Organic-Inorganic Hybrid Materials for Room Temperature Light-Activated Sub-ppm NO Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.1.1. Synthesis of Ru (II) Complex
2.1.2. Synthesis of Nanocrystalline SnO2 and In2O3
2.1.3. Synthesis of Hybrid Materials
- (i)
- in the form of powders to investigate optical properties and thermal stability. The RuITP organic dye was dissolved in methanol, and then 10 μL of the prepared solution was added dropwise to the weighed SnO2 or In2O3 powder. The obtained paste was dried at room temperature, the addition of each subsequent portion of the solution was carried out after complete evaporation of the solvent. The concentration of the solution was adjusted so that the Ru content in the hybrid material was 1 wt.%.
- (ii)
- in the form of thick films over specially designed micro-hotplates, provided with Pt contact electrodes and Pt contact heater to investigate gas sensor properties. Weighed nanocrystalline SnO2 or In2O3 powder was mixed with a vehicle (α-terpineol in ethanol). The paste obtained was deposited by microdroping technique (GeSim multi-dos 2 with NanoTipHV head) on the surface of microhotplate to cover the contact electrodes. Thick films were dried at 50 °C for 24 h and sintered at 300 °C for 24 h in air. The thickness of the films, estimated from the preliminary calibration carried out by scanning electron microscopy, was about 1 μm. The obtained films were functionalized by RuITP methanol solution and dried at 50 °C for 24 h in air.
2.2. Materials Characterization
3. Results and Discussion
3.1. Characteristics of Nanocrystalline Semiconductor Oxides
3.2. Characteristics of Ru (II) Heterocyclic Complex
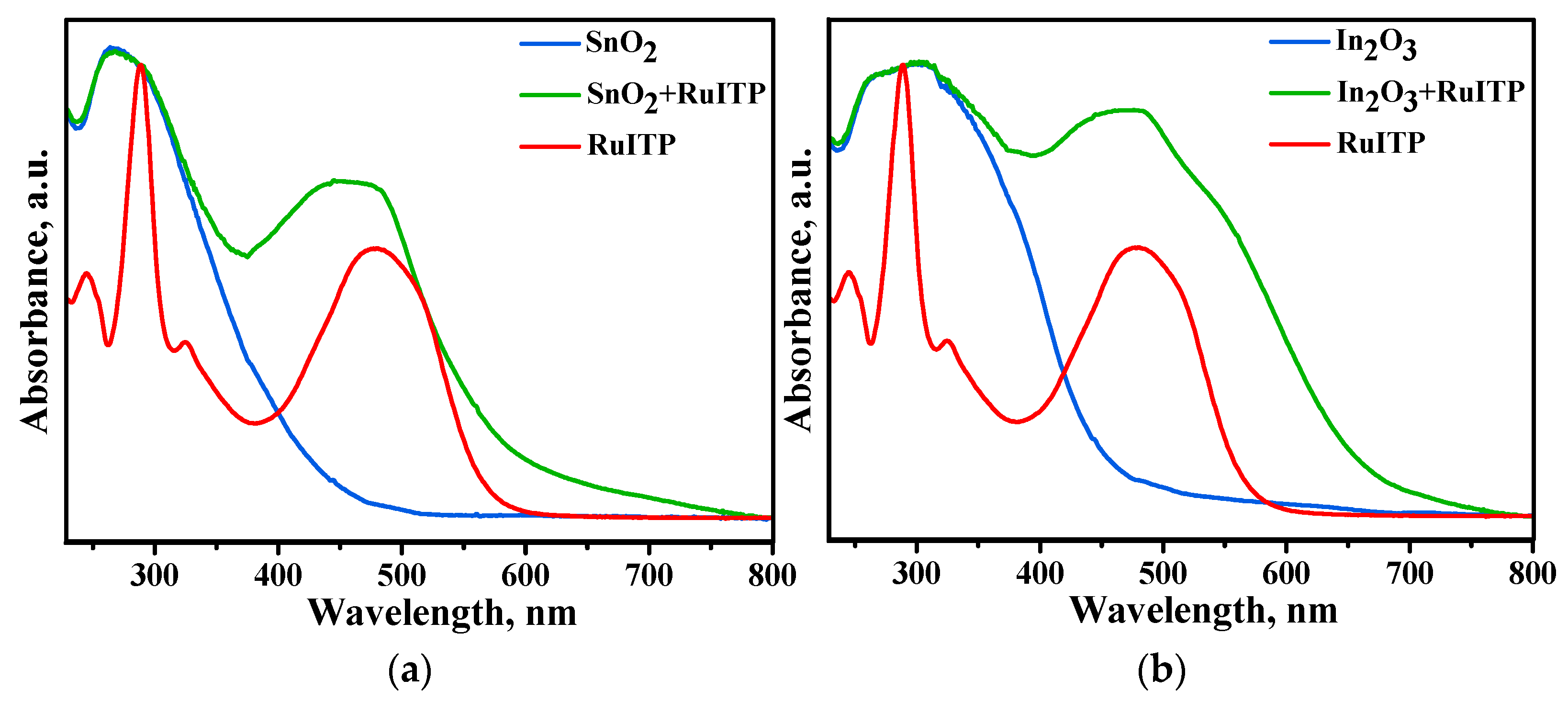
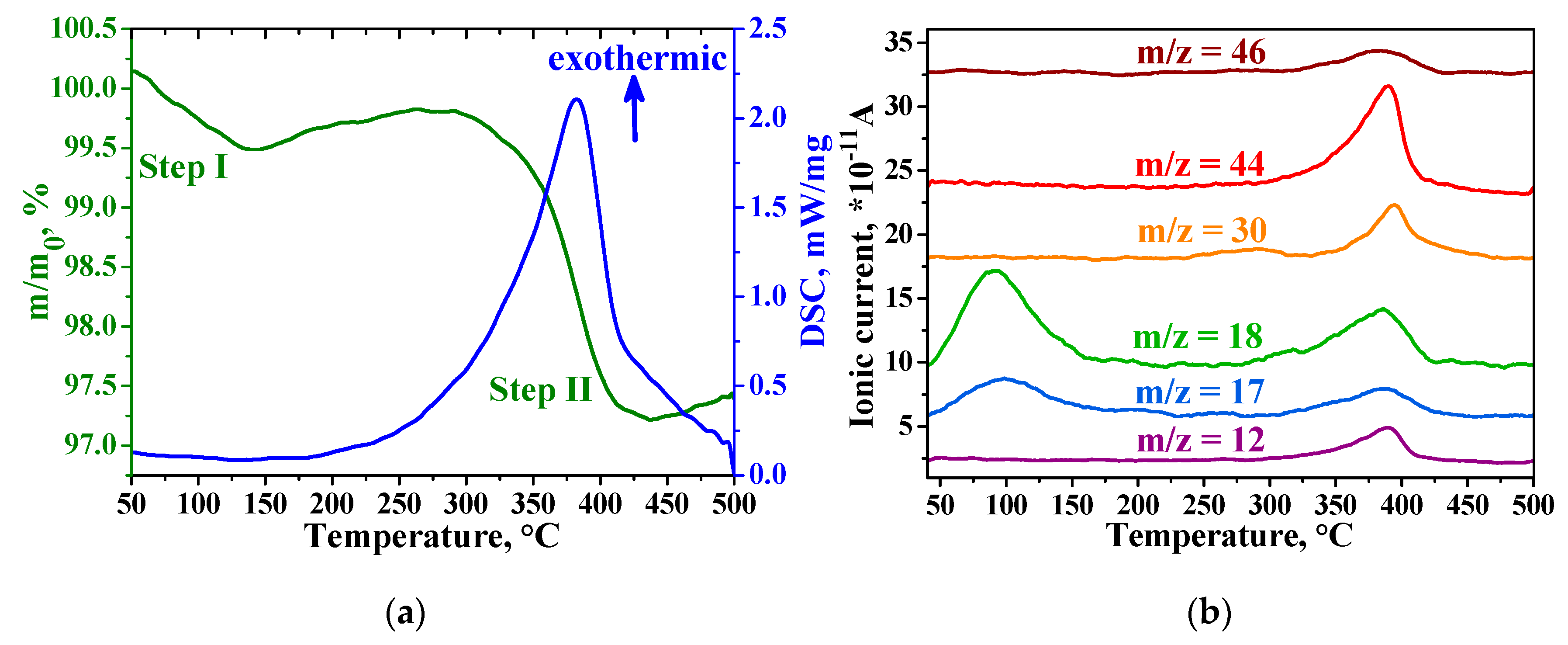
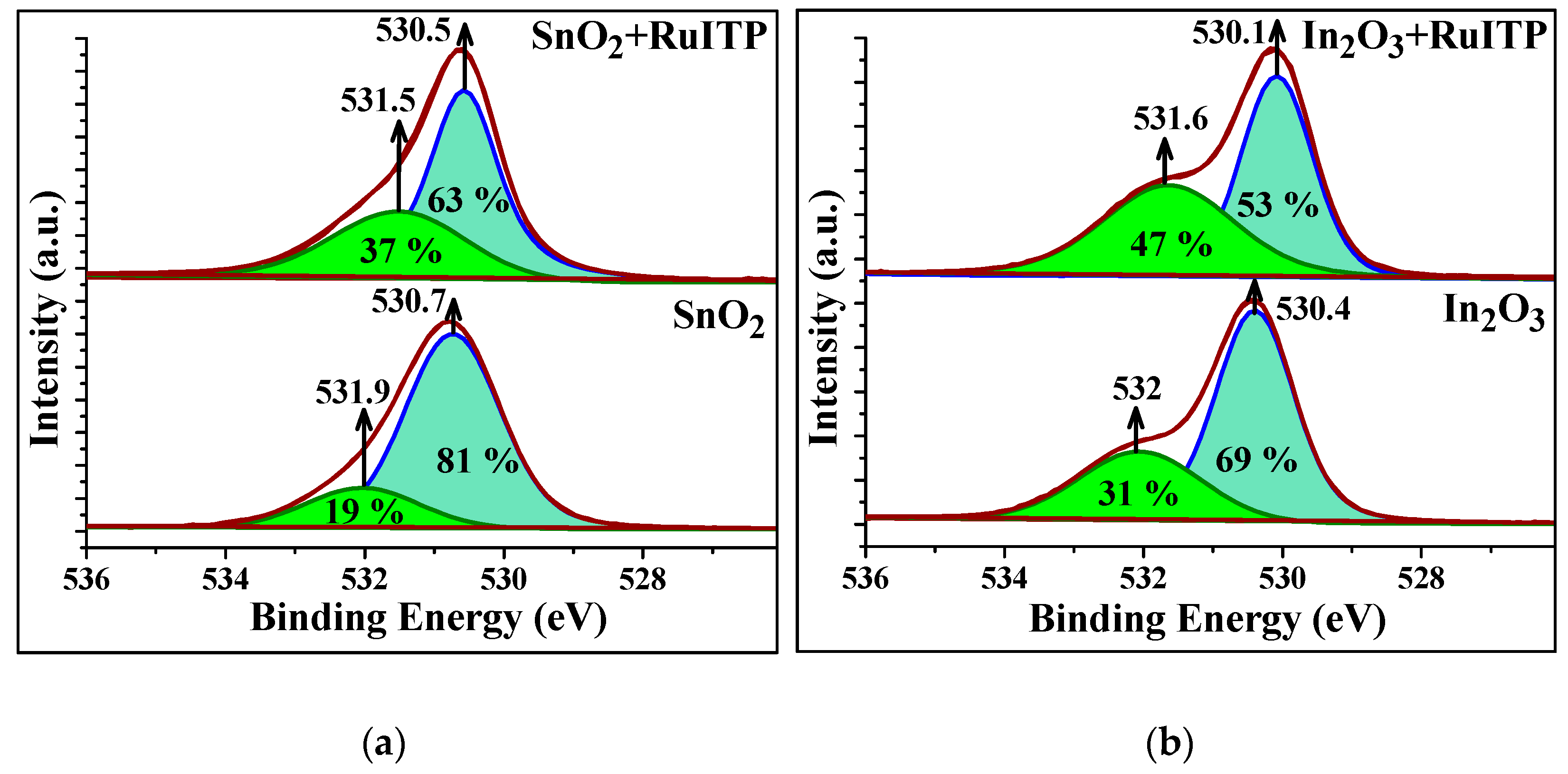
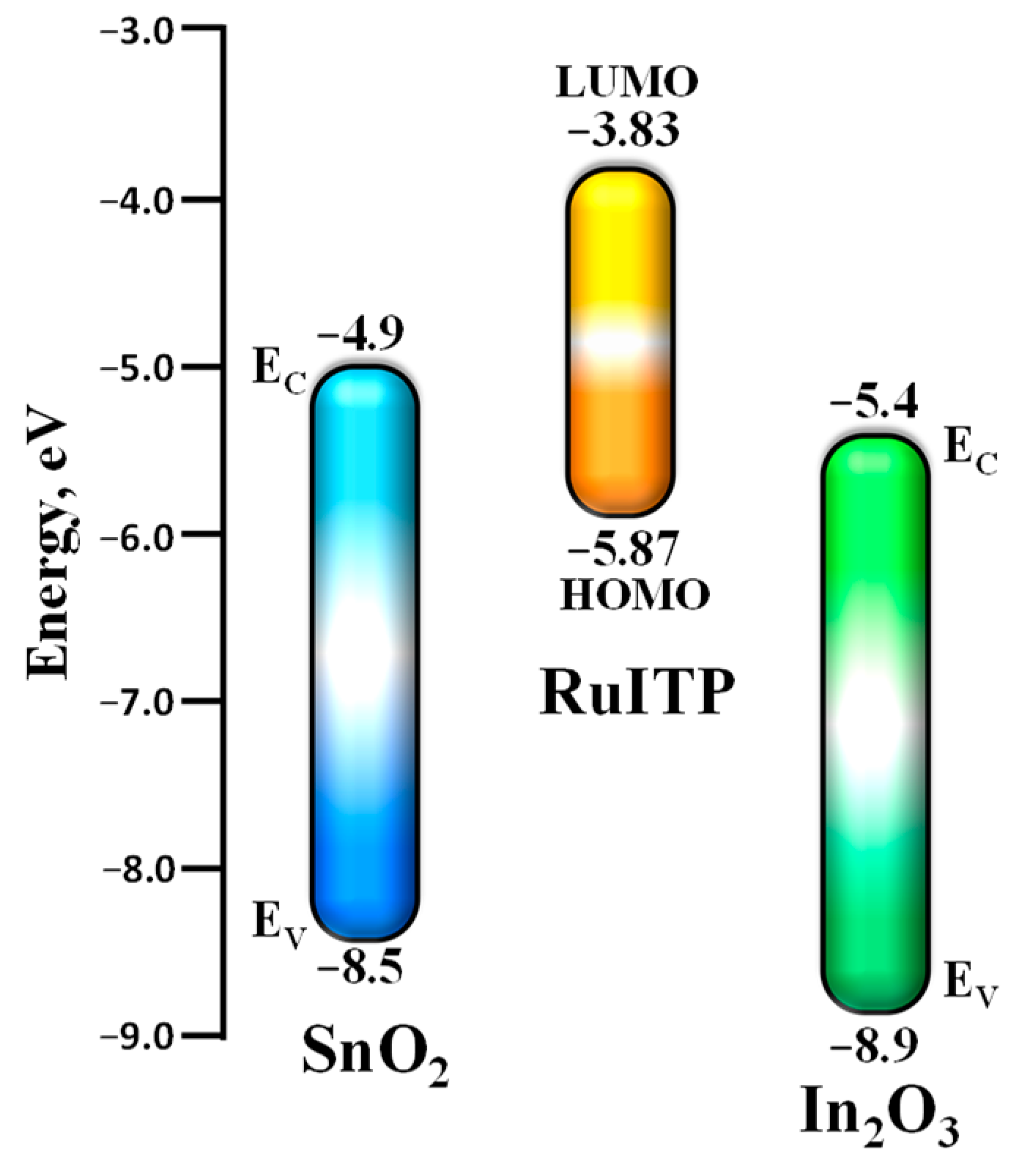
3.3. Characteristics of Hybrid Samples
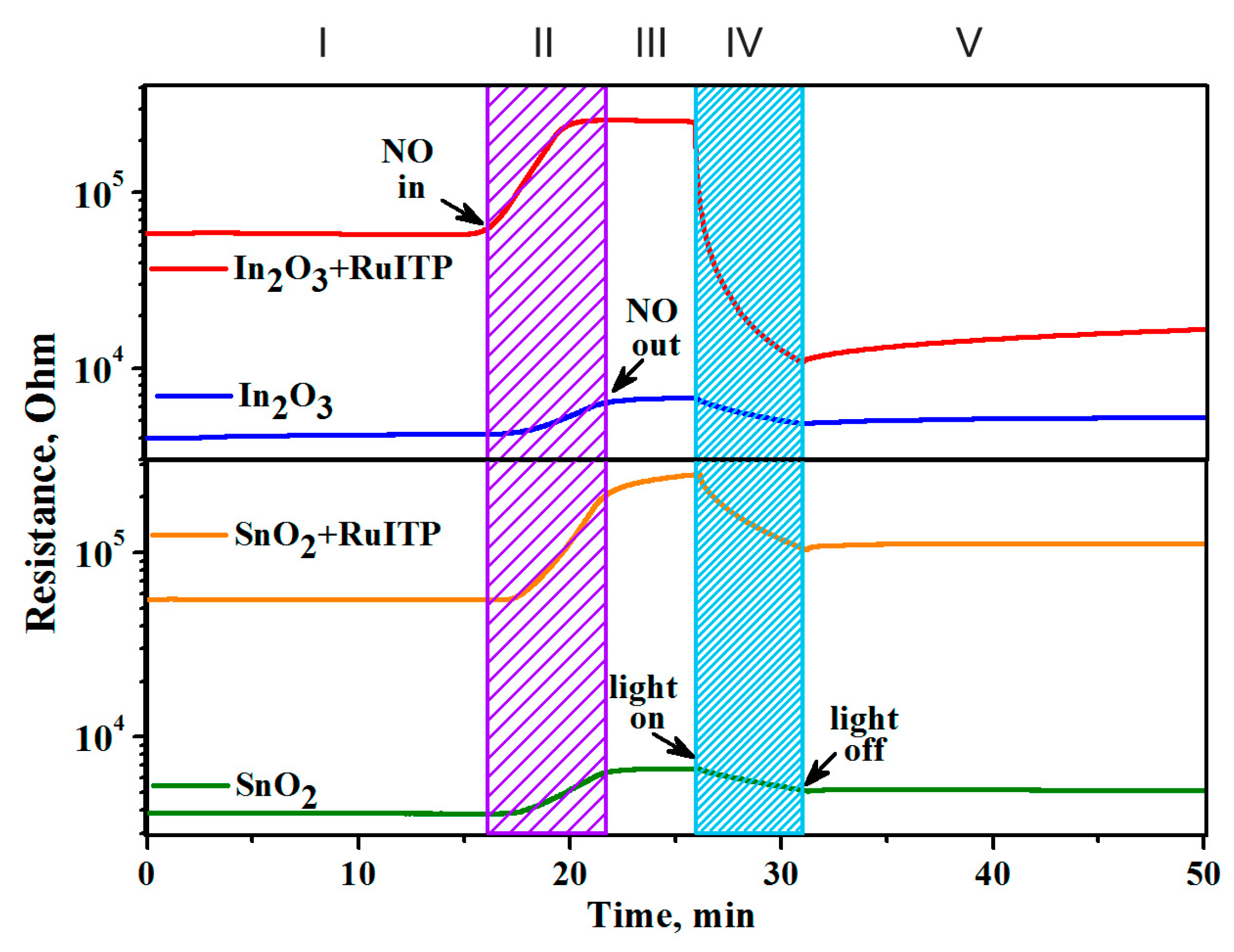
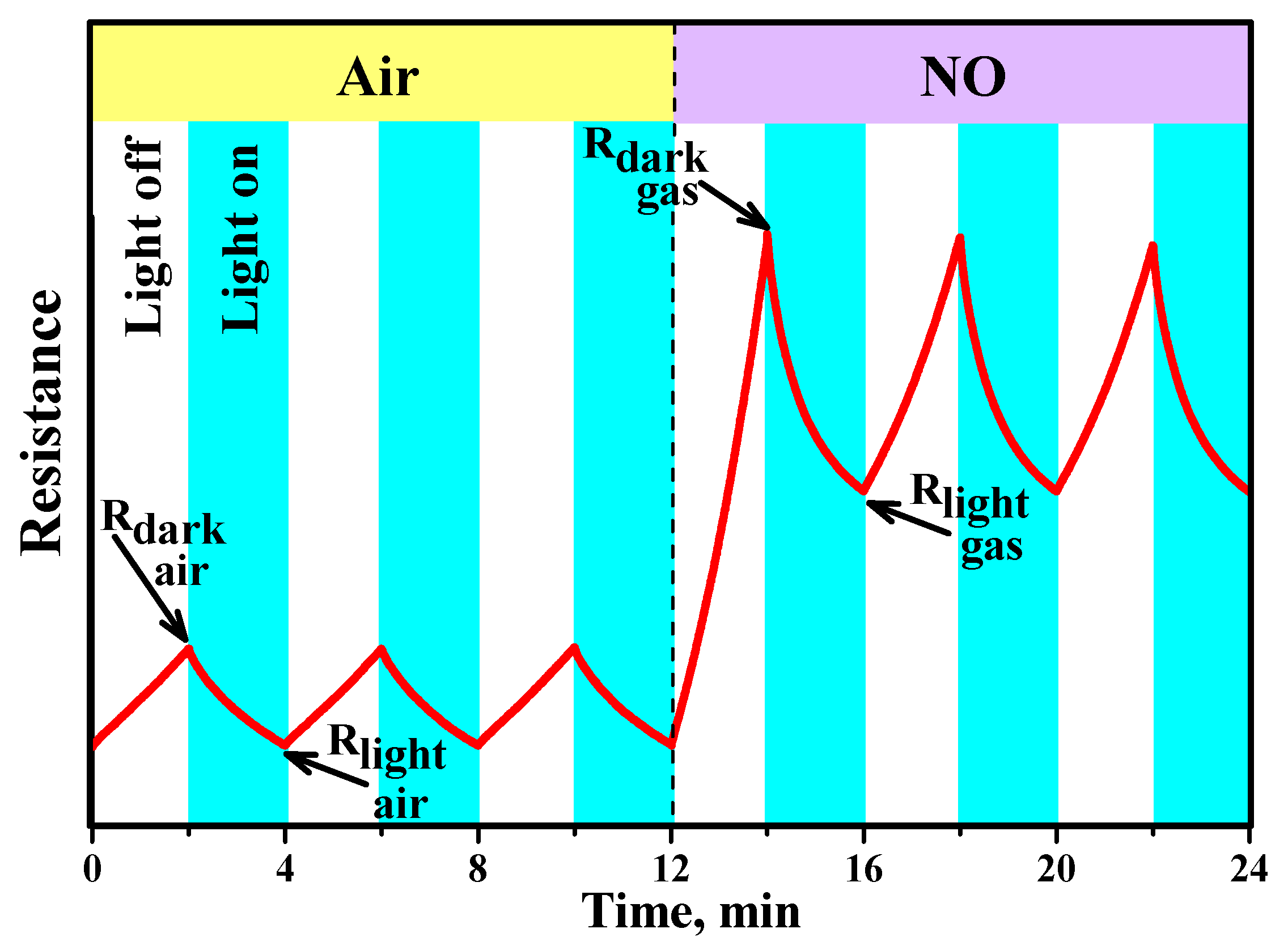
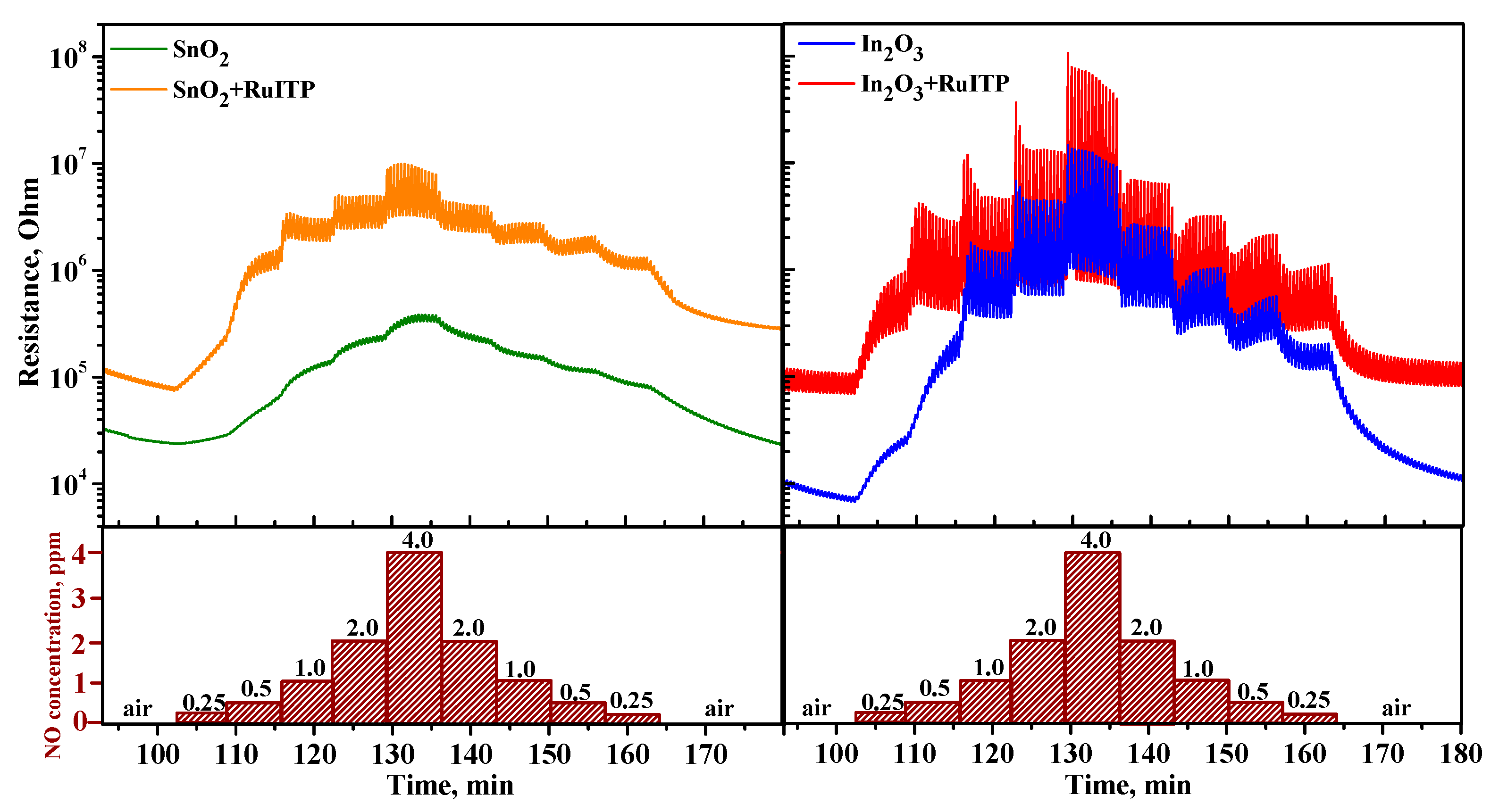
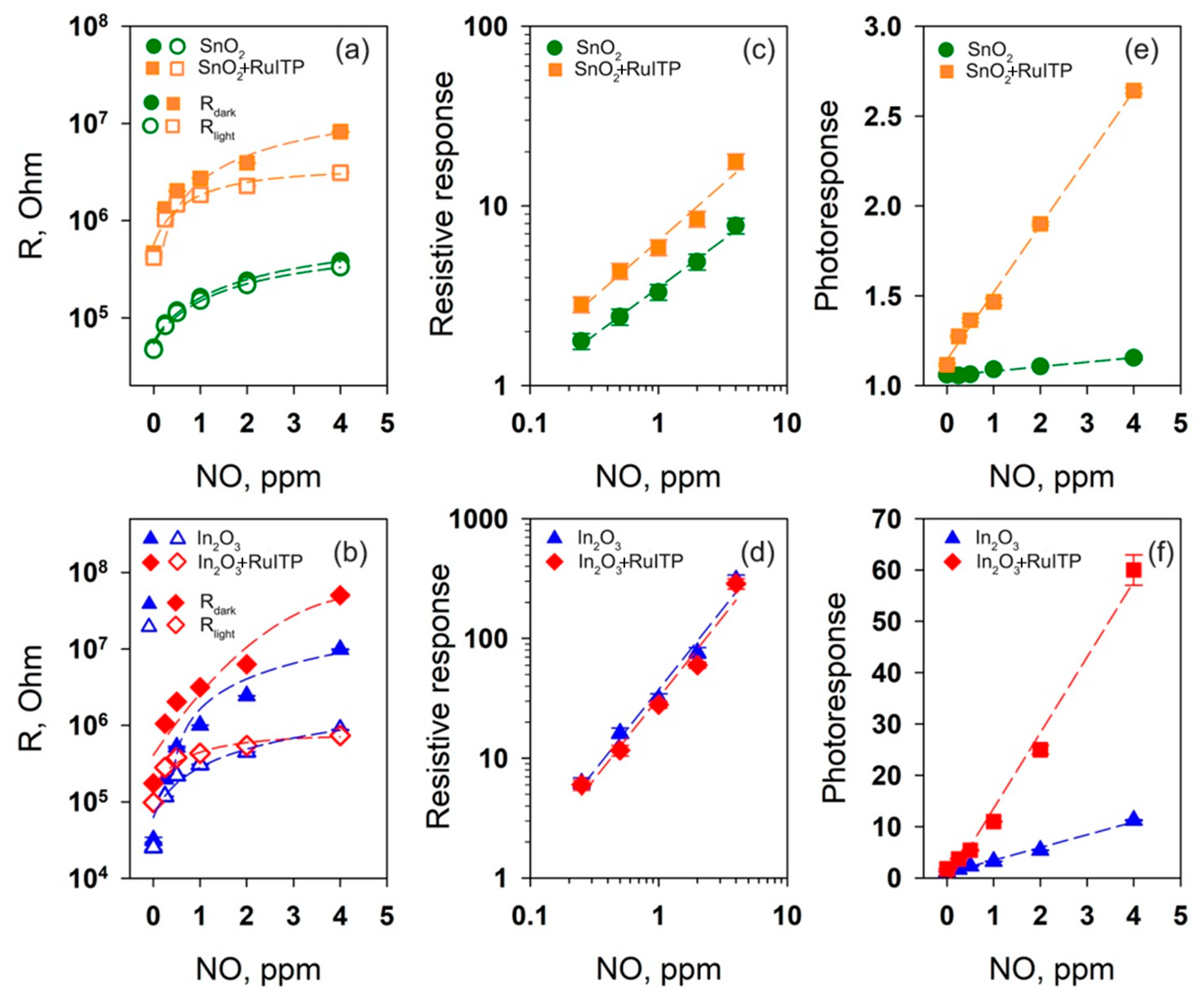
3.4. Gas Sensor Properties
- (i)
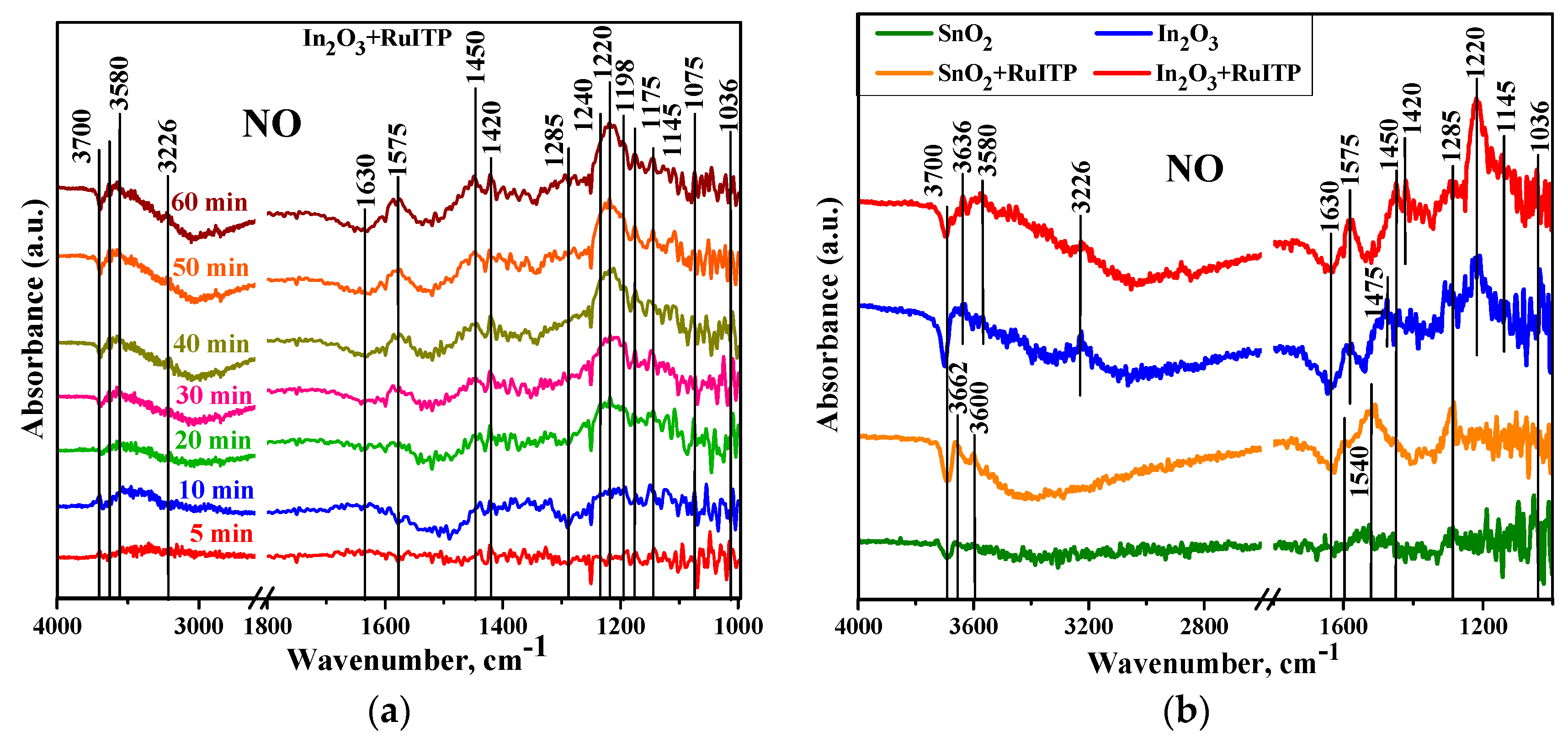
- The interaction of n-type semiconductor oxides with NO2 is accompanied by a decrease in electrical conductivity, since nitrogen dioxide is an electron acceptor. It was shown by Raman spectroscopy [58] that the conductivity of n-type metal oxides in the presence of NO2 correlates with the concentration of surface bidentate nitrite groups. Surface groups observed on DRIFT spectra [23] suggest that NO2 adsorption on n-type semiconductor oxides occurs in the following possible ways:NO2(gas) = NO2(ads),NO2(gas) + e− = NO2−(ads),2 NO2(gas) + ½ O2(gas) + 2 e− = NO2−(ads) + NO3−(ads),NO2−(ads) + ½ O2(gas) = NO3−(ads).
- (ii)
- At room temperature under dark conditions, the adsorption of NO2 on the surface of n-type semiconductor oxides is an irreversible process: when NO2 is removed from the atmosphere, there is no return of the conductivity (resistance) to its initial value in pure air [24,59]. The role of illumination probably consists in the photogeneration of holes that ensure the conversion of chemisorbed NO2− particles into physically adsorbed NO2, which can be desorbed from the surface of the semiconductor oxide even at room temperature.
- (iii)
- Usually, NO is also detected by various n-type semiconductor metal oxides as an oxidizing gas. Based on the results obtained by DRIFT method, the authors [23] suggested that NO sensing by semiconductor oxides is determined by the following reaction:NO(gas) + ½ O2(gas) + e− = NO2−(ads).
3.5. Influence of RuITP Complex on SnO2 and In2O3 Dark Resistance in Pure Air
3.6. Resistive Response in Dark Conditions
3.7. Effective Photoresponse
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). WHO Guidelines for Indoor Air Quality: Selected Pollutants. Available online: www.euro.who.int_data/assets/pdf_file/0009/128169/e94535/pdf (accessed on 20 November 2019).
- Rasmussen, R.E.; Mannix, R.C.; Oldham, M.J.; Phalen, R.F. Effects of nitrogen dioxide on respiratory tract clearance in the ferret. J. Toxicol. Environ. Health 1994, 41, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Witschi, H. Ozone, nitrogen dioxide and lung cancer: A review of some recent issues and problems. Toxicology 1988, 48, 1–20. [Google Scholar] [CrossRef]
- Morgenstern, V.; Zutavern, A.; Cyrys, J.; Brockow, I.; Koletzko, S.; Krämer, U.; Behrendt, H.; Herbarth, O.; Berg, A.; Bauer, C.P.; et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am. J. Respir. Crit. Care Med. 2008, 177, 1331–1337. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Chapter 20. Wet deposition. In Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 856–888. [Google Scholar]
- Warneck, P. Chapter 9. Nitrogen compounds in the troposphere. In Chemistry of the Natural Atmosphere, 2nd ed.; Academic Press: San Diego, CA, USA, 2000; Volume 71, pp. 511–586. [Google Scholar]
- Kharitonov, S.A.; Barnes, P.J. Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers 2002, 7, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, E.; Carraro, S. Exhaled NO and breath condensate. Pediatr. Respir. Rev. 2006, 7, 20–22. [Google Scholar] [CrossRef]
- Lu, Z.; Huang, W.; Wang, L.; Xu, N.; Ding, Q.; Cao, C. Exhaled nitric oxide in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2695–2705. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemoresistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Righettoni, M.; Ragnoni, A.; Guntner, A.; Loccioni, C.; Pratsinis, S.; Risby, T. Monitoring breath markers under controlled conditions. J. Breath Res. 2015, 9, 047101. [Google Scholar] [CrossRef]
- Ganas, K.; Loukidis, S.; Papatheodorou, G.; Panagou, P. Total nitrite/nitrate in expire breath condensate of patients with asthma. Resp. Med. 2001, 95, 649–654. [Google Scholar] [CrossRef]
- Marquis, B.T.; Vetelino, J.F. A semiconducting metal oxide sensor array for the detection of NOx and NH3. Sens. Actuators B Chem. 2001, 77, 100–110. [Google Scholar] [CrossRef]
- Lee, C.-T.; Lee, H.-Y.; Chiu, Y.-S. Performance improvement of nitrogen oxide gas sensors using Au catalytic metal on SnO2/WO3 complex nanoparticle sensing layer. IEEE Sens. J. 2016, 16, 7581–7585. [Google Scholar] [CrossRef]
- Yin, L.; Chen, D.; Fan, B.; Lu, H.; Wang, H.; Xu, H.; Yang, D.; Shao, G.; Zhang, R. Enhanced selective response to nitric oxide (NO) of Au-modified tungsten trioxide nanoplates. Mater. Chem. Phys. 2013, 143, 461–469. [Google Scholar] [CrossRef]
- Tomchenko, A.A.; Khatko, V.V.; Emelianov, I.L. WO3 thick-film gas sensors. Sens. Actuators B Chem. 1998, 46, 8–14. [Google Scholar] [CrossRef]
- Zhao, J.; Tan, R.; Shen, W.; Yang, Y.; Guo, Y.; Li, J.; Zhou, Z.; Jian, J.; Song, W. Highly selective Sn2O3-based sensors for NO detection. Mater. Lett. 2012, 84, 94–96. [Google Scholar] [CrossRef]
- Li, F.; Chen, Y.; Ma, J. Porous SnO2 nanoplates for highly sensitive NO detection. J. Mater. Chem. A 2014, 2, 7175–7178. [Google Scholar] [CrossRef]
- Singkammo, S.; Wisitsoraat, A.; Jaruwongrangsee, K.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Roles of catalytic PtO2 nanoparticles on nitric oxide sensing mechanisms of flame-made SnO2 nanoparticles. Appl. Surf. Sci. 2018, 458, 281–292. [Google Scholar] [CrossRef]
- Wu, M.-R.; Li, W.-Z.; Tung, C.-Y.; Huang, C.-Y.; Chiang, Y.-H.; Liu, P.-L.; Horng, R.-H. NO gas sensor based on ZnGa2O4 epilayer grown by metalorganic chemical vapor deposition. Sci. Rep. 2019, 9, 7459. [Google Scholar] [CrossRef]
- Verma, V.P.; Das, S.; Hwang, S.; Choi, H.; Jeon, M.; Choi, W. Nitric oxide gas sensing at room temperature by functionalized single zinc oxide nanowire. Mater. Sci. Eng. B 2010, 171, 45–49. [Google Scholar] [CrossRef]
- Samanta, C.; Ghatak, A.; Raychaudhuri, A.K.; Ghosh, B. ZnO/Si nanowires heterojunction array based nitric oxide (NO) gas sensor with noise-limited detectivity approaching 10 ppb. Nanotechnology 2019, 30, 305501. [Google Scholar] [CrossRef]
- Yang, L.; Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.; Khmelevsky, N.; Gaskov, A. Quasi similar routes of NO2 and NO sensing by nanocrystalline WO3: Evidence by in situ drift spectroscopy. Sensors 2019, 19, 3405. [Google Scholar] [CrossRef]
- Chizhov, A.S.; Rumyantseva, M.N.; Vasiliev, R.B.; Filatova, D.G.; Drozdov, K.A.; Krylov, I.V.; Abakumov, A.M.; Gaskov, A.M. Visible light activated room temperature gas sensors based on nanocrystalline ZnO sensitized with CdSe quantum dots. Sens. Actuators B Chem. 2014, 205, 305–312. [Google Scholar] [CrossRef]
- Chizhov, A.; Rumyantseva, M.; Vasiliev, R.; Filatova, D.; Drozdov, K.; Krylov, I.; Marchevsky, A.; Karakulina, O.; Abakumov, A.; Gaskov, A. Visible light activation of room temperature NO2 gas sensors based on ZnO, SnO2 and In2O3 sensitized with CdSe quantum dots. Thin Solid Films 2016, 618, 253–262. [Google Scholar] [CrossRef]
- Chizhov, A.; Vasiliev, R.; Rumyantseva, M.; Krylov, I.; Drozdov, K.; Batuk, M.; Hadermann, J.; Abakumov, A.; Gaskov, A. Light-activated sub-ppm NO2 detection by hybrid ZnO/QD nanomaterials vs. charge localization in core-shell QD. Front. Mater. 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Rumyantseva, M.; Nasriddinov, A.; Vladimirova, S.; Fedorova, O.; Tokarev, S.; Krylov, I.; Drozdov, K.; Baranchikov, A.; Gaskov, A. Photosensitive organic-inorganic hybrid materials for room temperature gas sensor applications. Nanomaterials 2018, 8, 671. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Qin, P.; Zeng, Q.; Song, H.; Lei, M.; Mwangi, J.J.N.; Wang, D.; Xie, T. Improvement of formaldehyde sensitivity of ZnO nanorods by modifying with Ru(dcbpy)2(NCS)2. Sens. Actuators B 2011, 160, 39–45. [Google Scholar] [CrossRef]
- Dongare, P.; Myron, B.D.B.; Wang, L.; Thompson, D.W.; Meyer, T.J. [Ru(bpy)3]2+ * revisited. Is it localized or delocalized? How does it decay? Coord. Chem. Rev. 2017, 345, 86–107. [Google Scholar] [CrossRef]
- Hussain, M.; Islam, A.; Bedja, I.; Gupta, R.K.; Hanb, L.; El-Shafei, A. A comparative study of Ru(II) cyclometallated complexes versus thiocyanated heteroleptic complexes: Thermodynamic force for efficient dye regeneration in dye-sensitized solar cells and how low could it be? Phys. Chem. Chem. Phys. 2014, 16, 14874–14881. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Shi, D.; Zhang, J.; Wang, M.; Jing, X.; Baker, R.H.; Wang, P.; Zakeeruddin, S.; Graetzel, M. Enhance the Optical Absorptivity of Nanocrystalline TiO2 Film with High Molar Extinction Coefficient Ruthenium Sensitizers for High Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2008, 130, 10720–10728. [Google Scholar] [CrossRef]
- Balasingam, S.K.; Lee, M.; Kang, M.G.; Jun, Y. Improvement of dye-sensitized solar cells toward the broader light harvesting of the solar spectrum. Chem. Commun. 2013, 49, 1471–1487. [Google Scholar] [CrossRef]
- Aghazad, S.; Nazeeruddin, M.K. Ruthenium Complexes as Sensitizers in Dye-Sensitized Solar Cells. Inorganics 2018, 6, 52. [Google Scholar] [CrossRef]
- Rumyantseva, M.; Makeeva, E.; Gaskov, A.; Shepel, N.; Peregudova, S.; Khoroshutin, A.; Tokarev, S.; Fedorova, O. H2S sensing by hybrids based on nanocrystalline SnO2 functionalized with Cu(II) organometallic complexes: The role of the ligand platform. Nanomaterials 2017, 7, 384. [Google Scholar] [CrossRef] [PubMed]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization on Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Peercy, P.S.; Morosin, B. Pressure and temperature dependences of the Raman-active phonons in SnO2. Phys. Rev. B 1973, 7, 2779–2786. [Google Scholar] [CrossRef]
- Garcia-Tecedor, M.; Maestre, D.; Cremades, A.; Piqueras, J. Growth and characterization of Cr doped SnO2 microtubes with resonant cavity modes. J. Mater. Chem. C 2016, 4, 5709–5716. [Google Scholar] [CrossRef]
- Caoshui, X.; Yonghong, X.; Hong, Z.; Yuheng, Z.; Yulong, L. Investigation of Raman spectrum for nano-SnO2. Sci. China Ser. A 1997, 40, 1222–1227. [Google Scholar]
- Ferreira, C.S.; Santos, P.L.; Bonacin, J.A.; Passos, R.R.; Pocrifka, L.A. Rice husk reuse in the preparation of SnO2/SiO2 nanocomposite. Mat. Res. 2015, 18, 639–643. [Google Scholar] [CrossRef]
- Abello, L.; Bochu, B.; Gaskov, A.; Koudryavtseva, S.; Lucazeau, G.; Roumyantseva, M. Structural characterization of nanocrystalline SnO2 by X-ray and Raman spectroscopy. J. Solid State Chem. 1998, 135, 78–85. [Google Scholar] [CrossRef]
- Dieguez, A.; Romano-Rodriguez, A.; Vila, A.; Morante, J.R. The complete Raman spectrum of nanometric SnO2 particles. J. Appl. Physics. 2001, 90, 1550–1557. [Google Scholar] [CrossRef]
- Liu, L.Z.; Li, T.H.; Wu, X.L.; Shen, J.C.; Chu, P.K. Identification of oxygen vacancy types from Raman spectra of SnO2 nanocrystals. J. Raman Spectrosc. 2012, 43, 1423–1426. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Rumyantseva, M.; Marikutsa, A.; Gaskov, A.; Lee, J.H.; Kim, J.H.; Kim, J.Y.; Kim, S.S.; Kim, H.W. Sub-ppm Formaldehyde Detection by n-n TiO2@SnO2 Nanocomposites. Sensors 2019, 19, 3182. [Google Scholar] [CrossRef]
- White, W.B.; Keramidas, V.G. Vibrational spectra of oxides with the C-type rare earth oxide structure. Spectrochim. Acta 1972, 28, 501–509. [Google Scholar] [CrossRef]
- Kranert, C.; Schmidt-Grund, R.; Grundmann, M. Raman active phonon modes of cubic In2O3. Phys. Status Solidi RRL 2014, 8, 554–559. [Google Scholar] [CrossRef]
- Garcia-Domene, B.; Ortiz, H.M.; Gomis, O.; Sans, J.A.; Manjon, F.J.; Munoz, A.; Rodrıguez-Hernandez, P.; Achary, S.N.; Errandonea, D.; Martınez-Garcıa, D.; et al. High-pressure lattice dynamical study of bulk and nanocrystalline In2O3. J. Appl. Phys. 2012, 112, 123511. [Google Scholar] [CrossRef]
- Arooj, S.; Xu, T.; Hou, X.; Wang, Y.; Tong, J.; Chu, R.; Liu, B. Green emission of indium oxide via hydrogen treatment. RSC Adv. 2018, 8, 11828–11833. [Google Scholar] [CrossRef]
- Yin, W.; Su, J.; Cao, M.; Ni, C.; Cloutier, C.G.; Huang, Z.; Ma, X.; Ren, L.; Hu, C.; Wei, B. In(OH)3 and In2O3 micro/nanostructures: Controllable NaOAc-assisted microemulsion synthesis and Raman properties. J. Phys. Chem. C 2009, 113, 19493–19499. [Google Scholar] [CrossRef]
- Baszczuk, A.; Jasiorski, M.; Nyk, M.; Hanuza, J.; Mączka, M.; Stręk, W. Luminescence properties of europium activated SrIn2O4. J. Alloy. Compd. 2005, 394, 88–92. [Google Scholar] [CrossRef]
- Kim, W.J.; Pradhan, D.; Sohn, Y. Fundamental nature and CO oxidation activities of indium oxide nanostructures: 1D-wires, 2D-plates and 3D-cubes and donuts. J. Mater. Chem. A 2013, 1, 10193. [Google Scholar] [CrossRef]
- Juris, A.; Balzani, V.; Barigelletti, F.; Campagna, S.; Belser, P.; Zelewsky, A. Ru(II) polypyridine complexes: Photophysics, photochemistry, eletrochemistry, and chemiluminescence. Coord. Chem. Rev. 1988, 84, 85–277. [Google Scholar] [CrossRef]
- Calzaferri, G.; Rytz, R. Electronic Transition Oscillator Strength by the Extended Hueckel Molecular Orbital Method. J. Phys. Chem. 1995, 99, 12141–12150. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Photophysics, photochemistry and solar energy conversion with tris(bipyridyl)ruthenium(II) and its analogues. Coord. Chem. Rev. 1982, 46, 159–244. [Google Scholar] [CrossRef]
- Minaev, B.; Minaeva, V.; Vahtras, O. Electronic structure and spectra of dyes for sensitizing nanocrystalline solar cells. In Proceedings of the Nano-Sol-Net International Symposium: Trends in Organic Electronics and Hybrid Photovoltaic’s, Eforie Nord, Romania, 12–14 June 2008. [Google Scholar]
- Minaev, B.; Minaeva, V.; Baryshnikov, G.; Girtu, M.; Agren, H. IR, Raman and UV-Visible spectra of the Ru(II) cyano complexes studied by DFT. Mol. Simul. 2011, 37, 670–677. [Google Scholar] [CrossRef]
- Silverstein, D.W.; Milojevich, C.B.; Camden, J.P.; Jensen, L. Investigation of linear and nonlinear Raman scattering for isotopologues of Ru(bpy)32+. J. Phys. Chem. C 2013, 117, 20855–20866. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Bard, A.J. Photochemistry of Tris(2,2’-bipyridyl) ruthenium(II) in colloidal clay suspensions. J. Phys. Chem. 1984, 88, 5519–5526. [Google Scholar] [CrossRef]
- Sergent, N.; Epifani, M.; Comini, E.; Faglia, G.; Pagnier, T. Interactions of nanocrystalline tin oxide powder with NO2: A Raman spectroscopic study. Sens. Actuators B 2007, 126, 1–5. [Google Scholar] [CrossRef]
- Ilin, A.; Martyshov, M.; Forsh, E.; Forsh, P.; Rumyantseva, M.; Abakumov, A.; Gaskov, A.; Kashkarov, P. UV effect on NO2 sensing properties of nanocrystalline In2O3. Sens. Actuators B Chem. 2016, 231, 491–496. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I. Identification of neutral and charged NxOy surface species by IR spectroscopy. Catal. Rev. Sci. Eng. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Azambre, B.; Zenboury, L.; Delacroix, F.; Weber, J.V. Adsorption of NO and NO2 on ceria–zirconia of composition Ce0.69Zr0.31O2: A DRIFTS study. Catal. Today 2008, 137, 278–282. [Google Scholar] [CrossRef]
- Hess, C.; Ozensoy, E.; Yi, C.W.; Goodman, D.W. NO Dimer and Dinitrosyl formation on Pd (111): From ultra-high-vacuum to elevated pressure conditions. J. Am. Chem. Soc. 2006, 128, 2988–2994. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Hadjiivanov, K.; Knözinger, H.; Tsyntsarski, B.; Dimitrov, L. Effect of water on the reduction of NOx with propane on Fe-ZSM-5. An FTIR mechanistic study. Catal. Lett. 1999, 62, 35–40. [Google Scholar] [CrossRef]
- Kantcheva, M.; Bushev, V.; Hadjiivanov, K. Nitrogen dioxide adsorption on deuteroxylated titania (anatase). J. Chem. Soc. Faraday Trans. 1992, 88, 3087–3089. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Klissurski, D.; Bushev, V. IR spectroscopic study of NO2 adsorption on chromia. J. Chem. Soc. Faraday Trans. 1995, 91, 149–153. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Klissurski, D.; Ramis, G.; Busca, G. Fourier transform IR study of NOx adsorption on a CuZSM-5 DeNOx catalyst. Appl. Catal. B Environ. 1996, 7, 251–267. [Google Scholar] [CrossRef]
- Djonev, B.; Tsyntsarski, B.; Klissurski, D.; Hadjiivanov, K. IR spectroscopic study of NOx adsorption and NOx–O2 coadsorption on Co2+/SiO2 catalysts. J. Chem. Soc. Faraday Trans. 1997, 93, 4055–4063. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons Ltd: West Sussex, UK, 2001. [Google Scholar]
- Cui, X.; Zhou, J.; Ye, Z.; Chen, H.; Li, L.; Ruan, M.; Shi, J. Selective catalytic oxidation of ammonia to nitrogen over mesoporous CuO/RuO2 synthesized by co-nanocasting-replication method. J. Catal. 2010, 270, 310–317. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Zhou, X.P.; Au, C.T. A mini-review on ammonia decomposition catalysts for on-site generation of hydrogen for fuel cell applications. Appl. Catal. A 2004, 277, 1–9. [Google Scholar] [CrossRef]
- Lorenzut, B.; Montini, T.; Pavel, C.C.; Comotti, M.; Vizza, F.; Bianchini, C.; Fornasiero, P. Embedded Ru@ZrO2 Catalysts for H2 Production by Ammonia Decomposition. ChemCatChem 2010, 2, 1096–1106. [Google Scholar] [CrossRef]
- Hong, Z.; Wang, Z.; Li, X. Catalytic oxidation of nitric oxide (NO) over different catalysts: An overview. Catal. Sci. Technol. 2017, 7, 3440–3452. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Gaskov, A. Specific interaction of PdOx- and RuOy modified tin dioxide with CO and NH3 gases: Kelvin probe and DRIFT studies. J. Phys. Chem. C 2015, 119, 24342–24350. [Google Scholar] [CrossRef]
- Marikutsa, A.; Krivetskiy, V.; Yashina, L.; Rumyantseva, M.; Konstantinova, E.; Ponzoni, A.; Comini, E.; Abakumov, A.; Gaskov, A. Catalytic impact of RuOx clusters to high ammonia sensitivity of tin dioxide. Sens. Actuators B Chem. 2012, 175, 186–193. [Google Scholar] [CrossRef]
- Robertson, J. Electronic structure of SnO2, GeO2, PbO2, TeO2 and MgF2. J. Phys. C Solid State Phys. 1979, 12, 4767–4772. [Google Scholar] [CrossRef]
- Klein, A. Electronic properties of In2O3 surfaces. Appl. Phys. Lett. 2000, 77, 2009–2011. [Google Scholar] [CrossRef]
- Ganose, A.M.; Scanlon, D.O. Band gap and work function tailoring of SnO2 for improved transparent conducting ability in photovoltaic. J. Mater. Chem. C 2016, 4, 1467–1475. [Google Scholar] [CrossRef]
- Tvrdy, K.; Frantsuzov, P.A.; Kamat, P.V. Photoinduced electron transfer from semiconductor quantum dots to metal oxide nanoparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Prades, J.D.; Cirera, A.; Morante, J.R. First-Principles Study of NOx and SO2 Adsorption onto SnO2 (110). J. Electrochem. Soc. 2007, 154, H675–H680. [Google Scholar] [CrossRef]
- Prades, J.D.; Cirera, A.; Morante, J.R. Ab initio calculations of NO2 and SO2 chemisorption onto non-polar ZnO surfaces. Sens. Actuators B 2009, 142, 179–184. [Google Scholar] [CrossRef]
- Wang, C.Y.; Becker, R.W.; Passow, T.; Pletsche, W.; Kohler, K.; Cimalla, V.; Ambacher, O. Photon-stimulated sensor based on indium oxide nanoparticles I: Wide-concentration-range ozone monitoring in air. Sens. Actuators B 2011, 152, 235–240. [Google Scholar] [CrossRef]
- Konvalina, G.; Haik, H. Sensors for Breath Testing: From Nanomaterials to Comprehensive Disease Detection. Acc. Chem. Res. 2014, 47, 66–76. [Google Scholar] [CrossRef]
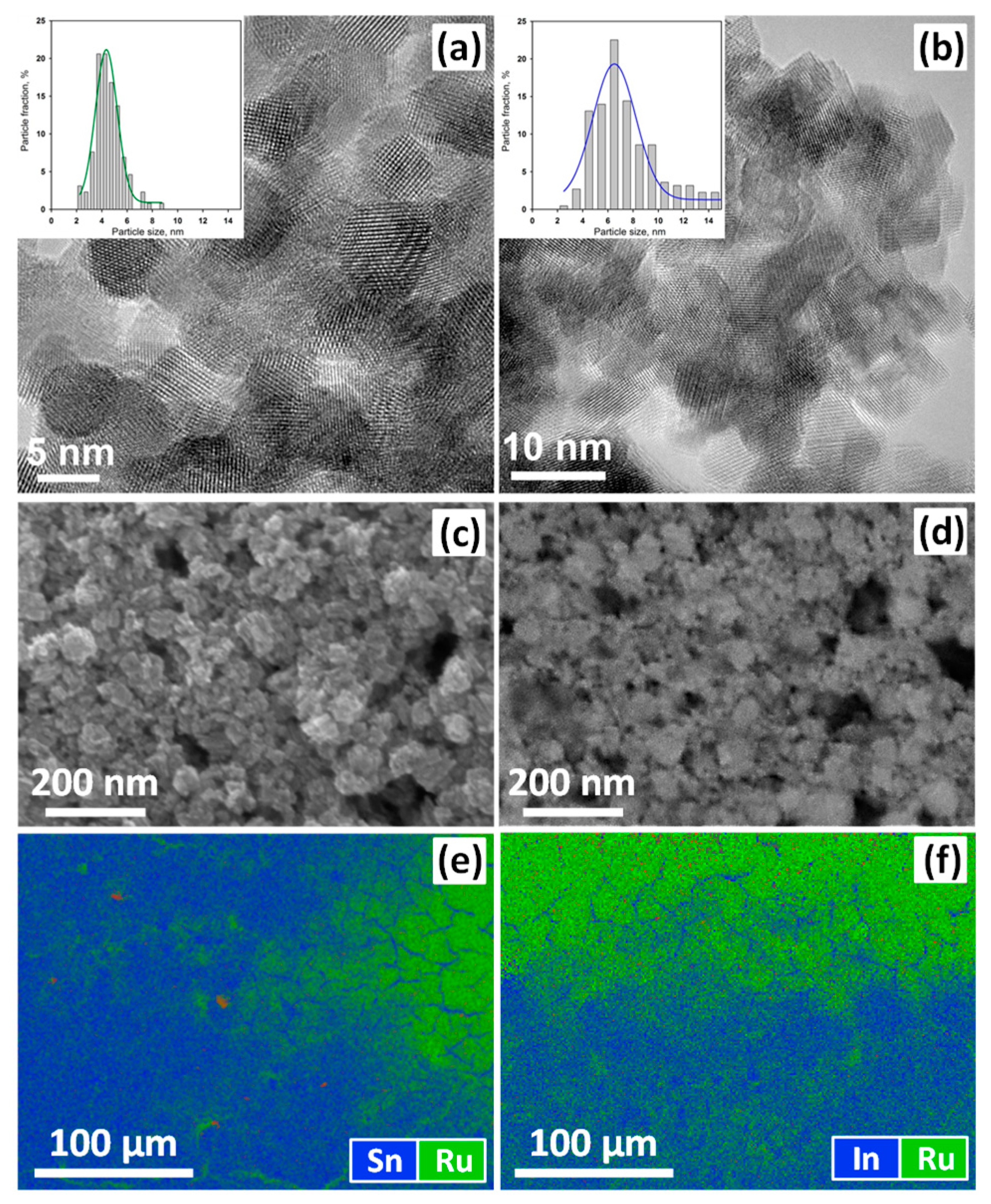
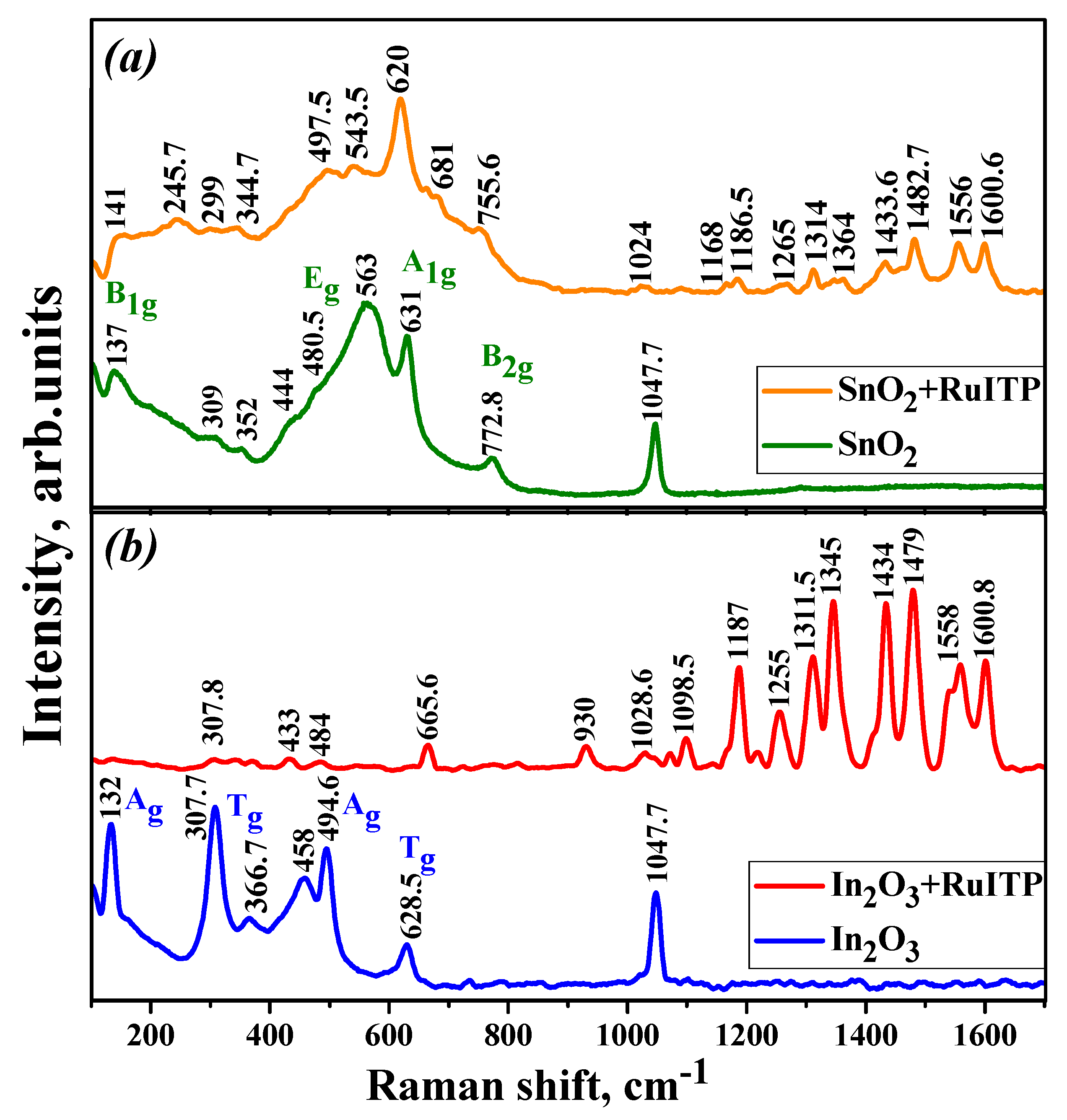
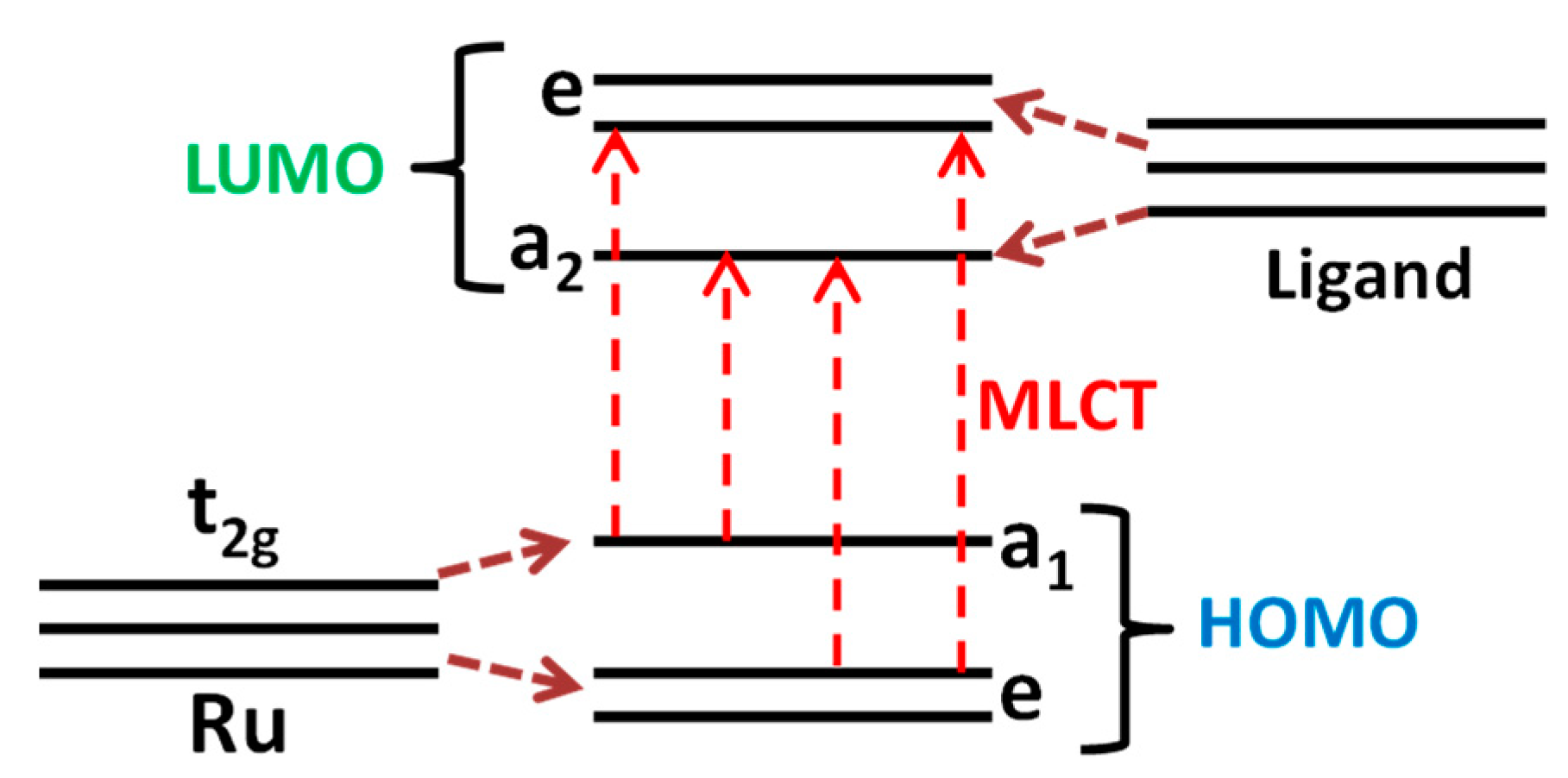
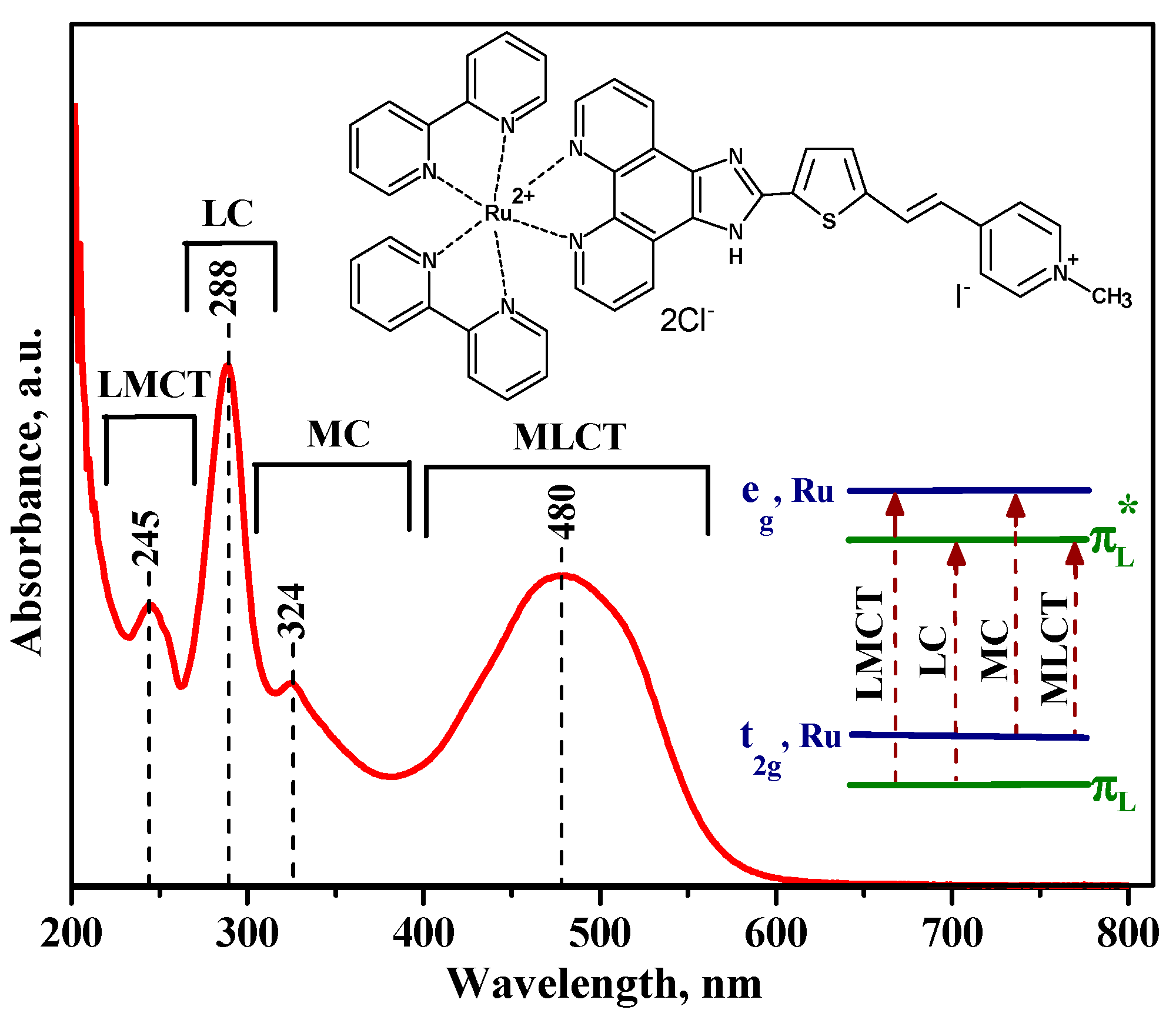

| Sample | Phase Composition | dXRD (a), nm | dTEM (b), nm | Ssurf (c), m2/g | Average Pore Diameter, nm |
|---|---|---|---|---|---|
| SnO2 | SnO2, cassiterite | 4 ± 1 | 4 ± 1 | 115 ± 5 | 3–5; 70–80 |
| In2O3 | In2O3, bixbyite | 7 ± 1 | 7 ± 2 | 90 ± 5 | 3–4 |
| SnO2 | In2O3 | SnO2+RuITP | In2O3+RuITP |
|---|---|---|---|
| 137 (B1g) | 132 (Ag) | 141 (B1g) | 307.8 (Tg) |
| 309, 352 (IR Eu) | 307.7 (Tg) | 245.7 (IR) | 433 (surface mode) |
| 444 (IR B1u) | 366.7 (Tg) | 299, 344.7 (IR Eu) | 484 (Ag) |
| 480.5 (Eg) | 458 (surface mode) | 497.5 (Eg) | 665.6 (Tg) |
| 563 (surface mode) | 494.6 (Ag) | 543.5 (surface mode) | 930 (ring breathing) |
| 631 (A1g) | 628.5 (Tg) | 620 (A1g) | 1028.6 (δ(ring) + ν(Ru-N)) |
| 772.8 (B2g) | 681 (Ru-N str., ring breathing) | 1071.4, 1098.5 (δ(CH) + ν(ring)) | |
| 755.6 (B2g), ring breathing) | 1187 (δ(CH)) | ||
| 1024 (δ(ring) + ν(Ru-N)) | 1218, 1255 (ν(C=N) + ν(C=C) + δ(CH)) | ||
| 1168, 1186.5 (δ(CH)) | 1311.5, 1345 (ν(C-C) + δ(CH)) | ||
| 1265 (ν(C=N) + ν(C=C) + δ(CH)) | 1434 (δ(CH) + ν(C=N) chel.) | ||
| 1314, 1364 (ν(C-C) + δ(CH)) | 1479 (ν(C-C), in phase) | ||
| 1433.6 (δ(CH) + ν(C=N) chel.) | 1540, 1558 (ν(C=C), ν(C=N)) | ||
| 1482.7 (ν(C-C), in phase) | 1600.8 (ν(C=C), ν(C-C)) | ||
| 1556 (ν(C=C), ν(C=N)) | |||
| 1600.6 (ν(C=C), ν(C-C)) |
| Sample | Wavenumber, cm−1 | Assignments |
|---|---|---|
| SnO2/SnO2+RuITP | 1050, 1285, 1540 | monodentate NO3− |
| 1450 | monodentate NO2− | |
| 1592 | chelating bidentate NO3− | |
| 1630 | bending H2O | |
| 3600–3700 | ‘‘free’’ OH groups | |
| In2O3/In2O3+RuITP | 1036, 1240, 1285 | monodentate NO3− |
| 1145, 1175 | chelating bidentate NO2− | |
| 1198, 1220 | bridging bidentate NO2− | |
| 1420 | nitro compound NO2− | |
| 1075, 1450, 1475 | monodentate NO2− | |
| 1575 | chelating bidentate NO3− | |
| 1630 | bending H2O | |
| 3226 | H-bonded OH groups | |
| 3580–3700 | ‘‘free’’ OH groups |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasriddinov, A.; Rumyantseva, M.; Shatalova, T.; Tokarev, S.; Yaltseva, P.; Fedorova, O.; Khmelevsky, N.; Gaskov, A. Organic-Inorganic Hybrid Materials for Room Temperature Light-Activated Sub-ppm NO Detection. Nanomaterials 2020, 10, 70. https://doi.org/10.3390/nano10010070
Nasriddinov A, Rumyantseva M, Shatalova T, Tokarev S, Yaltseva P, Fedorova O, Khmelevsky N, Gaskov A. Organic-Inorganic Hybrid Materials for Room Temperature Light-Activated Sub-ppm NO Detection. Nanomaterials. 2020; 10(1):70. https://doi.org/10.3390/nano10010070
Chicago/Turabian StyleNasriddinov, Abulkosim, Marina Rumyantseva, Tatyana Shatalova, Sergey Tokarev, Polina Yaltseva, Olga Fedorova, Nikolay Khmelevsky, and Alexander Gaskov. 2020. "Organic-Inorganic Hybrid Materials for Room Temperature Light-Activated Sub-ppm NO Detection" Nanomaterials 10, no. 1: 70. https://doi.org/10.3390/nano10010070
APA StyleNasriddinov, A., Rumyantseva, M., Shatalova, T., Tokarev, S., Yaltseva, P., Fedorova, O., Khmelevsky, N., & Gaskov, A. (2020). Organic-Inorganic Hybrid Materials for Room Temperature Light-Activated Sub-ppm NO Detection. Nanomaterials, 10(1), 70. https://doi.org/10.3390/nano10010070








