Effect of Nanoparticles with Different Chemical Nature on the Stability and Rheology of Acrylamide Sodium Acrylate Copolymer/Chromium (III) Acetate Gel for Conformance Control Operations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Gel Preparation
2.2.2. Rheological Tests
2.2.3. Syneresis Measurements
2.2.4. Sydansk’s Code
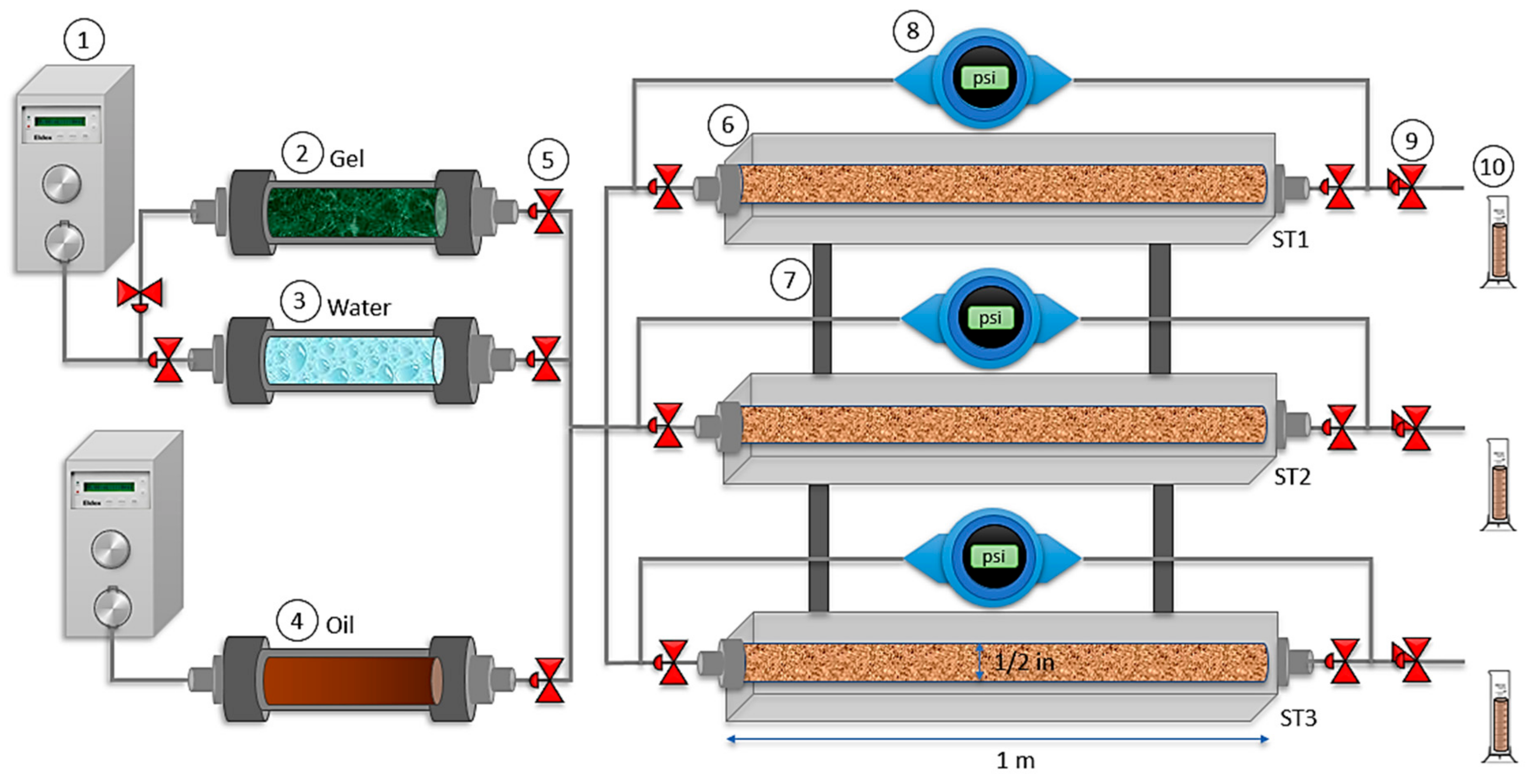
2.2.5. Displacement Test in a Triple Parallel Slim Tube System
3. Results and Discussion
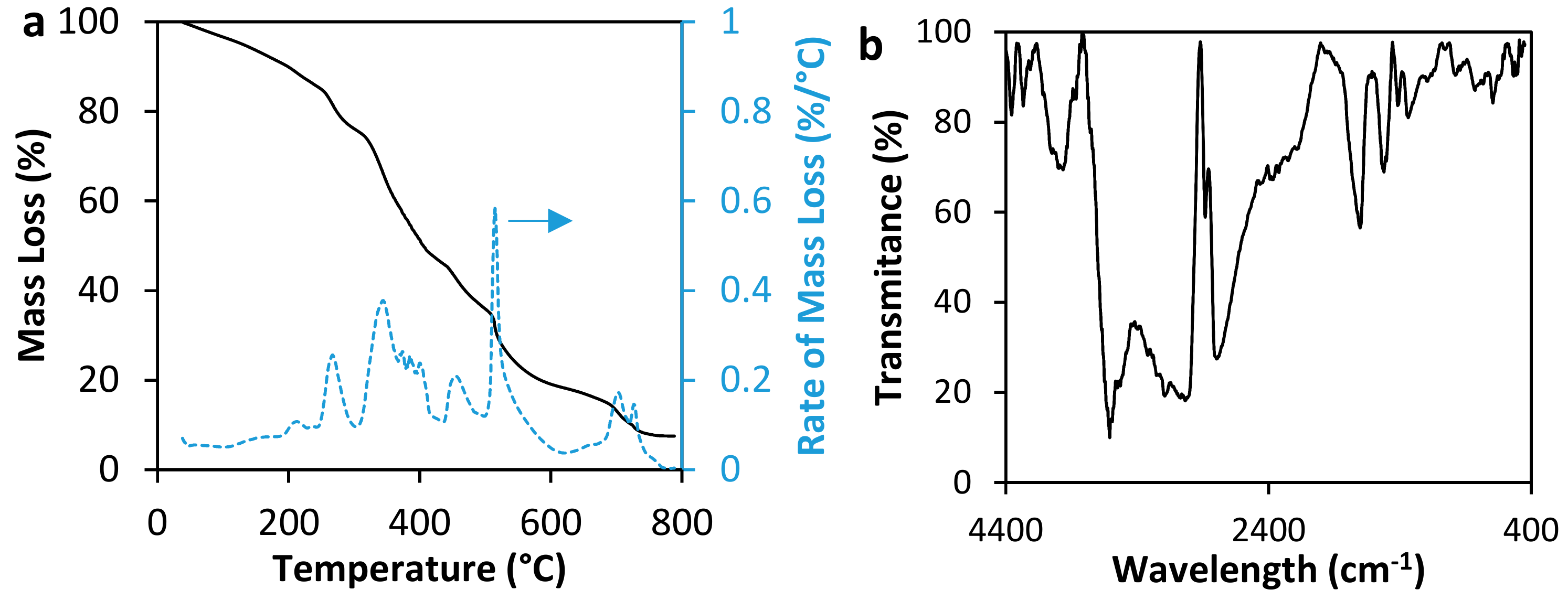
3.1. Materials Characterization
3.2. Rheological Measurements
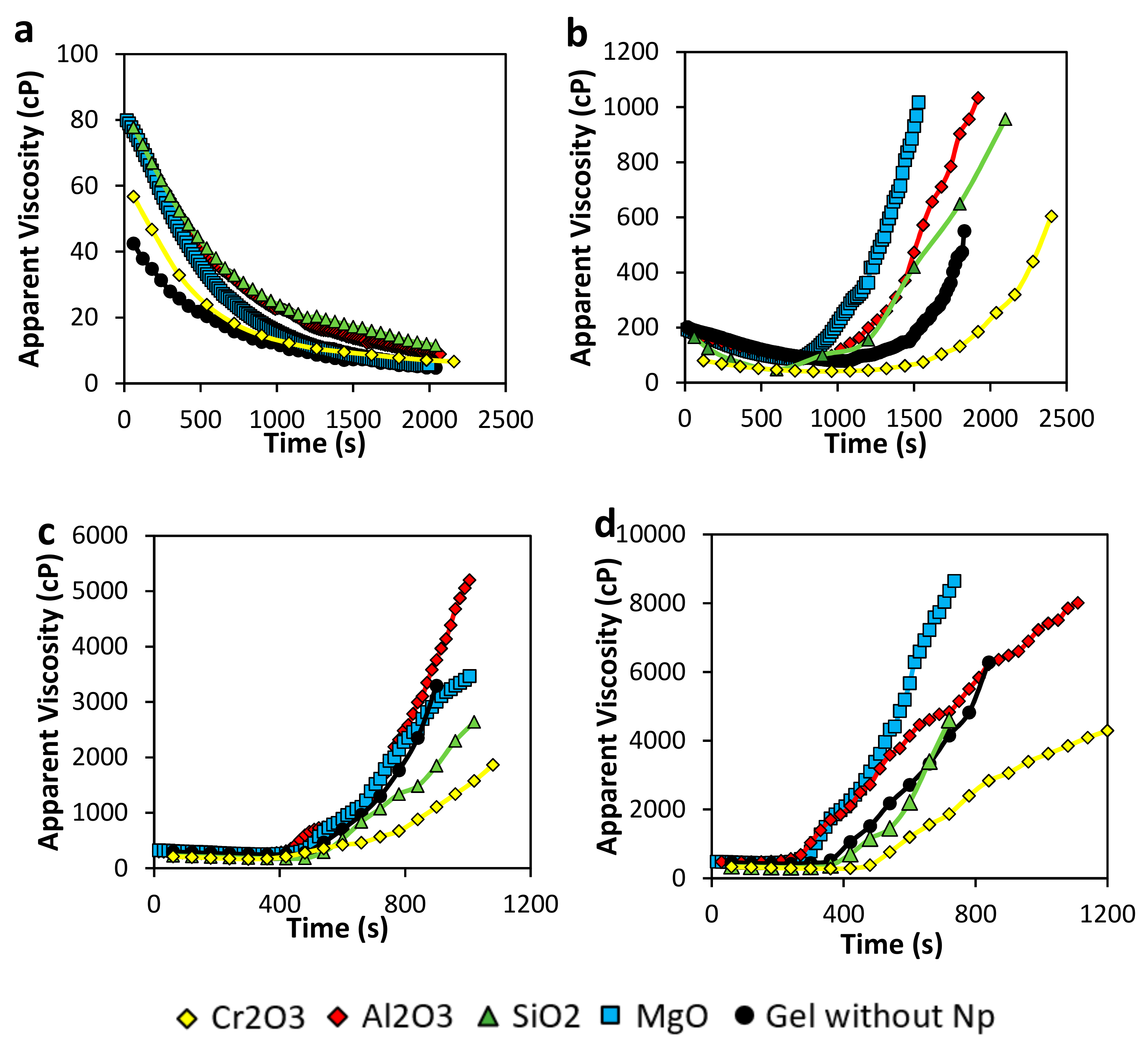
3.2.1. Gelation Time
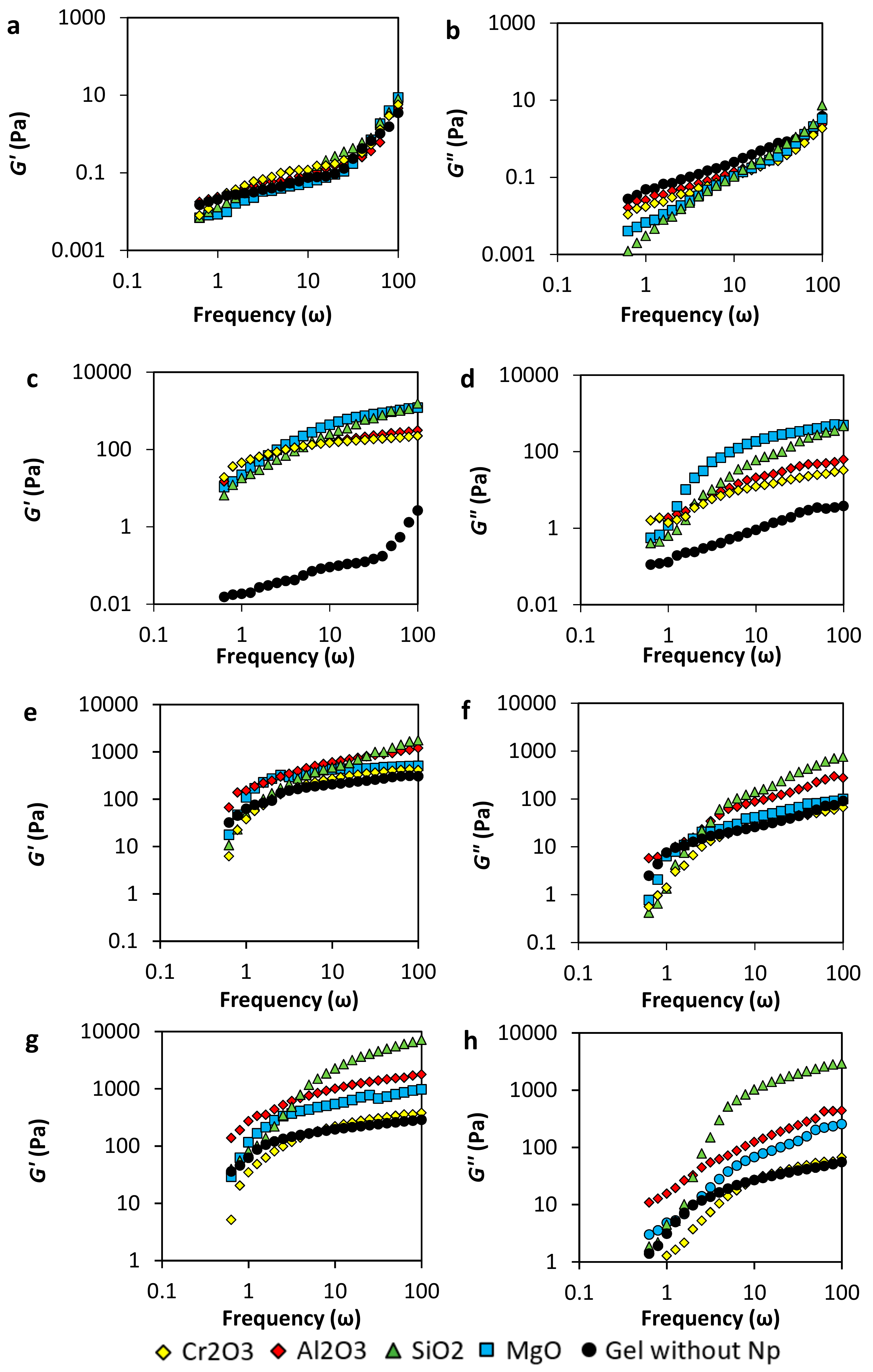
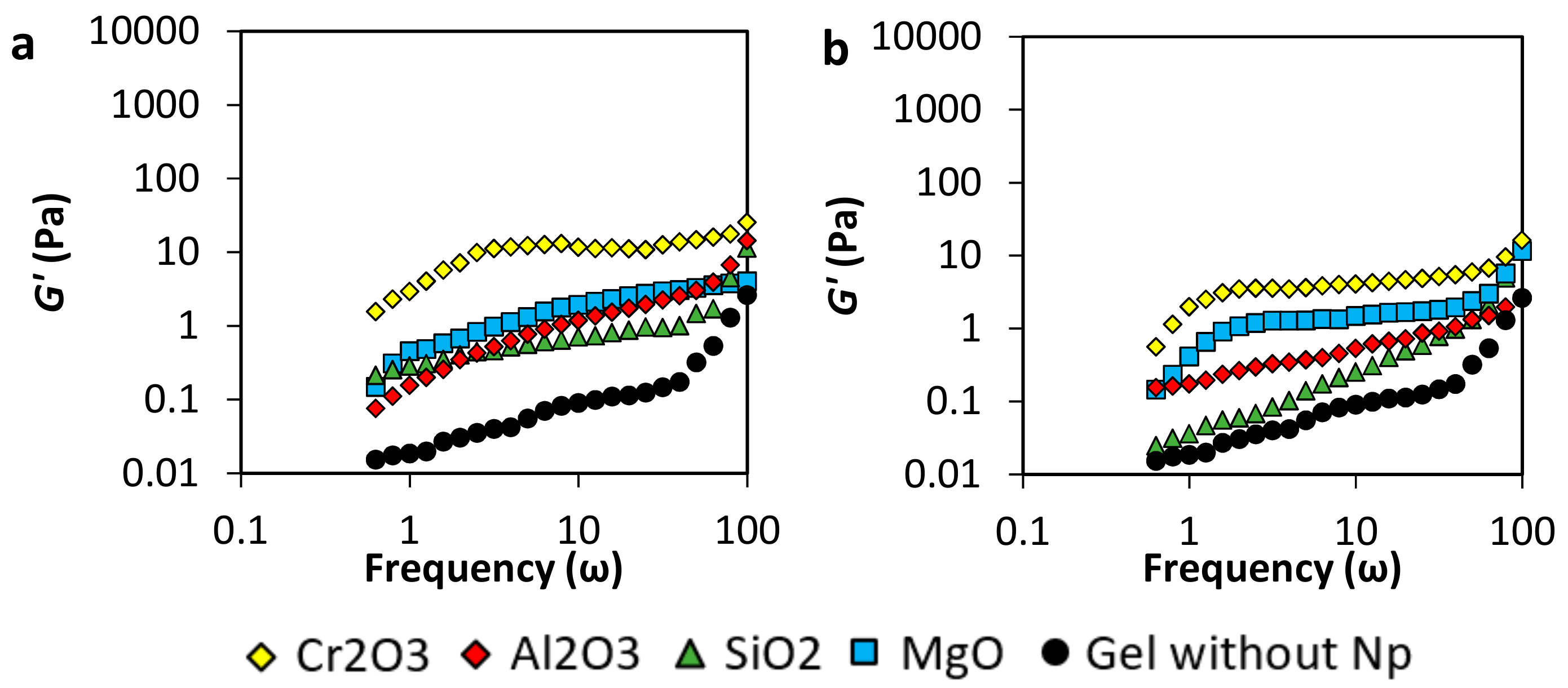
3.2.2. Viscoelastic Modulus (G’ and G”)
3.3. Syneresis Behavior
3.4. Gelation Kineticks by Sydansk’s Code
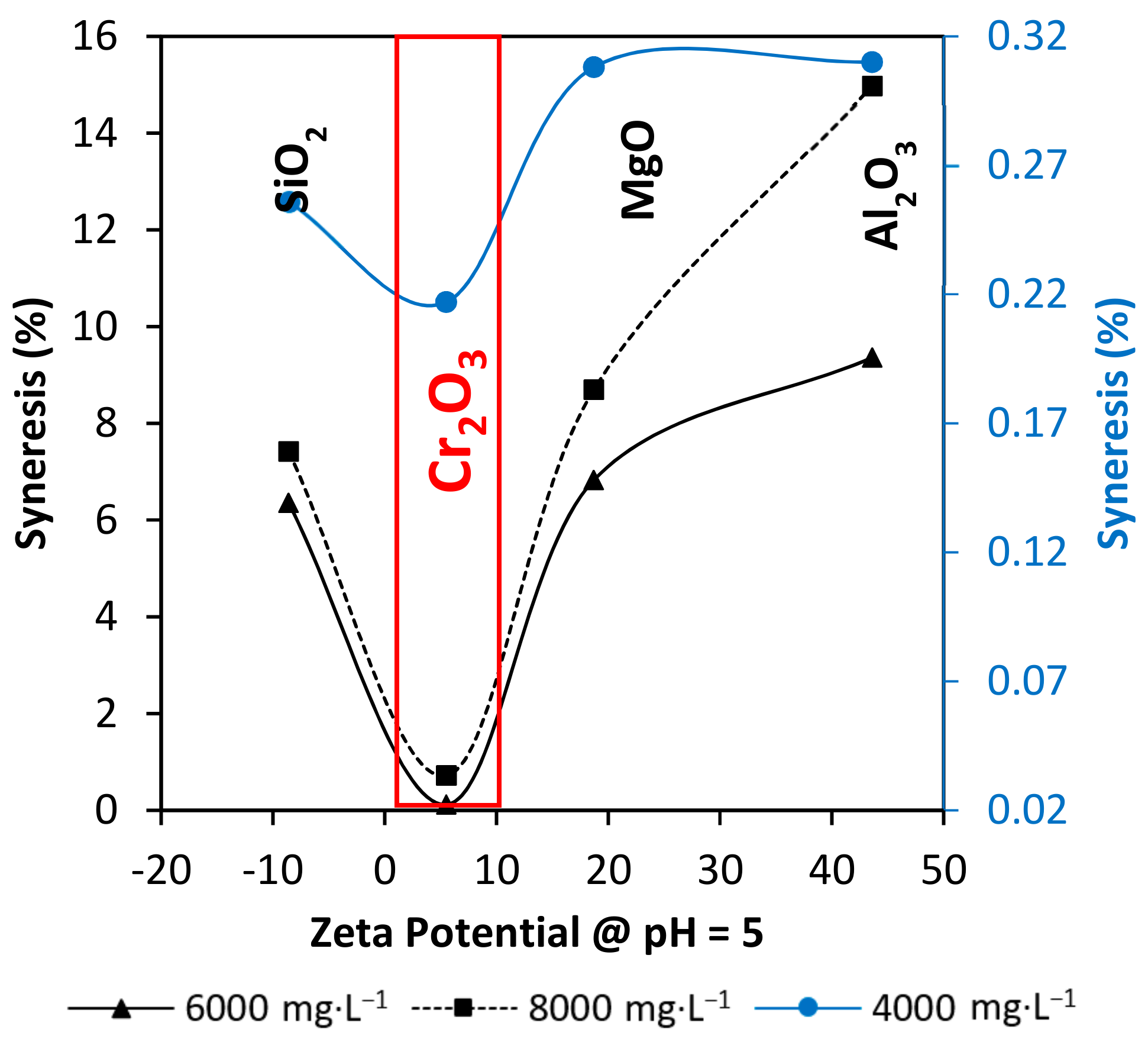
3.5. Syneresys Prediction through Nanoparticles Zeta Potential
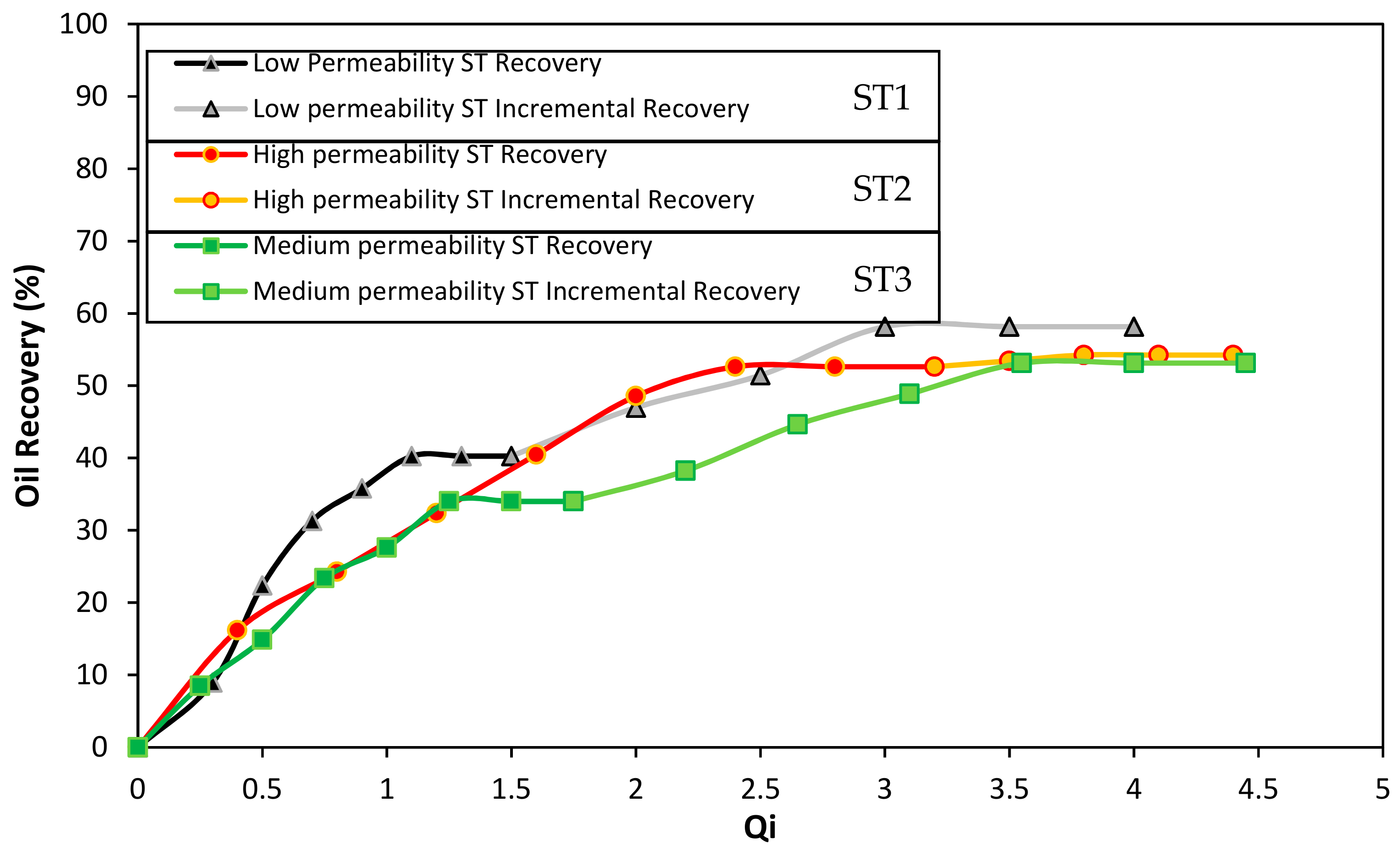
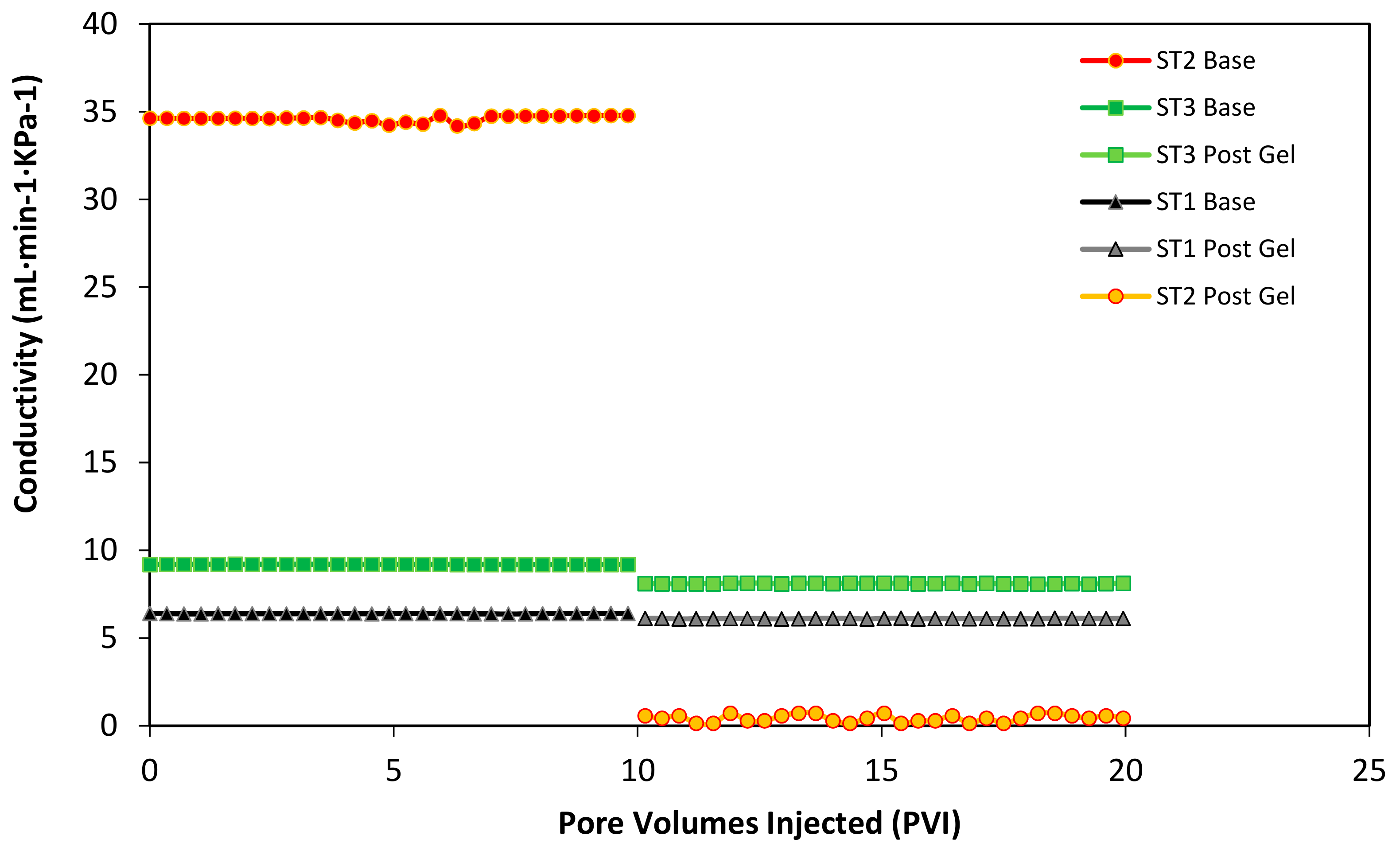
3.6. Displacement Test Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdulbaki, M.; Huh, C.; Sepehrnoori, K.; Delshad, M.; Varavei, A. A critical review on use of polymer microgels for conformance control purposes. J. Pet. Sci. Eng. 2014, 122, 741–753. [Google Scholar] [CrossRef]
- Borling, D.; Chan, K.; Hughes, T.; Sydansk, R. Pushing out the oil with conformance control. Oilfield Rev. 1994, 6, 44–58. [Google Scholar]
- Dang, T.; Chen, Z.; Nguyen, T.; Bae, W.; Chung, T.; Tu, T. The development and optimization of a polymer conformance control technology in mature reservoirs: Laboratory experiments vs. Field scale simulation. Energy Sources Part A Recovery Util. Environ. Eff. 2014, 36, 1219–1233. [Google Scholar] [CrossRef]
- Enick, R.M.; Olsen, D.K.; Ammer, J.R.; Schuller, W. Mobility and Conformance Control for CO2 EOR via Thickeners, Foams, and Gels—A Literature Review of 40 Years of Research and Pilot Tests. In Proceedings of the the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Sydansk, R.D. Reservoir Conformance Improvement; Society of Petroleum Engineers: London, UK, 2011. [Google Scholar]
- Sheng, J. Enhanced Oil Recovery; Gulf Proffesional Publishing: Walthan, MA, USA, 2013. [Google Scholar]
- Medina, O.E.; Olmos, C.; Lopera, S.H.; Cortés, F.B.; Franco, C.A. Nanotechnology Applied to Thermal Enhanced Oil Recovery Processes: A Review. Energies 2019, 12, 4671. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, B.; Hou, J. Polymer Gel Systems for Water Management in High-Temperature Petroleum Reservoirs: A Chemical Review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Liu, J.; Seright, R. Rheology of gels used for conformance control in fractures. SPE J. 2001, 6, 120–125. [Google Scholar] [CrossRef]
- Broseta, D.; Marquer, O.; Blin, N.; Zaitoun, A. Rheological screening of low-molecular-weight polyacrylamide/chromium (III) acetate water shutoff gels. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 3–5 April 2000. [Google Scholar]
- Grattoni, C.A.; Al-Sharji, H.H.; Yang, C.; Muggeridge, A.H.; Zimmerman, R.W. Rheology and permeability of crosslinked polyacrylamide gel. J. Colloid Interface Sci. 2001, 240, 601–607. [Google Scholar] [CrossRef]
- Karimi, S.; Kazemi, S.; Kazemi, N. Syneresis measurement of the HPAM-Cr (III) gel polymer at different conditions: An experimental investigation. J. Nat. Gas Sci. Eng. 2016, 34, 1027–1033. [Google Scholar] [CrossRef]
- Seright, R. Washout of Cr (III)-Acetate-HPAM Gels from Fractures. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 5–7 February 2003. [Google Scholar]
- Vargas-Vasquez, S.; Romero-Zerón, L.; MacMillan, B. Analysis of syneresis of HPAM/Cr (II) and HPAM/Cr (III) acetate gels through 1H nuclear magnetic resonance, bottle testing, and UV-vis spectroscopy. Pet. Sci. Technol. 2009, 27, 1727–1743. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Bryant, S.L.; Rabaioli, M.; Lockhart, T.P. Influence of syneresis on permeability reduction by polymer gels. SPE Prod. Facil. 1996, 11, 209–215. [Google Scholar] [CrossRef]
- Nguyen, N.; Tu, T.; Bae, W.; Dang, C.; Chung, T.; Nguyen, H. Gelation time optimization for an HPAM/chromium acetate system: The successful key of conformance control technology. Energy Sources Part A Recovery Util. Environ. Eff. 2012, 34, 1305–1317. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part B: Effects of nanoparticles on flooding. Int. Nano Lett. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Bera, A.; Belhaj, H. Application of nanotechnology by means of nanoparticles and nanodispersions in oil recovery-A comprehensive review. J. Nat. Gas Sci. Eng. 2016, 34, 1284–1309. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part A: Effects of nanoparticles on interfacial tension. Int. Nano Lett. 2016, 6, 129–138. [Google Scholar] [CrossRef]
- Crews, J.B.; Gomaa, A.M. Nanoparticle associated surfactant micellar fluids: An alternative to crosslinked polymer systems. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Negin, C.; Ali, S.; Xie, Q. Application of nanotechnology for enhancing oil recovery—A review. Petroleum 2016, 2, 324–333. [Google Scholar] [CrossRef]
- Rankin, K.; Nguyen, Q. Conformance Control through In-situ Gelation of Silica Nanoparticles. In Proceedings of the Technical 2014 NSTI Nanotechology Conference & Expo, Washington, DC, USA, 15–19 June 2014. [Google Scholar]
- Jayarambabu, N.; Kumari, S.; Rao, V.; Prabhu, Y. Enhancement of Growth In Maize By Biogenic-Synthesized Mgo Nanoparticles. Int. J. Pure Appl. Zool. 2016, 4, 262–270. [Google Scholar]
- Ogolo, N.; Olafuyi, O.; Onyekonwu, M. Enhanced oil recovery using nanoparticles. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 8–11 April 2012. [Google Scholar]
- Onyekonwu, M.O.; Ogolo, N.A. Investigating the use of nanoparticles in enhancing oil recovery. In Proceedings of the Nigeria Annual International Conference and Exhibition, Calabar, Nigeria, 31 July–7 August 2010. [Google Scholar]
- Agista, M.N.; Guo, K.; Yu, Z. A State-of-the-Art Review of Nanoparticles Application in Petroleum with a Focus on Enhanced Oil Recovery. Appl. Sci. 2018, 8, 871. [Google Scholar] [CrossRef]
- Ma, L.; Wang, S.; Long, Y.; Zhu, C.; Yang, H.; Yang, T.; Liu, X.; Li, X.; Bai, B.; Kang, W. Novel Environmentally Benign Hydrogel: Nano-Silica Hybrid Hydrolyzed Polyacrylamide/Polyethyleneimine Gel System for Conformance Improvement in High Temperature High Salinity Reservoir. In Proceedings of the SPE Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 13–16 November 2017. [Google Scholar]
- Dijvejin, Z.A.; Ghaffarkhah, A.; Sadeghnejad, S.; Sefti, M.V. Effect of silica nanoparticle size on the mechanical strength and wellbore plugging performance of SPAM/chromium (III) acetate nanocomposite gels. Polym. J. 2019, 51, 693. [Google Scholar] [CrossRef]
- Pérez-Robles, S.; Cortés, F.B.; Franco, C.A. Effect of the nanoparticles in the stability of hydrolyzed polyacrylamide/resorcinol/formaldehyde gel systems for water shut-off/conformance control applications. J. Appl. Polym. Sci. 2019, 136, 47568. [Google Scholar] [CrossRef]
- Barnes, H.A. Linear Viscoelasticty and Time Effects. In A Handbook of Elementary Rheology; Printers, C., Ed.; University of Wales Aberystwyth: Aberystwyth, UK, 2000. [Google Scholar]
- Sydansk, R.D. Hydraulic Fracturing Process Using a Polymer Gel. U.S. Patent 4,779,680, 25 October 1988. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies; John Wiley & Sons Ltd: Chichester, UK, 2004. [Google Scholar]
- Giraldo, L.J.; Giraldo, M.A.; Llanos, S.; Maya, G.; Zabala, R.D.; Nassar, N.N.; Franco, C.A.; Alvarado, V.; Cortés, F.B. The effects of SiO2nanoparticles on the thermal stability and rheological behavior of hydrolyzed polyacrylamide based polymeric solutions. J. Pet. Sci. Eng. 2017, 159, 841–852. [Google Scholar] [CrossRef]
- Magalhães, A.S.G.; Neto, M.P.A.; Bezerra, M.N.; Ricardo, N.M.P.S.; Feitosa, J.P.A. Application of ftir in the determination of acrylate content in poly(sodium acrylate-CO-acrylamide) superabsorbent hydrogels. Quim. Nova 2012, 35, 1464–1467. [Google Scholar] [CrossRef]
- Craciun, G.; Ighigeanu, D.; Manaila, E.; Stelescu, M.D. Synthesis and Characterization of Poly(Acrylamide-Co-Acrylic Acid) Flocculant Obtained by Electron Beam Irradiation. Mater. Res. 2015, 18, 984–993. [Google Scholar] [CrossRef]
- Huacai, G.; Wan, P.; Dengke, L. Graft copolymerization of chitosan with acrylic acid under microwave irradiation and its water absorbency. Carbohydr. Polym. 2006, 66, 372–378. [Google Scholar] [CrossRef]
- Nuno-Donlucas, S.; Rhoton, A.; Corona-Galvan, S.; Puig, J.; Kaler, E. Emulsion copolymerization of styrene and sodium acrylate. Polym. Bull. 1993, 30, 207–214. [Google Scholar] [CrossRef]
- Witten, T.A. Structured fluids. Phys. Today 1990, 43, 21–28. [Google Scholar] [CrossRef]
- Sydansk, R.D. Polymers, Gels, Foams, and Resins. In Petroleum Engineering Handbook Vol. V (B); Society of Petroleum Engineers: Richardson, TX, USA, 2007. [Google Scholar]

| Material | D50 (nm) | Zeta Potential pH ~ 5 | Point of Zero Charge | SBET (m2/g) |
|---|---|---|---|---|
| Al2O3 | 35 | 43 | 9 | 43 |
| MgO | 80 | 18 | 11 | 21 |
| Cr2O3 | 60 | 5 | 7 | 19 |
| SiO2 | 11 | −8 | 3 | 380 |
| Gel Strength Code | Gel Description |
|---|---|
| 1 | Gel flows as polymer upon inversion. |
| 2 | Gel flows slightly slower than the polymer solution upon inversion. |
| 3 | Gel flows very slowly and does not fully leave the tube upon inversion. |
| 4 | When the bottle is inverted, the bubble barely makes it to the top of the tube. |
| 5 | When inverted, the bubble flows very slow and hardly makes it to the top of the bottle. |
| 6 | When inverted, the bubble does not make it to the top of the bottle |
| 7 | When inverted, the bubble makes it than a halfway to the top. |
| 8 | The bubble hardly moves off from the bottom of the tube. |
| 9 | When inverted, the gel surface is barely disturbed. |
| 10 | The gel surface remains flat. |
| [Polymer] | 2 h | 4 h | 8 h | 24 h | 48 h | 1st Week | 2nd Week | 3rd Week | 4th Week | |
|---|---|---|---|---|---|---|---|---|---|---|
| Base Gel | 2000 | n | n | n | n | n | n | n | n | n |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 4000 | n | n | s | s | s | s | s | s | s | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 6000 | s | s | s | g | g | g | g | g | g | |
| 1 | 1 | 2 | 4 | 4 | 6 | 6 | 6 | 6 | ||
| 8000 | s | s | s | g | g | g | g | g | g | |
| 2 | 3 | 3 | 5 | 7 | 7 | 7 | 7 | 7 | ||
| Al2O3 | 2000 | n | n | n | n | n | n | n | n | n |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 4000 | s | s | s | s | s | s | s | s | s | |
| 1 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||
| 6000 | s | s | g | g | g | g | g | g | g | |
| 1 | 2 | 4 | 4 | 9 | 9 | 9 | 9 | 9 | ||
| 8000 | s | s | g | g | g | e | e | e | e | |
| 2 | 2 | 3 | 5 | 8 | 10 | 10 | 10 | 10 | ||
| SiO2 | 2000 | n | n | n | n | n | n | n | n | n |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 4000 | s | s | s | s | s | s | s | s | s | |
| 1 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||
| 6000 | s | s | g | g | g | g | g | g | g | |
| 1 | 2 | 3 | 4 | 7 | 7 | 7 | 7 | 7 | ||
| 8000 | s | s | g | g | g | g | g | g | g | |
| 2 | 2 | 3 | 5 | 9 | 9 | 9 | 9 | 9 | ||
| MgO | 2000 | n | n | n | n | n | n | n | n | n |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 4000 | s | s | s | s | s | s | s | s | s | |
| 1 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||
| 6000 | s | s | g | g | g | g | g | g | g | |
| 1 | 3 | 4 | 4 | 8 | 8 | 8 | 8 | 8 | ||
| 8000 | s | s | g | g | g | e | e | e | e | |
| 2 | 2 | 3 | 5 | 8 | 10 | 10 | 10 | 10 | ||
| Cr2O3 | 2000 | n | n | n | n | n | n | n | n | n |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 4000 | n | n | s | s | s+ | s+ | s+ | s+ | s+ | |
| 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||
| 6000 | s | s | s | g | g | g | g | g | g | |
| 1 | 2 | 3 | 4 | 4 | 7 | 7 | 7 | 7 | ||
| 8000 | s | s | s | g | g | g | g | g | g | |
| 2 | 2 | 2 | 5 | 8 | 8 | 8 | 8 | 8 | ||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Conductivity (mL·min−1·KPa−1) | ST1 Low Permeability | ST2 High Permeability | ST3 Medium Permeability |
|---|---|---|---|
| Before | 6.382 | 34.519 | 9.282 |
| After | 6.092 | 7.252 × 10−2 | 8.122 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Robles, S.; Matute, C.A.; Lara, J.R.; Lopera, S.H.; Cortés, F.B.; Franco, C.A. Effect of Nanoparticles with Different Chemical Nature on the Stability and Rheology of Acrylamide Sodium Acrylate Copolymer/Chromium (III) Acetate Gel for Conformance Control Operations. Nanomaterials 2020, 10, 74. https://doi.org/10.3390/nano10010074
Pérez-Robles S, Matute CA, Lara JR, Lopera SH, Cortés FB, Franco CA. Effect of Nanoparticles with Different Chemical Nature on the Stability and Rheology of Acrylamide Sodium Acrylate Copolymer/Chromium (III) Acetate Gel for Conformance Control Operations. Nanomaterials. 2020; 10(1):74. https://doi.org/10.3390/nano10010074
Chicago/Turabian StylePérez-Robles, Saray, Cristian A. Matute, Jeison R. Lara, Sergio H. Lopera, Farid B. Cortés, and Camilo A. Franco. 2020. "Effect of Nanoparticles with Different Chemical Nature on the Stability and Rheology of Acrylamide Sodium Acrylate Copolymer/Chromium (III) Acetate Gel for Conformance Control Operations" Nanomaterials 10, no. 1: 74. https://doi.org/10.3390/nano10010074
APA StylePérez-Robles, S., Matute, C. A., Lara, J. R., Lopera, S. H., Cortés, F. B., & Franco, C. A. (2020). Effect of Nanoparticles with Different Chemical Nature on the Stability and Rheology of Acrylamide Sodium Acrylate Copolymer/Chromium (III) Acetate Gel for Conformance Control Operations. Nanomaterials, 10(1), 74. https://doi.org/10.3390/nano10010074







