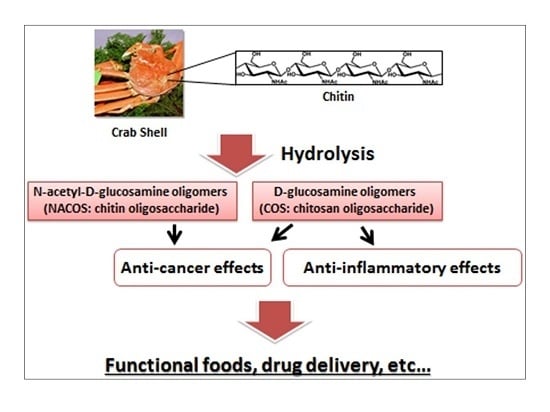
Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides
Abstract
:1. Introduction
2. NACOS, COS, and Their Derivatives as Anti-Cancer Agents
2.1. Anti-Cancer Activities of NACOS and COS
| Preparation | Cells or Model | Major Results | Ref. |
|---|---|---|---|
| COS | HT-29 (in vitro) | Increased QR and GST activities and GSH levels; Inhibited ODC activities and COX-2 expression | [9] |
| COS | HT-29 (in vitro) | Inhibited NO production and iNOS expression | [10] |
| COS | MDA-MB-231 (in vitro) | Reduced MMP-9 secretion and activities | [12] |
| COS | HepG2 (in vitro) | Reduced cells in S-phase and decreased the rate of DNA synthesis; Upregulated p21 and downregulated PCNA, cyclin A and CDK-2 | [13] |
| COS | LLC (in vitro) | Inhibited MMP-9 | [13] |
| COS | HepG2 (in vivo) | Inhibited the tumor growth | [13] |
| COS | LCC (in vivo) | Inhibited the tumor growth and decreased the number of metastatic colonies | [13] |
| NACOS, COS | Meth-A (in vivo) | Enhanced acquired immunity | [14] |
| COS | PC-3, A549 (in vitro) | Suppressed cancer cell growth | [15] |
| CSO-SA | MCF-7, A549, Bel-7402 (in vitro) | Discovered anti-cancer activities of podophyllotoxin loaded on CSO-SA micelles | [16] |
| CSOSA-g-PEI | Hala, MCF-7 (in vitro) | CSOSA-g-PEI/plasmid suppressed tumor growth | [17] |
| CSOAA | FaDu (in vitro) | Showed cytotoxicity. DOX-loaded CSOAA-based nanoparticle was highly uptake | [18] |
| Gal-CSO/ATP | HepG2 (in vitro) | Gal-CSO/ATP nanoparticle showed high cytotoxicity | [19] |
| FA-PEG-COL | OVK18 #2 (in vitro) | FA-PEG-COL nanoparticles accumulated in tumors | [20] |
| FcCOS | – | The release of drug was enhanced in the oxidative condition and low pH | [21] |
2.2. Anti-Cancer Activities of COS Derivatives
3. Anti-Cancer Effects of NACOS and COS Following Oral Administration
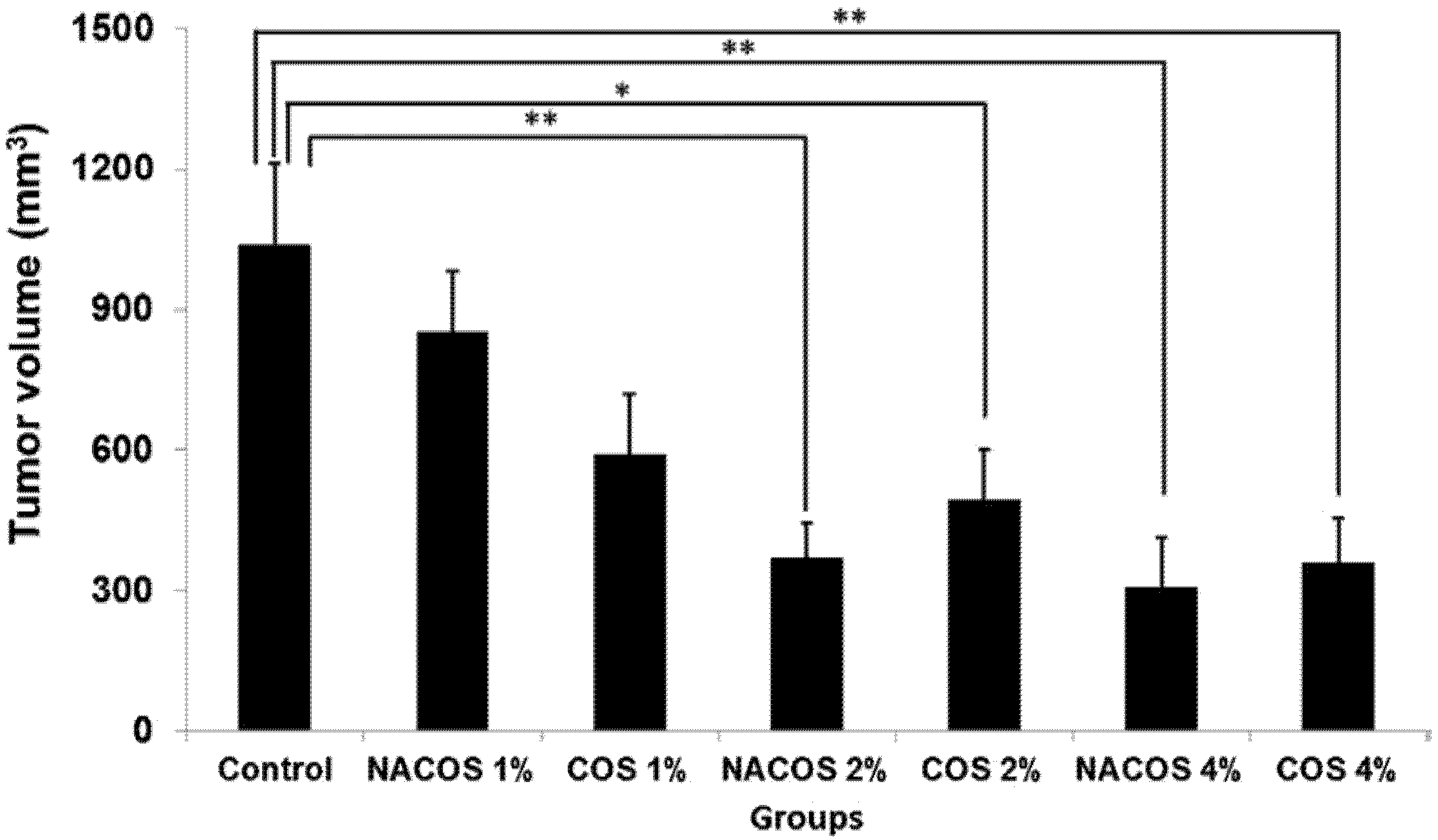
| Cytokines | Control | NACOS 4% | COS 4% |
|---|---|---|---|
| IFN-γ (pg/mL) | 0.6 ± 0.2 | 8.3 ± 0.5 ** | 8.4 ± 0.3 ** |
| IL-12 (pg/mL) | 11.2 ± 1.3 | 25.3 ± 3.5 ** | 23.5 ± 3.0 ** |
| TNF-α (pg/mL) | 13.9 ± 1.6 | 13.4 ± 1.3 | 12.7 ± 0.8 |
4. Anti-Inflammatory Activities of COS
| Cells or Model | Major Results | Ref. |
|---|---|---|
| RAW 264.7 cells (in vitro) | Exposured LPS-induced secration of TNF-α and IL-6; Decreased the LPS-induced secretion of NO | [33] |
| Acute renal failure model (in vivo) | Improved renal function and had antioxidant effects | [34] |
| Paw edema model (in vivo) | Sowed the anti-inflammatory effects according to the dose and MW dependent manner | [35] |
| Spesis model (in vivo) | Attenuated organ dysfunction and improved survival rate | [36] |
| BV2 microglial cells (in vitro) | Attenuated the production of NO and PGE2 by inhibiting iNOS and COX-2 expression; Decreased the expression levels of TNF-α, IL-6 and IL-1β. Suppressed the phosphorylations of JNK and p38MAPK | [37] |
| Asthma model (in vivo) | Reduced the mRNA expression and protein levels of IL-4, IL-5, IL-13 and TNF-α | [38] |
| Obese model (in vivo) | Reduced the weight gain by involving inflammatory response | [39] |
| L9 microglial cells (in vitro) | Abrogated NO production. Decreased the phosphorylation of p38 MAPK and inhibited activations of NF-κB and AP-1 | [40] |
| Autoimmune anterior uveitis model (in vitro) | Attenuated the clinical score; Decreased the inflammation mediators such as TNF-α, iNOS, MCP-1, RANTES | [41] |
| Endothelial cells (in vitro) | Suppressed the activation of NF-κB pathways | [42] |
| Endothelial cells (in vitro) | Reduced mRNA expression of E-selectin and ICAM-1 through the inhibition of p38 MAPK/ERK1/2 and NF-κB cascade | [43] |
5. Anti-Inflammatory Effects of COS for Inflammatory Bowel Disease
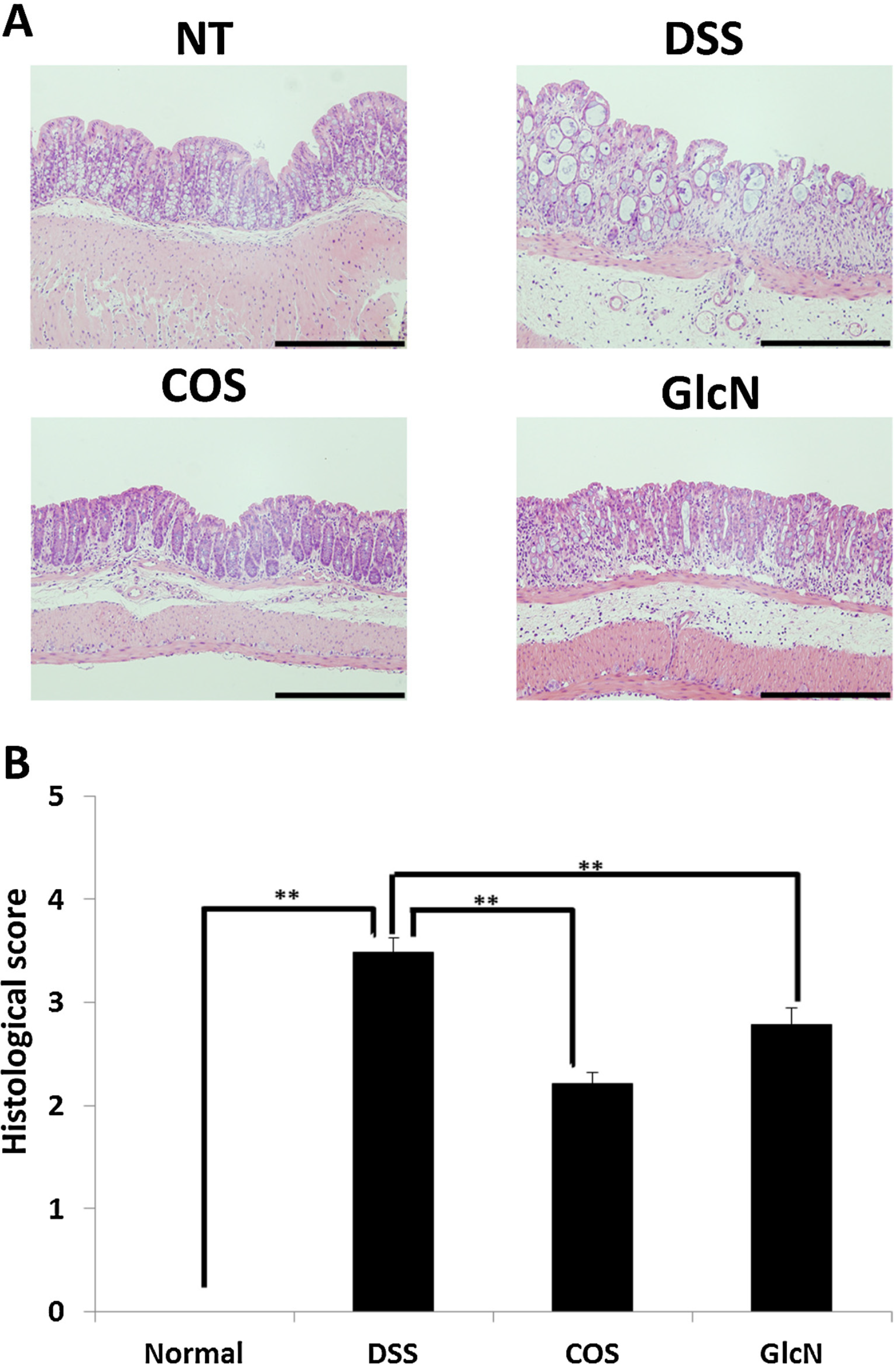
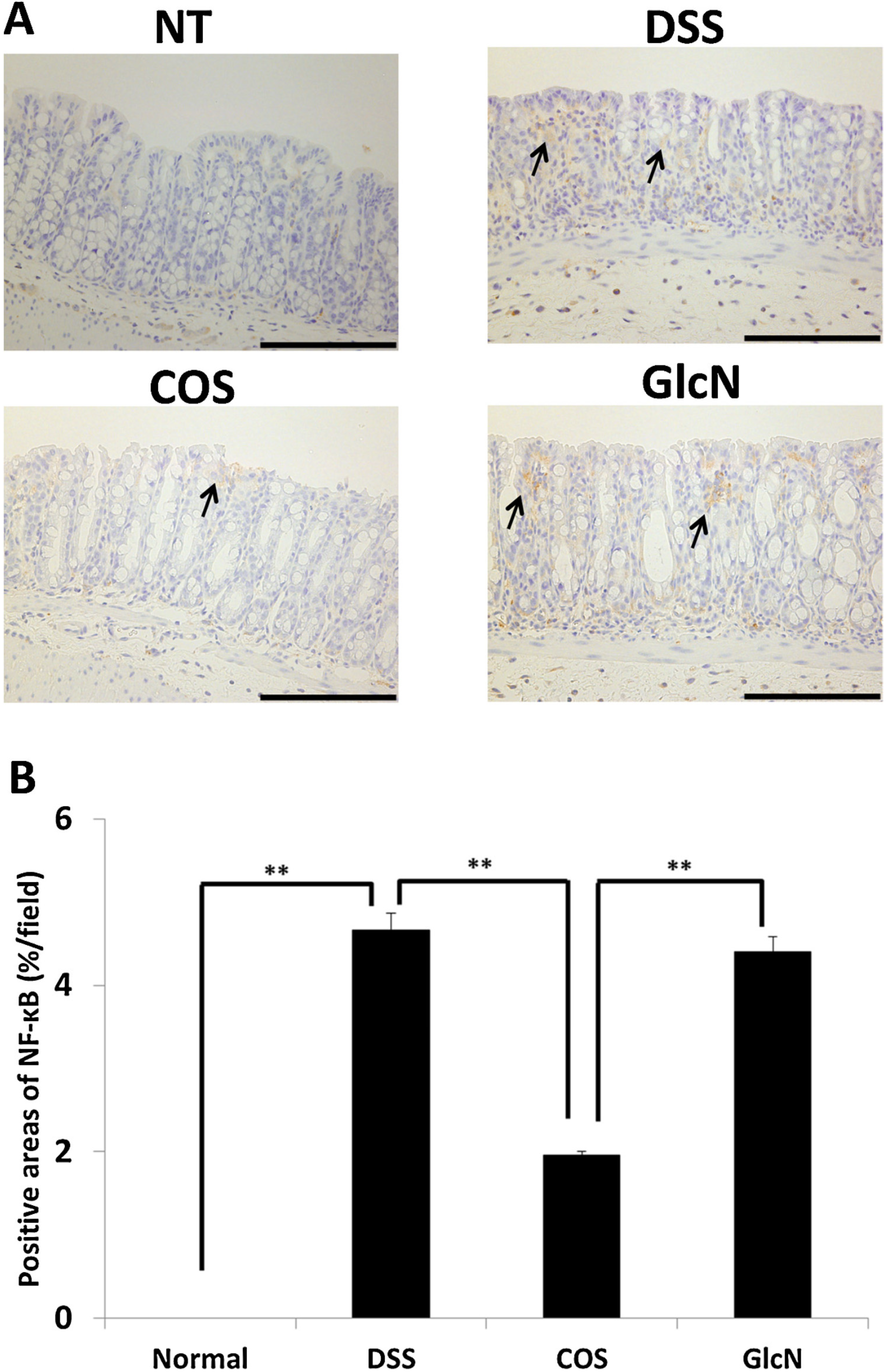
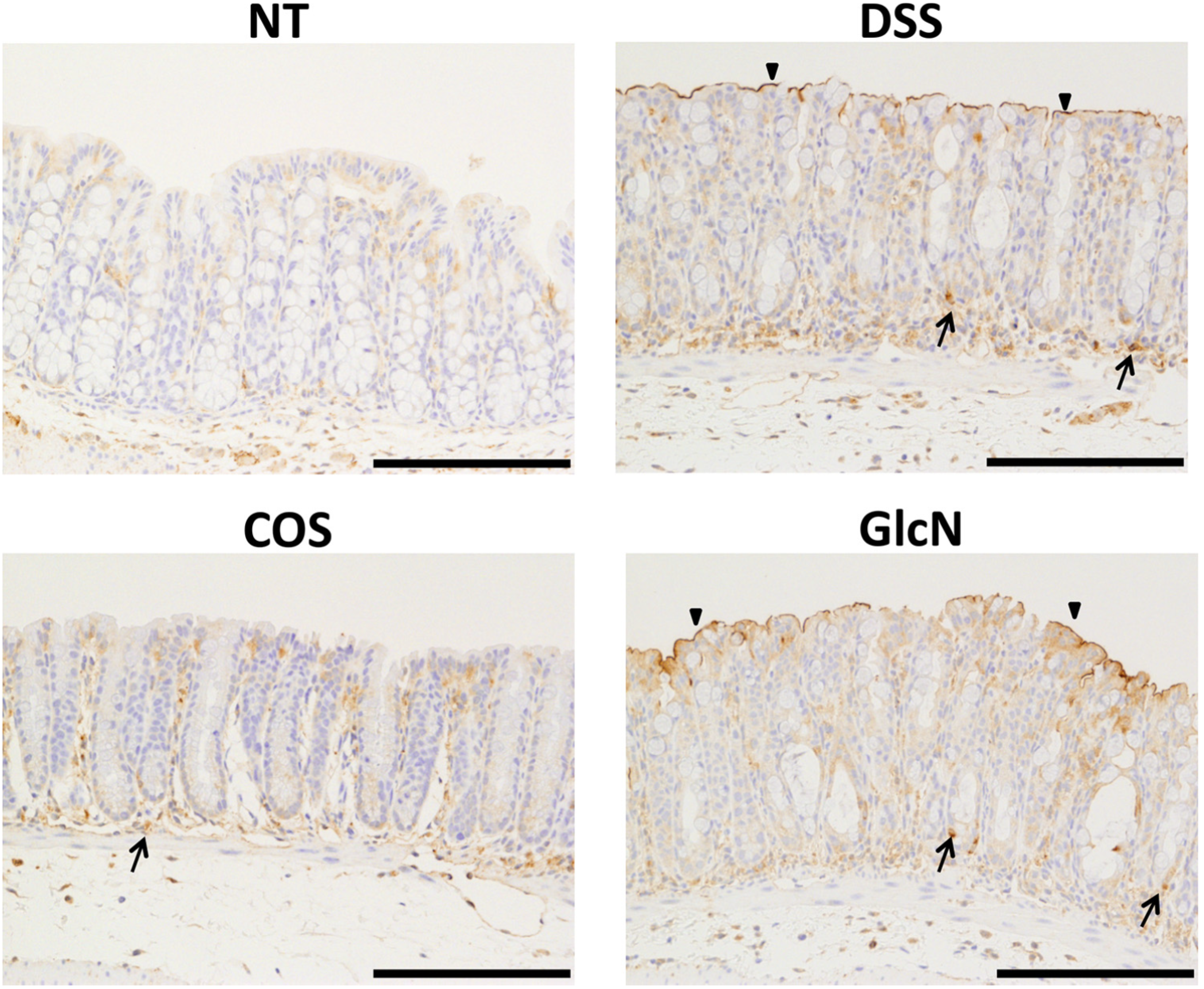
6. Next Step to Use NACOS, COS and Its Derivatives for Patient

Author Contributions
Conflicts of Interest
References
- Muzzarelli, R.A.A. Chitin Nanostructures in Living Organisms. In Chitin: Formation and Diagenesis; Gupta, N., Ed.; Springer: Dordrecht, The Netherlands, 2011; Volume 34, pp. 1–34. [Google Scholar]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and Biomedical Applications of Chitin and Chitosan Nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef]
- Kurita, K. Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Pillai, K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, Y.J.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and its derivatives: Preparation and biological applications. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Shahidi, F.; Kim, S.K. Preparation of chitin and chitosan oligomers and their applications in physiological functional foods. Food Rev. Int. 2000, 16, 159–176. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, M.M. Applications of chitin and its derivatives in biological medicine. Int. J. Mol. Sci. 2010, 11, 5152–5164. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.S.; Kim, M.K.; Shon, Y.H. Chemopreventive effect of chitosan oligosaccharide against colon carcinogenesis. J. Microbiol. Biotechnol. 2007, 17, 1546–1549. [Google Scholar] [PubMed]
- Nam, K.S.; Kim, M.K.; Shon, Y.H. Inhibition of proinflammatory cytokine-induced invasiveness of HT-29 cells by chitosan oligosaccharide. J. Microbiol. Biotechnol. 2007, 17, 2042–2045. [Google Scholar] [PubMed]
- Quan, H.; Zhu, F.; Han, X.; Xu, Z.; Zhao, Y.; Miao, Z. Mechanism of anti-angiogenic activities of chitooligosaccharides may be through inhibiting heparanase activity. Med. Hypotheses 2009, 73, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.S.; Shon, Y.H. Suppression of metastasis of human breast cancer cells by chitosan oligosaccharides. J. Microbiol. Biotechnol. 2009, 19, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.T.; Chen, M.H.; Chan, H.Y.; Jeng, J.H.; Wang, Y.J. Inhibitory effects of chitooligosaccharides on tumor growth and metastasis. Food Chem. Toxicol. 2009, 47, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Tokoro, A.; Tatewaki, N.; Mikami, T.; Suzuki, S.; Suzuki, M. Effect of NACOS-6 on lymphokine-activated killer cell (LAK) activity. Biotherapy 1989, 3, 51–54. [Google Scholar]
- Park, J.K.; Chung, M.J.; Choi, H.N.; Park, Y.I. Effects of the molecular weight and the degree of deacetylation of chitosan oligosaccharides on antitumor activity. Int. J. Mol. Sci. 2011, 12, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, X.; Jiang, X.H.; Hu, F.Q.; Du, Y.Z.; Zhu, Q.F.; Jin, C.S. In vitro antitumour activity of stearic acid-g-chitosan oligosaccharide polymeric micelles loading podophyllotoxin. J. Microencapsul. 2012, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.Q.; Chen, W.W.; Zhao, M.D.; Yuan, H.; Du, Y.Z. Effective antitumor gene therapy delivered by polyethylenimine-conjugated stearic acid-g-chitosan oligosaccharide micelles. Gene Ther. 2013, 20, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Termsarasab, U.; Cho, H.J.; Kim, D.H.; Chong, S.; Chung, S.J.; Shim, C.K.; Moon, H.T.; Kim, D.D. Chitosan oligosaccharide-arachidic acid-based nanoparticles for anti-cancer drug delivery. Int. J. Pharm. 2013, 441, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.L.; Du, Y.Z.; Yu, R.S.; Liu, P.; Shi, D.; Chen, Y.; Wang, Y.; Huang, F.F. Galactosylated chitosan oligosaccharide nanoparticles for hepatocellular carcinoma cell-targeted delivery of adenosine triphosphate. Int. J. Mol. Sci. 2013, 14, 15755–15766. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.; Yawata, T.; Honke, K. Efficient siRNA delivery and tumor accumulation mediated by ionically cross-linked folic acid-poly(ethylene glycol)-chitosan oligosaccharide lactate nanoparticles: For the potential targeted ovarian cancer gene therapy. Eur. J. Pharm. Sci. 2014, 52, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, L.; Li, Y.K.; Wang, C.Q. Oxidation and pH responsive nanoparticles based on ferrocene-modified chitosan oligosaccharide for 5-fluorouracil delivery. Carbohydr. Polym. 2014, 114, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Azuma, K.; Kurozumi, S.; Kiyose, M.; Osaki, T.; Tsuka, T.; Itoh, N.; Imagawa, T.; Minami, S.; Sato, K.; Okamoto, Y. Anti-tumor properties of orally administered glucosamine and N-acetyl-d-glucosamine oligomers in a mouse model. Carbohydr. Polym. 2014, 111, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Watanabe, T.; Suzuki, S.; Suzuki, M. Effect of N-acetylchitohexaose against Candida albicans infection of tumor-bearing mice. Microbiol. Immunol. 1990, 34, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kimura, Y. Antitumor effects of various low-molecular-weight chitosans are due to increased natural killer activity of intestinal intraepithelial lymphocytes in sarcoma 180-bearing mice. J. Nutr. 2004, 134, 945–950. [Google Scholar] [PubMed]
- Kimura, Y.; Okuda, H. Prevention by chitosan of myelotoxicity, gastrointestinal toxicity and immunocompetent organic toxicity induced by 5-fluorouracil without loss of antitumor activity in mice. Jpn. J. Cancer Res. 1999, 90, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, D.; Liu, H.; Yang, Q.; Yao, K.; Wang, X.; Wang, L.; Yang, X. Effect of low molecular weight chitosans on drug permeation through mouse skin: 1.Transdermal delivery of baicalin. J. Pharm. Sci. 2010, 99, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, S.A.; Beltrán, C.J.; Aguirre, I.M.; Silva, P.; Peralta, A.L.; Malinarich, F.; Hermoso, M.A. Toll-like receptors are key participants in innate immune response. Biol. Res. 2007, 40, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.A.; Hartl, D.; Liu, W.; Lee, C.G.; Elias, J.A. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J. Immunol. 2009, 181, 4279–4286. [Google Scholar] [CrossRef]
- Akira, S. Toll-like receptors: Lessons from knockout mice. Biochem. Soc. Trans. 2000, 28, 551–556. [Google Scholar] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 24, 783–801. [Google Scholar] [CrossRef]
- Kan, K. Therapeutic effect of a chitin oligosaccharide mixture by per os administration on human cancer. J. Chitin Chitosan Sci. 2014, 2, 205–208. [Google Scholar] [CrossRef]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Im, S.Y.; Kim, Y.H. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 358, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Kim, H.W.; Im, S.Y.; Lee, J.H.; Kim, Y.H. Effects of chitosan oligosaccharide (COS) on the glycerol-induced acute renal failure in vitro and in vivo. Food Chem. Toxicol. 2008, 46, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Spindola, H.; de Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-inflammatory activity of chitooligosaccharides in vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Bai, X.F.; Du, Y.G. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011, 11, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Bak, S.S.; Kim, S.K. Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. Int. J. Biol. Macromol. 2011, 49, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.J.; Park, J.K.; Park, Y.I. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int. Immunopharmacol. 2012, 12, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H.; Yang, H.P.; Chun, H.S. Chitooligosaccharide ameliorates diet-induced obesity in mice and affects adipose gene expression involved in adipogenesis and inflammation. Nutr. Res. 2012, 32, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Ma, P.; Xu, Q.S.; Bai, Q.H.; Gu, J.G.; Xi, H.; Du, Y.G.; Yu, C. Chitosan oligosaccharides suppress production of nitric oxide in lipopolysaccharide-induced N9 murine microglial cells in vitro. Glycoconj. J. 2012, 29, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Fang, I.M.; Yang, C.H.; Yang, C.M. Chitosan oligosaccharides attenuate ocular inflammation in rats with experimental autoimmune anterior uveitis. Mediat. Inflamm. 2014, 2014, 827–847. [Google Scholar]
- Li, Y.; Liu, H.; Xu, Q.S.; Du, Y.G.; Xu, J. Chitosan oligosaccharides block LPS-induced O-GlcNAcylation of NF-κB and endothelial inflammatory response. Carbohydr. Polym. 2014, 99, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Q.; Wei, P.; Cheng, L.; Peng, Q.; Li, S.; Yin, H.; Du, Y. Chitosan oligosaccharides downregulate the expression of E-selectin and ICAM-1 induced by LPS in endothelial cells by inhibiting MAP kinase signaling. Int. J. Mol. Med. 2014, 33, 392–400. [Google Scholar] [PubMed]
- Morrison, G.; Headon, B.; Gibson, P. Update in inflammatory bowel disease. Aust. Fam. Phys. 2009, 38, 956–961. [Google Scholar]
- Goh, K.; Xiao, S.D. Inflammatory bowel disease: A survey of the epidemiology in Asia. J. Dig. Dis. 2009, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.J.; DeMeo, M.T.; Keshavarzian, A.; Hamaker, B.R. Influence of dietary fiber on inflammatory bowel disease and colon cancer: Importance of fermentation pattern. Nutr. Rev. 2007, 65, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Kurozumi, S.; Kiyose, M.; Tsuka, T.; Murahata, Y.; Imagawa, T.; Itoh, N.; Minami, S.; Sato, K.; Okamoto, Y. Anti-inflammatory effects of orally administered glucosamine oligomer in an experimental model of inflammatory bowel disease. Carbohydr. Polym. 2015, 115, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Karrasch, T.; Jobin, T. NF-κB and the intestine: Friend or foe? Inflamm. Bowel. Dis. 2008, 14, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Elson, C.O.; Cong, Y.; McCracken, V.J.; Dimmitt, R.A.; Lorenz, R.G.; Weaver, C.T. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol. Rev. 2005, 206, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Grisham, M.B.; Pavlick, K.P.; Laroux, F.S.; Hoffman, J.; Bharwani, S.; Wolf, R.E. Nitric oxide and chronic gut inflammation: Controversies in inflammatory bowel disease. J. Investig. Med. 2002, 50, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Araujo, L.F.; Soeiro, A.M.; Fernandes, J.L.; Serrano, C.V., Jr. Cardiovascular events: A class effect by COX-2 inhibitors. Arq. Bras. Cardiol. 2005, 85, 222–229. [Google Scholar] [PubMed]
- Cross, R.K.; Wilson, K.T. Nitric oxide in inflammatory bowel disease. Inflamm. Bowel. Dis. 2003, 9, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Hogaboam, C.M.; Jacobson, K.; Collins, S.M.; Blennerhassett, M.G. The selective beneficial effects of nitric oxide inhibition in experimental colitis. Am. J. Physiol. 1995, 268, G673–G684. [Google Scholar] [PubMed]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Cárdeno, A.; de la Lastra, C.A. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem. Pharmacol. 2011, 82, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.R.; Villegas, I.; Sánchez-Hidalgo, M.; de la Lastra, C.A. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br. J. Pharmacol. 2006, 147, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Deng, H.; Du, Y.; Shi, X.; Wang, Q. Emerging chitin and chitosan nanofibrous materials for biomedical applications. Nanoscale 2014, 6, 9477–9493. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Gao, J.; Wang, L.; Zeng, L.; Liu, Y. Safety evaluation of short-term exposure to chitooligomers from enzymic preparation. Food Chem. Toxicol. 2006, 44, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Wang, Y.; Wang, X.; Wang, Q.; Chen, M. Advances in self-assembled chitosan nanomaterials for drug delivery. Biotechnol. Adv. 2014, 32, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Du, Y.; Fan, L.; Liu, H.; Wang, X. Structures and properties of chitosan-starch-sodium benzoate blend films. J. Wuhan Univ. (Nat. Sci. Ed.) 2003, 49, 725–730. [Google Scholar]
- Wang, Q.; Zhang, N.; Hu, X.; Yang, J.; Du, Y. Chitosan/polyethylene glycol blend fibers and their properties for drug controlled release. J. Biomed. Mater. Res. A 2008, 85, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dong, Z.; Duand, Y.; Kennedy, J.F. Controlled release of ciprofloxacin hydrochloride from chitosan/polyethylene glycol blend films. Carbohydr. Polym. 2007, 69, 336–343. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, N.; Hu, X.; Yang, J.; Du, Y. Chitosan/starch fibers and their properties for drug controlled release. Eur. J. Pharm. Biopharm. 2007, 66, 398–404. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides. J. Funct. Biomater. 2015, 6, 33-49. https://doi.org/10.3390/jfb6010033
Azuma K, Osaki T, Minami S, Okamoto Y. Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides. Journal of Functional Biomaterials. 2015; 6(1):33-49. https://doi.org/10.3390/jfb6010033
Chicago/Turabian StyleAzuma, Kazuo, Tomohiro Osaki, Saburo Minami, and Yoshiharu Okamoto. 2015. "Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides" Journal of Functional Biomaterials 6, no. 1: 33-49. https://doi.org/10.3390/jfb6010033
APA StyleAzuma, K., Osaki, T., Minami, S., & Okamoto, Y. (2015). Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides. Journal of Functional Biomaterials, 6(1), 33-49. https://doi.org/10.3390/jfb6010033