Decapsulation of Dextran by Destruction of Polyelectrolyte Microcapsule Nanoscale Shell by Bacillus subtilis Bacteria
Abstract
1. Introduction
2. Materials and Methods
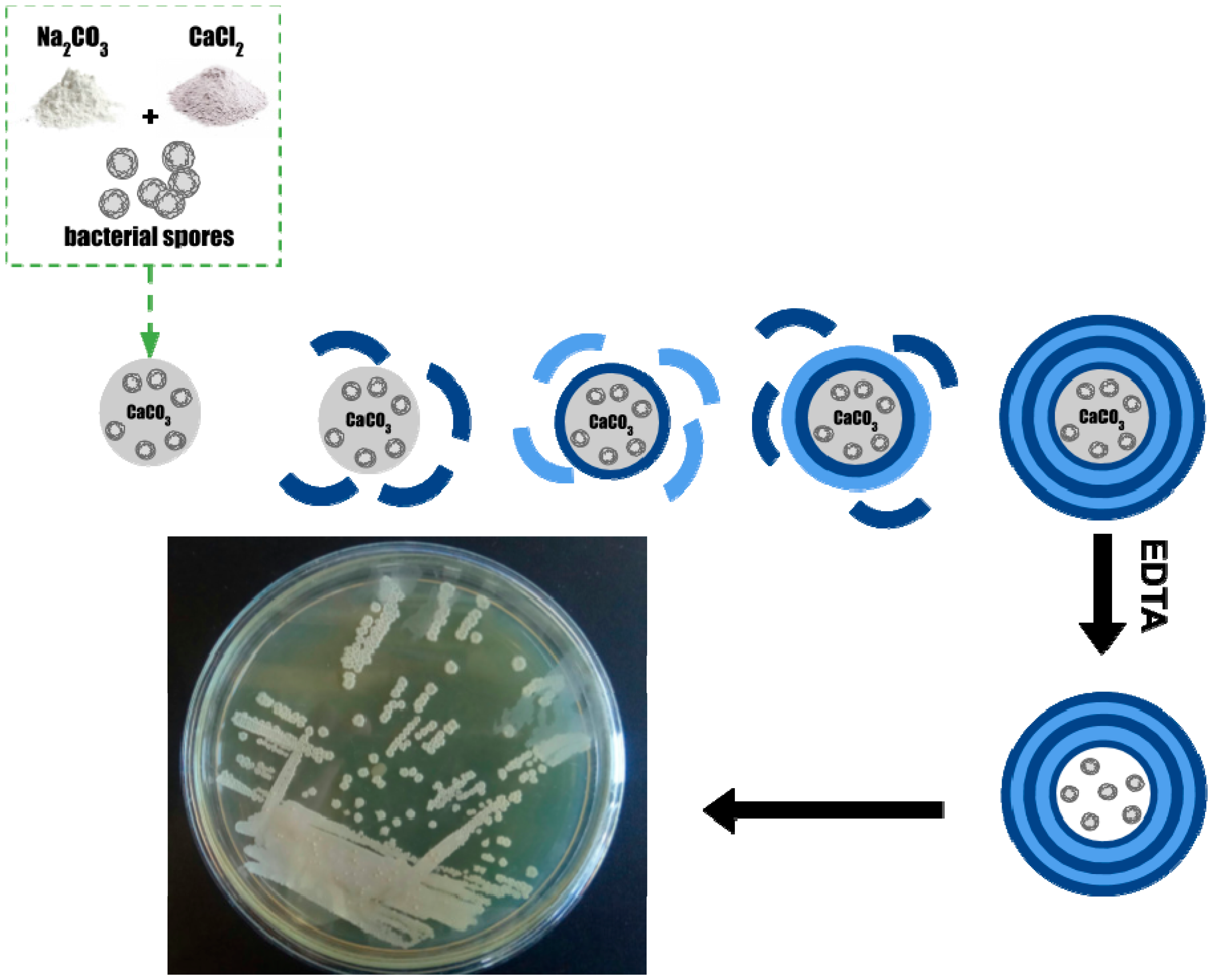
- Microspherolytes: To a 0.33 M CaCl2 solution vigorously agitated on a magnetic stirrer, an equal volume of 0.33 M Na2CO3 solution was rapidly added.
- Microspherolytes with spores: To a 0.33 M CaCl2 solution, containing 1.5 × 108 bacterial spores vigorously agitated on a magnetic stirrer, an equal volume of 0.33 M Na2CO3 solution was rapidly added.
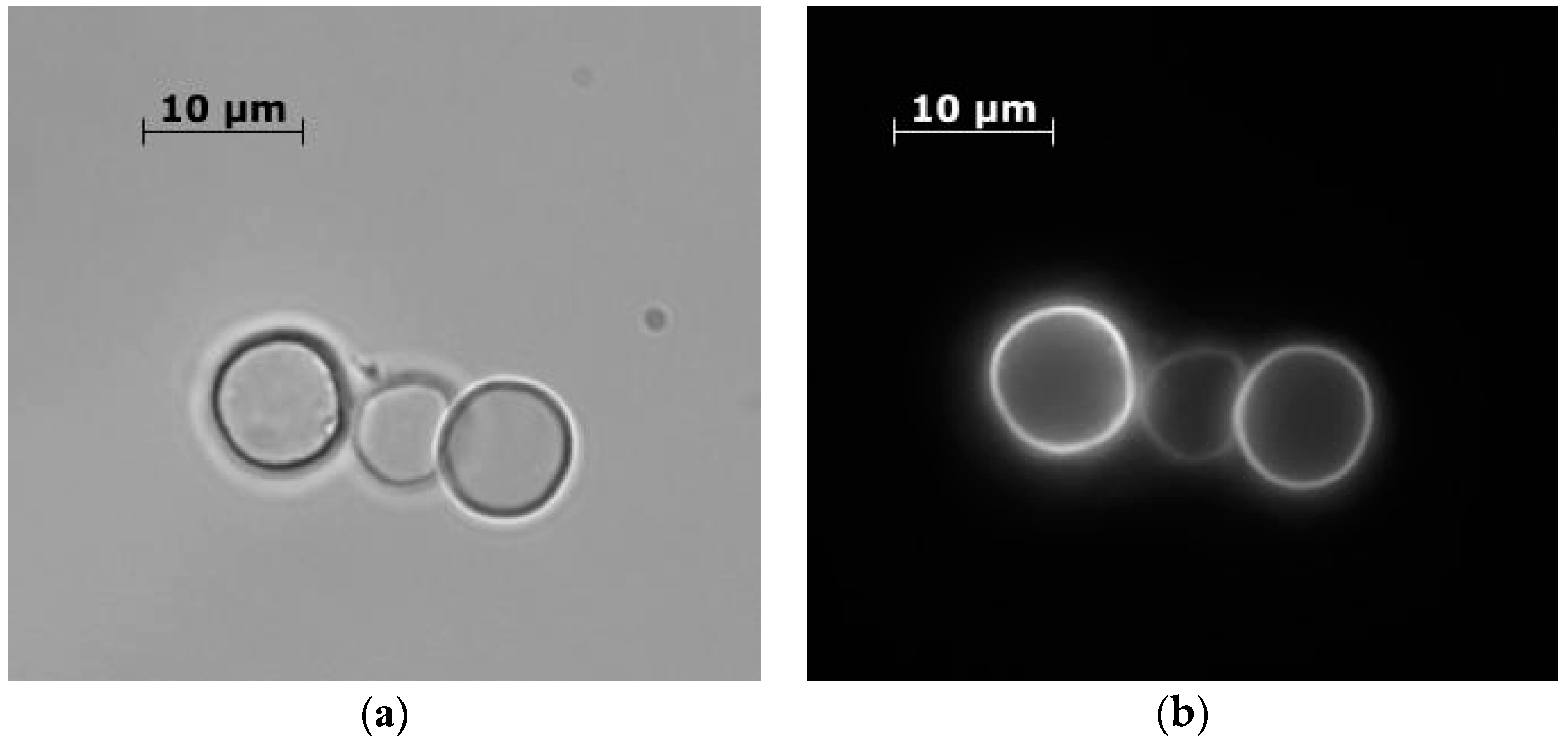
- Microspherolytes with spores and dextrain-FITC: To a 0.33 M CaCl2 solution, containing 1.5 × 108 bacterial spores vigorously agitated on a magnetic stirrer, an equal volume of 0.33 M Na2CO3 solution, containing 6 mg/mL of FITC-dextran, was rapidly added.
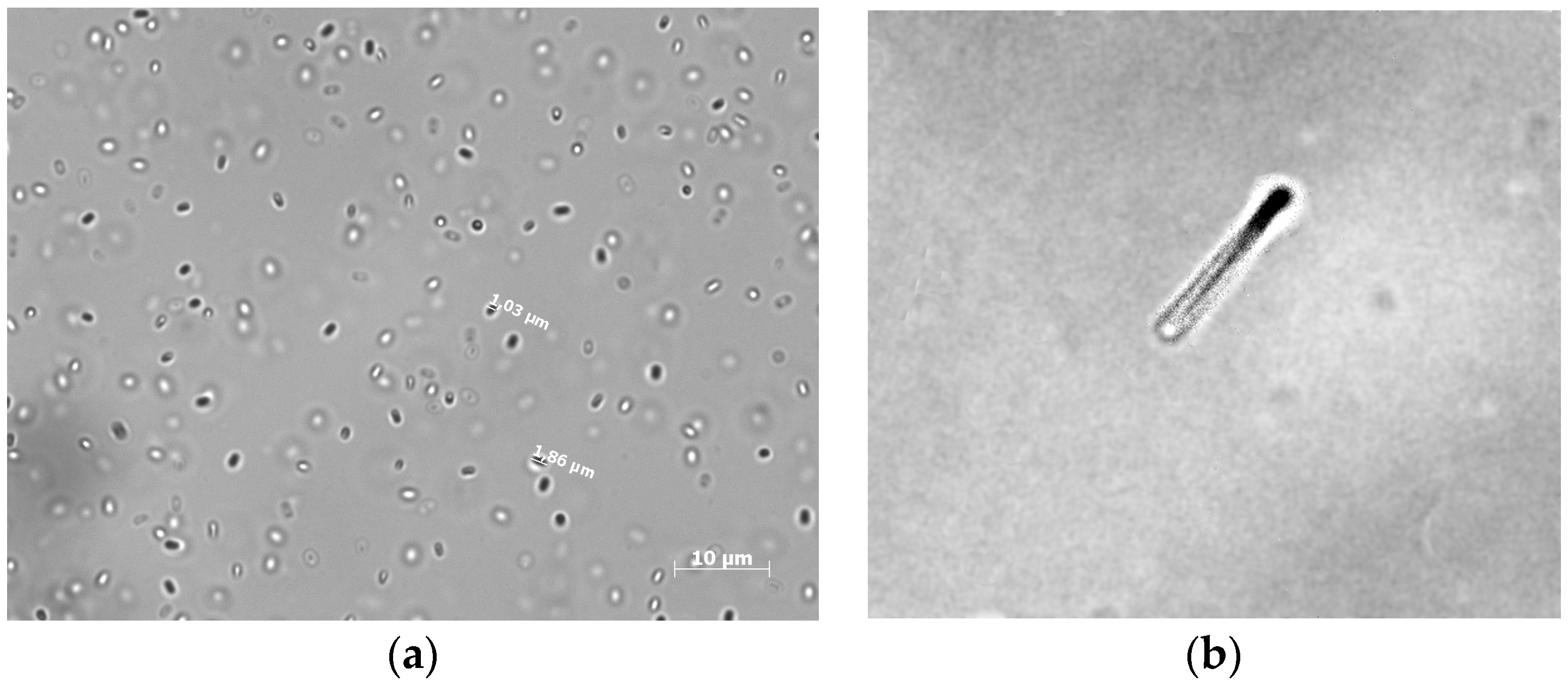
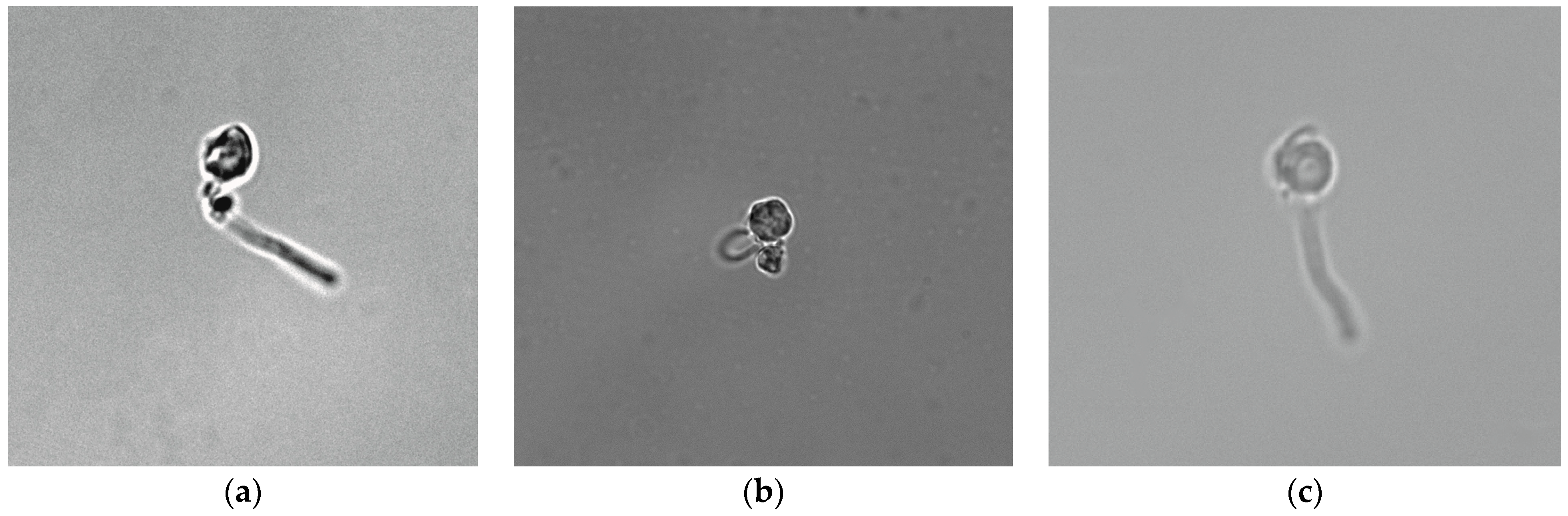
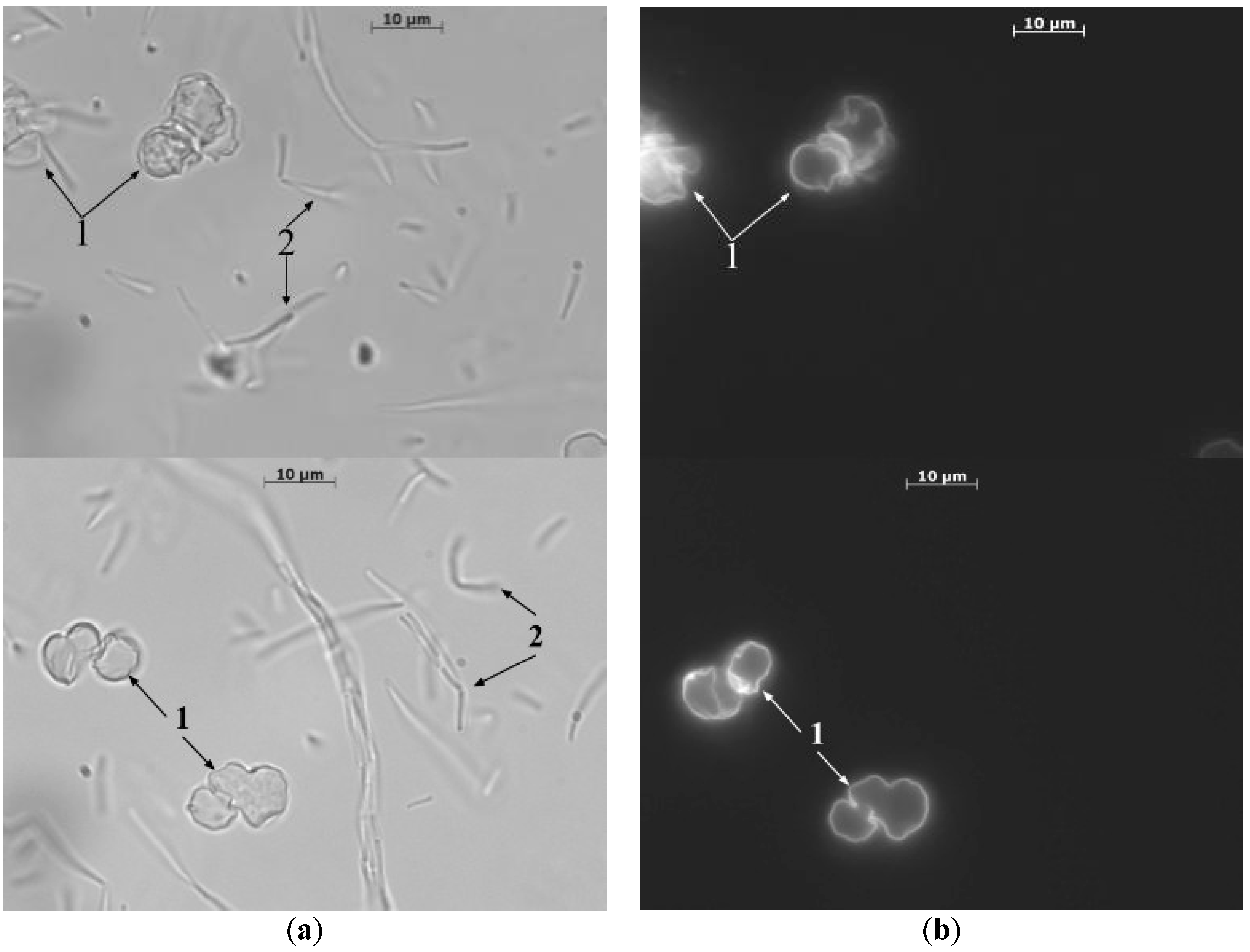
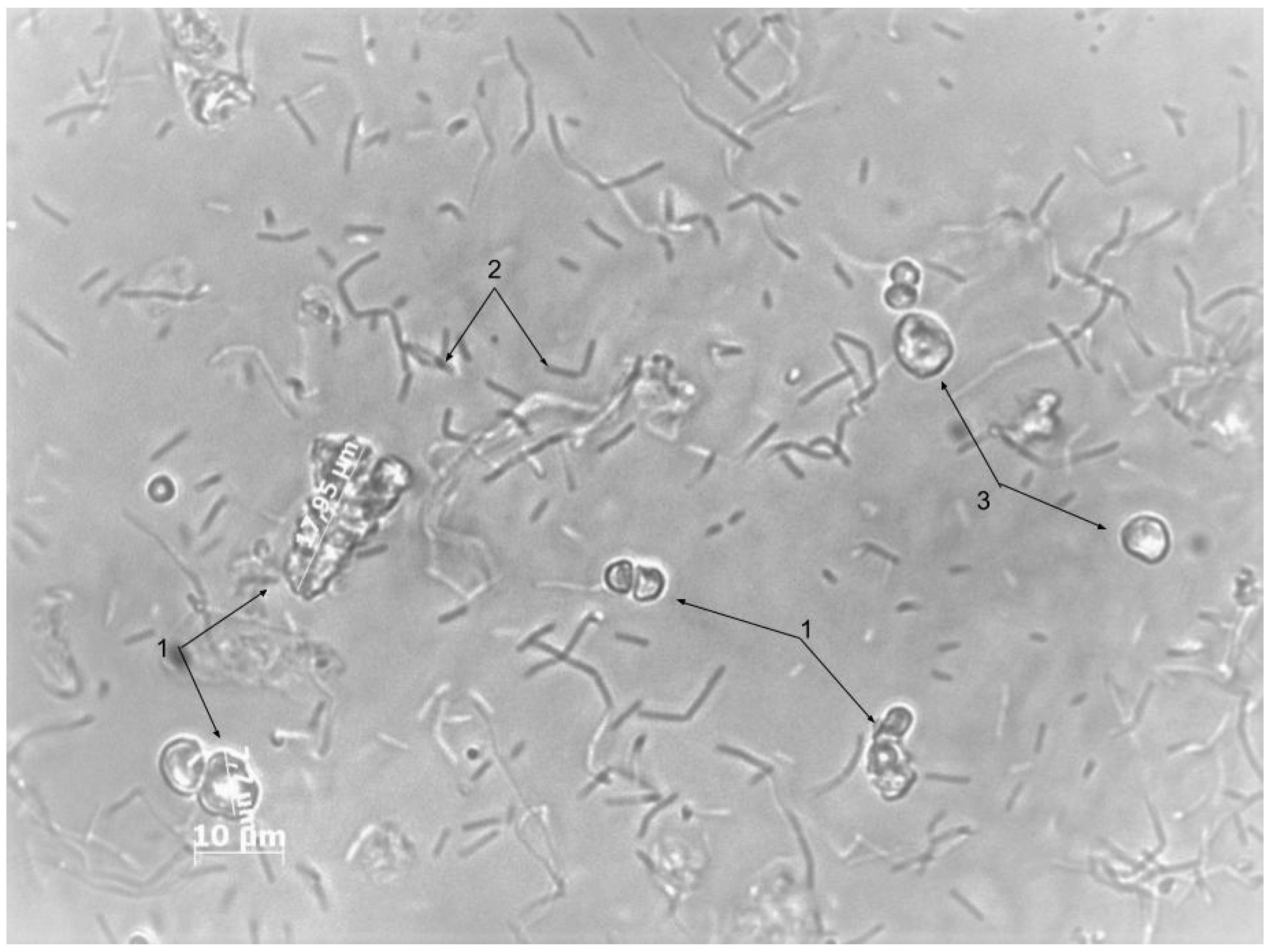
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew. Chemie Int. Ed. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Sukhorukov, B.; Saburova, E.A.; Shabarchina, L.; Dubrovsky, A.T.S. Microdiagnostic and Method of Determining the Concentration of an Analyte Under Analysis. Russian Patent 2316769, 27 September 2006. [Google Scholar]
- Sukhorukov, B.I.; Tikhonenko, S.A.; Saburova, E.A.; Dubrovskii, A.V.; Dybovskaya, Y.N.; Shabarchina, L.I. Protein-filled polyelectrolyte microcapsules in the design of enzymic microdiagnostics. Biophysics 2007, 52, 575–581. [Google Scholar] [CrossRef]
- De Geest, B.G.; Déjugnat, C.; Sukhorukov, G.B.; Braeckmans, K.; De Smedt, S.C.; Demeester, J. Self-rupturing microcapsules. Adv. Mater. 2005, 17, 2357–2361. [Google Scholar] [CrossRef]
- Muñoz Javier, A.; Kreft, O.; Semmling, M.; Kempter, S.; Skirtach, A.G.; Bruns, O.T.; del Pino, P.; Bedard, M.F.; Rädler, J.; Käs, J.; et al. Uptake of colloidal polyelectrolyte-coated particles and polyelectrolyte multilayer capsules by living cells. Adv. Mater. 2008, 20, 4281–4287. [Google Scholar] [CrossRef]
- Borodina, T.N.; Rumsh, L.D.; Kunizhev, S.M.; Sukhorukov, G.B.; Vorozhtsov, G.N.; Feldman, B.M.; Markvicheva, E.A. Polyelectrolyte microcapsules as the systems for delivery of biologically active substances. Biochem. Suppl. Ser. B Biomed. Chem. 2008, 2, 88–93. [Google Scholar]
- Sukhorukov, G.B.; Antipov, A.A.; Voigt, A.; Donath, E.; Mhwald, H. pH-controlled macromolecule encapsulation in and release from polyelectrolyte multilayer nanocapsules. Macromol. Rapid Commun. 2001, 22, 44–46. [Google Scholar] [CrossRef]
- Marchenko, I.V.; Plotnikov, G.S.; Baranov, A.N.; Saletskii, A.M.; Bukreeva, T.V. Formation and destruction of polyelectrolyte microcapsules modified by rhodamine 6G. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2010, 4, 95–98. [Google Scholar] [CrossRef]
- Gulyaev, Y.V.; Cherepenin, V.A.; Vdovin, V.A.; Taranov, I.V.; Sukhorukov, G.B.; Gorin, D.A.; Khomutov, G.B. Decapsulation of polyelectrolyte nanocomposite microcapsules by pulsed microwave effect. J. Commun. Technol. Electron. 2015, 60, 1286–1290. [Google Scholar] [CrossRef]
- Demina, P.A.; Degtyareva, E.V.; Kuzmicheva, G.M.; Bukreeva, T.V. Polyelectrolyte microcapsules modified with nanosized titanium dioxide for targeted drug delivery. Chem. Technol. Inorg. Mater. 2014, 4, 73–79. [Google Scholar]
- Skirtach, A.G.; Antipov, A.A.; Shchukin, D.G.; Sukhorukov, G.B. Remote Activation of Capsules Containing Ag Nanoparticles and IR Dye by Laser Light. Langmuir 2004, 20, 6988–6992. [Google Scholar] [CrossRef] [PubMed]
- Bukreeva, T.A.; Orlova, O.; Sulyanov, S.V.; Grigoriev, Y.; Dorovatovskii, P. A New approach to modification of polyelectrolyte capsule shells by magnetite nanoparticles. Crystallogr. Rep. 2011, 56, 880–883. [Google Scholar] [CrossRef]
- Tu, L.; He, Y.; Shan, C.; Wu, Z. Preparation of microencapsulated Bacillus subtilis SL-13 seed coating agents and their effects on the growth of cotton seedlings. Biomed. Res. Int. 2016, 2016, 1–7. [Google Scholar]
- Balkundi, S.S.; Veerabadran, N.G.; Eby, D.M.; Johnson, G.R.; Lvov, Y.M. Encapsulation of bacterial spores in nanoorganized polyelectrolyte shells. Langmuir 2009, 25, 14011–14016. [Google Scholar] [CrossRef] [PubMed]
- Arys, X.; Jonas, A.M.; Laguitton, B.; Laschewsky, A.; Legras, R.; Wischerhoff, E. Ultrathin multilayers made by alternate deposition of ionenes and polyvinylsulfate: From unstable to stable growth. Thin Solid Films 1998, 327–329, 734–738. [Google Scholar] [CrossRef]
- Dubas, S.T.; Schlenoff, J.B. Factors controlling the growth of polyelectrolyte multilayers. Macromolecules 1999, 32, 8153–8160. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Donath, E.; Lichtenfeld, H.; Knippel, E.; Knippel, M.; Budde, A.; Möhwald, H. Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloids Surfaces A Physicochem. Eng. Asp. 1998, 137, 253–266. [Google Scholar] [CrossRef]
- Garrity, G.; De Vos, P.; Jones, D.; Kreig, N.; Ludwig, W.; Rainey, F.; Schleifer, K.; Whitman, W.B. Bergey’s Manual of Systematic Bacteriology: Volume 3: The Firmicutes; Springer: Cham, Switzerland, 2010. [Google Scholar]
- Riesenman, P.J.; Nicholson, W.L. Role of the spore coat layers in Bacillus subtilis spore resistance to hydrogen peroxide, artificial UV-C, UV-B, and solar UV radiation. Appl. Environ. Microbiol. 2000, 66, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Atrih, A.; Foster, S.J. Analysis of the role of bacterial endospore cortex structure in resistance properties and demonstration of its conservation amongst species. J. Appl. Microbiol. 2001, 91, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.K.M.; Uyen, N.Q.; Hong, H.A.; Duc, L.H.; Hoa, T.T.; Serra, C.R.; Henriques, A.O.; Cutting, S.M. The intestinal life cycle of Bacillus subtilis and close relatives. J. Bacteriol. 2006, 188, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.R. Bacillus subtilis and its relatives: Molecular biological and industrial workhorses. Trends Biotechnol. 1992, 10, 247–256. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musin, E.V.; Kim, A.L.; Dubrovskii, A.V.; Kudryashova, E.B.; Tikhonenko, S.A. Decapsulation of Dextran by Destruction of Polyelectrolyte Microcapsule Nanoscale Shell by Bacillus subtilis Bacteria. Nanomaterials 2020, 10, 12. https://doi.org/10.3390/nano10010012
Musin EV, Kim AL, Dubrovskii AV, Kudryashova EB, Tikhonenko SA. Decapsulation of Dextran by Destruction of Polyelectrolyte Microcapsule Nanoscale Shell by Bacillus subtilis Bacteria. Nanomaterials. 2020; 10(1):12. https://doi.org/10.3390/nano10010012
Chicago/Turabian StyleMusin, Egor V., Aleksandr L. Kim, Alexey V. Dubrovskii, Ekaterina B. Kudryashova, and Sergey A. Tikhonenko. 2020. "Decapsulation of Dextran by Destruction of Polyelectrolyte Microcapsule Nanoscale Shell by Bacillus subtilis Bacteria" Nanomaterials 10, no. 1: 12. https://doi.org/10.3390/nano10010012
APA StyleMusin, E. V., Kim, A. L., Dubrovskii, A. V., Kudryashova, E. B., & Tikhonenko, S. A. (2020). Decapsulation of Dextran by Destruction of Polyelectrolyte Microcapsule Nanoscale Shell by Bacillus subtilis Bacteria. Nanomaterials, 10(1), 12. https://doi.org/10.3390/nano10010012




