Catalytic Performance of Toluene Combustion over Pt Nanoparticles Supported on Pore-Modified Macro-Meso-Microporous Zeolite Foam
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalytic Characterization
2.3. Catalytic Evaluation
3. Results and Discussion
3.1. Catalytic Performance
3.1.1. Catalytic Combustion of Toluene over Pt/ZF-D, Pt/ZF, Pt/ZSM-5-D, and Pt/ZSM-5
3.1.2. Catalytic Combustion of Toluene over xPt/ZF-D (Where x = 0.1%, 0.5%, 1%, 2%Pt Loadings)
3.2. Catalysts Characterization
3.2.1. Structural and Morphology Analysis
3.2.2. Surface Compositions Analysis
3.2.3. Pore Size Distribution Analysis
3.2.4. The Mechanism of Toluene Diffusion in the Hierarchical Porous Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Azalim, S.; Franco, M.; Brahmi, R.; Giraudon, J.-M.; Lamonier, J.-F. Removal of oxygenated volatile organic compounds by catalytic oxidation over Zr–Ce–Mn catalysts. J. Hazard. Mater. 2011, 188, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, W.; Zhang, T.; Zhang, Y.; Jiang, L.; Yin, S. Facile fabrication of shape-controlled CoxMnyOβ nanocatalysts for benzene oxidation at low temperatures. Chem. Commun. 2018, 54, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Hu, F.; Li, J. Hierarchical Core–Shell Al2O3@Pd-CoAlO Microspheres for Low-Temperature Toluene Combustion. ACS Catal. 2016, 6, 3433–3441. [Google Scholar] [CrossRef]
- Liu, G.; Ji, J.; Huang, H.; Xie, R.; Feng, Q.; Shu, Y.; Zhan, Y.; Fang, R.; He, M.; Liu, S.; et al. UV/H2O2: An efficient aqueous advanced oxidation process for VOCs removal. Chem. Eng. J. 2017, 324, 44–50. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, C.; Peng, H.; Peng, C.; Zhang, L.; Xu, X.; Liu, W.; Wang, Z.; Zhang, N.; Wang, X. Enhanced toluene combustion performance over Pt loaded hierarchical porous MOR zeolite. Chem. Eng. J. 2018, 334, 10–18. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Gas phase adsorption of volatile organic compounds onto titanium dioxide photocatalysts. Chem. Eng. J. 2018, 337, 60–73. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Peng, R.; Li, S.; Sun, X.; Ren, Q.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Size effect of Pt nanoparticles on the catalytic oxidation of toluene over Pt/CeO2 catalysts. Appl. Catal. B Environ. 2018, 220, 462–470. [Google Scholar] [CrossRef]
- Deng, J.; He, S.; Xie, S.; Yang, H.; Liu, Y.; Guo, G.; Dai, H. Ultralow Loading of Silver Nanoparticles on Mn2O3 Nanowires Derived with Molten Salts: A High-Efficiency Catalyst for the Oxidative Removal of Toluene. Environ. Sci. Technol. 2015, 49, 11089–11095. [Google Scholar] [CrossRef]
- Xu, X.; Wang, P.; Xu, W.; Wu, J.; Chen, L.; Fu, M.; Ye, D. Plasma-catalysis of metal loaded SBA-15 for toluene removal: Comparison of continuously introduced and adsorption-discharge plasma system. Chem. Eng. J. 2016, 283, 276–284. [Google Scholar] [CrossRef]
- Bai, B.; Li, J. Positive Effects of K+ Ions on Three-Dimensional Mesoporous Ag/Co3O4 Catalyst for HCHO Oxidation. ACS Catal. 2014, 4, 2753–2762. [Google Scholar] [CrossRef]
- Zou, J.-P.; Wu, D.-D.; Luo, J.; Xing, Q.-J.; Luo, X.-B.; Dong, W.-H.; Luo, S.-L.; Du, H.-M.; Suib, S.L. A Strategy for One-Pot Conversion of Organic Pollutants into Useful Hydrocarbons through Coupling Photodegradation of MB with Photoreduction of CO2. ACS Catal. 2016, 6, 6861–6867. [Google Scholar] [CrossRef]
- Xie, S.; Liu, Y.; Deng, J.; Zhao, X.; Yang, J.; Zhang, K.; Han, Z.; Arandiyan, H.; Dai, H. Effect of transition metal doping on the catalytic performance of Au–Pd/3DOM Mn2O3 for the oxidation of methane and o-xylene. Appl. Catal. B Environ. 2017, 206, 221–232. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, T.; Xu, X.; Xiao, P.; Li, J. Pt nanoparticles supported on SBA-15: Synthesis, characterization and applications in heterogeneous catalysis. Appl. Catal. B Environ. 2013, 130, 197–217. [Google Scholar] [CrossRef]
- Chantaravitoon, P.; Chavadej, S.; Schwank, J. Temperature-programmed desorption of methanol and oxidation of methanol on Pt–Sn/Al2O3 catalysts. Chem. Eng. J. 2004, 97, 161–171. [Google Scholar] [CrossRef]
- Liu, G.; Tian, Y.; Zhang, B.; Wang, L.; Zhang, X. Catalytic combustion of VOC on sandwich-structured Pt@ZSM-5 nanosheets prepared by controllable intercalation. J. Hazard. Mater. 2019, 367, 568–576. [Google Scholar] [CrossRef]
- Tang, H.; Wei, J.; Liu, F.; Qiao, B.; Pan, X.; Li, L.; Liu, J.; Wang, J.; Zhang, T. Strong Metal-Support Interactions between Gold Nanoparticles and Nonoxides. J. Am. Chem. Soc. 2016, 138, 56–59. [Google Scholar] [CrossRef]
- Wang, G.; Xu, S.; Wang, L.; Liu, Z.; Dong, X.; Wang, L.; Zheng, A.; Meng, X.; Xiao, F.S. Fish-in-hole: Rationally positioning palladium into traps of zeolite crystals for sinter-resistant catalysts. Chem. Commun. (Camb.) 2018, 54, 3274–3277. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Q.; Chen, F.; Zhang, L.; Pan, S.; Bian, C.; Zheng, X.; Meng, X.; Xiao, F.-S. Aluminium-rich Beta zeolite-supported platinum nanoparticles for the low-temperature catalytic removal of toluene. J. Mater. Chem. A 2015, 3, 5556–5562. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Zhang, J.; Bian, C.; Pan, S.; Chen, F.; Meng, X.; Zheng, X.; Gao, X.; Xiao, F.-S. Superior performance in catalytic combustion of toluene over mesoporous ZSM-5 zeolite supported platinum catalyst. Catal. Today 2015, 258, 190–195. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Sun, H.; Peng, P.; Wang, Y.; Li, C.; Subhan, F.; Bai, P.; Xing, W.; Zhang, Z.; Liu, Z.; Yan, Z. Preparation, scale-up and application of meso-ZSM-5 zeolite by sequential desilication–dealumination. J. Porous Mater. 2017, 24, 1513–1525. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.; Tang, X.; Zhao, S.; Yang, Z.; Ma, Y.; Feng, T.; Cui, X. Behaviors and kinetics of toluene adsorption-desorption on activated carbons with varying pore structure. J. Environ. Sci. (China) 2018, 67, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, J.; Chen, F.; Meng, X.; Zheng, X.; Gao, X.; Xiao, F.-S. Enhanced performance in catalytic combustion of toluene over mesoporous Beta zeolite-supported platinum catalyst. Appl. Catal. B Environ. 2013, 140, 199–205. [Google Scholar] [CrossRef]
- Rajappa, C.; Yashonath, S. Levitation effect and its relationship with the underlying potential energy landscape. J. Chem. Phys. 1999, 110, 5960–5968. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.S.; Park, Y.S.; Yoon, K.B. Synthesis of Large Monolithic Zeolite Foams with Variable Macropore Architectures. Adv. Mater. 2001, 13, 1259–1263. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, M.; Ren, Q.; Mo, S.; Peng, R.; Yan, D.; Fu, M.; Chen, L.; Wu, J.; Ye, D. Design of 3-dimensionally self-assembled CeO2 hierarchical nanosphere as high efficiency catalysts for toluene oxidation. Chem. Eng. J. 2019, 369, 18–25. [Google Scholar] [CrossRef]
- Hu, X.; Yang, M.; Fan, D.; Qi, G.; Wang, J.; Wang, J.; Yu, T.; Li, W.; Shen, M. The role of pore diffusion in determining NH3 SCR active sites over Cu/SAPO-34 catalysts. J. Catal. 2016, 341, 55–61. [Google Scholar] [CrossRef]
- Chen, C.; Chen, F.; Zhang, L.; Pan, S.; Bian, C.; Zheng, X.; Meng, X.; Xiao, F.S. Importance of platinum particle size for complete oxidation of toluene over Pt/ZSM-5 catalysts. Chem. Commun. (Camb) 2015, 51, 5936–5938. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Zhang, J.; Pan, S.; Bian, C.; Wang, L.; Chen, F.; Meng, X.; Zheng, X.; Gao, X.; et al. Superior Performance in Catalytic Combustion of Toluene over KZSM-5 Zeolite Supported Platinum Catalyst. Catal. Lett. 2014, 144, 1851–1859. [Google Scholar] [CrossRef]
- Armaroli, T.; Simon, L.J.; Digne, M.; Montanari, T.; Bevilacqua, M.; Valtchev, V.; Patarin, J.; Busca, G. Effects of crystal size and Si/Al ratio on the surface properties of H-ZSM-5 zeolites. Appl. Catal. A Gen. 2006, 306, 78–84. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kim, Y.-W.; Jun, K.-W.; Viswanadham, N.; Bae, J.W.; Park, H.-S. Textural Properties and Catalytic Applications of ZSM-5 Monolith Foam for Methanol Conversion. Catal. Lett. 2009, 129, 408–415. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Jae, J.; Shi, J.; Fan, W.; Huber, G.W. Production of Renewable Aromatic Compounds by Catalytic Fast Pyrolysis of Lignocellulosic Biomass with Bifunctional Ga/ZSM-5 Catalysts. Angew. Chemie Int. Ed. 2012, 51, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulou, E.F.; Stefanidis, S.; Kalogiannis, K.; Psarras, A.C.; Delimitis, A.; Triantafyllidis, K.S.; Lappas, A.A. Pilot-scale validation of Co-ZSM-5 catalyst performance in the catalytic upgrading of biomass pyrolysis vapours. Green Chem. 2014, 16, 662–674. [Google Scholar] [CrossRef]
- Janssens, T.V.W. A new approach to the modeling of deactivation in the conversion of methanol on zeolite catalysts. J. Catal. 2009, 264, 130–137. [Google Scholar] [CrossRef]
- Xu, J.; Xu, X.-C.; Ouyang, L.; Yang, X.-J.; Mao, W.; Su, J.; Han, Y.-F. Mechanistic study of preferential CO oxidation on a Pt/NaY zeolite catalyst. J. Catal. 2012, 287, 114–123. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Wu, H.; Liu, Y.; Li, X.; Jiang, J.; He, M.; Wu, P. Postsynthesis of mesoporous MOR-type titanosilicate and its unique catalytic properties in liquid-phase oxidations. J. Catal. 2011, 281, 263–272. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Ren, F.; Du, J.; Li, R. Hierarchical architectures of ZSM-5 nanocrystalline aggregates with particular catalysis for lager molecule reaction. Microporous Mesoporous Mater. 2017, 240, 22–30. [Google Scholar] [CrossRef]
- Bertrand-Drira, C.; Cheng, X.-W.; Cacciaguerra, T.; Trens, P.; Melinte, G.; Ersen, O.; Minoux, D.; Finiels, A.; Fajula, F.; Gerardin, C. Mesoporous mordenites obtained by desilication: Mechanistic considerations and evaluation in catalytic oligomerization of pentene. Microporous Mesoporous Mater. 2015, 213, 142–149. [Google Scholar] [CrossRef]
- Gargiulo, N.; Caputo, D.; Totarella, G.; Lisi, L.; Cimino, S. Me-ZSM-5 monolith foams for the NH3 -SCR of NO. Catal. Today 2018, 304, 112–118. [Google Scholar] [CrossRef]
- Zhao, L.; Shen, B.; Gao, J.; Xu, C. Investigation on the mechanism of diffusion in mesopore structured ZSM-5 and improved heavy oil conversion. J. Catal. 2008, 258, 228–234. [Google Scholar] [CrossRef]
- Zhang, D.F.; Sun, L.D.; Yin, J.L.; Yan, C.H. Low-Temperature Fabrication of Highly Crystalline SnO2 Nanorods. Adv. Mater. 2003, 15, 1022–1025. [Google Scholar] [CrossRef]
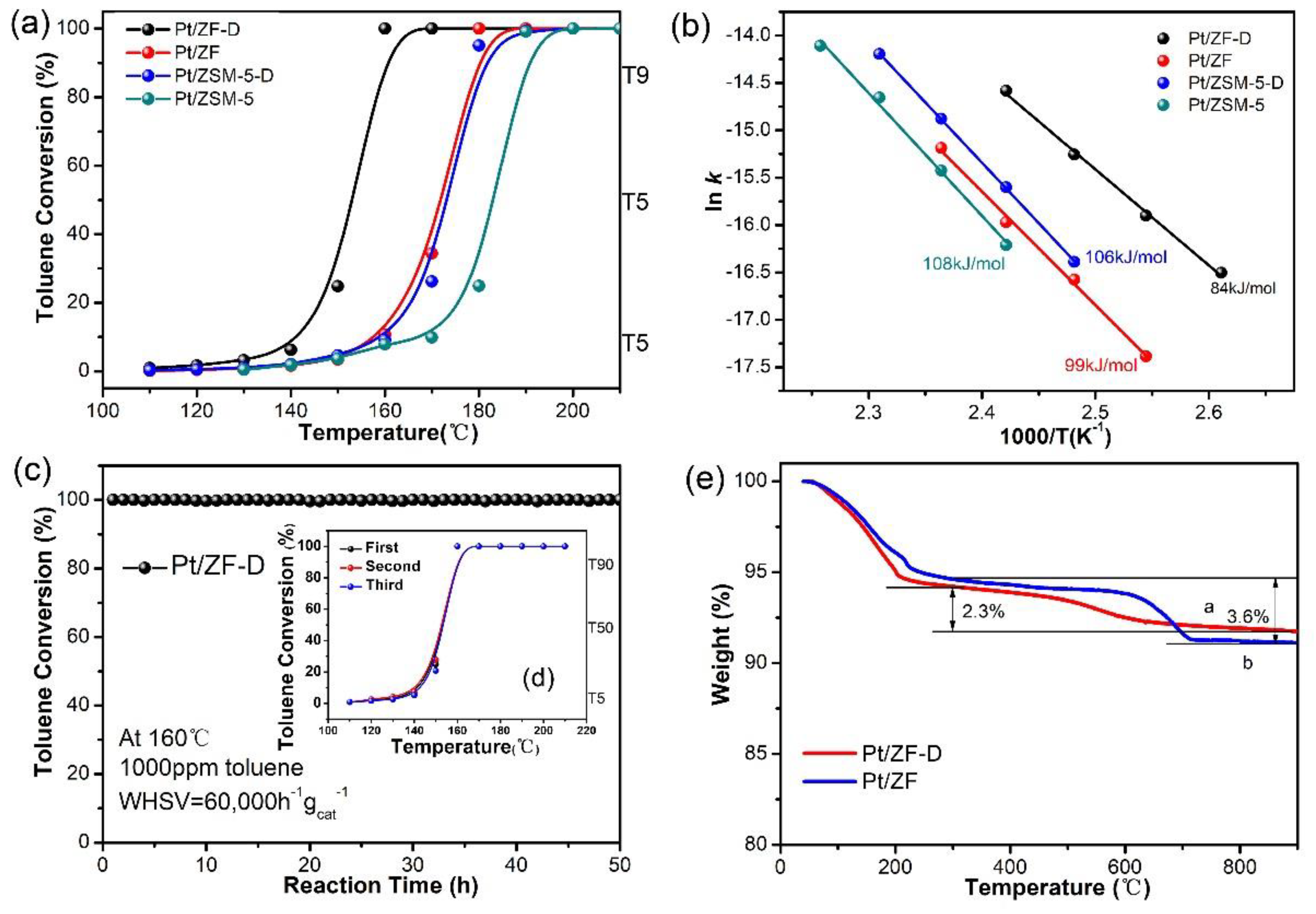
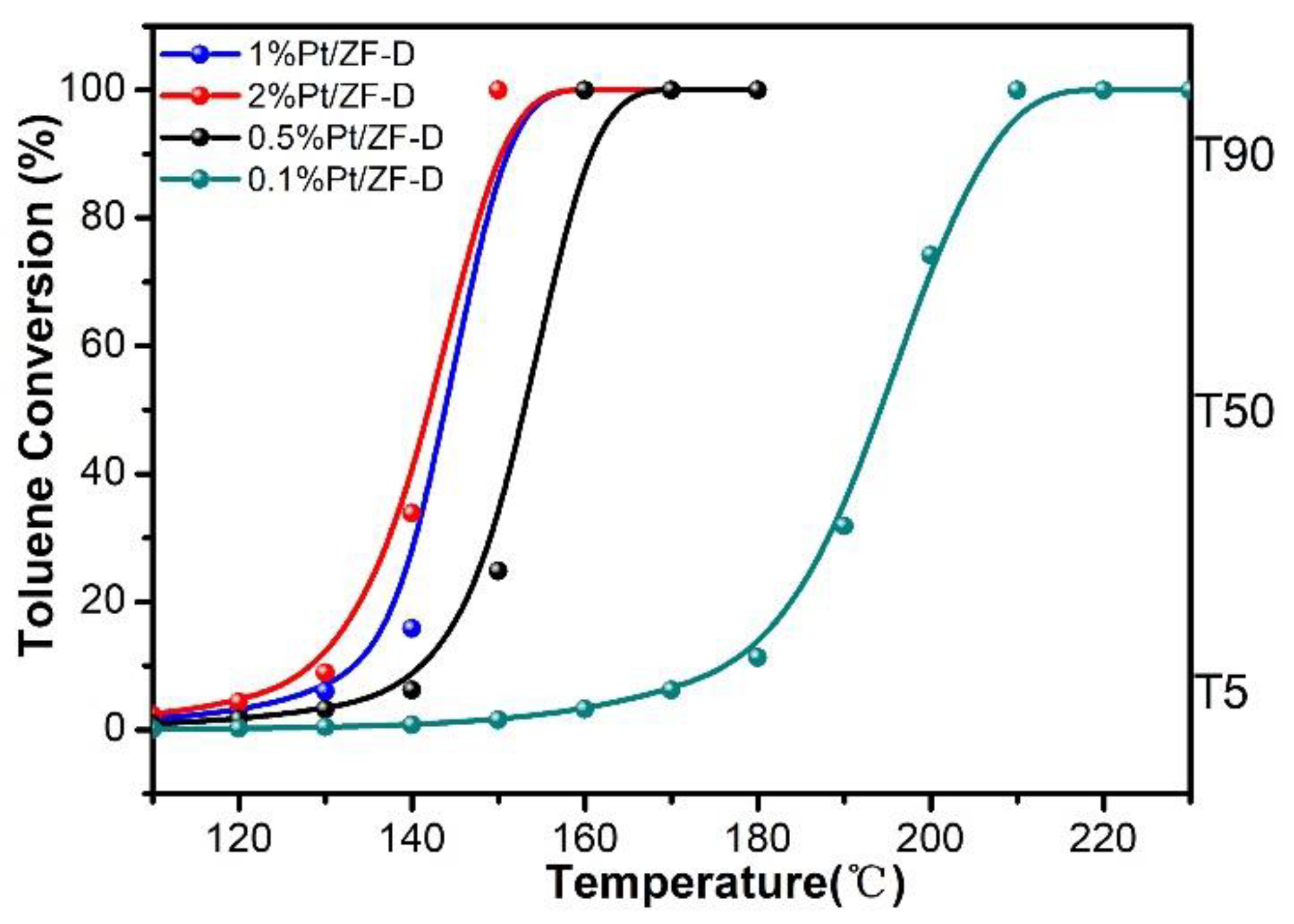
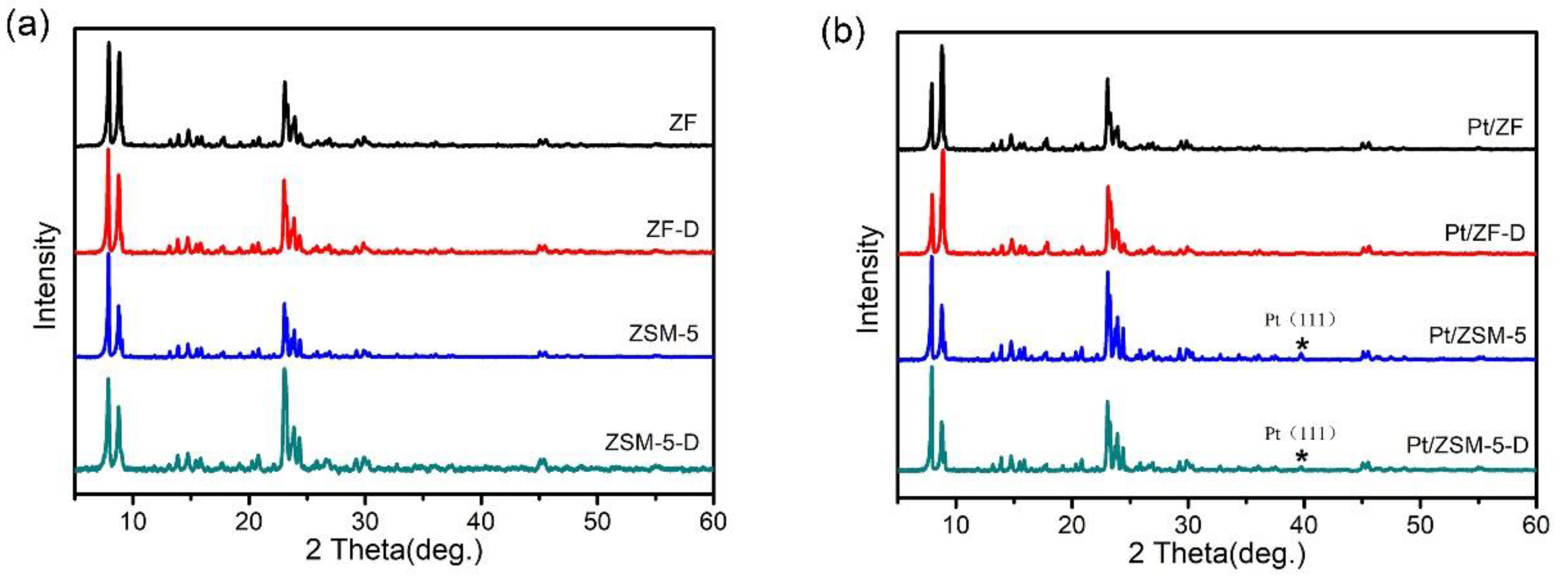

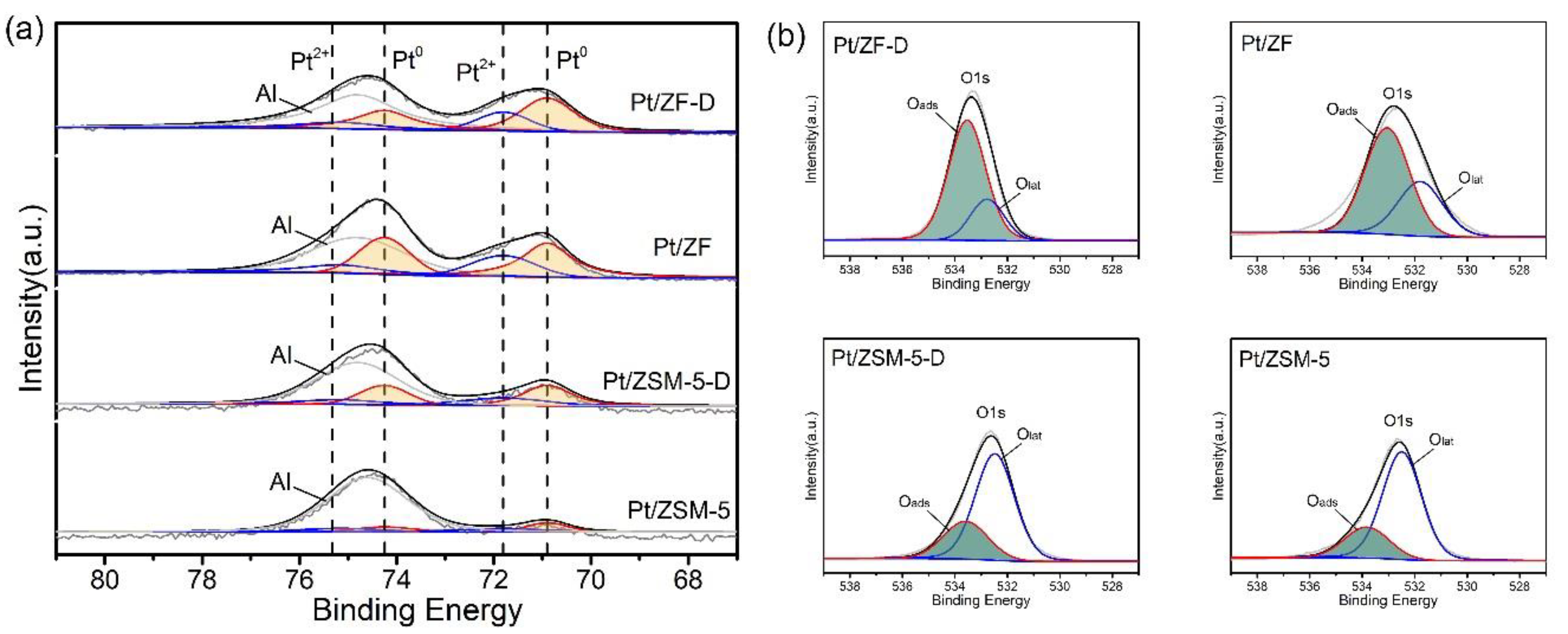


| Catalysts | Activities (°C) a | Ref. | Pt loadings b,c | ||
|---|---|---|---|---|---|
| T5 | T50 | T90 | |||
| 2%Pt/ZF-D | 122 | 141 | 148 | This work | 2.054% b |
| 1%Pt/ZF-D | 126 | 144 | 148 | This work | 1.02% b |
| 0.5%Pt/ZF-D | 135 | 152 | 158 | This work | 0.52% b |
| 0.1%Pt/ZF-D | 166 | 194 | 205 | This work | 0.084% b |
| Pt/HPMOR | - | 175 | 190 | [5] | 1% c |
| Pt-1.9/ZSM-5 | 138 | 147 | 155 | [29] | 1% c |
| Pt-1.9/ZSM-5 | 161 | 172 | 175 | [29] | 0.5% c |
| Pt@PZN-2 | - | - | 176 | [16] | 0.45% c |
| Pt-R/Beta-H | 121 | 186 | 195 | [24] | - |
| Pt/HZSM-5–60 | 165 | 182 | 205 | [30] | 0.5% c |
| Catalysts | T90 (°C) | Ea (kJ/mol) a | Pt loadings b/% | Oads/Olat c | P(Pt0) d | Pt particle size e | Pt dispersion f |
|---|---|---|---|---|---|---|---|
| Pt/ZF-D | 158 | 84 | 0.084 | 3.38 | 67.3% | 5.23 nm | 21.5% |
| Pt/ZF | 178 | 99 | 0.58 | 1.38 | 65.1% | 6.89 nm | 16.3% |
| Pt/ZSM-5-D | 179 | 107 | 1.02 | 0.38 | 62.2% | 30 nm | 4.5% |
| Pt/ZSM-5 | 188 | 109 | 2.05 | 0.33 | 46.6% | 50 nm | 2.2% |
| Samples | SBETa (m2/g) | Sextb (m2/g) | Vtotalc (cm3/g) | Vmicrod (cm3/g) | Vmesoe (cm3/g) |
|---|---|---|---|---|---|
| ZF | 329 | 303 | 0.2749 | 0.059 | 0.2209 |
| ZF-D | 402 | 364 | 0.4382 | 0.021 | 0.4172 |
| ZSM-5 | 383 | 195 | 0.2551 | 0.097 | 0.1581 |
| ZSM-5-D | 419 | 266 | 0.2938 | 0.079 | 0.2148 |
| Pt/ZF | 287 | 230 | 0.2518 | 0.033 | 0.2188 |
| Pt/ZF-D | 390 | 275 | 0.3089 | 0.061 | 0.2469 |
| Pt/ZSM-5 | 378 | 186 | 0.2548 | 0.099 | 0.1558 |
| Pt/ZSM-5-D | 390 | 192 | 0.2595 | 0.100 | 0.1595 |
| Sample | P(mbar) | Dads × 10−19 (m2/s) | R a | SD b |
|---|---|---|---|---|
| Pt/ZF-D | 2 | 82.5 | 0.999 | 0.011 |
| Pt/ZF | 2 | 39.6 | 0.999 | 0.0092 |
| Pt/ZSM-5-D | 2 | 4.06 | 0.996 | 0.136 |
| Pt/ZSM-5 | 2 | 3.45 | 0.997 | 0.267 |
| Pt/ZF-D | 1 | 69.5 | 0.999 | 0.003 |
| Pt/ZF | 1 | 26.8 | 0.999 | 0.008 |
| Pt/ZSM-5-D | 1 | 4.79 | 0.999 | 0.0098 |
| Pt/ZSM-5 | 1 | 4.06 | 0.997 | 0.259 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, S.; Zhang, M.; Mo, S.; Cheng, H.; Fu, M.; Chen, P.; Chen, L.; Shi, W.; Ye, D. Catalytic Performance of Toluene Combustion over Pt Nanoparticles Supported on Pore-Modified Macro-Meso-Microporous Zeolite Foam. Nanomaterials 2020, 10, 30. https://doi.org/10.3390/nano10010030
Zou S, Zhang M, Mo S, Cheng H, Fu M, Chen P, Chen L, Shi W, Ye D. Catalytic Performance of Toluene Combustion over Pt Nanoparticles Supported on Pore-Modified Macro-Meso-Microporous Zeolite Foam. Nanomaterials. 2020; 10(1):30. https://doi.org/10.3390/nano10010030
Chicago/Turabian StyleZou, Sibei, Mingyuan Zhang, Shengpeng Mo, Hairong Cheng, Mingli Fu, Peirong Chen, Limin Chen, Wei Shi, and Daiqi Ye. 2020. "Catalytic Performance of Toluene Combustion over Pt Nanoparticles Supported on Pore-Modified Macro-Meso-Microporous Zeolite Foam" Nanomaterials 10, no. 1: 30. https://doi.org/10.3390/nano10010030
APA StyleZou, S., Zhang, M., Mo, S., Cheng, H., Fu, M., Chen, P., Chen, L., Shi, W., & Ye, D. (2020). Catalytic Performance of Toluene Combustion over Pt Nanoparticles Supported on Pore-Modified Macro-Meso-Microporous Zeolite Foam. Nanomaterials, 10(1), 30. https://doi.org/10.3390/nano10010030







