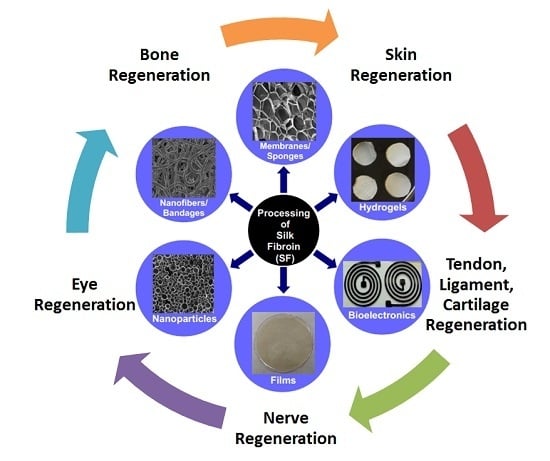
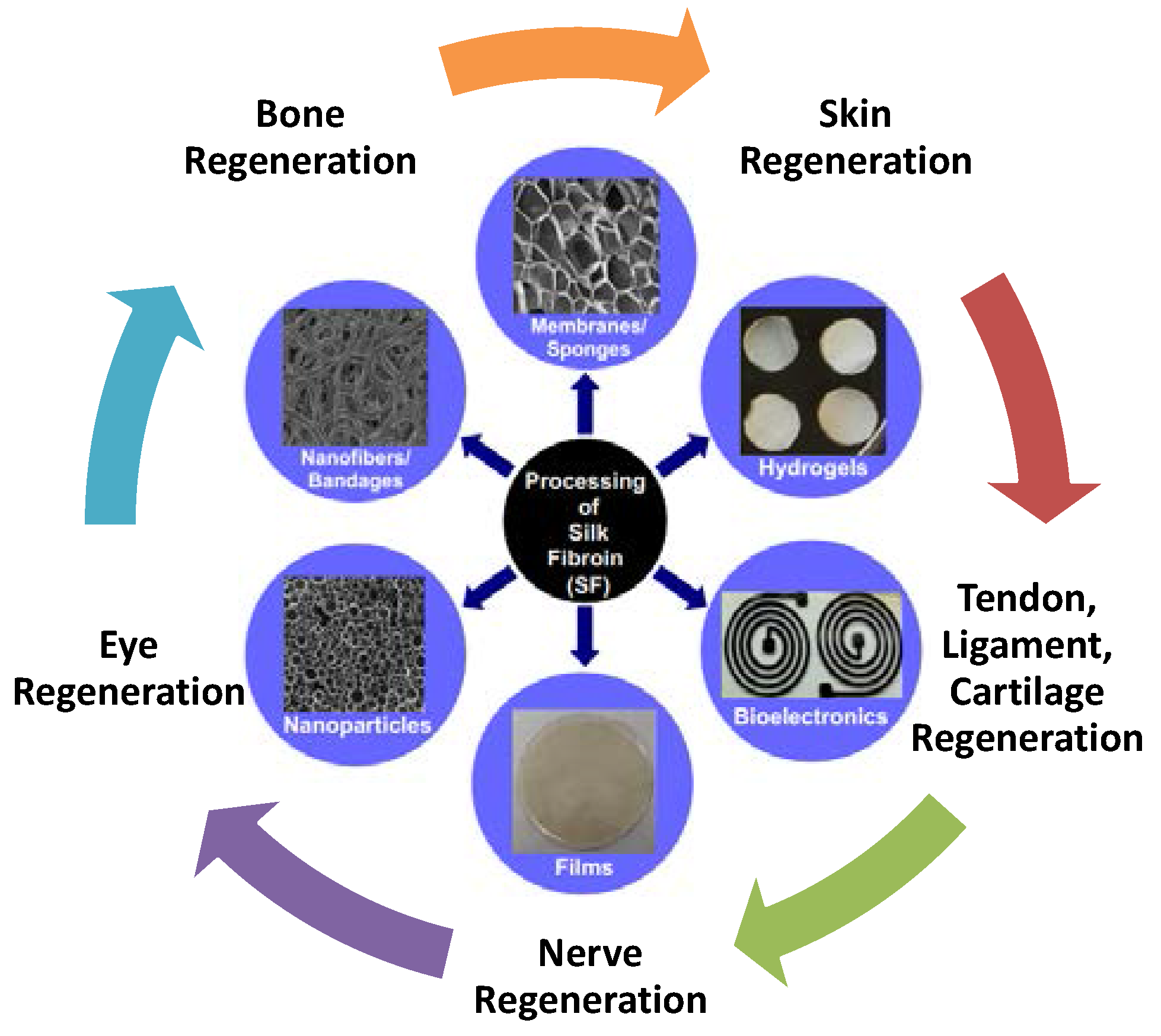
Tissue Regeneration: A Silk Road
Abstract
:1. Introduction
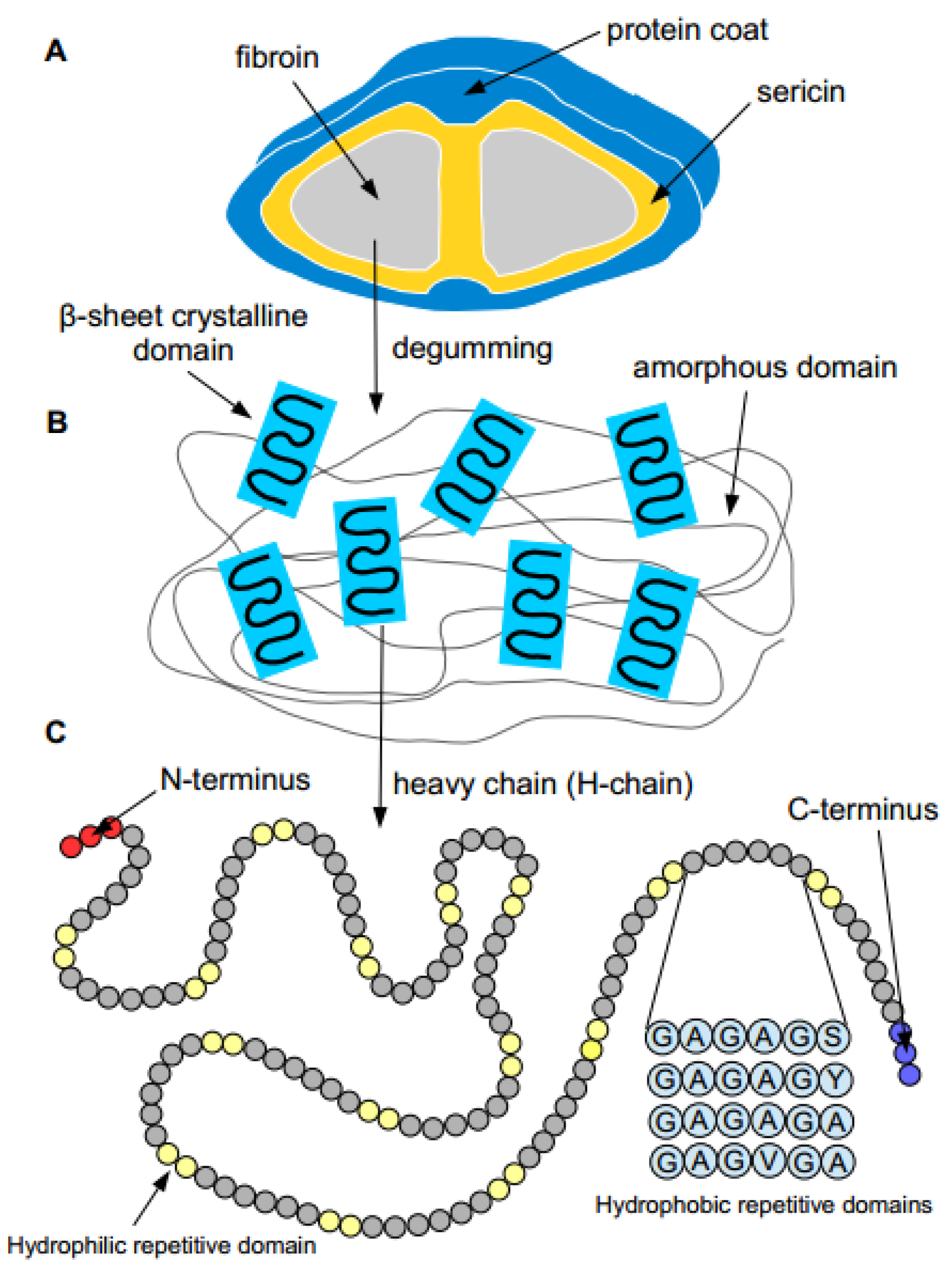
2. Properties of Silk Proteins
3. Silk Materials for Tissue Regenerations
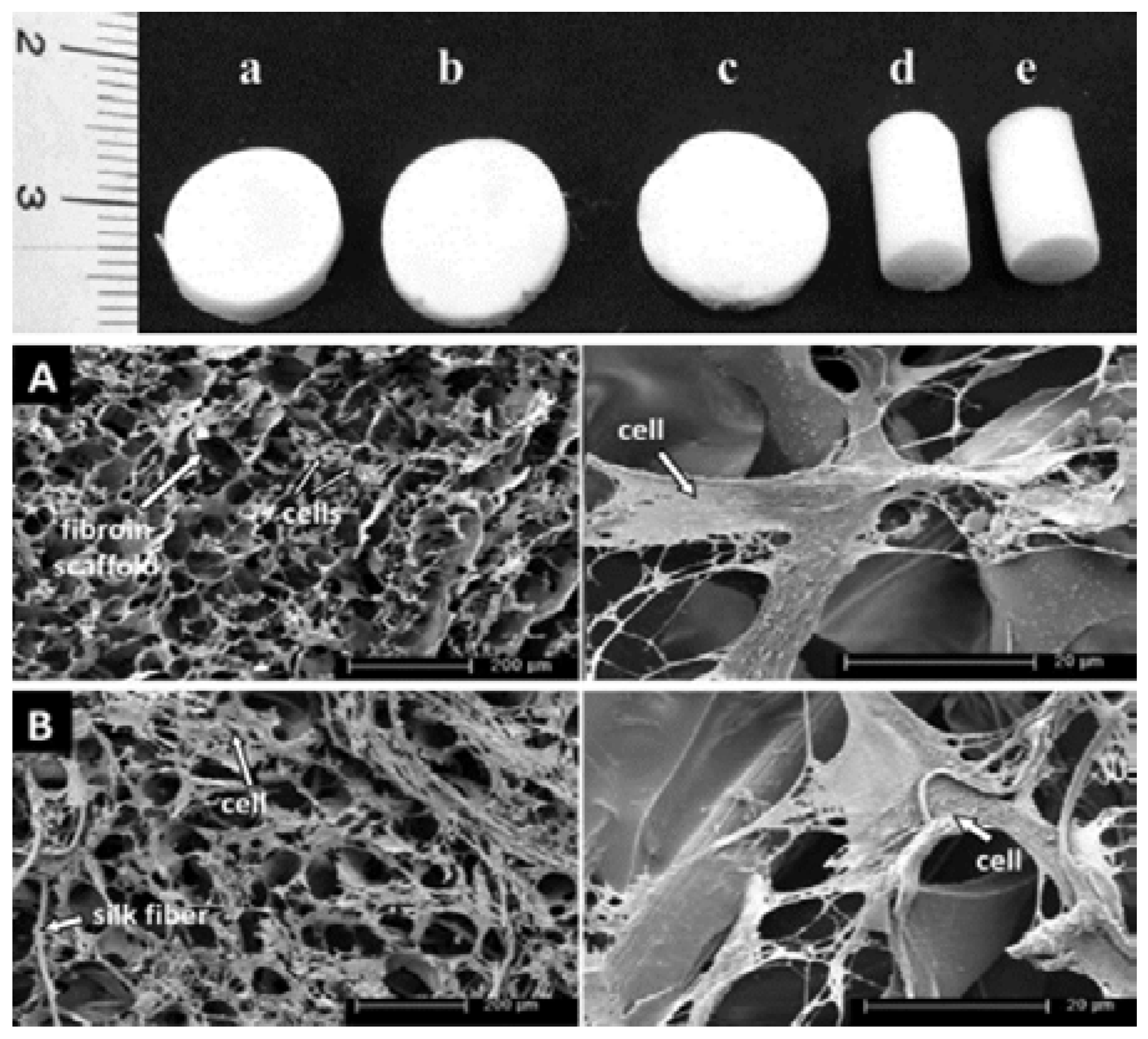
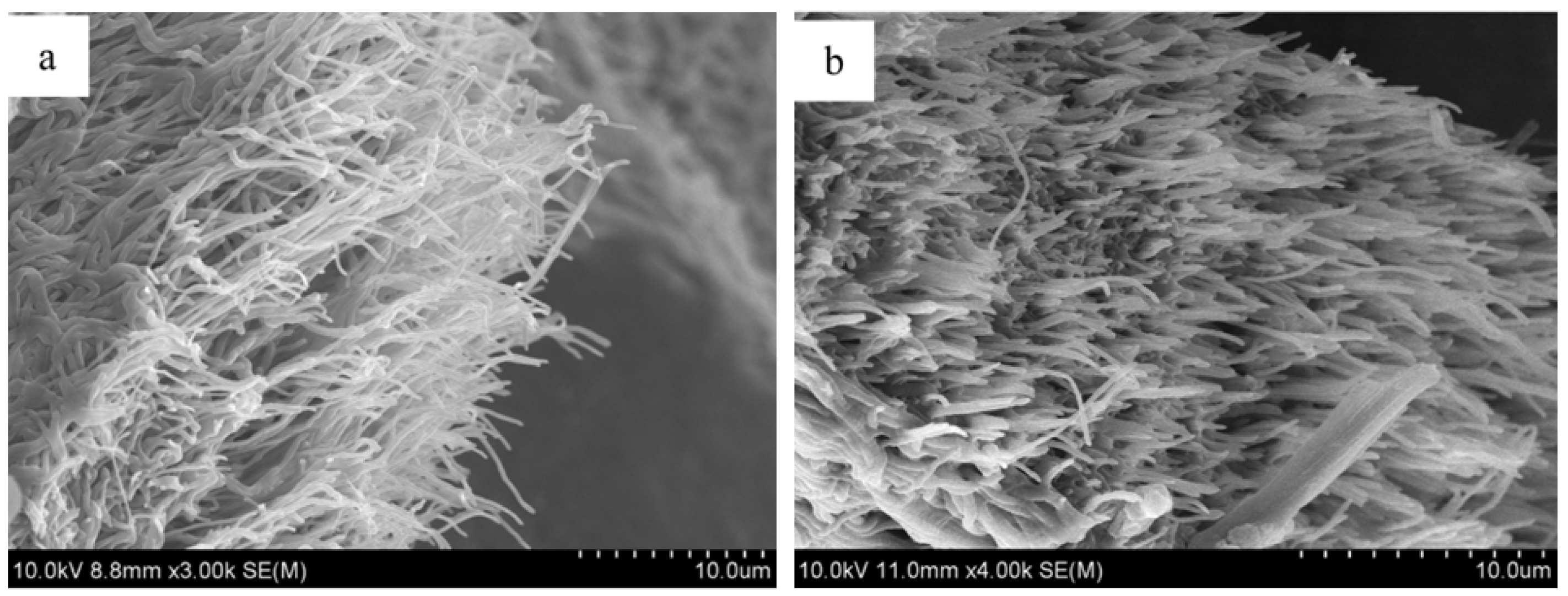
3.1. Silk for Bone Regeneration
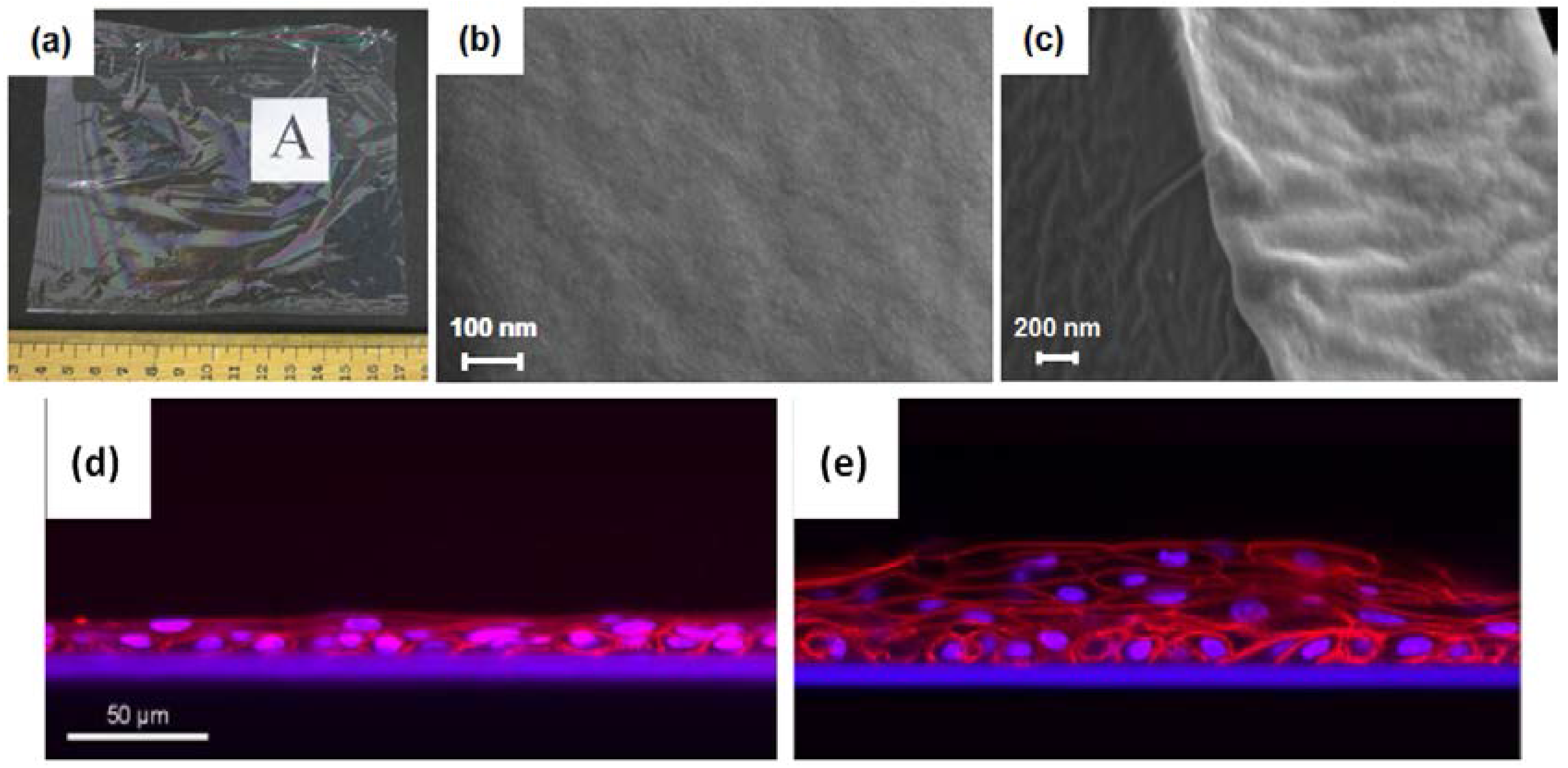
3.2. Silk for Eye Regeneration
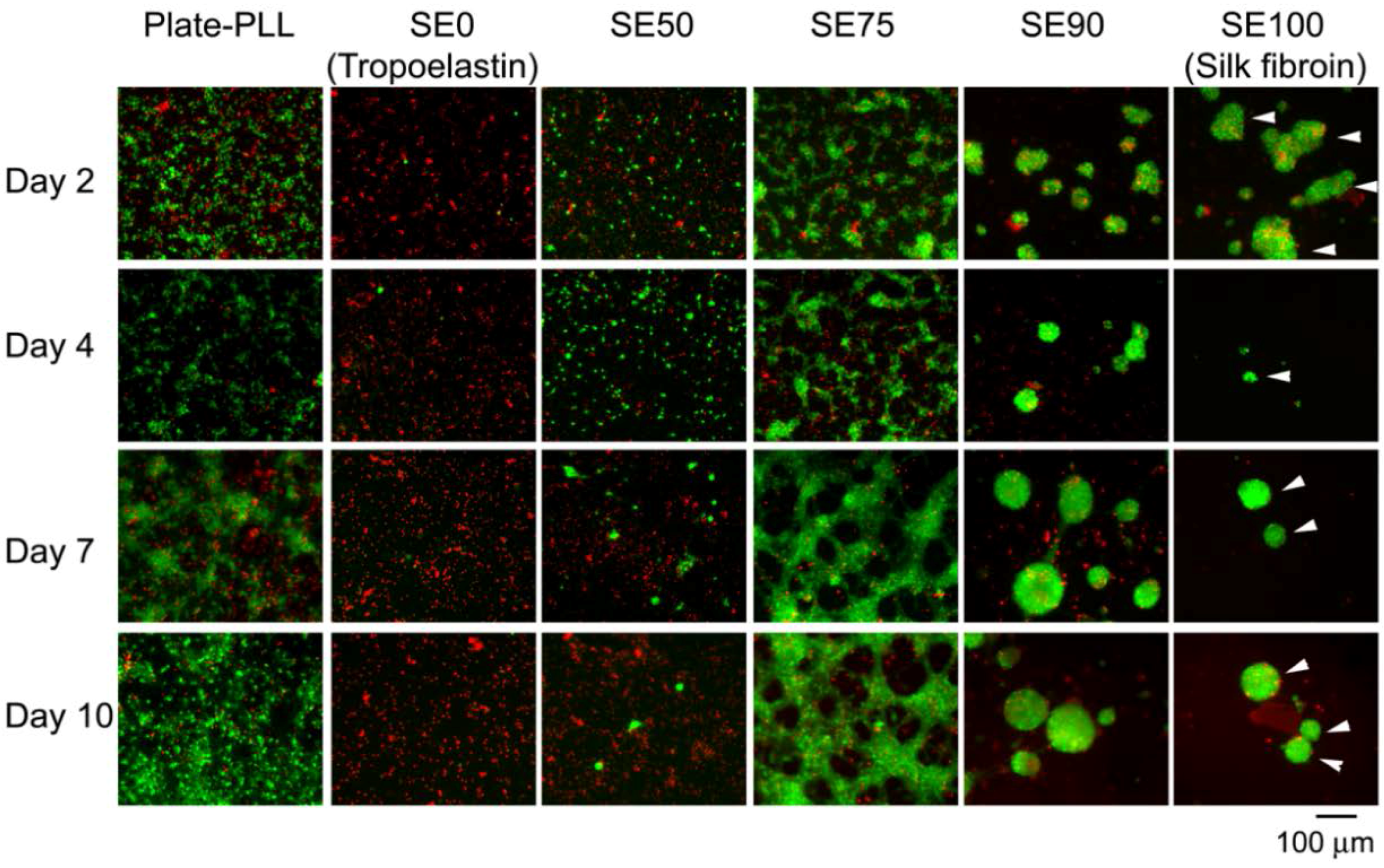
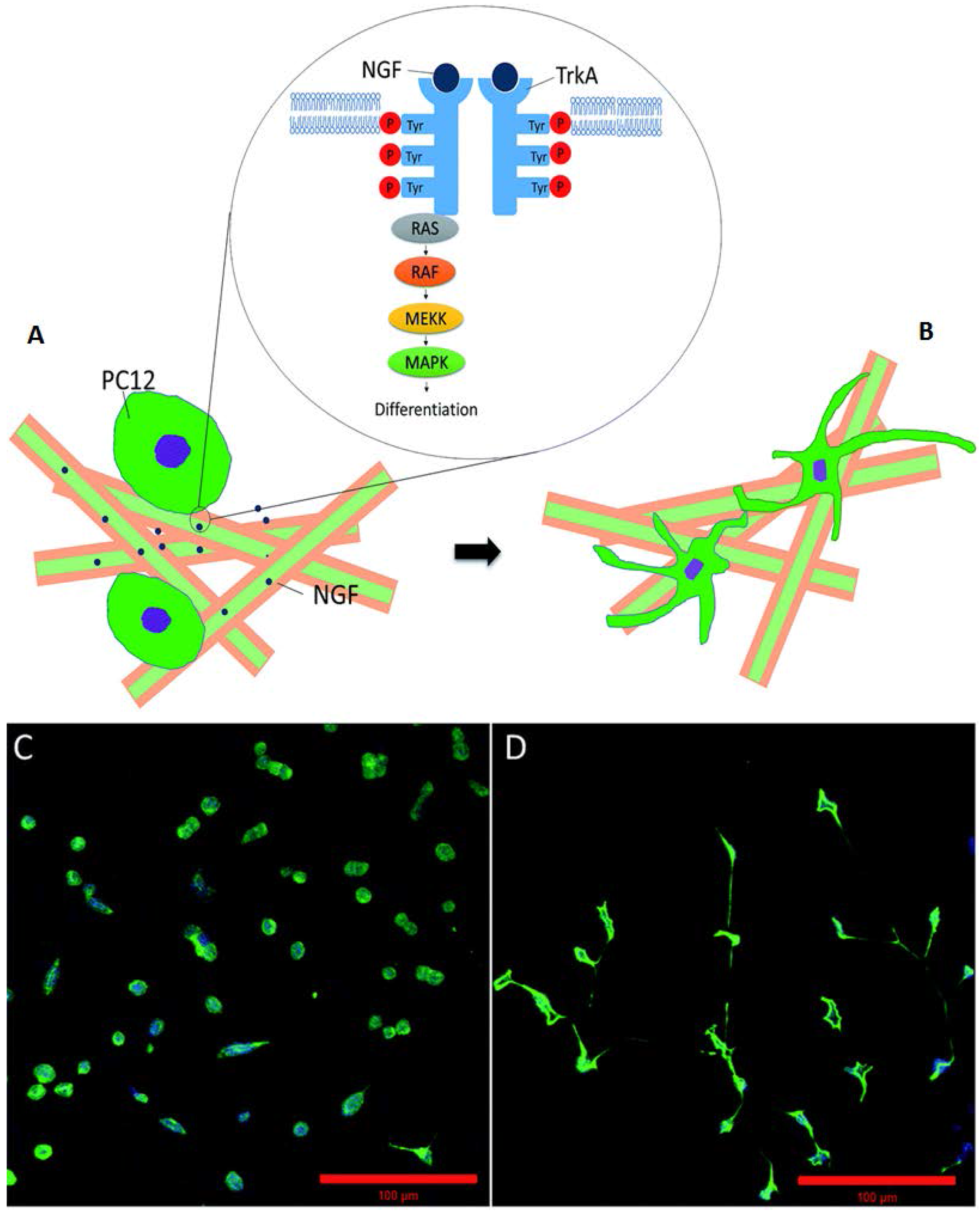
3.3. Silk for Nerve Regeneration
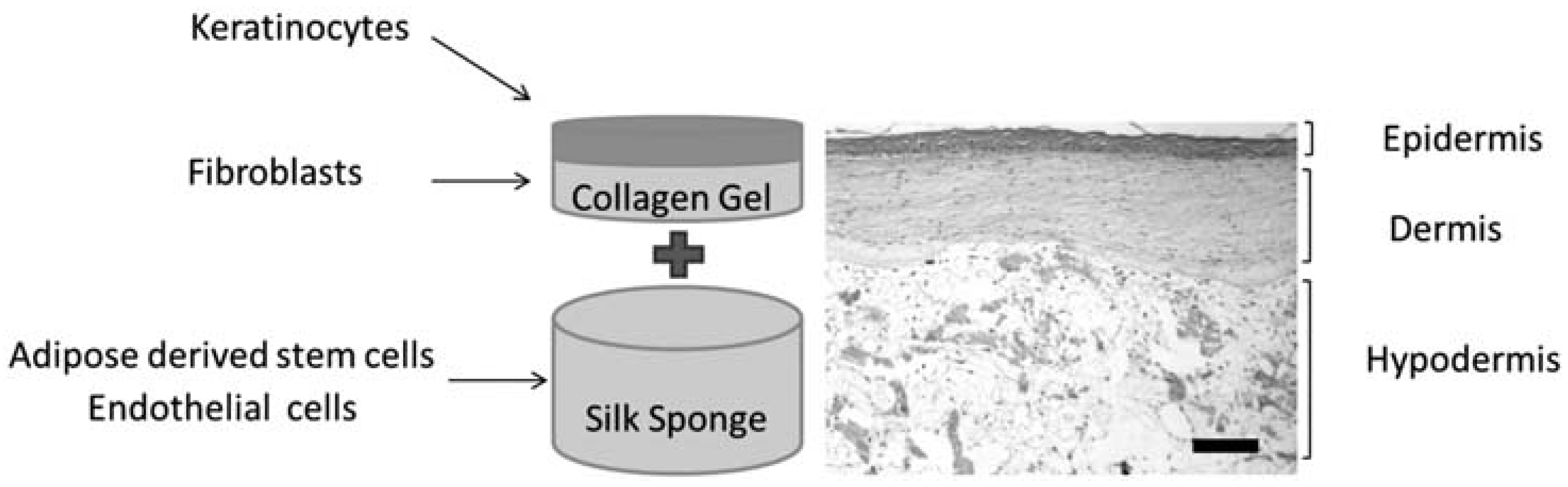
3.4. Silk for Skin Regeneration
3.5. Silk for Tendon and Ligament Regeneration
3.6. Silk for Cartilage Regeneration
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kundu, B.; Kurland, N.E.; Yadavalli, V.K.; Kundu, S.C. Isolation and processing of silk proteins for biomedical applications. Int. J. Biol. Macromol. 2014, 70, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Kurland, N.E.; Bano, S.; Patra, C.; Engel, F.B.; Yadavalli, V.K.; Kundu, S.C. Silk proteins for biomedical applications: Bioengineering perspectives. Prog. Polym. Sci. 2014, 39, 251–267. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Rajkhowa, R.; Wang, X.; Devi, D. Milled non-mulberry silk fibroin microparticles as biomaterial for biomedical applications. Int. J. Biol. Macromol. 2015, 81, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Kundu, S.C. A non-mulberry silk fibroin protein based 3D in vitro tumor model for evaluation of anticancer drug activity. Adv. Funct. Mater. 2012, 22, 4778–4788. [Google Scholar] [CrossRef]
- Musson, D.S.; Naot, D.; Chhana, A.; Matthews, B.G.; McIntosh, J.D.; Lin, S.T.; Choi, A.J.; Callon, K.E.; Dunbar, P.R.; Lesage, S. In vitro evaluation of a novel non-mulberry silk scaffold for use in tendon regeneration. Tissue Eng. Part A 2015, 21, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.-T.; Zhang, Y.-Q. Processing and characterization of silk sericin from bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C 2016, 61, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Kundu, S.C. Osteogenic and adipogenic differentiation of rat bone marrow cells on non-mulberry and mulberry silk gland fibroin 3D scaffolds. Biomaterials 2009, 30, 5019–5030. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Yao, K.; Lin, S.; Yang, Z.; Li, X.; Xie, H.; Qing, T.; Gao, L. Poly(d, l-lactic acid) surfaces modified by silk fibroin: Effects on the culture of osteoblast in vitro. Biomaterials 2002, 23, 1153–1160. [Google Scholar] [CrossRef]
- Partlow, B.P.; Hanna, C.W.; Rnjak-Kovacina, J.; Moreau, J.E.; Applegate, M.B.; Burke, K.A.; Marelli, B.; Mitropoulos, A.N.; Omenetto, F.G.; Kaplan, D.L. Highly tunable elastomeric silk biomaterials. Adv. Funct. Mater. 2014, 24, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Naskar, D.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Non-mulberry silk fibroin grafted poly(ε-caprolactone) nanofibrous scaffolds mineralized by electrodeposition: An optimal delivery system for growth factors to enhance bone regeneration. RSC Adv. 2016, 6, 26835–26855. [Google Scholar] [CrossRef]
- Cai, Z.-X.; Mo, X.-M.; Zhang, K.-H.; Fan, L.-P.; Yin, A.-L.; He, C.-L.; Wang, H.-S. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk sericin: A versatile material for tissue engineering and drug delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Kundu, S.C. Silk sericin/polyacrylamide in situ forming hydrogels for dermal reconstruction. Biomaterials 2012, 33, 7456–7467. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Siritientong, T.; Kanokpanont, S.; Srichana, T. Formulation and characterization of silk sericin–pva scaffold crosslinked with genipin. Int. J. Biol. Macromol. 2010, 47, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Wantanasiri, P.; Ratanavaraporn, J.; Yamdech, R.; Aramwit, P. Fabrication of silk sericin/alginate microparticles by electrohydrodynamic spraying technique for the controlled release of silk sericin. J. Electrostat. 2014, 72, 22–27. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Hosseinkhani, H.; Shokrgozar, M.A.; Mao, C.; Yang, M.; Farokhi, M. Silk as a potential candidate for bone tissue engineering. J. Control. Release 2015, 215, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.; Hoyer, B.; Solati-Hashjin, M.; Lode, A.; Nosoudi, N.; Samadikuchaksaraei, A.; Gelinsky, M. Fabrication and characterization of regenerated silk scaffolds reinforced with natural silk fibers for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.; Bhumiratana, S.; Yan, L.-P.; Oliveira, A.L.; Gimble, J.M.; Rockwood, D.; Kaplan, D.L.; Sousa, R.A.; Reis, R.L.; Vunjak-Novakovic, G. Development of silk-based scaffolds for tissue engineering of bone from human adipose-derived stem cells. Acta Biomater. 2012, 8, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Bhumiratana, S.; Grayson, W.L.; Castaneda, A.; Rockwood, D.N.; Gil, E.S.; Kaplan, D.L.; Vunjak-Novakovic, G. Nucleation and growth of mineralized bone matrix on silk-hydroxyapatite composite scaffolds. Biomaterials 2011, 32, 2812–2820. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Gu, Z.; Li, C.; Franco, C.; Wang, J.; Li, L.; Meredith, N.; Ye, Q.; Wan, C. A novel bioceramic scaffold integrating silk fibroin in calcium polyphosphate for bone tissue-engineering. Ceram. Int. 2016, 42, 2386–2392. [Google Scholar] [CrossRef]
- Xu, M.; Li, H.; Zhai, D.; Chang, J.; Chen, S.; Wu, C. Hierarchically porous nagelschmidtite bioceramic–silk scaffolds for bone tissue engineering. J. Mater. Chem. B 2015, 3, 3799–3809. [Google Scholar] [CrossRef]
- Park, S.Y.; Ki, C.S.; Park, Y.H.; Jung, H.M.; Woo, K.M.; Kim, H.J. Electrospun silk fibroin scaffolds with macropores for bone regeneration: An in vitro and in vivo study. Tissue Eng. Part A 2010, 16, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; He, J.; Sang, F.; Ding, B.; Chen, L.; Cui, S.; Li, K.; Han, Q.; Tan, W. Coaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite–tussah silk fibroin nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2016, 58, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Naskar, D.; Maiti, T.K.; Bhattacharya, D.; Das, P.; Nandi, S.K.; Kundu, S.C. Potential of non-mulberry silk protein fibroin blended and grafted poly (є-caprolactone) nanofibrous matrices for in vivo bone regeneration. Colloids Surf. B 2016, 143, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Naskar, D.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Non-mulberry silk fibroin grafted poly(є-caprolactone)/nano hydroxyapatite nanofibrous scaffold for dual growth factor delivery to promote bone regeneration. J. Colloid Interface Sci. 2016, 472, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Wei, R.-H.; Zhao, S.-Z. Evaluation of corneal cell growth on tissue engineering materials as artificial cornea scaffolds. Int. J. Ophthalmol. 2013, 6, 873–878. [Google Scholar] [PubMed]
- Meller, D.; Pauklin, M.; Thomasen, H.; Westekemper, H.; Steuhl, K.-P. Amniotic membrane transplantation in the human eye. Dtsch. Arztebl. Int. 2011, 108, 243–248. [Google Scholar] [PubMed]
- Liu, J.; Lawrence, B.D.; Liu, A.; Schwab, I.R.; Oliveira, L.A.; Rosenblatt, M.I. Silk fibroin as a biomaterial substrate for corneal epithelial cell sheet generationsf as a biomaterial substrate. Investig. Ophthalmol. Visual. Sci. 2012, 53, 4130–4138. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Dawson, R.A.; Chirila, T.V.; Shadforth, A.; Hogerheyde, T.A.; Edwards, G.A.; Harkin, D.G. Treatment of silk fibroin with poly(ethylene glycol) for the enhancement of corneal epithelial cell growth. J. Funct. Biomater. 2015, 6, 345–366. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.D.; Marchant, J.K.; Pindrus, M.A.; Omenetto, F.G.; Kaplan, D.L. Silk film biomaterials for cornea tissue engineering. Biomaterials 2009, 30, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Madden, P.W.; Lai, J.N.; George, K.A.; Giovenco, T.; Harkin, D.G.; Chirila, T.V. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials 2011, 32, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Bray, L.J.; Suzuki, S.; Harkin, D.G.; Chirila, T.V. Incorporation of exogenous rgd peptide and inter-species blending as strategies for enhancing human corneal limbal epithelial cell growth on bombyx mori silk fibroin membranes. J. Funct. Biomater. 2013, 4, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Nandi, S.; Naskar, D.; Guha, R.; Chowdhury, S.; Pradhan, N.; Kundu, S.C.; Konar, A. Non-mulberry silk fibroin biomaterial for corneal regeneration. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Dong, P.; Huang, D.; Mei, L.; Xia, Y.; Wang, Z.; Pan, X.; Li, G.; Wu, C. Fabrication and characterization of silk fibroin-coated liposomes for ocular drug delivery. Eur. J. Pharm. Biopharm. 2015, 91, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, X.; Ding, F.; Zhang, P.; Liu, J.; Gu, X. Biocompatibility evaluation of silk fibroin with peripheral nerve tissues and cells in vitro. Biomaterials 2007, 28, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, V.; Stahl, K.; Gomis-Perez, C.; Toffanin, S.; Sagnella, A.; Torp, R.; Kaplan, D.L.; Ruani, G.; Omenetto, F.G.; Zamboni, R. Biofunctional silk/neuron interfaces. Adv. Funct. Mater. 2012, 22, 1871–1884. [Google Scholar] [CrossRef]
- Gu, Y.; Zhu, J.; Xue, C.; Li, Z.; Ding, F.; Yang, Y.; Gu, X. Chitosan/silk fibroin-based, schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials 2014, 35, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tang-Schomer, M.D.; Huang, W.; Xia, X.X.; Weiss, A.S.; Kaplan, D.L. Charge-tunable autoclaved silk-tropoelastin protein alloys that control neuron cell responses. Adv. Funct. Mater. 2013, 23, 3875–3884. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Prabhakaran, M.P.; Hu, J.; Chen, M.; Besenbacher, F.; Ramakrishna, S. Coaxial electrospun poly (lactic acid)/silk fibroin nanofibers incorporated with nerve growth factor support the differentiation of neuronal stem cells. RSC Adv. 2015, 5, 49838–49848. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, K.; Xing, Y.; Yu, Q. Lysine-doped polypyrrole/spider silk protein/poly (L-lactic) acid containing nerve growth factor composite fibers for neural application. Mater. Sci. Eng. C 2015, 56, 564–573. [Google Scholar] [CrossRef] [PubMed]
- White, J.D.; Wang, S.; Weiss, A.S.; Kaplan, D.L. Silk–tropoelastin protein films for nerve guidance. Acta Biomater. 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Lewandowska, K.; Michalska, M.; Walczak, M. Characterization of silk fibroin 3D composites modified by collagen. J. Mol. Liq. 2016, 215, 323–327. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Gomes, A.C.; Cavaco-Paulo, A. Novel silk fibroin/elastin wound dressings. Acta Biomater. 2012, 8, 3049–3060. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Sow, W.T.; Devi, D.; Ng, K.W.; Mandal, B.B.; Cho, N.-J. Silk fibroin–keratin based 3D scaffolds as a dermal substitute for skin tissue engineering. Integr. Biol. 2015, 7, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, F.A.; Ju, H.W.; Lee, J.M.; Moon, B.M.; Park, H.J.; Lee, O.J.; Kim, J.-H.; Kim, D.-K.; Park, C.H. 3D electrospun silk fibroin nanofibers for fabrication of artificial skin. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Chutipakdeevong, J.; Ruktanonchai, U.R.; Supaphol, P. Process optimization of electrospun silk fibroin fiber mat for accelerated wound healing. J. Appl. Polym. Sci. 2013, 130, 3634–3644. [Google Scholar] [CrossRef]
- Sharma, C.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Fabrication of quaternary composite scaffold from silk fibroin, chitosan, gelatin, and alginate for skin regeneration. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Bellas, E.; Seiberg, M.; Garlick, J.; Kaplan, D.L. In vitro 3D full-thickness skin-equivalent tissue model using silk and collagen biomaterials. Macromol. Biosci. 2012, 12, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Yin, Z.; Shen, W.L.; Chen, X.; Heng, B.C.; Zou, X.H.; Ouyang, H.W. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials 2010, 31, 9438–9451. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Kumar, P.S.; Deepthi, S.; Chennazhi, K.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Sahoo, S.; Toh, S.L.; Goh, J.C. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials 2010, 31, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Blum, B.E.; Burgess, A.V. Special segment: Soft tissue matrices—One form of acellular human dermis for use in tendon and ligament repairs in the foot and ankle. Foot Ankle Spec. 2009, 2, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yin, Z.; Chen, J.-L.; Liu, H.-H.; Shen, W.-L.; Fang, Z.; Zhu, T.; Ji, J.; Ouyang, H.-W.; Zou, X.-H. Scleraxis-overexpressed human embryonic stem cell–derived mesenchymal stem cells for tendon tissue engineering with knitted silk-collagen scaffold. Tissue Eng. Part A 2014, 20, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Chen, X.; Chen, J.; Yin, Z.; Heng, B.C.; Chen, W.; Ouyang, H.-W. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials 2010, 31, 7239–7249. [Google Scholar] [CrossRef] [PubMed]
- Naghashzargar, E.; Farè, S.; Catto, V.; Bertoldi, S.; Semnani, D.; Karbasi, S.; Tanzi, M.C. Nano/micro hybrid scaffold of PCL or P3HB nanofibers combined with silk fibroin for tendon and ligament tissue engineering. J. Appl. Biomater. Funct. Mater. 2015, 13, 156–168. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Sahoo, S.; Ng, K.S.; Chen, K.; Toh, S.L.; Goh, J.C.H. Enhanced osteoinductivity and osteoconductivity through hydroxyapatite coating of silk-based tissue-engineered ligament scaffold. J. Biomed. Mater. Res. Part A 2013, 101, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Tuli, R.; Li, W.-J.; Tuan, R.S. Current state of cartilage tissue engineering. Arthritis Res. Ther. 2003, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Q.; Cheng, N.; Tao, X.; Zhang, Z.; Sun, X.; Zhang, Q. Collagen/silk fibroin composite scaffold incorporated with plga microsphere for cartilage repair. Mater. Sci. Eng. C 2016, 61, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ai, C.; Zhan, Z.; Zhang, P.; Wan, F.; Chen, J.; Hao, W.; Wang, Y.; Yao, J.; Shao, Z. Enhanced fibroblast cellular ligamentization process to polyethylene terepthalate artificial ligament by silk fibroin coating. Artif. Organs 2015. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Nguyen, Q.T.; Chen, A.C.; Kaplan, D.L.; Sah, R.L.; Kundu, S.C. Potential of 3-D tissue constructs engineered from bovine chondrocytes/silk fibroin-chitosan for in vitro cartilage tissue engineering. Biomaterials 2011, 32, 5773–5781. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jao, D.; Mou, X.; Hu, X. Tissue Regeneration: A Silk Road. J. Funct. Biomater. 2016, 7, 22. https://doi.org/10.3390/jfb7030022
Jao D, Mou X, Hu X. Tissue Regeneration: A Silk Road. Journal of Functional Biomaterials. 2016; 7(3):22. https://doi.org/10.3390/jfb7030022
Chicago/Turabian StyleJao, Dave, Xiaoyang Mou, and Xiao Hu. 2016. "Tissue Regeneration: A Silk Road" Journal of Functional Biomaterials 7, no. 3: 22. https://doi.org/10.3390/jfb7030022
APA StyleJao, D., Mou, X., & Hu, X. (2016). Tissue Regeneration: A Silk Road. Journal of Functional Biomaterials, 7(3), 22. https://doi.org/10.3390/jfb7030022