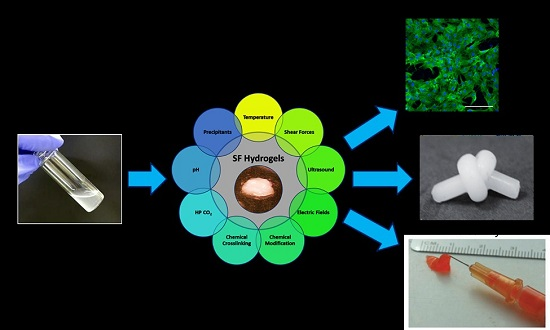
Processing Techniques and Applications of Silk Hydrogels in Bioengineering
Abstract
:1. Introduction
2. Characteristics of Silk Proteins
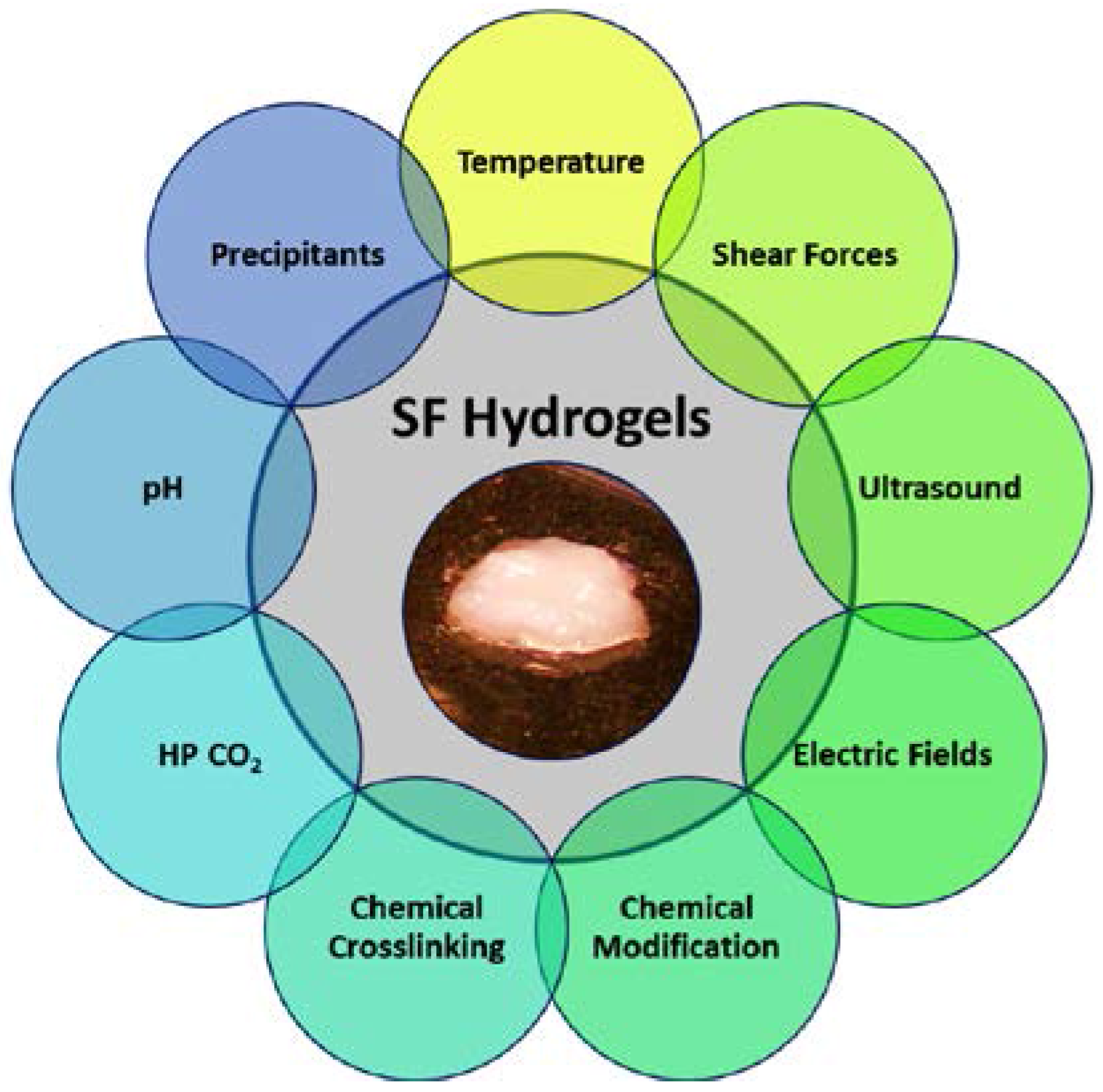
3. Regulation of Silk Protein Gelation
3.1. Chemical Methods
3.1.1. Precipitating Agents
3.1.2. pH
3.1.3. Solution Acidification via Hugh Pressure CO2
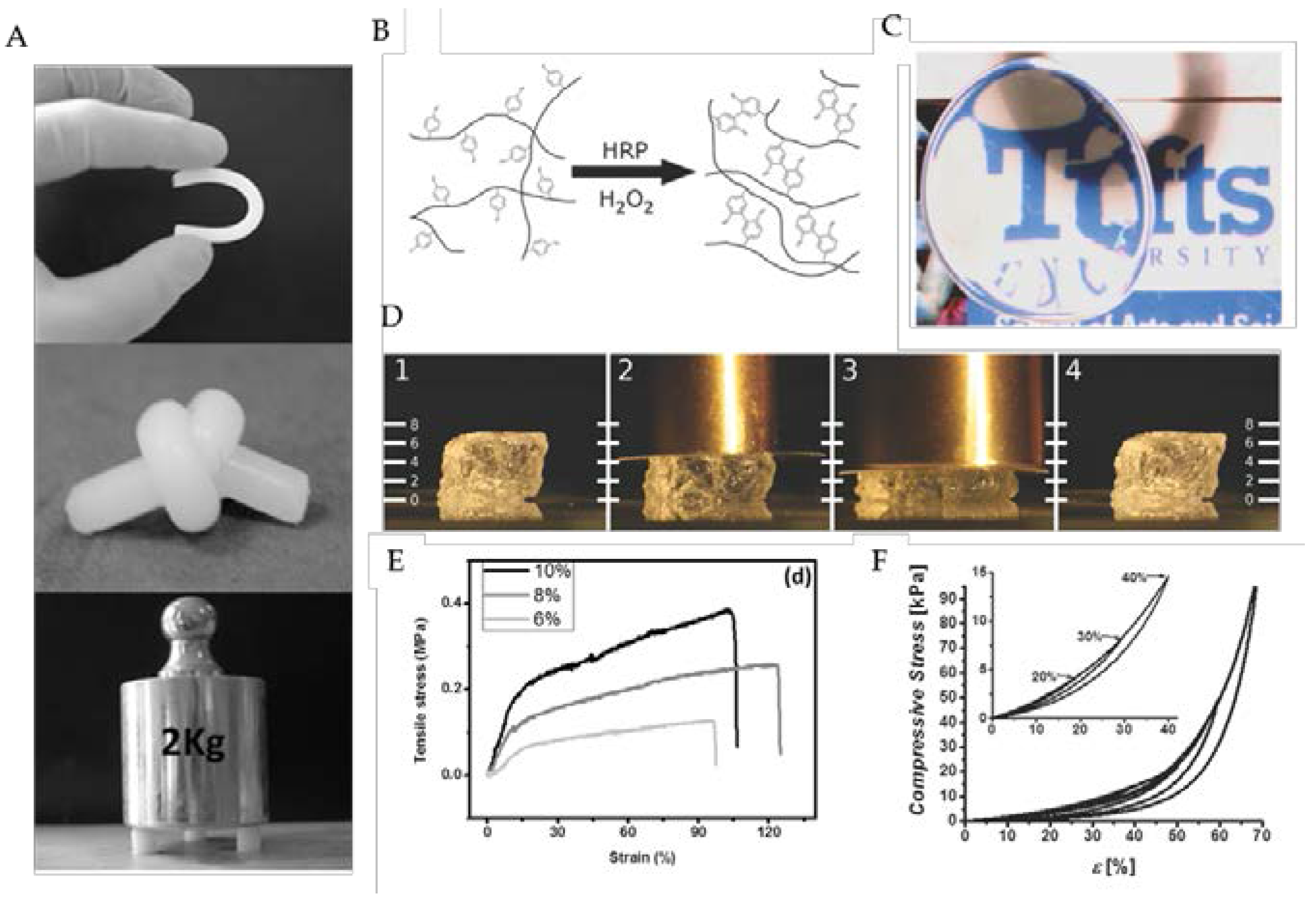
3.1.4. Chemical Stabilization
3.1.5. Chemical Modification of SF
3.2. Physical Methods
3.2.1. Temperature
3.2.2. Shear Forces
3.2.3. Ultrasound
3.2.4. Electric Field
3.3. Blend Hydrogels
3.3.1. Synthetic Polymers
3.3.2. Natural Polymers
3.4. Genetically Engineered Silks for Hydrogel Formation
4. Silk Hydrogels in Bioengineering and Medicine
4.1. Tissue Engineering and Regenerative Medicine
4.1.1. Hydrogels for Hard Tissue Engineering
4.1.1.1. Bone
4.1.1.2. Cartilage
4.1.2. Hydrogels for Soft Tissue Engineering
4.1.2.1. Neuronal Regeneration
4.1.2.2. Vascular Regeneration
4.2. Controlled Release
5. Future Directions and Recommendations
Author Contributions
Conflicts of Interest
References
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Kirschner, C.M. Hydrogels in Healthcare: From Static to Dynamic Material Microenvironments. Acta Mater. 2013, 61, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar]
- Seo, S.-J.; Mahapatra, C.; Singh, R.K.; Knowles, J.C.; Kim, H.-W. Strategies for osteochondral repair: Focus on scaffolds. J. Tissue Eng. 2014, 5, 2041731414541850. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based Biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Shao, Z.; Vollrath, F. Surprising strength of silkworm silk. Nature 2002, 418, 741. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Moy, R.L.; Lee, A.; Zalka, A. Commonly used suture materials in skin surgery. Am. Fam. Phys. 1991, 44, 2123–2128. [Google Scholar]
- Kundua, B.; Kurlandb, N.E.; Banoa, S.; Patrac, C.; Engelc, F.B.; Vamsi, K.; Yadavalli, V.; Kundu, S.C. Silk proteins for biomedical applications: Bioengineering perspectives. Prog. Polym. Sci. 2014, 39, 251–267. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.L. Silk Biomaterials. In Comprehensive Biomaterials; Ducheyne, P., Healy, K., Hutmacher, D.E., Grainger, D.W., Kirkpatrick, C.J., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; Volume 2, pp. 207–219. [Google Scholar]
- Hu, X.; Kaplan, D.; Cebe, P. Determining β-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Motta, A.; Foss, C.; Migliaresi, C. Tailoring Silk-Based Matrices for Tissue Engineering. In Tailored Polymer Architectures for Pharmaceutical and Biomedical Applications; ACS Symposium Series Volume 1135; Scholz, C., Kresseler, J., Eds.; American Chemical Society: Washington, DC, USA, 2013; Chapter 17; pp. 281–299. Available online: http://pubs.acs.org/isbn/9780841227989 (accessed on 8 July 2013). [CrossRef]
- Yamada, H.; Igarashi, Y.; Takasu, Y.; Saito, H.; Tsubouchi, K. Identification of fibroin-derived peptides enhancing the proliferation of cultured human skin fibroblasts. Biomaterials 2004, 25, 467–472. [Google Scholar] [CrossRef]
- Sun, W.; Incitti, T.; Migliaresi, C.; Quattrone, A.; Casarosa, S.; Motta, A. Genipin-crosslinked gelatin–silk fibroin hydrogels for modulating the behaviour of pluripotent cells. J. Tissue Eng. Regen. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Floren, M.; Bonani, W.; Dharmarajan, A.; Motta, A.; Migliaresi, C.; Tan, W. Human mesenchymal stem cells cultured on silk hydrogels with variable stiffness and growth factor differentiate into mature smooth muscle cell phenotype. Acta Biomater. 2016, 31, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Gahanati, S.; Unger, S.R.; Webber, M.J.; Barbeck, M.; Orth, C.; Kirkpatrick, J.A.; Booms, P.; Motta, A.; Migliaresi, C.; Sader, R.A.; et al. Scaffold vascularization in vivo driven by primary human osteoblasts in concert with host inflammatory cells. Biomaterials 2011, 32, 8150–8160. [Google Scholar] [CrossRef] [PubMed]
- Stoppato, M.; Stevens, H.Y.; Carletti, E.; Migliaresi, C.; Motta, A.; Guldberg, R.E. Effects of Silk Fibroin Fiber Incorporation on Mechanical Properties, Endothelial Cell Colonization and Vascularization of PDLLA Scaffolds. Biomaterials 2013, 34, 4573–4581. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.E.; Ghanaati, S.; Orth, C.; Sartoris, A.; Barbeck, M.; Halstenberg, S.; Motta, A.; Migliaresi, C.; Kirkpatric, C.J. The rapid anastomosis between prevascularized networks on silk fibroin scaffolds generated in vitro with cocultures of human microvascular endothelial and osteoblast cells and the host vasculature. Biomaterials 2010, 31, 6959–6967. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Orth, C.; Wang, H.; Barbeck, M.; Unger, R.; Eblenkamp, M.; Gotte, H.; Kunkel, M.; Motta, A.; Migliaresi, C.; et al. Fine-Tuning Scaffolds for Tissue Regeneration: Effects of Formic acid Processing on Tissue Reaction to Silk Fibroin. J. Tissue Eng. Regen. Med. 2010, 4, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Yigit, S.; Dinjaski, N.; Kaplan, D.L. Fibrous proteins: At the crossroads of genetic engineering and biotechnological applications. Biotechnol. Bioeng. 2016, 113, 913–929. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A. Introduction to protein crystallization. Methods 2004, 34, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Cudney, B.; Mcpherson, A. Polymeric Precipitants for the Crystallization of Macromolecules. Biochem. Biophys. Res. Commun. 1995, 207, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Römer, L.M.; Scheibel, T.R. Polymeric materials based on silk proteins. Polymer 2008, 49, 4309–4327. [Google Scholar] [CrossRef]
- Kim, U.J.; Park, J.; Li, C.; Jin, H.J.; Valluzzi, R.; Kaplan, D.L. Structure and properties of silk hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Knight, D.P.; Vollrath, F. Rheological characterization of nephila spidroin solution. Biomacromolecules 2002, 3, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Lammel, A.S.; Hu, X.; Park, S.; Kaplan, D.L.; Scheibel, T.R. Controlling silk fibroin particle features for drug delivery. Biomaterials 2010, 31, 4583–4591. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.L. Characterization of protein and peptide stability and solubility in non-aqueous solvents. Curr. Pharm. Biotechnol. 2000, 1, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Rammensee, S.; Huemmerich, D.; Hermanson, K.D.; Scheibel, T.; Bausch, A.R. Rheological characterization of hydrogels formed by recombinantly produced spider silk. Appl. Phys. A 2006, 82261–82264. [Google Scholar] [CrossRef]
- Numata, K.; Katashima, T.; Sakai, T. State of water, molecular structure, and cytotoxicity of silk hydrogels. Biomacromolecules 2011, 12, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Nema, S.; Teagarden, D. Protein-aggregation–pathways and influencing factors. Int. J. Pharm. 2010, 390, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hou, J.; Li, M.; Wang, J.; Kaplan, D.L.; Lu, S. Sodium dodecyl sulfate-induced rapid gelation of silk fibroin. Acta Biomater. 2012, 8, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T.; Watanabe, A.; Tsuchiya, T.; Ikoma, R.; Hidaka, M.; Sugihara, M. New Oral Dosage Form for Elderly Patients: Preparation and Characterization of Silk Fibroin Gel. Chem. Pharm. Bull. 1995, 43, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.; Migliaresi, C.; Faccioni, F.; Torricelli, P.; Fini, M.; Giardino, R. Fibroin hydrogels for biomedical applications: Preparation, characterization and in vitro cell culture studies. J. Biomater. Sci. Polym. Ed. 2004, 15, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.-D.; Nahm, J.-H.; Park, J.-S.; Moon, J.-Y.; Cho, C.-S.; Yeo, J.-H. Effects of poloxamer on the gelation of silk fibroin. Macromol. Rapid Commun. 2000, 21, 788–791. [Google Scholar] [CrossRef]
- Ayub, Z.; Arai, M.; Hirabayashi, K. Mechanism of the gelation of fibroin solution. Biosci. Biotechnol. Biochem. 1993, 57, 1910–1912. [Google Scholar] [CrossRef]
- Matsumoto, A.; Chen, J.; Collette, A.L.; Kim, U.J.; Altman, G.H.; Cebe, P.; Kaplan, D.J. Mechanisms of silk fibroin sol-gel transitions. J. Phys. Chem. B 2006, 110, 21630–21638. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.E.; Knight, D.P.; Porter, P.; Vollrath, F. pH Induced Changes in the Rheology of Silk Fibroin Solution from the Middle Division of Bombyx mori Silkworm. Biomacromolecules 2004, 5, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Floren, M.L.; Spilimbergo, S.; Motta, A.; Migliaresi, C. Carbon Dioxide Induced Silk Protein Gelation. Biomacromolecules 2012, 13, 2060–2072. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Wray, L.S.; Hu, X.; Gallego, J.; Georgakoudi, I.; Omenetto, F.G.; Schmidt, D.; Kaplan, D.L. Effect of processing on silk-based biomaterials: Reproducibility and biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99B, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Partlow, B.P.; Hanna, C.W.; Rnjak-Kovacina, J.; Moreau, J.E.; Applegate, M.B.; Burke, K.A.; Marelli, B.; Mitropoulos, A.N.; Omenetto, F.G.; Kaplan, D.L. Highly Tunable Elastomeric Silk Biomaterials. Adv. Funct. Mater. 2014, 24, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.R.; kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 23, 6443–6450. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.R.; St. Jhon, P.; Kaplan, D.L. Modification of Silk Fibroin Using Diazonium Coupling Chemistry and the Effects on hMSC Proliferation and Differentiation. Biomaterials 2008, 29, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Urry, D.W. Molecular machines: How motion and other functions of living organisms can resukt from reversible chemical changes. Angew. Chem. Int. Ed. Engl. 1993, 32, 819–841. [Google Scholar] [CrossRef]
- Nagarkar, S.; Nicolai, T.; Chassenieux, C.; Lele, A. Structure and gelation mechanism of silk hydrogels. Phys. Chem. Chem. Phys. 2010, 12, 3834–3844. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Nafaile, C.; Metzner, A.B.; Wissbrun, K.F. Analysis of stress-induced phase separations in polymer solutions. Macromolecules 1984, 17, 1187–1195. [Google Scholar] [CrossRef]
- Ji, H.; Helfand, E. Concentration Fluctuations in Sheared Polymer Solutions. Macromolecules 1995, 28, 3869–3880. [Google Scholar] [CrossRef]
- Yucel, T.; Cebe, P.; Kaplan, D.L. Vortex-Induced Injectable Silk Fibroin Hydrogels. Biophys. J. 2009, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Wang, S.; Zhao, J.; Xu, L.; Zhu, C.; Zeng, D.; Chen, J.; Zhang, Z.; Kaplan, D.; et al. The use of injectable sonication-induced silk hydrogel for VEGF165 and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials 2011, 32, 9415–9424. [Google Scholar] [CrossRef] [PubMed]
- Paulusse, J.M.J.; Sijbesma, R.P. Ultrasound in polymer chemistry: Revival of an established technique. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5445–5453. [Google Scholar] [CrossRef]
- Wang, X.; Kluge, J.A.; Leisk, G.G.; Kaplan, D.L. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Leisk, G.G.; Lo, T.J.; Yucel, T.; Lu, Q.; Kaplan, D.L. Electrogelation for Protein Adhesives. Adv. Mater. 2010, 22, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Z.; Lu, S.Z.; Wu, Z.Y.; Tan, K.; Minoura, N.; Kuga, S. Structure and properties of silk fibroin-poly(vinyl alcohol) gel. Int. J. Biol. Macromol. 2002, 30, 89–94. [Google Scholar] [CrossRef]
- Kweon, H.Y.; Park, S.H.; Yeo, J.H.; Lee, Y.W.; Cho, C.S. Preparation of semi-interpenetrating polymer networks composed of silk fibroin and poly(ethylene glycol) macromer. J. Appl. Polym. Sci. 2001, 80, 1848–1853. [Google Scholar] [CrossRef]
- Yoo, M.K.; Kweon, H.Y.; Lee, K.G.; Lee, H.C.; Cho, C.S. Preparation of semi-interpenetrating polymer networks composed of silk fibroin and poloxamer macromer. Int. J. Biol. Macromol. 2004, 34, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Frankowski, D.J.; Spontak, R.J.; Hudson, S.M. Swelling behavior and morphological evolution of mixed gelatin/silk fibroin hydrogels. Biomacromolecules 2005, 6, 3079–3087. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; He, J.; Nichol, J.W.; Wang, L.; Hutson, C.B.; Wang, B.; Khademhosseini, A. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater. 2011, 7, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.; Bacakova, L.; Newman, C.; Hategan, A.; Griffin, M.; Discher, D. Substrate compliance versus ligand density in cellon gel responses. Biophys. J. 2004, 86, 617–628. [Google Scholar] [CrossRef]
- Gordon, T.D.; Schloesser, L.; Humphries, D.E.; Spector, M. Effects of the degradation rate of collagen matrices on articular chondrocyte proliferation and biosynthesis in vitro. Tissue Eng. 2004, 10, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Hu, K.; Feng, Q.L.; Cui, F. Fibroin/collagen hybrid hydrogels with crosslinking method: Preparation, properties, and cytocompatibility. J. Biomed. Mater. Res. A 2008, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lu, Q.; Sun, L.; Cebe, P.; Wang, X.; Zhang, X.; Kaplan, D.L. Biomaterials from ultrasonication-induced silk fibroin-hyaluronic acid hydrogels. Biomacromolecules 2010, 11, 3178–3188. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Int. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Van Beek, M.; Jones, L.; Sheardown, H. Hyaluronic acid containing hydrogels for the reduction of protein adsorption. Biomaterials 2008, 29, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Yang, Y.; Shao, Z. Physically Crosslinked Biocompatible Silk-Fibroin-Based Hydrogels with High Mechanical Performance. Adv. Funct. Mater. 2015. [Google Scholar] [CrossRef]
- Gong, Z.; Yang, Y.; Ren, Q.; Chen, X.; Shao, Z. Injectable thixotropic hydrogel comprising regenerated silk fibroin and hydroxypropylcellulose. Soft Matter 2012, 8, 2875–2883. [Google Scholar] [CrossRef]
- Klock, G.; Pfeffermann, A.; Ryser, C.; Grohn, P.; Kuttler, B.; Hahn, H.J.; Zimmermann, U. Biocompatibility of mannuronic acid-rich alginates. Biomaterials 1997, 18, 707–713. [Google Scholar] [CrossRef]
- Ziv, K.; Nuhn, H.; Ben-Haim, Y.; Sasportas, L.S.; Kempen, P.J.; Niedringhaus, T.P.; Hrynyk, M.; Sinclair, R.; Barron, A.E.; Gambhir, S.S. A tunable silk-alginate hydrogel scaffold for stem cell culture and transplantation. Biomaterials 2014, 35, 3736–3743. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Kobashi, K.; Aburada, M. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol. Pharm. Bull. 1994, 17, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Motta, A.; Rodrigues, M.T.; Pinheiro, A.F.M.; Gomes, M.E.; Mano, J.F.; Reis, R.L.; Migliaresi, C. Novel Genipin-Cross-Linked Chitosan/Silk Fibroin Sponges for Cartilage Engineering Strategies. Biomacromolecules 2008, 9, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Branden, C.; Tooze, C. Introduction to Protein Structure; Taylor & Francis: Thames, Oxfordshire, UK, 1999; pp. 283–298. [Google Scholar]
- Sarikaya, M.; Tamerler, C.; Jen, A.K.; Schulten, K.; Baneyx, F. Molecular biomimetics: Nanotechnology through biology. Nat. Mater. 2003, 2, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Urry, D.W.; Luan, C.H.; Parker, T.M.; Gowda, D.C.; Prasad, K.U.; Reid, M.C.; Safavy, A. Temperature of polypeptide inverse temperature transition depends on mean residue hydrophobicity. J. Am. Chem. Soc. 1991, 113, 4346–4348. [Google Scholar] [CrossRef]
- Xia, X.X.; Xu, Q.B.; Hu, X.; Qin, G.; Kaplan, D.L. Tunable self-assembly of genetically engineered silk-elastin-like protein polymers. Biomacromolecules 2011, 12, 3844–3850. [Google Scholar] [CrossRef] [PubMed]
- Megeed, Z.; Cappello, J.; Ghandehari, H. Genetically engineered silk-elastinlike protein polymers for controlled drug delivery. Adv. Drug Deliv. Rev. 2002, 54, 1075–1091. [Google Scholar] [CrossRef]
- Dinjaski, N.; Kaplan, D.L. Recombinant protein blends: Silk beyond natural design. Curr. Opin. Biotechnol. 2015, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.W.; Rollett, A.; Kaplan, D.L. Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics. Expert Opin. Drug Deliv. 2015, 12, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 14, 920–926. [Google Scholar] [CrossRef]
- Fini, M.; Motta, A.; Torricelli, P.; Niccoli Aldini, N.; Tschon, M.; Giardino, R.; Migliaresi, C. The healing of confined critical size cancellous defects in the presence of silk fibroin hydrogel. Biomaterials 2005, 26, 3527–3536. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.H.; Yodmuang, S.; Wang, X.; Sun, L.; Kaplan, D.L. Silk hydrogel for cartilage tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Yodmuang, S.; Mcnamara, S.L.; Nover, A.B.; Mandal, B.B.; Agarwal, M.; Kelly, T.A.; Chao, P.H.; Hung, C.; Kaplan, D.L. Silk microfiber-reinforced silk hydrogel composites for functional cartilage tissue repair. Acta Biomater. 2015, 11, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Cho, H.; Gil, E.S.; Mandal, B.B.; Min, B.H.; Kaplan, D.L. Silk-fibrin/hyaluronic acid composite gels for nucleus pulposus tissue regeneration. Tissue Eng. Part A 2011, 17, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Incitti, T.; Migliaresi, C.; Quattrone, A.; Casarosa, S.; Motta, A. Viability and neuronal differentiation of neural stem cells encapsulated in silk fibroin hydrogel functionalized with an IKVAV peptide. J. Tissue Eng. Regen. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Seib, F.P.; Pritchard, E.M.; Kaplan, D.L. Self-Assembling Doxorubicin Silk Hydrogels for the Focal Treatment of Primary Breast Cancer. Adv. Funct. Mater. 2013, 23, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lim, J.; Teoh, S.-H. Review: Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2013, 31, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Samee, M.; Kasugai, S.; Kondo, H.; Ohya, K.; Shimokawa, H.; Kuroda, S. Bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF) transfection to human periosteal cells enhances osteoblast differentiation and bone formation. J. Pharmacol. Sci. 2008, 108, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Suh, J.-K.; Aroen, A.; Muzzonigro, T.S.; DiSilvestro, M.; Fu, F.H. Injury and repair of articular cartilage: Related scientific issues. Oper. Tech. Orthop. 1997, 7, 270–278. [Google Scholar] [CrossRef]
- Lima, E.G.; Bian, L.; Ng, K.W.; Mauck, R.L.; Byers, B.A.; Tuan, R.S.; Ateshian, G.A.; Hung, C.T. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-β3. Osteoarthr. Cartil. 2007, 15, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Vink, R.; Bullock, M.R. Traumatic brain injury: Therapeutic challenges and new directions. Neurotherapeutics 2010, 7, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Fuehrmann, T.; Gerardo-Nava, J.; Brook, G.A. Central nervous system. In Tissue Engineering: From Lab to Clinic; Pallua, N., Suscheck, V., Eds.; Springer: Berlin/Heidenberg, Germany, 2011; pp. 221–244. [Google Scholar]
- Cheng, T.Y.; Chen, M.H.; Chang, W.H.; Huang, M.Y.; Wang, T.W. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials 2013, 34, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, K.D.; Xu, J.Q.; Murphy, S.L.; Miniño, A.M.; Kung, H.C. Deaths: Final data for 2009. Natl. Vital Stat. Rep. 2011, 60, 1–117. [Google Scholar] [PubMed]
- Weintraub, W.S.; Jones, E.L.; Craver, J.M.; Guyton, R.A. Frequency of repeat coronary-bypass or coronary angioplasty after coronary-artery bypasssurgery using saphenous venous grafts. Am. J. Cardiol. 1994, 73, 103–112. [Google Scholar] [CrossRef]
- Krawiec, J.T.; Vorp, D.A. Adult stem cell-based tissue engineered blood vessels: A review. Biomaterials 2012, 33, 3388–3400. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.E.; Bhatia, S.N. Micromechanical control of cell–cell interactions. Proc. Natl. Acad. Sci. USA 2007, 104, 5722–5726. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Saha, K.; Bogatyrev, S.R.; Yang, J.; Hook, A.L.; Kalcioglu, Z.I.; Cho, S.; Mitalipova, M.; Pyzocha, N.; Rojas, F.; et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 2010, 9, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Chen, J.P.; Leu, Y.L.; Wang, H. Characterization and evaluation of silk protein hydrogels for drug delivery. Chem. Pharm. Bull. 2006, 54, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Mann, J.K.; Kundu, S.C. Silk fibroin/gelatin multilayered films as a model system for controlled drug release. Eur. J. Pharm. Sci. 2009, 37, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tibbitt, M.W.; Basta, L.; Anseth, K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014, 13, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Nuttelman, C.R.; Tripodi, M.C.; Anseth, K.S. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005, 24, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Koshy, S.T.; Branco da Cunha, C.; Shin, J.; Verbeke, C.S.; Allison, K.H.; Mooney, D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014, 13, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, Y.; Partlow, B.P.; Golding, A.S.; Tseng, P.; Coburn, J.; Applegate, M.B.; Moreau, J.E.; Omenetto, F.G.; Kaplan, D.L. Bio-functionalized silk hydrogel microfluidic systems. Biomaterials 2016, 93, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Applegate, M.B.; Coburn, J.; Partlow, B.P.; Moreau, J.E.; Mondia, J.P.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Laser-based three-dimensional multiscale micropatterning of biocompatible hydrogels for customized tissue engineering scaffolds. Proc. Natl. Acad. Sci. USA 2015, 112, 12052–12057. [Google Scholar] [CrossRef] [PubMed]


| SF Hydrogel Fabrication | Target Regeneration | Methods | Comments | Ref. |
|---|---|---|---|---|
| SF-gelatin blend prepared by sonication and chemical crosslinking by genipin | Neuronal tissue | Mouse embryonic stem cells (mESCs) were seeded and kept in knockout serum replacement (KSR) for 15 days |
| [14] |
| SF hydrogel prepared by CO2 treatment | Vascular Smooth muscle | Culturing of hMSCs within SF hydrogels of variable stiffness and combined with 10 ng/mL TGF-β1 |
| [15] |
| VEGF165 and BMP-2 growth factors were encapsulated in 1 mL of 5.0 wt % SF solution and stabilized via sonication (25% amplitude) for 30 s | Bone | Evaluate in situ forming SF hydrogels combined with dual growth factors for rabbit maxillary sinus floor augmentation |
| [49] |
| Addition of 1 M citric acid to 2% w/v SF water solution and treated at 50 °C overnight | Bone | In vitro and in vivo response of injectable SF hydrogels toward osteoblast culture and implantation in critical-size defects of rabbit distal femurs |
| [78] |
| Sonicated SF hydrogels of various protein concentration | Cartilage | Encapsulated primary calf chondrocytes into SF hydrogels of different protein concentration and compared against a porous silk scaffold control |
| [79] |
| Composite hydrogel combining silk microfibers with sonicated SF hydrogel | Cartilage | Prepared SF-fiber blend hydrogels to mimic fiber morphology found in native cartilage |
| [80] |
| SF-HA blend hydrogel prepared by sonication | Nucleus Pulpous (NP) | Encapsulation of human chondrocytes |
| [81] |
| SF modified with IKVAV peptide and stabilized by sonication | Neuronal tissue | Encapsulation of human neural stem cells |
| [82] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floren, M.; Migliaresi, C.; Motta, A. Processing Techniques and Applications of Silk Hydrogels in Bioengineering. J. Funct. Biomater. 2016, 7, 26. https://doi.org/10.3390/jfb7030026
Floren M, Migliaresi C, Motta A. Processing Techniques and Applications of Silk Hydrogels in Bioengineering. Journal of Functional Biomaterials. 2016; 7(3):26. https://doi.org/10.3390/jfb7030026
Chicago/Turabian StyleFloren, Michael, Claudio Migliaresi, and Antonella Motta. 2016. "Processing Techniques and Applications of Silk Hydrogels in Bioengineering" Journal of Functional Biomaterials 7, no. 3: 26. https://doi.org/10.3390/jfb7030026
APA StyleFloren, M., Migliaresi, C., & Motta, A. (2016). Processing Techniques and Applications of Silk Hydrogels in Bioengineering. Journal of Functional Biomaterials, 7(3), 26. https://doi.org/10.3390/jfb7030026