Simulations of Graphene Nanoribbon Field Effect Transistor for the Detection of Propane and Butane Gases: A First Principles Study
Abstract
1. Introduction
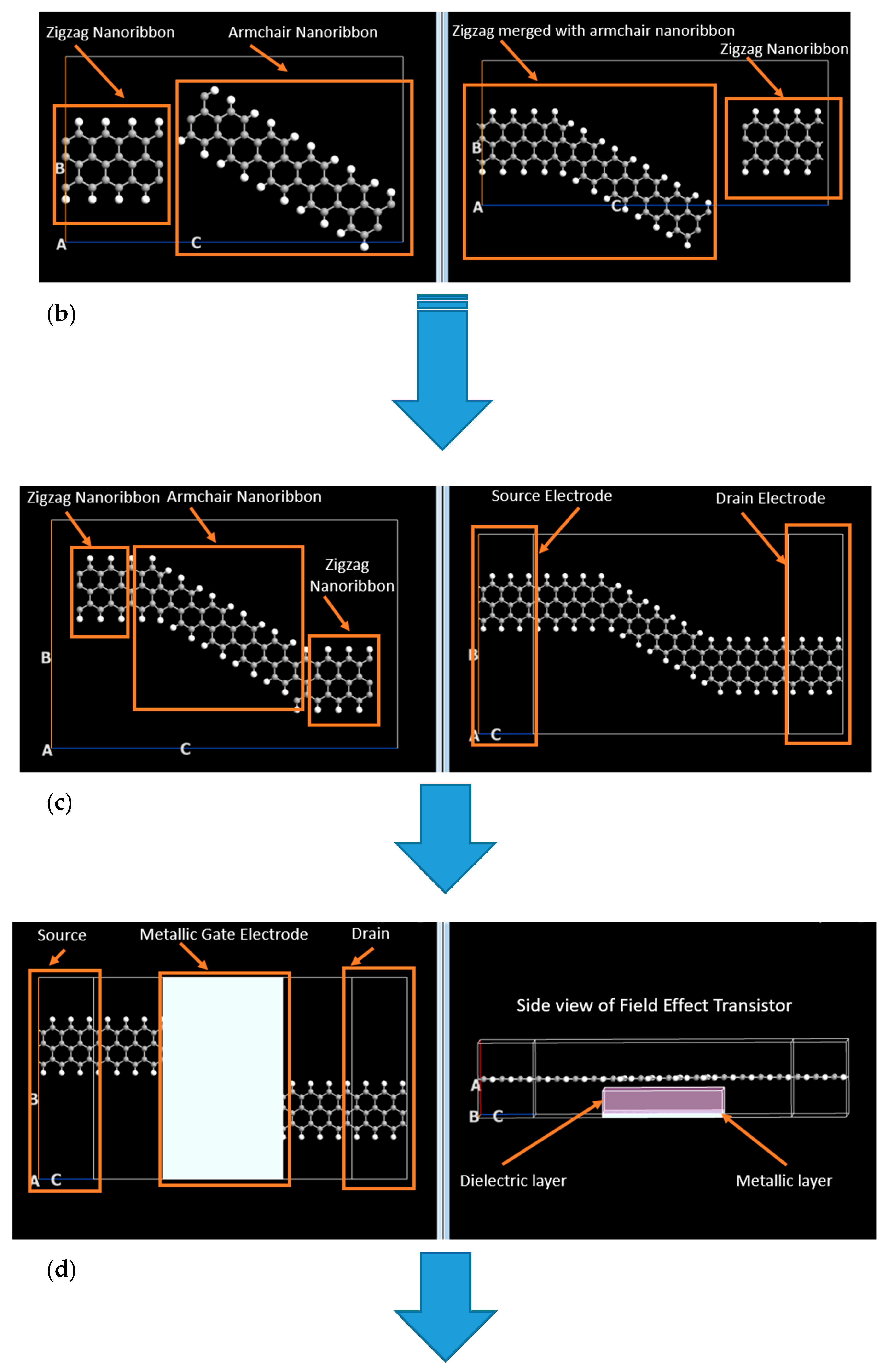
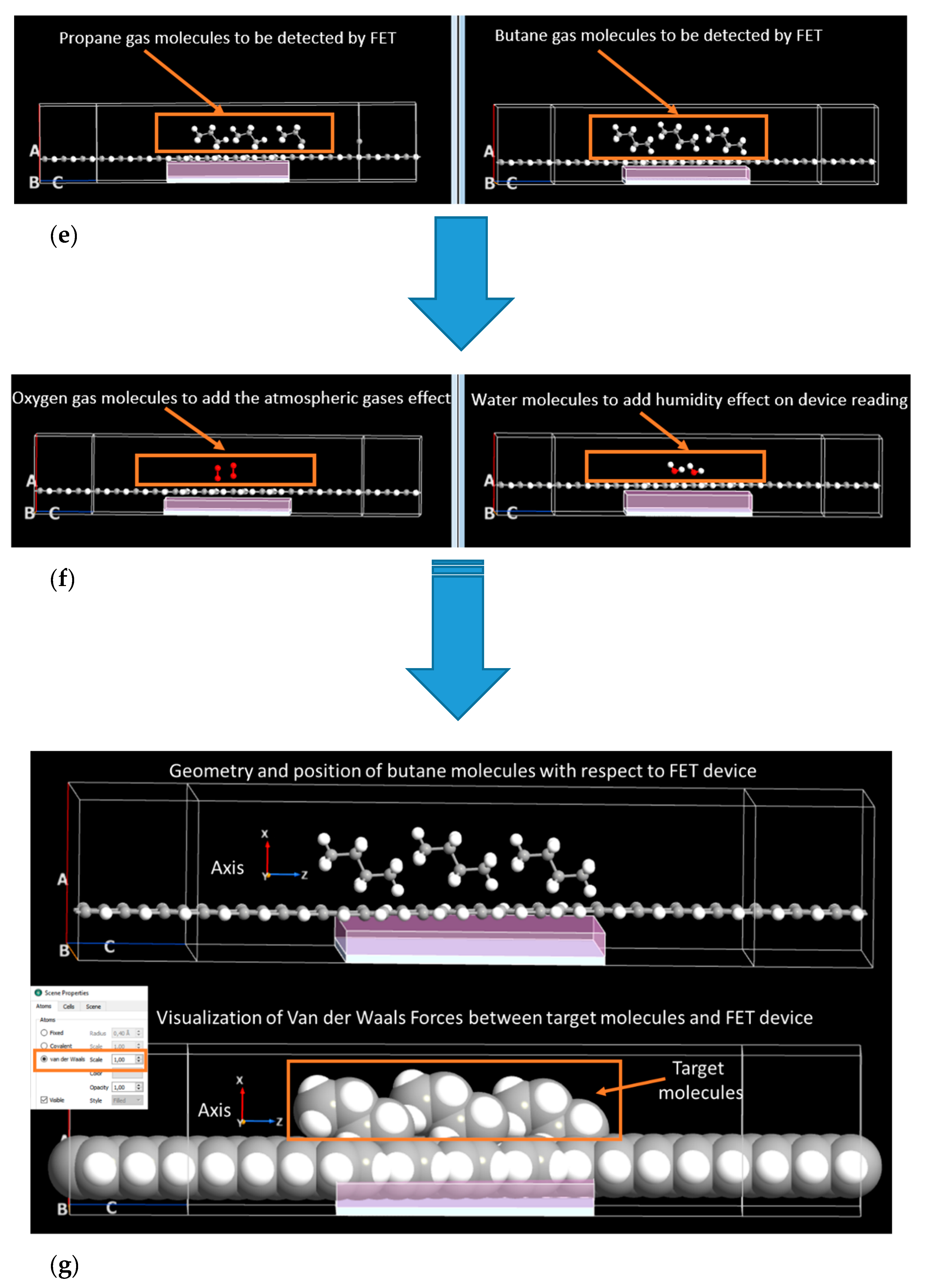
2. Materials and Methods
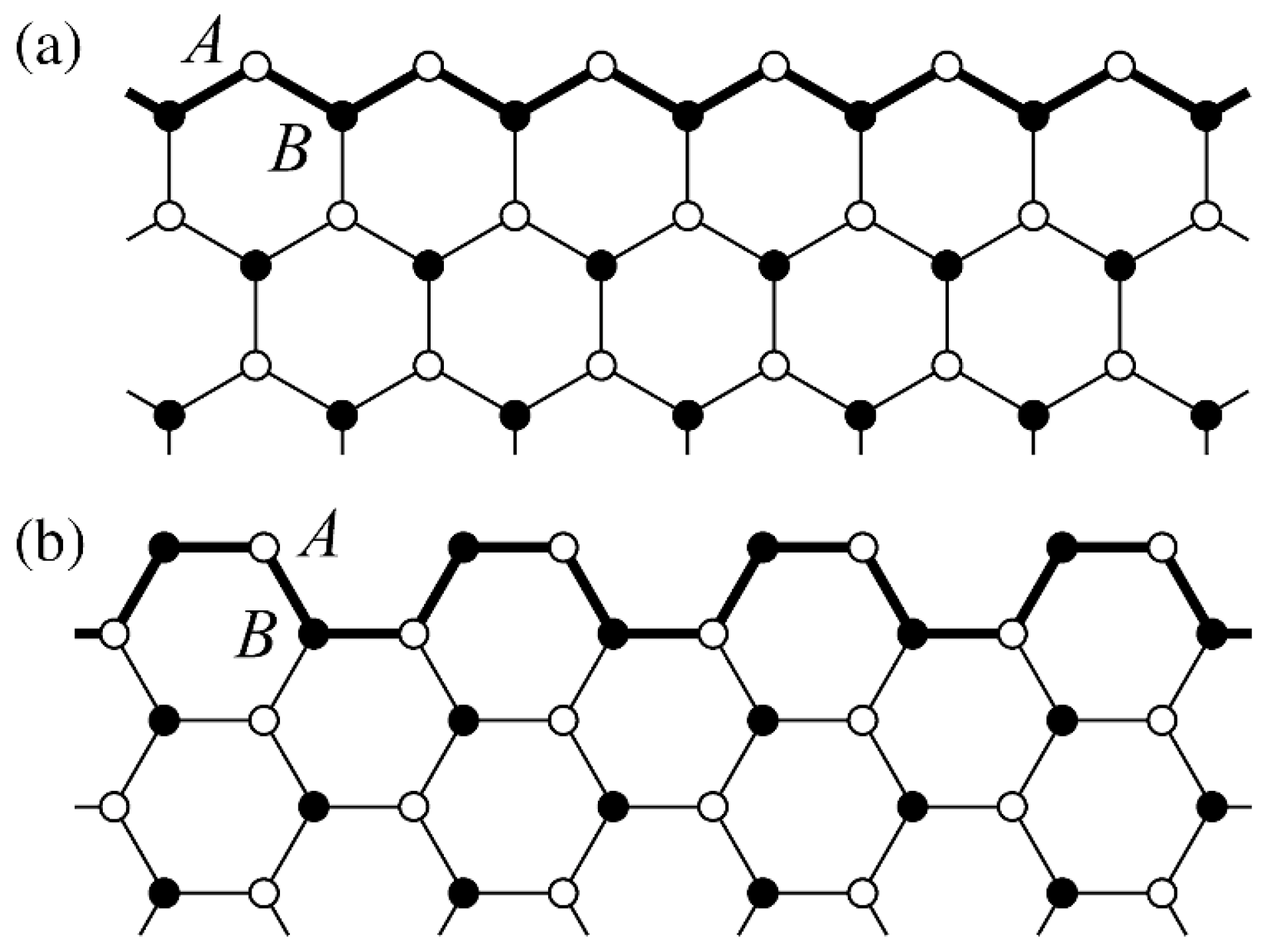
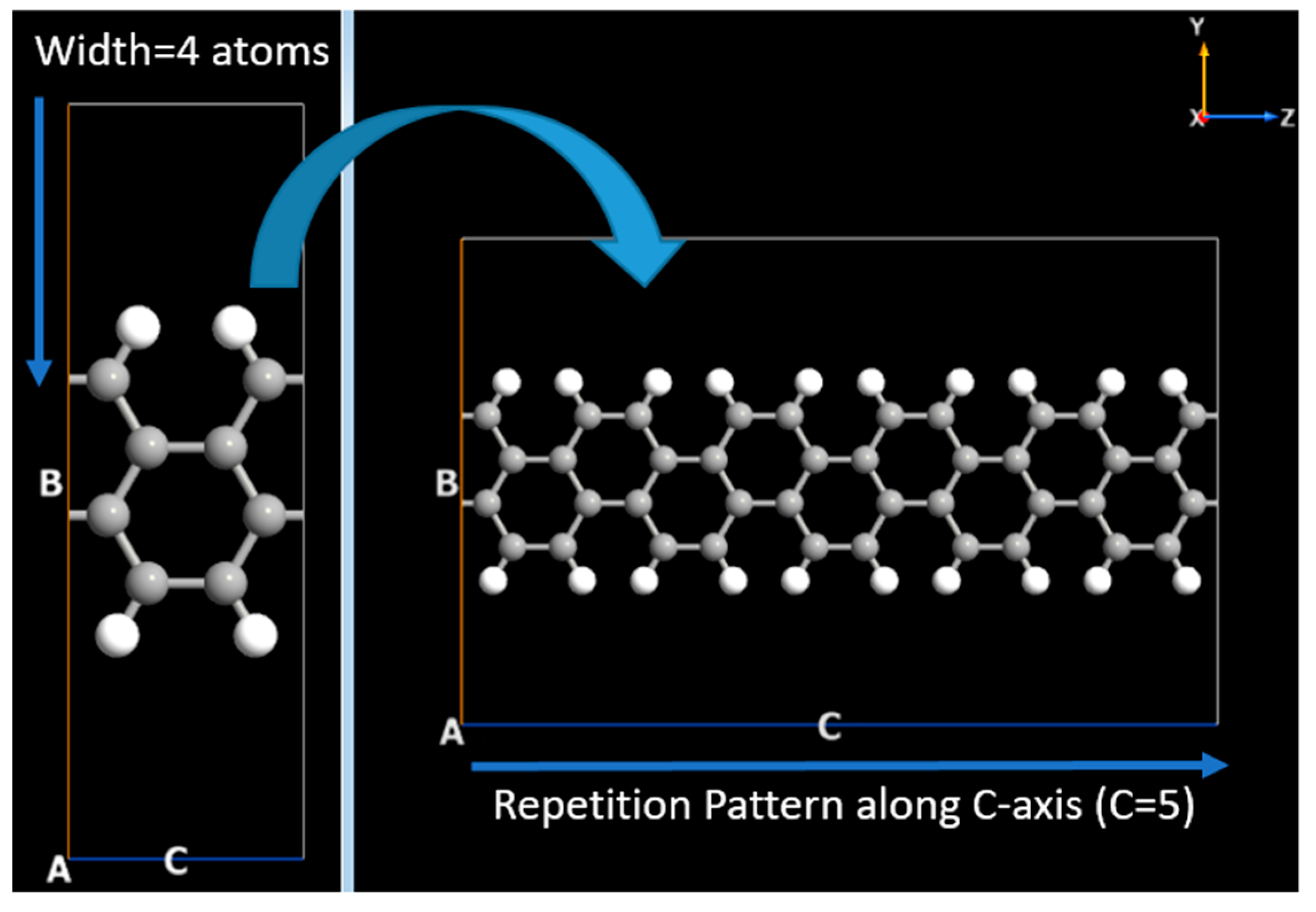
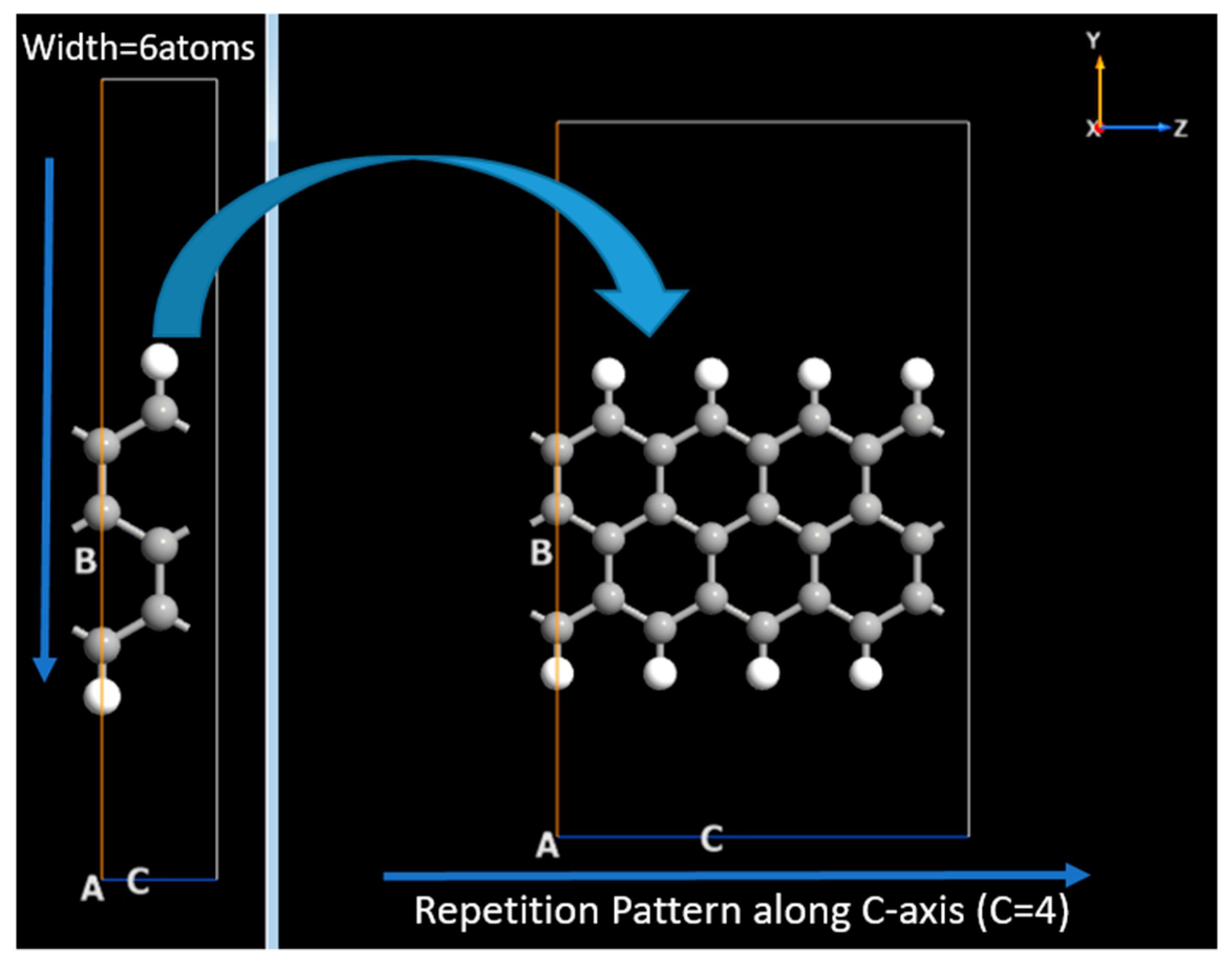
Graphene Armchair and Zigzag Nanoribbons
3. Results and Discussion
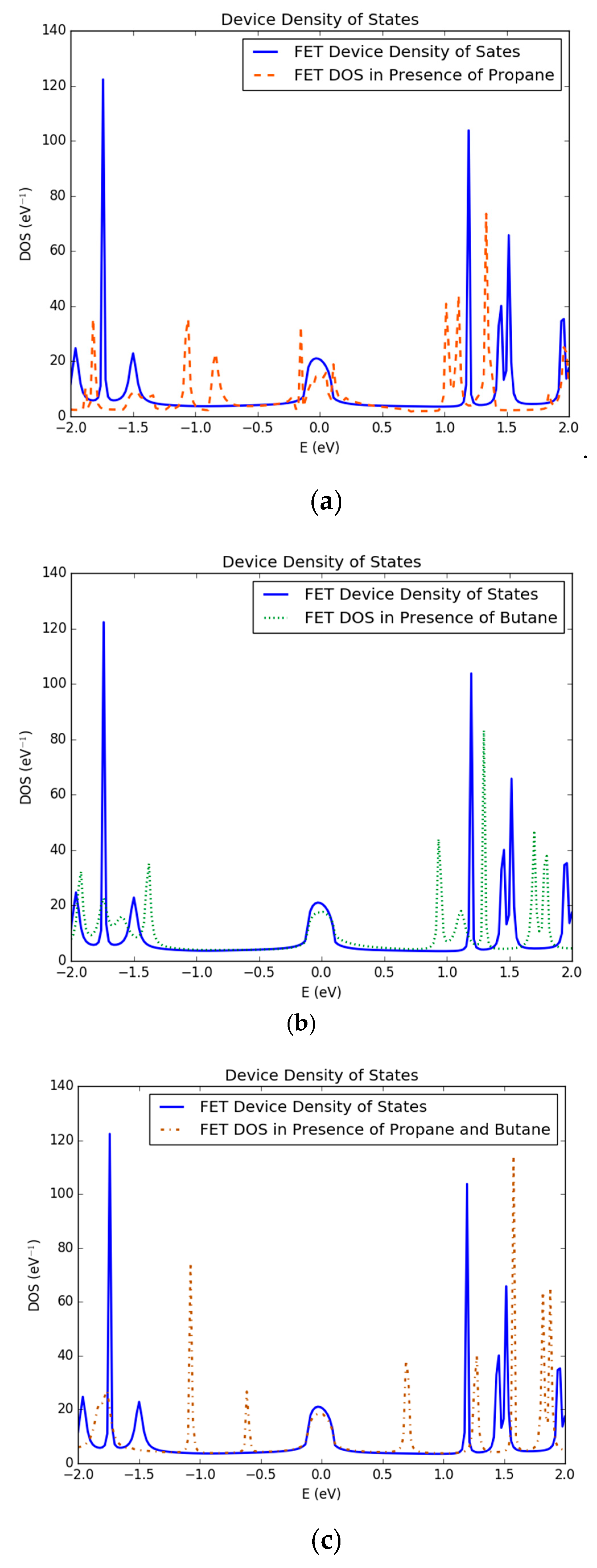
3.1. Density of States of Simulated Graphene Nanoribbon Field Effect Transistor Device
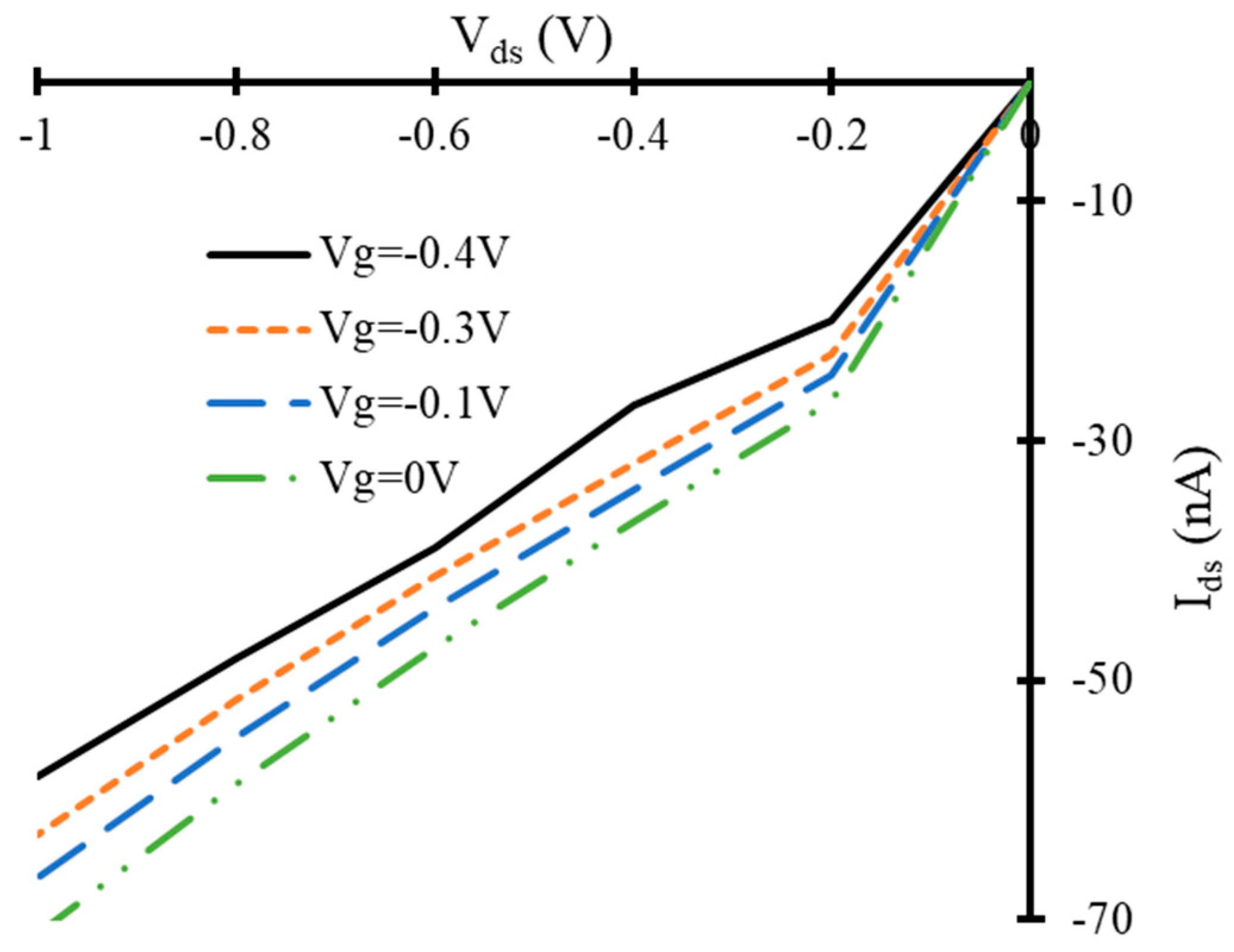
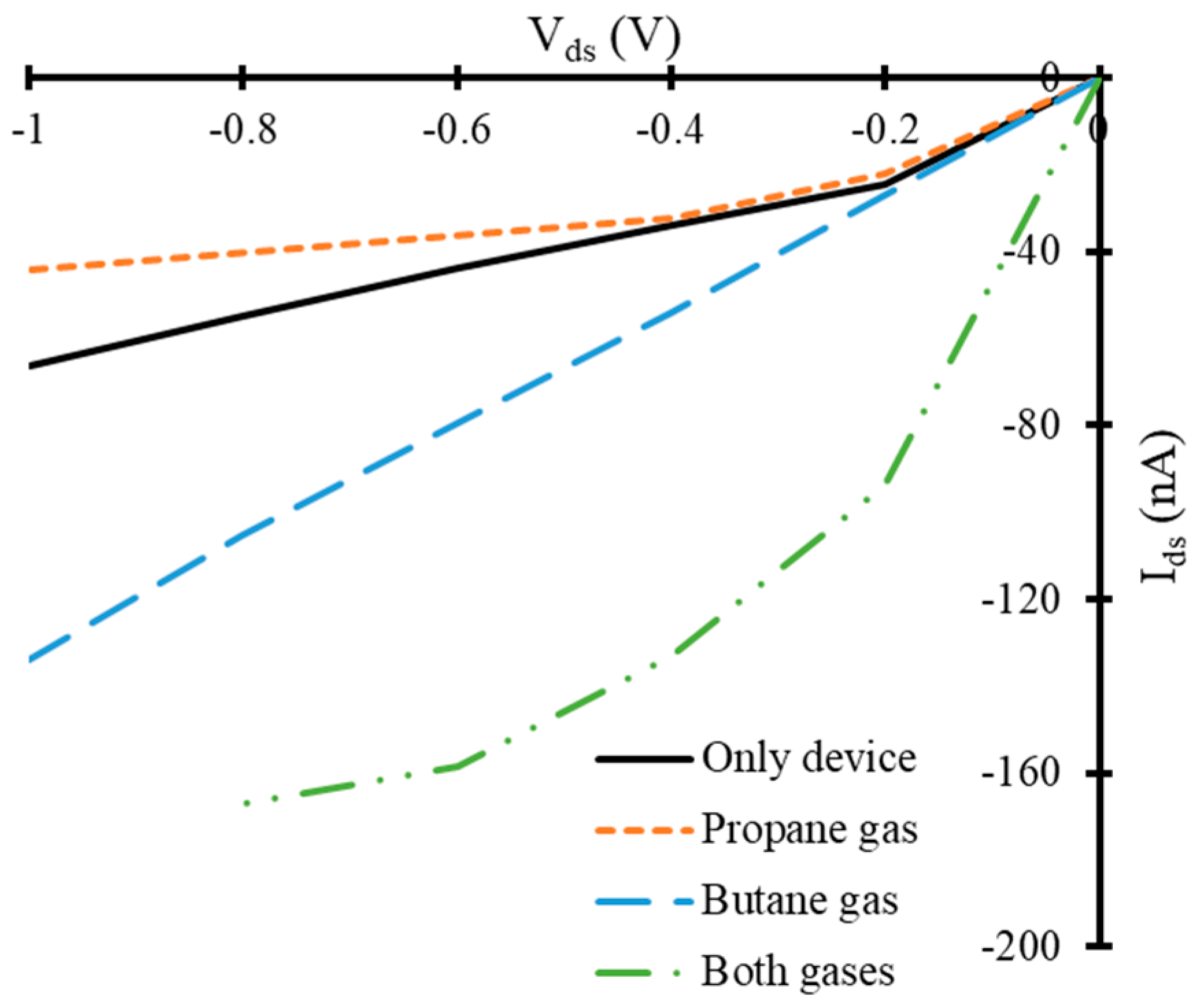
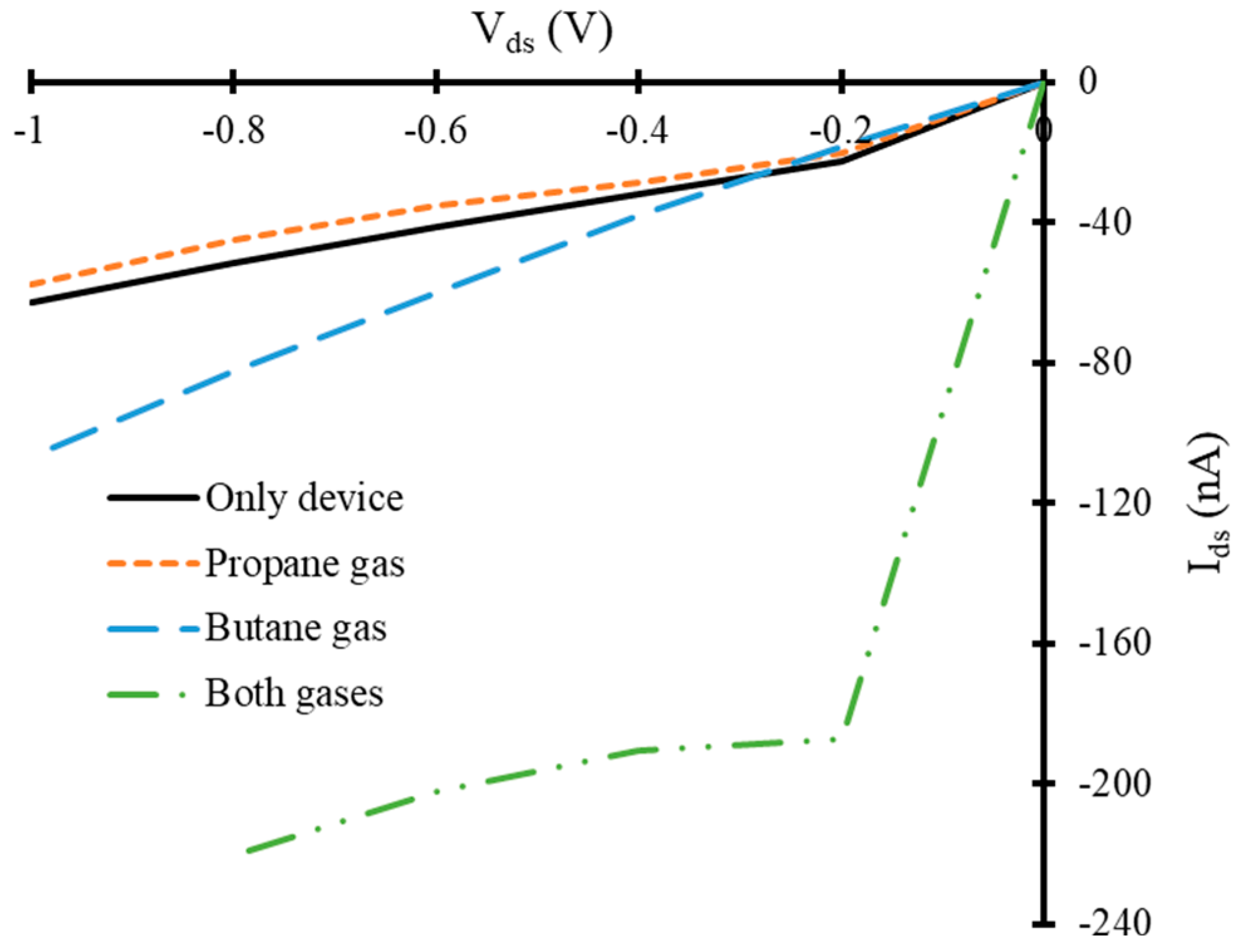
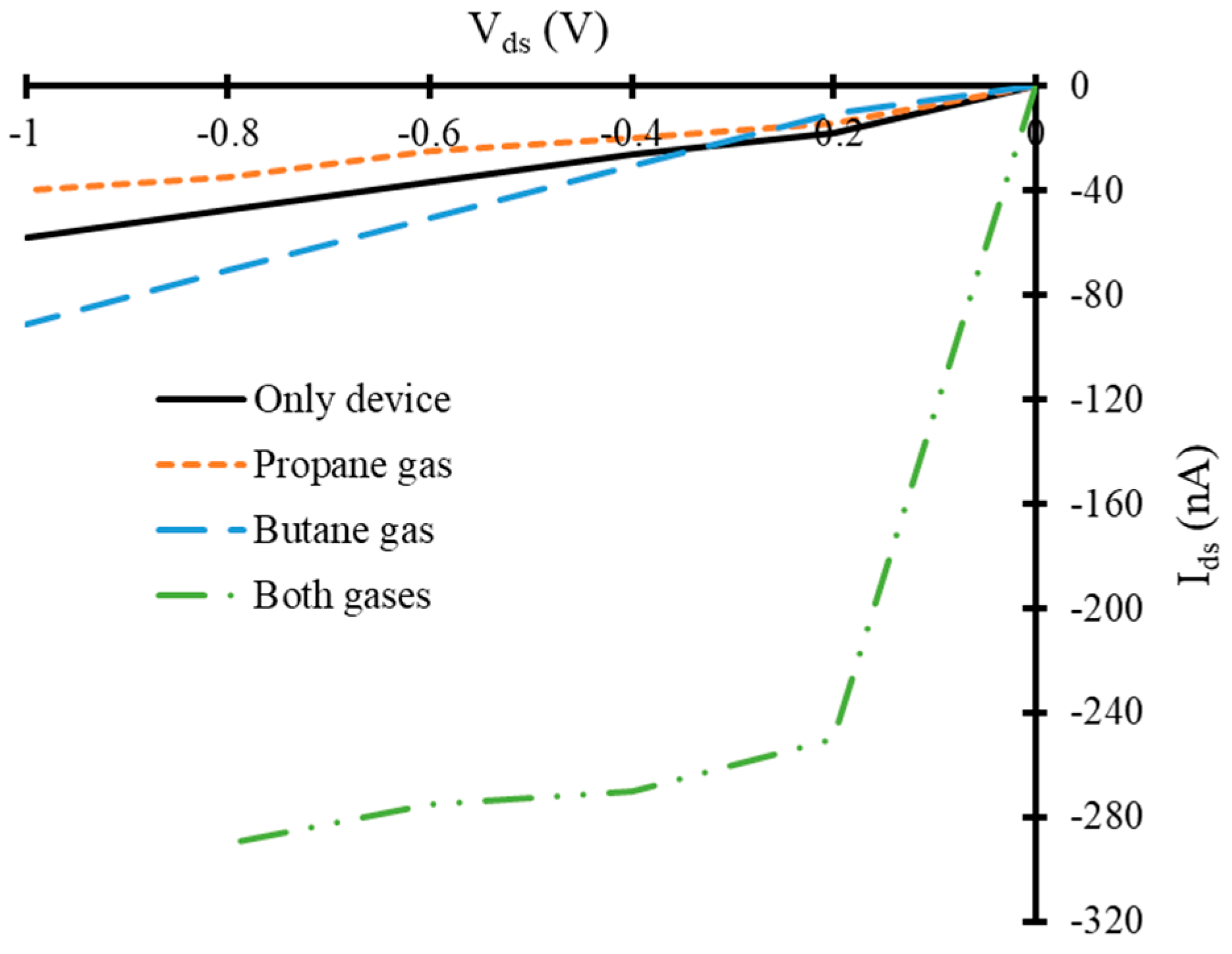
3.2. Current-Voltage Characteristics of Simulated Graphene Nanoribbon Field Effect Transistor Device in Presence of Only Propane and Butane Molecules
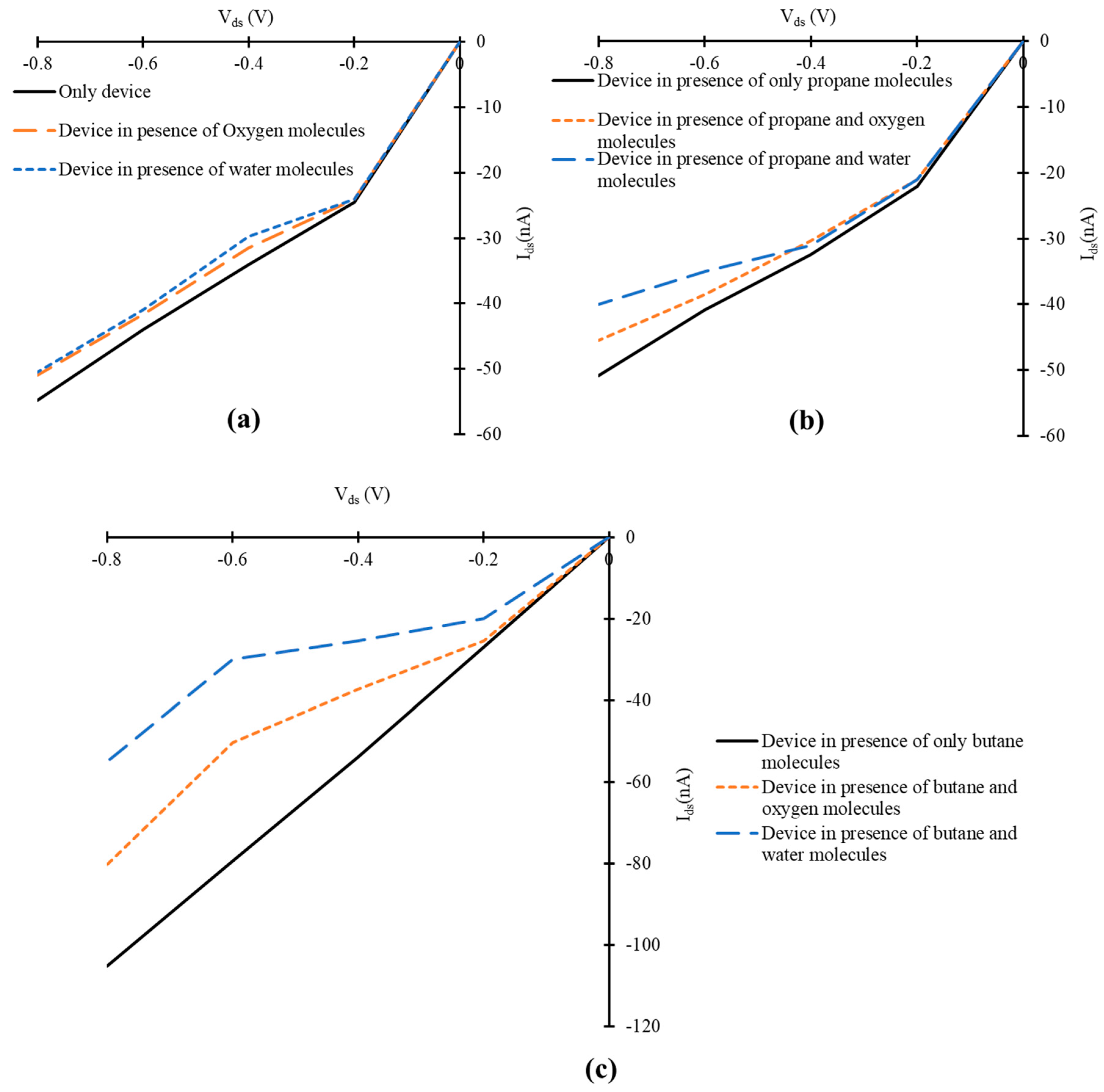
3.3. Influence of Oxygen and Water Molecules on the Current-voltage Characteristics of Simulated Graphene Nanoribbon Field Effect Transistor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joshi, S.; Bhatt, V.D.; Wu, H.; Becherer, M.; Lugli, P. Flexible lactate and glucose sensors using electrolyte-gated carbon nanotube field effect transistor for non-invasive real-time monitoring. IEEE Sens. J. 2017, 17, 4315–4321. [Google Scholar] [CrossRef]
- Hosseini-Golgoo, S.M.; Salimi, F.; Saberkari, A.; Rahbarpour, S. Comparison of information content of temporal response of chemoresistive gas sensor under three different temperature modulation regimes for gas detection of different feature reduction methods. J. Phys. 2017, 939, 012005. [Google Scholar] [CrossRef]
- Moseley, P.T. Solid state gas sensors. Meas. Sci. Technol. 1997, 8, 223–237. [Google Scholar] [CrossRef]
- Simonetta, C.; Forleo, A.; Francioso, L.; Rella, R.; Siciliano, P.; Spadavecchia, J.; Presicce, D.S.; Taurino, A.M.; Forleo, D.A. Solid state gas sensors: State of the art and future activities. J. Optoelectron. Adv. Mater. 2003, 5, 1335–1348. [Google Scholar]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Horn, P.M. Low-frequency fluctuations in solids: 1/f noise. Rev. Mod. Phys. 1981, 53, 497–516. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Dutta, P.K.; Akbar, S.A. Oxygen sensors: Materials, methods, designs and applications. J. Mater. Sci. 2003, 38, 4271–4282. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy: Emphasizing Solid Materials and Systems; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Park, C.O.; Akbar, S.A.; Weppner, W. Ceramic electrolytes and electrochemical sensors. J. Mater. Sci. 2003, 38, 4639–4660. [Google Scholar] [CrossRef]
- Liu, Y.; Parisi, J.; Sun, X.; Lei, Y. Solid-state gas sensors for high temperature applications-a review. J. Mater. Chem. A 2014, 2, 9919–9943. [Google Scholar] [CrossRef]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: State of the art and approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Jiménez-Cadena, G.; Riu, J.; Rius, F.X. Gas sensors based on nanostructured materials. Analyst 2007, 132, 1083–1099. [Google Scholar]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I. Dtection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced Graphene Oxide Molecular Sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Goldsmith, B.R.; Kybert, N.J.; Johnson, A.T.C. DNA Decorated Graphene Chemical Sensors. Appl. Phys. Lett. 2010, 97, 83107. [Google Scholar] [CrossRef]
- Pearce, R.; Iakimov, T.; Andersson, M.; Hultman, L.; Spetz, A.L.; Yakimova, R. Epitaxially grown graphene based gas sensors for ultra sensitive NO2 detection. Sens. Actuators B Chem. 2011, 155, 451–455. [Google Scholar] [CrossRef]
- Ohno, K.; Maehashi, K.; Matsumoto, K. Chemical and biological sensing applications based on graphene field-effect transistors. Biosens. Bioelectron. 2010, 26, 1727–1730. [Google Scholar] [CrossRef]
- Syu, Y.-C.; Hsu, W.-E.; Lin, C.-T. Review—Field effect transistor biosensing: Devices and clinical application. ECS J. Solid State Sci. Technol. 2018, 7, Q3196–Q3207. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Im, J.P.; Lim, S.Y.; Kwon, J.Y.; Lee, S.M.; Moon, S.E. MEMS-based NO2 gas sensor using ZnO nano-rods for low-power IoT application. J. Korean Phys. Soc. 2017, 70, 924–928. [Google Scholar] [CrossRef]
- Bogue, R. Recent developments in MEMS sensors: A review of applications, markets and technologies. Sens. Rev. 2013, 33, 300–304. [Google Scholar] [CrossRef]
- Morales-Naraez, E.; Merkoci, A. Graphene oxide as an optical biosensing platform. Adv. Mater. 2012, 24, 3298–3308. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent Advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Latif, U.; Dickert, F.L. Graphene hybrid materials in gas sensing applications. Sensors 2015, 15, 30504–30524. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice. Mater. Sci. Eng. B 2007, 139, 1–13. [Google Scholar] [CrossRef]
- Rumyantsev, S.; Liu, G.; Potyrailo, R.A.; Balandin, A.A.; Shur, M.S. Selective sensing of individual gases using graphene devices. IEEE Sens. J. 2013, 13, 2818–2822. [Google Scholar] [CrossRef]
- Natgaz. Propane vs. Butane. Available online: http://www.natgaz.com.lb/all-about-gaz/propane-vs-butane (accessed on 10 September 2019).
- National Research Council USA. Butane: Acute exposure guide levels. In Acute Exposure Guideline Levels for Selected Airborne Chemicals; National Academies Press (US): Washington, DC, USA, 2012; Volume 12. Available online: https://www.ncbi.nlm.nih.gov/books/NBK201460/olume12 (accessed on 10 September 2019).
- Yan, Q.; Huang, B.; Yu, J.; Zheng, F.; Zang, J.; Wu, J.; Gu, B.; Liu, F.; Duan, W. Intrinsic current-voltage characteristics of graphene nanoribbon transistors and effect of edge doping. Nano Lett. 2007, 7, 1469–1473. [Google Scholar] [CrossRef]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2433–2454. [Google Scholar] [CrossRef]
- Rashid, M.H.; Koel, A.; Rang, T. Simulations of propane and butane gas sensor based on pristine armchair graphene nanoribbon. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bucharest, Romania, 7–9 March 2018; Volume 362. [Google Scholar]
- Narendar, V.; Gupta, S.K.; Saxena, S. First principle study of doped graphene for FET applications. Silicon 2019, 11, 277–286. [Google Scholar] [CrossRef]
- Siddique, S.; Mukhtar, H. Probing the performance of glucose oxidase treated graphene-based field effect transistors. J. Nanosci. Nanotechnol. 2019, 19, 7442–7446. [Google Scholar] [CrossRef]
- Hasan, N.; Hou, B.; Radadia, A.D. Ion sensing with solution-gated graphene field-effect sensors in the frequency domain. IEEE Sens. J. 2019, 19, 8758–8766. [Google Scholar] [CrossRef]
- Tsang, D.K.H.; Lieberthal, T.J.; Watts, C.; Dunlop, E.L.; Ramadan, S.; Hernandez, A.E.D.R.; Klien, N. Chemically functionalised graphene FET biosensor for the label-free sensing of exosomes. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- QuantumWise User Manual, ATK-DFT Calculator. Available online: https://docs.quantumwise.com/v2017/manuals/ATKDFT.html (accessed on 14 October 2019).
- High Performance Computing. Available online: https://www.ttu.ee/support-structure/it-services/services-4/high-performance-computing-2/ (accessed on 15 October 2019).
- Poklonski, N.A.; Kislyakov, E.F.; Vyrko, S.A.; Bubel’, O.N.; Ratkevich, S.V. Electronic band structure and magnetic states of zigzag graphene nanoribbons: Quantum chemical calculations. J. Nanophotonics 2012, 6, 61712. [Google Scholar] [CrossRef]
- Owens, F.J. Electronic and magnetic properties of armchair and zigzag graphene nanoribbons. J. Chem. Phys. 2008, 128, 194701. [Google Scholar] [CrossRef] [PubMed]
- Quantumwise, Building a Graphene Nanoribbon Transistor. Available online: https://docs.quantumwise.com/v2017/tutorials/vnl_graphene_transistor/vnl_graphene_transistor.html (accessed on 16 October 2019).
- Yavari, F.; Koratkar, N. Graphene-based chemical sensors. J. Phys. Chem. Lett. 2012, 3, 1746–1753. [Google Scholar] [CrossRef]
- Bekduz, B.; Kampermann, L.; Mertin, W.; Punckt, C.; Aksay, I.A.; Bacher, G. Influence of the atmospheric species on the electrical properties of functionalized graphene sheets. RCS Adv. 2018, 8, 42073–42079. [Google Scholar] [CrossRef]
- Melios, C.; Centeno, A.; Zurutuza, A.; Panchal, V.; Giusca, C.E.; Spencer, S.; Silva, S.R.P.; Kazakova, O. Effects of humidity on the electronic properties of graphene prepared by chemical vapour deposition. Carbon 2016, 103, 273–280. [Google Scholar] [CrossRef]
- What Effect Does Humidity Have on Graphene Sensors? Available online: https://www.azosensors.com/article.aspx?ArticleID=1323 (accessed on 3 December 2019).
- Yao, Y.; Chen, X.; Zhu, J.; Zeng, B.; Wu, Z.; Li, X. The effect of ambient humidity on the electrical properties of graphene oxide films. Nanoscale Res. Lett. 2012, 7, 363. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Elgammal, K.; Fan, X.; Lemme, M.C.; Delin, A.; Rasander, M.; Bergqvit, L.; Schroder, S.; Fischer, A.C.; Niklaus, F.; et al. Graphene-based CO2 sensing and its cross-sensitivity with humidity. RSC Adv. 2017, 7, 22329–22339. [Google Scholar] [CrossRef]
- Eugster, W.; Kling, G.W. Performance of low-cost methane sensor for ambient concentration measurements in preliminary studies. Atmos. Meas. Tech. 2012, 5, 1925–1934. [Google Scholar] [CrossRef]
- Tao, C.; Li, X.; Yang, J.; Shi, Y. Optical fiber sensing element based on luminescence quenching of silica nanowires modified with cryptophane-A for the detection of methane. Sens. Actuators B Chem. 2011, 156, 553–558. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Liu, B. Room Temperature Methane Sensor Based on Graphene Nanosheets/Polyaniline Nanocomposite Thin Film. IEEE Sens. 2013, 13, 777–782. [Google Scholar] [CrossRef]
- Roy, S.; Sarkar, C.K.; Bhattacharyya, P. A highly sensitive methane sensor with nickel alloy microheater on micromachined Si substrate. Solid State Electron. 2012, 76, 84–90. [Google Scholar] [CrossRef]
- Haridas, D.; Gupta, V. Enhanced response characteristics of SnO2 thin film based sensors loaded with Pd clusters for methane detection. Sens. Actuators B Chem. 2012, 166, 156–164. [Google Scholar] [CrossRef]
- Prasad, A.K.; Amirthapandian, S.; Dhara, S.; Dash, S.; Murali, N.; Tyagi, A.K. Novel single phase vanadium dioxide nanostructured films for methane sensing near room temperature. Sens. Actuators B Chem. 2014, 191, 252–256. [Google Scholar] [CrossRef]
- Vuong, N.M.; Hieu, N.M.; Hieu, H.N.; Yi, H.; Kim, D.; Han, Y.S.; Kim, M. Ni2O3-decorated SnO2 particulate films for methane gas sensors. Sens. Actuators B Chem. 2014, 192, 327–333. [Google Scholar] [CrossRef]
- Gong, G.; Zhu, H. Development of highly sensitive sensor system for methane utilizing cataluminescence. Luminescence 2015, 31, 183–189. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhao, Y.; Wang, Q. Measurement of methane concentration with cryptophane E infiltrated photonic crystal microcavity. Sens. Actuators B Chem. 2015, 209, 431–437. [Google Scholar] [CrossRef]
- Yang, D.; Yang, N.; Ni, J.; Xiao, J.; Jiang, J.; Liang, Q.; Ren, T.; Chen, X. First-principles approach to design and evaluation of graphene as methane sensors. Mater. Des. 2017, 119, 401. [Google Scholar] [CrossRef]
- Humayun, M.T.; Divan, R.; Stan, L.; Rosenmann, D.; Gosztola, D.; Paul, L.G.; Solomon, A.; Paprotny, I. Ubiquitous low-cost functionalized multi-walled carbon nanotube sensors for distributed methane leak detection. IEEE Sens. J. 2016, 16, 8692–8699. [Google Scholar] [CrossRef]
- Humayun, M.T.; Divan, R.; Liu, Y.; Gundel, L.; Solomon, P.; Paprotny, I. Novel chemoresistive CH4 sensor with 10 ppm sensitivity based on multiwalled carbon nanotubes functionalized with SnO2 nanocrystals. J. Vac. Sci. Technol. A Vac. Surf. Films 2016, 34, 01A131. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.H.; Koel, A.; Rang, T. Simulations of Graphene Nanoribbon Field Effect Transistor for the Detection of Propane and Butane Gases: A First Principles Study. Nanomaterials 2020, 10, 98. https://doi.org/10.3390/nano10010098
Rashid MH, Koel A, Rang T. Simulations of Graphene Nanoribbon Field Effect Transistor for the Detection of Propane and Butane Gases: A First Principles Study. Nanomaterials. 2020; 10(1):98. https://doi.org/10.3390/nano10010098
Chicago/Turabian StyleRashid, Muhammad Haroon, Ants Koel, and Toomas Rang. 2020. "Simulations of Graphene Nanoribbon Field Effect Transistor for the Detection of Propane and Butane Gases: A First Principles Study" Nanomaterials 10, no. 1: 98. https://doi.org/10.3390/nano10010098
APA StyleRashid, M. H., Koel, A., & Rang, T. (2020). Simulations of Graphene Nanoribbon Field Effect Transistor for the Detection of Propane and Butane Gases: A First Principles Study. Nanomaterials, 10(1), 98. https://doi.org/10.3390/nano10010098




