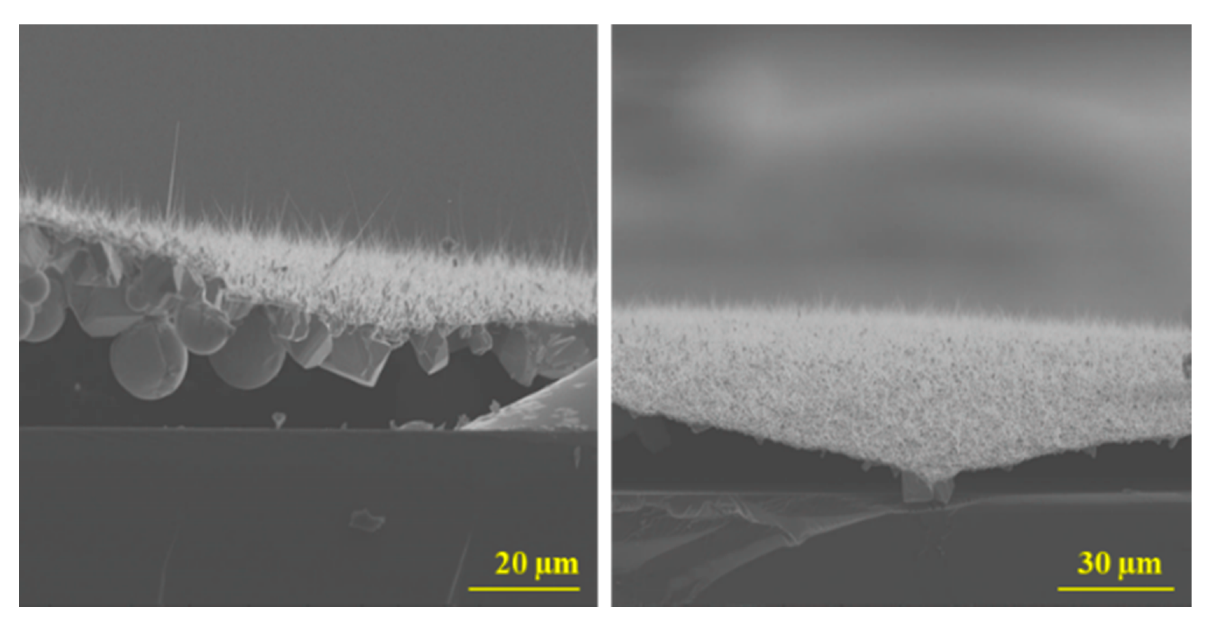
3.1. Growth Morphologies of Ga2O3 on Different Substrates
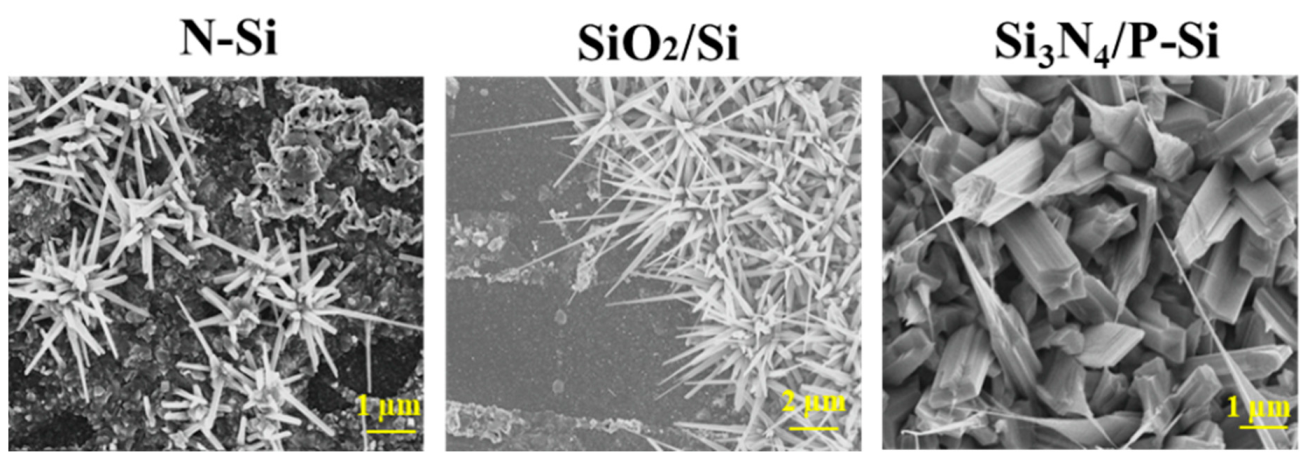
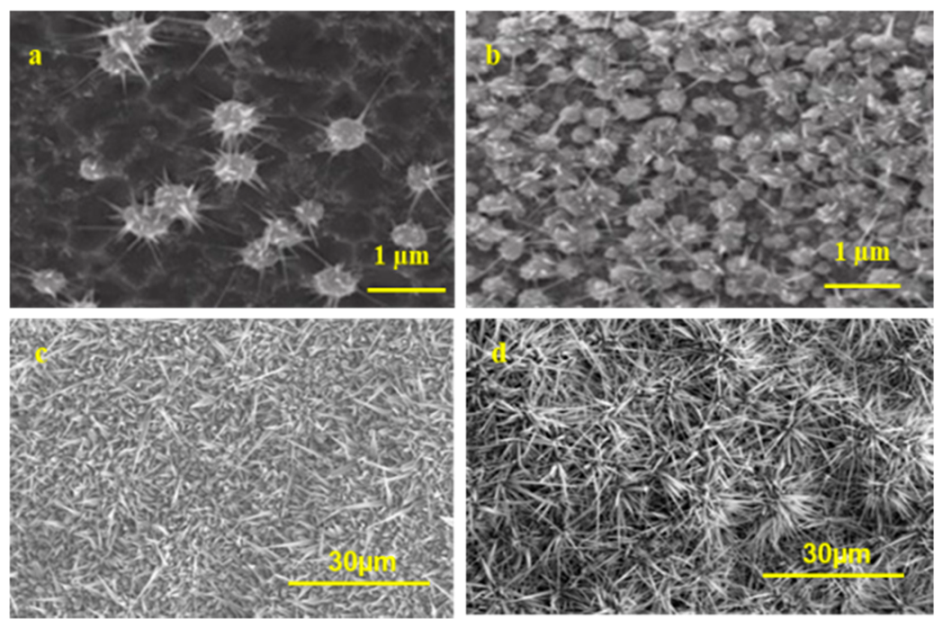
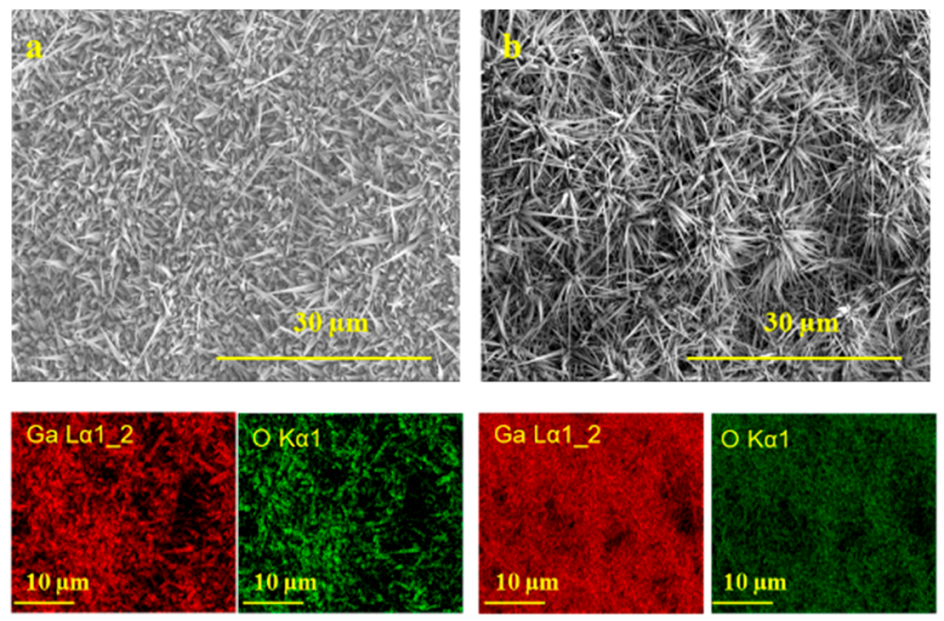
3.2. Effect of Ag Catalyst on the Growth Morphologies of Ga2O3
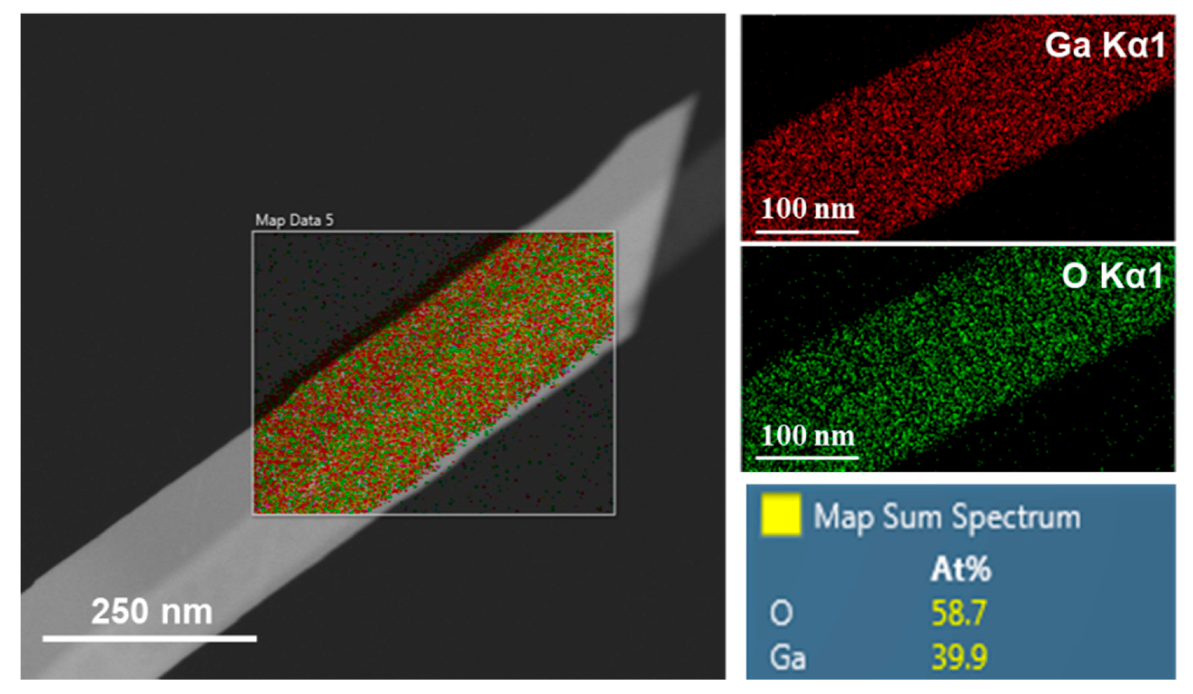
3.3. Elemental Analysis of Ga2O3 Nanowires
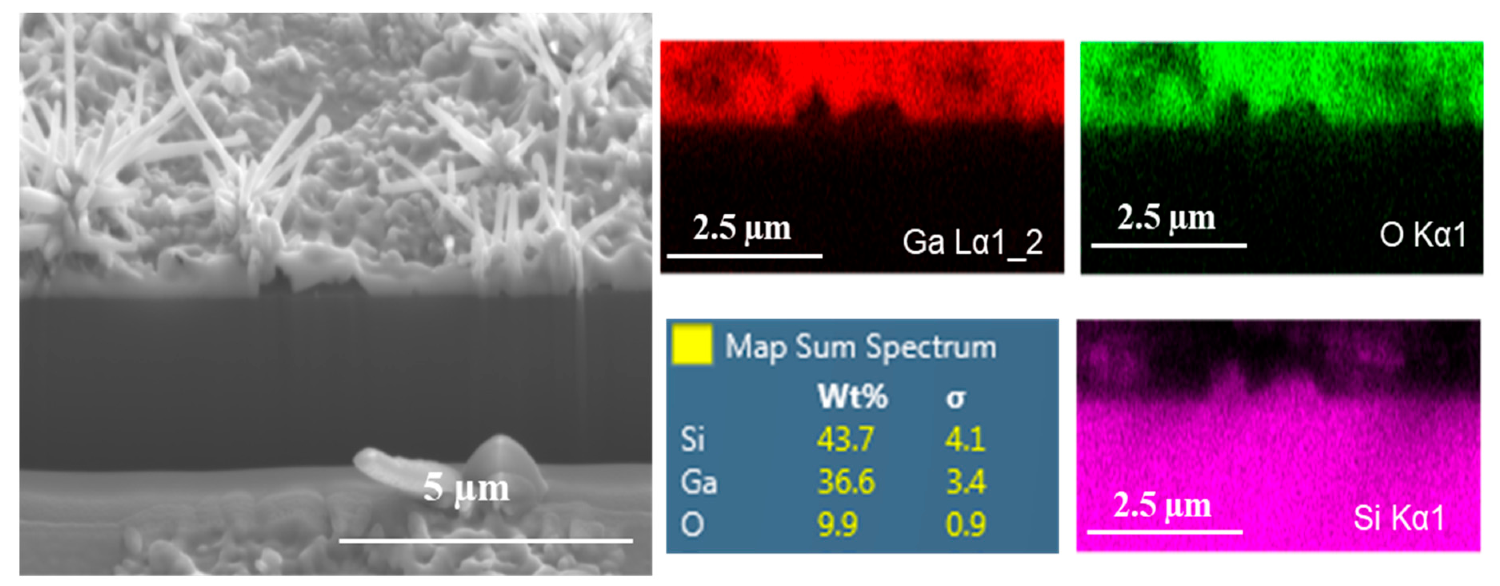
3.4. Incongruities at the Substrate/Ga2O3 Interface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pearton, S.J.; Yang, J.; Carey, P.H.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef] [Green Version]
- Alhalaili, B.; Bunk, R.; Vidu, R.; Islam, M.S. Dynamics Contributions to the Growth Mechanism of Ga2O3 Thin Film and NWs Enabled by Ag Catalyst. Nanomaterials 2019, 9, 1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, D.; Wang, Q.; Wu, Z.; Li, P.; Lei, M.; Tang, W. Magnetic anisotropy and deep ultraviolet photoresponse characteristics in Ga2O3: Cr vermicular nanowire thin film nanostructure. RSC Adv. 2015, 5, 12894–12898. [Google Scholar] [CrossRef]
- Wang, B.; Look, D.; Leedy, K.D. Deep level defects in β-Ga2O3 pulsed laser deposited thin films and Czochralski-grown bulk single crystals by thermally stimulated techniques. J. Appl. Phys. 2019, 125, 105103. [Google Scholar] [CrossRef]
- Ghose, S.; Rahman, S.; Rojas-Ramirez, J.S.; Caro, M.; Droopad, R.; Arias, A.; Nedev, N. Structural and optical properties of ?-Ga2O3 thin films grown by plasma-assisted molecular beam epitaxy. J. Vac. Sci. Technol. B 2016, 34, 2. [Google Scholar] [CrossRef]
- Ngo, T.S.; Le, D.D.; Song, J.H.; Hong, S.-K. Growth and characterization of gallium oxide films grown with nitrogen by plasma-assisted molecular-beam epitaxy. Thin Solid Films 2019, 682, 93–98. [Google Scholar] [CrossRef]
- Pallister, P.J.; Buttera, S.C.; Barry, S.T. Self-seeding gallium oxide nanowire growth by pulsed chemical vapor deposition. Phys. Status Solidi (a) 2015, 212, 1514–1518. [Google Scholar] [CrossRef]
- He, T.; Li, C.; Zhang, X.; Ma, Y.; Cao, X.; Shi, X.; Sun, C.; Li, J.; Song, L.; Zeng, C.; et al. Metalorganic Chemical Vapor Deposition Heteroepitaxial β-Ga2O3 and Black Phosphorus Pn Heterojunction for Solar-Blind Ultraviolet and Infrared Dual-Band Photodetector. Phys. Status Solidi (a) 2019, 217, 1900861. [Google Scholar] [CrossRef]
- Zhao, Y.; Frost, R.L.; Yang, J.; Martens, W.N. Size and Morphology Control of Gallium Oxide Hydroxide GaO(OH), Nano- to Micro-Sized Particles by Soft-Chemistry Route without Surfactant. J. Phys. Chem. C 2008, 112, 3568–3579. [Google Scholar] [CrossRef]
- Reddy, L.S.; Ko, Y.H.; Yu, J.S. Hydrothermal Synthesis and Photocatalytic Property of beta-Ga2O3 Nanorods. Nanoscale Res. Lett. 2015, 10, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Bae, H.J.; Yoo, T.H.; Yoon, Y.; Lee, I.G.; Kim, J.P.; Cho, B.J. High-Aspect Ratio β-Ga2O3 Nanorods via Hydrothermal Synthesis. Nanomaterials 2018, 8, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boschi, F.; Bosi, M.; Berzina, T.; Buffagni, E.; Ferrari, C.; Fornari, R. Hetero-epitaxy of epsilon-Ga2O3 layers by MOCVD and ALD. J. Cryst. Growth 2016, 443, 25–30. [Google Scholar] [CrossRef]
- Feng, Q.; Li, F.; Dai, B.; Jia, Z.; Xie, W.; Xu, T.; Lu, X.; Tao, X.; Zhang, J.; Hao, Y. The properties of gallium oxide thin film grown by pulsed laser deposition. Appl. Surf. Sci. 2015, 359, 847–852. [Google Scholar] [CrossRef]
- Mi, W.; Luan, C.; Li, Z.; Zhao, C.; Xiao, H.; Ma, J. Synthesis of monoclinic structure gallium oxide epitaxial film on MgAl6O10 (100). Mater. Lett. 2013, 107, 83–85. [Google Scholar] [CrossRef]
- Mi, W.; Luan, C.N.; Li, Z.; Zhao, C.S.; Feng, X.J.; Ma, J. Ultraviolet-green photoluminescence of beta-Ga2O3 films deposited on MgAl6O10 (100) substrate. Opt. Mater. 2013, 35, 2624–2628. [Google Scholar] [CrossRef]
- Feng, X.J.; Li, Z.; Mi, W.; Luo, Y.; Ma, J. Mg-doped beta-Ga2O3 films with tunable optical band gap prepared on MgO (110) substrates by metal-organic chemical vapor deposition. Mater. Sci. Semicond. Process. 2015, 34, 52–57. [Google Scholar] [CrossRef]
- Weng, W.Y.; Hsueh, T.J.; Chang, S.J.; Huang, G.J.; Hsueh, H.T. A beta-Ga2O3 Solar-Blind Photodetector Prepared by Furnace Oxidization of GaN Thin Film. IEEE Sens. J. 2011, 11, 999–1003. [Google Scholar] [CrossRef]
- Mazeina, L.; Perkins, F.K.; Bermudez, V.M.; Arnold, S.P.; Prokes, S.M. Functionalized Ga2O3Nanowires as Active Material in Room Temperature Capacitance-Based Gas Sensors. Langmuir 2010, 26, 13722–13726. [Google Scholar] [CrossRef]
- Singh, P.; Simanjuntak, F.M.; Wu, Y.-C.; Kumar, A.; Zan, H.-W.; Tseng, T.Y. Sensing performance of gas sensors fabricated from controllably grown ZnO-based nanorods on seed layers. J. Mater. Sci. 2020, 55, 8850–8860. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Wu, B.-K.; Chern, M.-Y. Schottky Photodiode Fabricated from Hydrogen-Peroxide-Treated ZnO Nanowires. Appl. Phys. Express 2013, 6, 054103. [Google Scholar] [CrossRef]
- Singh, P.; Simanjuntak, F.M.; Kumar, A.; Tseng, T.Y. Resistive switching behavior of Ga doped ZnO-nanorods film conductive bridge random access memory. Thin Solid Films 2018, 660, 828–833. [Google Scholar] [CrossRef]
- Alhalaili, B.; Vidu, R.; Islam, M.S. The Growth of Ga2O3 Nanowires on Silicon for Ultraviolet Photodetector. Sensors 2019, 19, 5301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.; Yang, Z.X.; Zhou, W.M.; Zhang, Y.F. Direct synthesis of beta gallium oxide nanowires, nanobelts, nanosheets and nanograsses by microwave plasma. Solid State Commun. 2006, 137, 177–181. [Google Scholar] [CrossRef]
- Sajanlal, P.R.; Sreeprasad, T.S.; Samal, A.K.; Pradeep, T. Anisotropic nanomaterials: Structure, growth, assembly, and functions. Nano Rev. 2011, 2, 53. [Google Scholar] [CrossRef] [PubMed]
- Azulay, A.R.; Turkulets, Y.; Del Gaudio, D.; Goldman, R.S.; Shalish, I. Why do nanowires grow with their c-axis vertically-aligned in the absence of epitaxy? Sci. Rep. 2020, 10, 6554. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sunkara, M.K. Direct Synthesis of Gallium Oxide Tubes, Nanowires, and Nanopaintbrushes. J. Am. Chem. Soc. 2002, 124, 12288–12293. [Google Scholar] [CrossRef]
- Smeltzer, W.W.; Tollefson, E.L.; Cambron, A. Adsorption of Oxygen by a Silver Catalyst. Can. J. Chem. 1956, 34, 1046–1060. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, G.I.N.; Bowmaker, G.A.; Metson, J.B. Oxygen chemisorption on an electrolytic silver catalyst: A combined TPD and Raman spectroscopic study. Appl. Surf. Sci. 2003, 214, 36–51. [Google Scholar] [CrossRef]
- Zhou, Z.-Y.; Ma, Y.; Han, Q.-F.; Liu, Y.-L. Solubility, permeation, and capturing of impurity oxygen in Au/Ag: A comparative investigation from first-principles. Comput. Mater. Sci. 2016, 114, 79–85. [Google Scholar] [CrossRef]
- Askeland, D.R.; Fulay, P.P.; Wright, W.J. Science and Engineering of Materials, 6th ed.; CL-Engineering: Stamford, CT, USA, 2011. [Google Scholar]
- Müller, C.M.; Spolenak, R. Microstructure evolution during dewetting in thin Au films. Acta Mater. 2010, 58, 6035–6045. [Google Scholar] [CrossRef]
- Strobel, S.; Kirkendall, C.; Chang, J.-B.; Berggren, K.K. Sub-10 nm structures on silicon by thermal dewetting of platinum. Nanotechnology 2010, 21, 505301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinkevich, M.; Aldinger, F. Thermodynamic Assessment of the Gallium-Oxygen System. J. Am. Ceram. Soc. 2004, 87, 683–691. [Google Scholar] [CrossRef]
- Gould, A.L.; Kadkhodazadeh, S.; Wagner, J.B.; Catlow, C.R.A.; Logsdail, A.J.; Di Vece, M. Understanding the Thermal Stability of Silver Nanoparticles Embedded in a-Si. J. Phys. Chem. C 2015, 119, 23767–23773. [Google Scholar] [CrossRef] [Green Version]
- Varley, J.B.; Weber, J.R.; Janotti, A.; van de Walle, C.G. Oxygen vacancies and donor impurities in β-Ga2O3. Appl. Phys. Lett. 2010, 97, 142106. [Google Scholar] [CrossRef]
- Vj, L.; Oh, J.; Nayak, A.P.; Katzenmeyer, A.M.; Gilchrist, K.H.; Grego, S.; Kobayashi, N.P.; Wang, S.-Y.; Talin, A.A.; Dhar, N.K.; et al. A Perspective on Nanowire Photodetectors: Current Status, Future Challenges, and Opportunities. IEEE J. Sel. Top. Quantum Electron. 2011, 17, 1002–1032. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhalaili, B.; Vidu, R.; Mao, H.; Islam, M.S. Comparative Study of Growth Morphologies of Ga2O3 Nanowires on Different Substrates. Nanomaterials 2020, 10, 1920. https://doi.org/10.3390/nano10101920
Alhalaili B, Vidu R, Mao H, Islam MS. Comparative Study of Growth Morphologies of Ga2O3 Nanowires on Different Substrates. Nanomaterials. 2020; 10(10):1920. https://doi.org/10.3390/nano10101920
Chicago/Turabian StyleAlhalaili, Badriyah, Ruxandra Vidu, Howard Mao, and M. Saif Islam. 2020. "Comparative Study of Growth Morphologies of Ga2O3 Nanowires on Different Substrates" Nanomaterials 10, no. 10: 1920. https://doi.org/10.3390/nano10101920
APA StyleAlhalaili, B., Vidu, R., Mao, H., & Islam, M. S. (2020). Comparative Study of Growth Morphologies of Ga2O3 Nanowires on Different Substrates. Nanomaterials, 10(10), 1920. https://doi.org/10.3390/nano10101920




