Nanotherapy Targeting miR-10b Improves Survival in Orthotopic Glioblastoma Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Therapeutic Synthesis
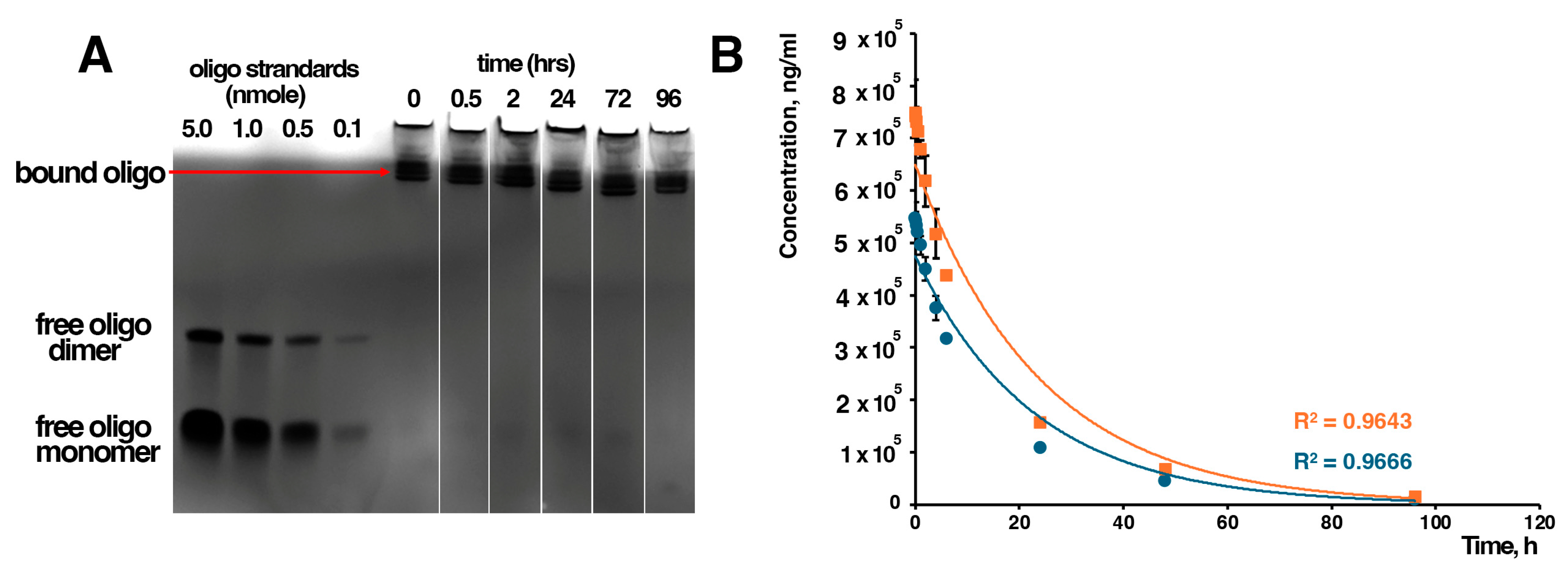
2.3. Analysis of Plasma Stability
2.4. Pharmacokinetics Studies
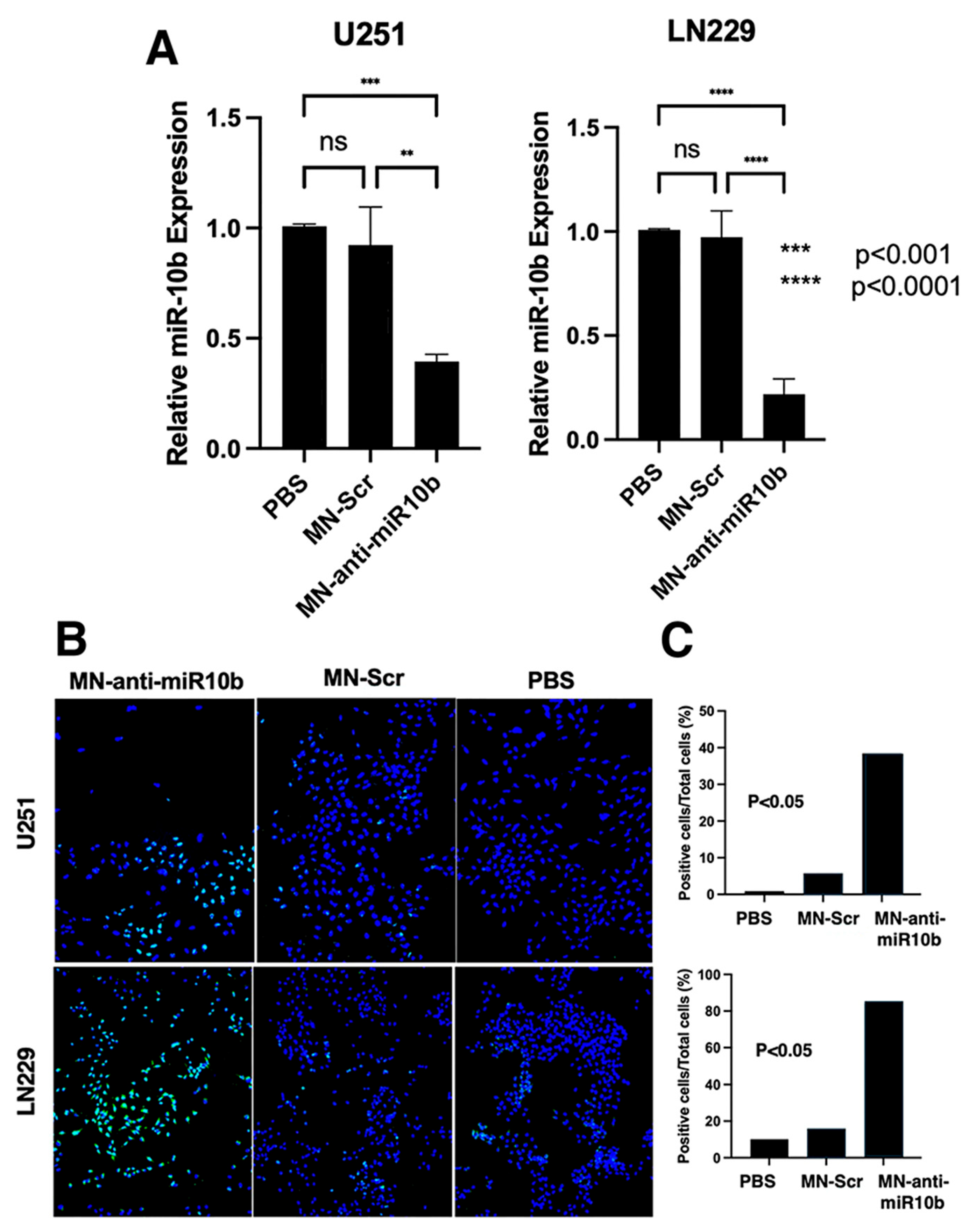
2.5. Cell Treatment and Real-Time Quantitative RT-qPCR
2.6. In Vitro Apoptosis Assays
2.7. Orthotopic Tumor Implantation
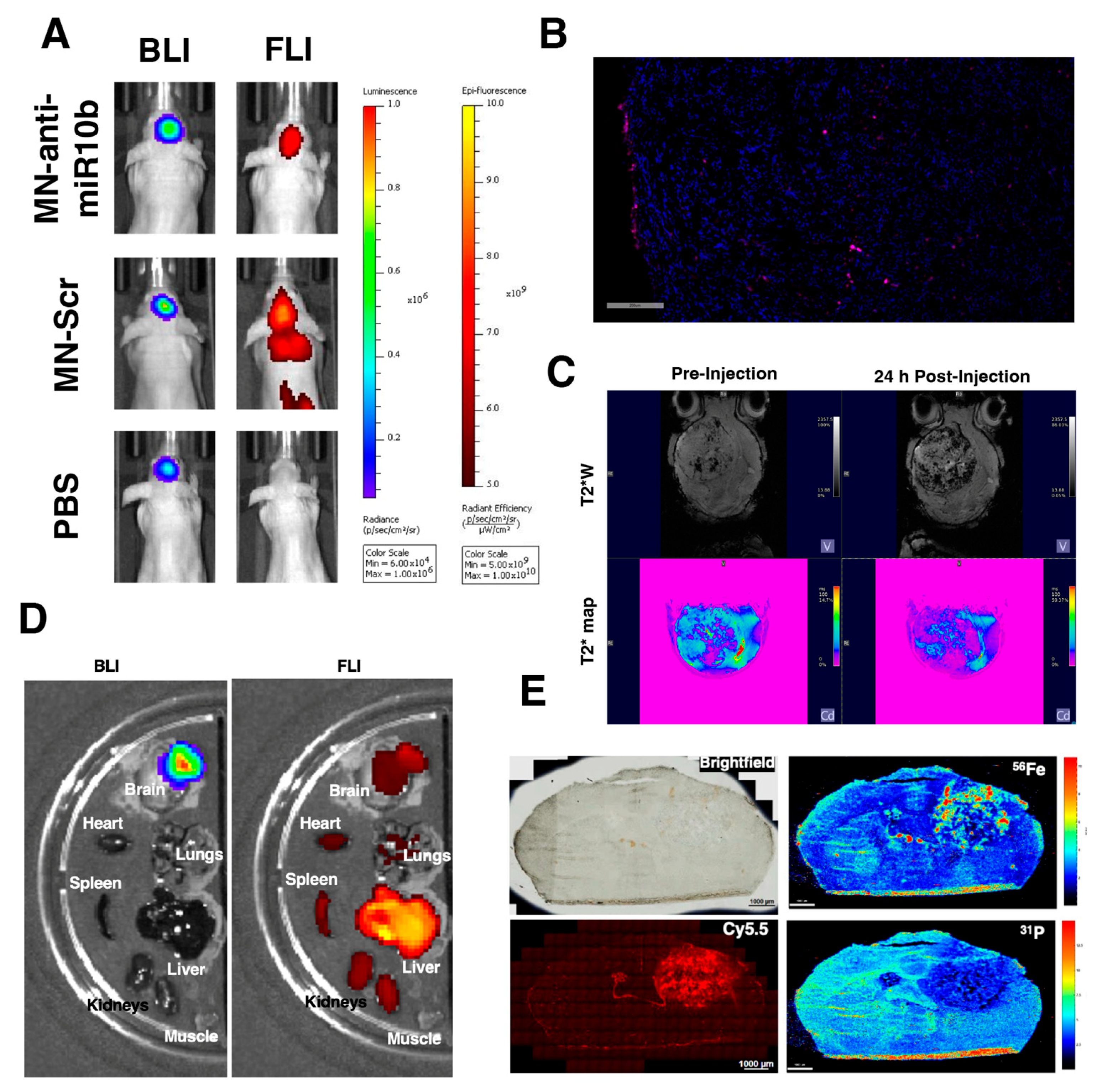
2.8. In Vivo Imaging of Tumor-Bearing Animals
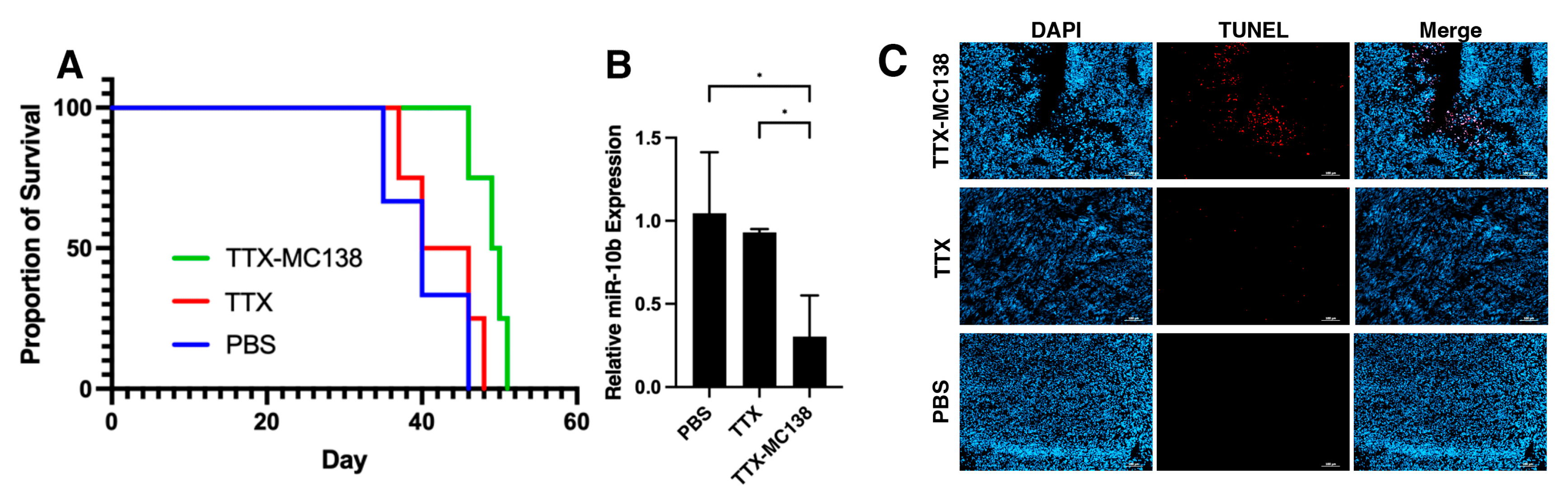
2.9. In Vivo Therapeutic Studies in Orthotopic GBM Models
2.10. RNA Isolation and Gene Expression Analysis
2.11. Laser Ablation Inductively Coupled Plasma Mass Spectrometry
2.12. Ex Vivo Fluorescence and Light Microscopy
2.13. Ex Vivo TUNEL Assay
2.14. Statistical Analysis
3. Results
3.1. Stability and Pharmacokinetics
3.2. In Vitro Studies with Established and Patient-Derived Human GBM Cell Lines
3.3. MN-Anti-miR10b Accumulates in Orthotopic GBM Tumors In Vivo
3.4. MN-Anti-miR10b Improves Survival in Orthotopic Models of Glioblastoma
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mondragon-Soto, M.; Rodriguez-Hernandez, L.A.; Moreno Jimenez, S.; Gomez Amador, J.L.; Gutierrez-Aceves, A.; Montano-Tello, H.; Reyes-Moreno, I.; Santos-Zambrano, J.; Castro-Martinez, E.; Gonzalez-Aguilar, A. Clinical, Therapeutic, and Prognostic Experience in Patients With Glioblastoma. Cureus 2022, 14, e29856. [Google Scholar] [CrossRef]
- Roda, D.; Veiga, P.; Melo, J.B.; Carreira, I.M.; Ribeiro, I.P. Principles in the Management of Glioblastoma. Genes 2024, 15, 501. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018, 20, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Gabriely, G.; Yi, M.; Narayan, R.S.; Niers, J.M.; Wurdinger, T.; Imitola, J.; Ligon, K.L.; Kesari, S.; Esau, C.; Stephens, R.M.; et al. Human glioma growth is controlled by microRNA-10b. Cancer Res. 2011, 71, 3563–3572. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yan, W.; Wang, Y.; Sun, G.; Luo, H.; Zhang, J.; Wang, X.; You, Y.; Yang, Z.; Liu, N. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011, 1389, 9–18. [Google Scholar] [CrossRef]
- Sasayama, T.; Nishihara, M.; Kondoh, T.; Hosoda, K.; Kohmura, E. MicroRNA-10b is overexpressed in malignant glioma and associated with tumor invasive factors, uPAR and RhoC. Int. J. Cancer 2009, 125, 1407–1413. [Google Scholar] [CrossRef]
- Guessous, F.; Alvarado-Velez, M.; Marcinkiewicz, L.; Zhang, Y.; Kim, J.; Heister, S.; Kefas, B.; Godlewski, J.; Schiff, D.; Purow, B.; et al. Oncogenic effects of miR-10b in glioblastoma stem cells. J. Neurooncol. 2013, 112, 153–163. [Google Scholar] [CrossRef]
- Gabriely, G.; Teplyuk, N.M.; Krichevsky, A.M. Context effect: microRNA-10b in cancer cell proliferation, spread and death. Autophagy 2011, 7, 1384–1386. [Google Scholar] [CrossRef]
- Lin, J.; Teo, S.; Lam, D.H.; Jeyaseelan, K.; Wang, S. MicroRNA-10b pleiotropically regulates invasion, angiogenicity and apoptosis of tumor cells resembling mesenchymal subtype of glioblastoma multiforme. Cell Death Dis. 2012, 3, e398. [Google Scholar] [CrossRef]
- Ma, C.; Wei, F.; Xia, H.; Liu, H.; Dong, X.; Zhang, Y.; Luo, Q.; Liu, Y.; Li, Y. MicroRNA-10b mediates TGF-beta1-regulated glioblastoma proliferation, migration and epithelial-mesenchymal transition. Int. J. Oncol. 2017, 50, 1739–1748. [Google Scholar] [CrossRef]
- Teplyuk, N.M.; Uhlmann, E.J.; Gabriely, G.; Volfovsky, N.; Wang, Y.; Teng, J.; Karmali, P.; Marcusson, E.; Peter, M.; Mohan, A.; et al. Therapeutic potential of targeting microRNA-10b in established intracranial glioblastoma: First steps toward the clinic. EMBO Mol. Med. 2016, 8, 268–287. [Google Scholar] [CrossRef]
- Teplyuk, N.M.; Uhlmann, E.J.; Wong, A.H.; Karmali, P.; Basu, M.; Gabriely, G.; Jain, A.; Wang, Y.; Chiocca, E.A.; Stephens, R.; et al. MicroRNA-10b inhibition reduces E2F1-mediated transcription and miR-15/16 activity in glioblastoma. Oncotarget 2015, 6, 3770–3783. [Google Scholar] [CrossRef]
- El Fatimy, R.; Subramanian, S.; Uhlmann, E.J.; Krichevsky, A.M. Genome Editing Reveals Glioblastoma Addiction to MicroRNA-10b. Mol. Ther. 2017, 25, 368–378. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, A.X.; Gartrell, R.D.; Silverman, A.M.; Aparicio, L.; Chu, T.; Bordbar, D.; Shan, D.; Samanamud, J.; Mahajan, A.; et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 2019, 25, 462–469. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, Y.W.; Brewer, G.; Jing, Q. MicroRNA degradation and turnover: Regulating the regulators. Wiley Interdiscip. Rev. RNA 2012, 3, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, G.; Zhuravlev, E.; Shender, V.; Nushtaeva, A.; Balakhonova, E.; Mozhaeva, E.; Kasakin, M.; Koval, V.; Lomzov, A.; Pavlyukov, M.; et al. Nucleotide Modifications Decrease Innate Immune Response Induced by Synthetic Analogs of snRNAs and snoRNAs. Genes 2018, 9, 531. [Google Scholar] [CrossRef]
- Paliwal, S.R.; Paliwal, R.; Vyas, S.P. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv. 2015, 22, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Lu, M. RNA Interference-Induced Innate Immunity, Off-Target Effect, or Immune Adjuvant? Front. Immunol. 2017, 8, 331. [Google Scholar] [CrossRef]
- Malhotra, M.; Sekar, T.V.; Ananta, J.S.; Devulapally, R.; Afjei, R.; Babikir, H.A.; Paulmurugan, R.; Massoud, T.F. Targeted nanoparticle delivery of therapeutic antisense microRNAs presensitizes glioblastoma cells to lower effective doses of temozolomide in vitro and in a mouse model. Oncotarget 2018, 9, 21478–21494. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Lachelt, U.; Wagner, E. Dynamic carriers for therapeutic RNA delivery. Proc. Natl. Acad. Sci. USA 2024, 121, e2307799120. [Google Scholar] [CrossRef]
- Ananta, J.S.; Paulmurugan, R.; Massoud, T.F. Nanoparticle-Delivered Antisense MicroRNA-21 Enhances the Effects of Temozolomide on Glioblastoma Cells. Mol. Pharm. 2015, 12, 4509–4517. [Google Scholar] [CrossRef]
- Ananta, J.S.; Paulmurugan, R.; Massoud, T.F. Tailored Nanoparticle Codelivery of antimiR-21 and antimiR-10b Augments Glioblastoma Cell Kill by Temozolomide: Toward a “Personalized” Anti-microRNA Therapy. Mol. Pharm. 2016, 13, 3164–3175. [Google Scholar] [CrossRef]
- Kucukturkmen, B.; Bozkir, A. Development and characterization of cationic solid lipid nanoparticles for co-delivery of pemetrexed and miR-21 antisense oligonucleotide to glioblastoma cells. Drug Dev. Ind. Pharm. 2018, 44, 306–315. [Google Scholar] [CrossRef]
- Kucukturkmen, B.; Devrim, B.; Saka, O.M.; Yilmaz, S.; Arsoy, T.; Bozkir, A. Co-delivery of pemetrexed and miR-21 antisense oligonucleotide by lipid-polymer hybrid nanoparticles and effects on glioblastoma cells. Drug Dev. Ind. Pharm. 2017, 43, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rabinovsky, R.; Deforzh, E.; Kobayashi, A.; Kuzkina, A.; Varghese, J.F.; Rai, D.; Korecka, J.A.; Khurana, V.; Murugaiyan, G.; et al. Lipid nanoparticle formulation for gene editing and RNA-based therapies for glioblastoma. Neuro Oncol. 2025, noaf162. [Google Scholar] [CrossRef]
- Tao, J.; Wang, Q.; Mendez-Dorantes, C.; Burns, K.H.; Chiarle, R. Frequency and mechanisms of LINE-1 retrotransposon insertions at CRISPR/Cas9 sites. Nat. Commun. 2022, 13, 3685. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Kim, B.; Robertson, N.; Mondal, S.K.; Medarova, Z.; Moore, A. Co-administration of temozolomide (TMZ) and the experimental therapeutic targeting miR-10b, profoundly affects the tumorigenic phenotype of human glioblastoma cells. Front. Mol. Biosci. 2023, 10, 1179343. [Google Scholar] [CrossRef] [PubMed]
- Savan, N.A.; Saavedra, P.V.; Halim, A.; Yuzbasiyan-Gurkan, V.; Wang, P.; Yoo, B.; Kiupel, M.; Sempere, L.; Medarova, Z.; Moore, A. Case report: MicroRNA-10b as a therapeutic target in feline metastatic mammary carcinoma and its implications for human clinical trials. Front. Oncol. 2022, 12, 959630. [Google Scholar] [CrossRef]
- Yigit, M.V.; Ghosh, S.K.; Kumar, M.; Petkova, V.; Kavishwar, A.; Moore, A.; Medarova, Z. Context-dependent differences in miR-10b breast oncogenesis can be targeted for the prevention and arrest of lymph node metastasis. Oncogene 2013, 32, 1530–1538. [Google Scholar] [CrossRef]
- Yoo, B.; Kavishwar, A.; Ross, A.; Wang, P.; Tabassum, D.P.; Polyak, K.; Barteneva, N.; Petkova, V.; Pantazopoulos, P.; Tena, A.; et al. Combining miR-10b-Targeted Nanotherapy with Low-Dose Doxorubicin Elicits Durable Regressions of Metastatic Breast Cancer. Cancer Res. 2015, 75, 4407–4415. [Google Scholar] [CrossRef]
- Yoo, B.; Kavishwar, A.; Wang, P.; Ross, A.; Pantazopoulos, P.; Dudley, M.; Moore, A.; Medarova, Z. Therapy targeted to the metastatic niche is effective in a model of stage IV breast cancer. Sci. Rep. 2017, 7, 45060. [Google Scholar] [CrossRef]
- deCarvalho, A.C.; Kim, H.; Poisson, L.M.; Winn, M.E.; Mueller, C.; Cherba, D.; Koeman, J.; Seth, S.; Protopopov, A.; Felicella, M.; et al. Discordant inheritance of chromosomal and extrachromosomal DNA elements contributes to dynamic disease evolution in glioblastoma. Nat. Genet. 2018, 50, 708–717. [Google Scholar] [CrossRef]
- Irtenkauf, S.M.; Sobiechowski, S.; Hasselbach, L.A.; Nelson, K.K.; Transou, A.D.; Carlton, E.T.; Mikkelsen, T.; deCarvalho, A.C. Optimization of Glioblastoma Mouse Orthotopic Xenograft Models for Translational Research. Comp. Med. 2017, 67, 300–314. [Google Scholar]
- Haim, O.; Agur, A.; Efrat, O.T.; Valdes, P.; Ram, Z.; Grossman, R. The clinical significance of radiological changes associated with gliadel implantation in patients with recurrent high grade glioma. Sci. Rep. 2023, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Islam, T.; Wolf, G. The pharmacokinetics of the lymphotropic nanoparticle MRI contrast agent ferumoxtran-10. Cancer Biomark. 2009, 5, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Marecos, E.; Bogdanov, A., Jr.; Weissleder, R. Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology 2000, 214, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Medarova, Z.; Pantazopoulos, P.; Dai, G.; Moore, A. Novel membrane-permeable contrast agent for brain tumor detection by MRI. Magn. Reson. Med. 2010, 63, 617–624. [Google Scholar] [CrossRef]
- Shah, M.Y.; Calin, G.A. MicroRNAs as therapeutic targets in human cancers. Wiley Interdiscip. Rev. RNA 2014, 5, 537–548. [Google Scholar] [CrossRef]
- Ma, L.; Teruya-Feldstein, J.; Weinberg, R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007, 449, 682–688. [Google Scholar] [CrossRef]
- Ciafre, S.A.; Galardi, S.; Mangiola, A.; Ferracin, M.; Liu, C.G.; Sabatino, G.; Negrini, M.; Maira, G.; Croce, C.M.; Farace, M.G. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem. Biophys. Res. Commun. 2005, 334, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhao, X.; Ming, J.; Liu, X.; Liu, D.; Jiang, C. Stepwise detection and evaluation reveal miR-10b and miR-222 as a remarkable prognostic pair for glioblastoma. Oncogene 2019, 38, 6142–6157. [Google Scholar] [CrossRef]
- Kim, B.D.; Mondal, S.K.; Kenyon, E.; Chen, M.; Mallett, C.L.; deCarvalho, A.C.; Medarova, Z.; Moore, A. Nanoparticle Delivery of an Oligonucleotide Payload in a Glioblastoma Multiforme Animal Model. J. Vis. Exp. 2024, 211, e66986. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.A.; Bago, A.G.; Neuwelt, E.A. Single-dose contrast agent for intraoperative MR imaging of intrinsic brain tumors by using ferumoxtran-10. AJNR Am. J. Neuroradiol. 2005, 26, 1084–1088. [Google Scholar] [PubMed]
- Ljubimova, J.Y.; Sun, T.; Mashouf, L.; Ljubimov, A.V.; Israel, L.L.; Ljubimov, V.A.; Falahatian, V.; Holler, E. Covalent nano delivery systems for selective imaging and treatment of brain tumors. Adv. Drug Deliv. Rev. 2017, 113, 177–200. [Google Scholar] [CrossRef]
- Le Fur, M.; Ross, A.; Pantazopoulos, P.; Rotile, N.; Zhou, I.; Caravan, P.; Medarova, Z.; Yoo, B. Radiolabeling and PET-MRI microdosing of the experimental cancer therapeutic, MN-anti-miR10b, demonstrates delivery to metastatic lesions in a murine model of metastatic breast cancer. Cancer Nanotechnol. 2021, 12, 16. [Google Scholar] [CrossRef]
- Bourrinet, P.; Bengele, H.H.; Bonnemain, B.; Dencausse, A.; Idee, J.M.; Jacobs, P.M.; Lewis, J.M. Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agent. Investig. Radiol. 2006, 41, 313–324. [Google Scholar] [CrossRef]
- Boros, E.; Bowen, A.M.; Josephson, L.; Vasdev, N.; Holland, J.P. Chelate-free metal ion binding and heat-induced radiolabeling of iron oxide nanoparticles. Chem. Sci. 2015, 6, 225–236. [Google Scholar] [CrossRef]
- Briley-Saebo, K.; Bjornerud, A.; Grant, D.; Ahlstrom, H.; Berg, T.; Kindberg, G.M. Hepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: Implications for magnetic resonance imaging. Cell Tissue Res. 2004, 316, 315–323. [Google Scholar] [CrossRef]
- Estevanato, L.L.; Lacava, L.M.; Carvalho, L.C.; Azevedo, R.B.; Silva, O.; Pelegrini, F.; Bao, S.N.; Morais, P.C.; Lacava, Z.G. Long-term biodistribution and biocompatibility investigation of dextran-coated magnetite nanoparticle using mice as the animal model. J. Biomed. Nanotechnol. 2012, 8, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Schlachter, E.K.; Widmer, H.R.; Bregy, A.; Lonnfors-Weitzel, T.; Vajtai, I.; Corazza, N.; Bernau, V.J.; Weitzel, T.; Mordasini, P.; Slotboom, J.; et al. Metabolic pathway and distribution of superparamagnetic iron oxide nanoparticles: In vivo study. Int. J. Nanomed. 2011, 6, 1793–1800. [Google Scholar] [CrossRef]
- Evgenov, N.V.; Medarova, Z.; Pratt, J.; Pantazopoulos, P.; Leyting, S.; Bonner-Weir, S.; Moore, A. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes 2006, 55, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- Daldrup, H.E.; Link, T.M.; Blasius, S.; Strozyk, A.; Konemann, S.; Jurgens, H.; Rummeny, E.J. Monitoring radiation-induced changes in bone marrow histopathology with ultra-small superparamagnetic iron oxide (USPIO)-enhanced MRI. J. Magn. Reson. Imaging 1999, 9, 643–652. [Google Scholar] [CrossRef]
- Simon, G.H.; Bauer, J.; Saborovski, O.; Fu, Y.; Corot, C.; Wendland, M.F.; Daldrup-Link, H.E. T1 and T2 relaxivity of intracellular and extracellular USPIO at 1.5T and 3T clinical MR scanning. Eur. Radiol. 2006, 16, 738–745. [Google Scholar] [CrossRef]
- Yoo, B.; Ghosh, S.K.; Kumar, M.; Moore, A.; Yigit, M.V.; Medarova, Z. Design of nanodrugs for miRNA targeting in tumor cells. J. Biomed. Nanotechnol. 2014, 10, 1114–1122. [Google Scholar] [CrossRef]
- Kim, J.; Siverly, A.N.; Chen, D.; Wang, M.; Yuan, Y.; Wang, Y.; Lee, H.; Zhang, J.; Muller, W.J.; Liang, H.; et al. Ablation of miR-10b Suppresses Oncogene-Induced Mammary Tumorigenesis and Metastasis and Reactivates Tumor-Suppressive Pathways. Cancer Res. 2016, 76, 6424–6435. [Google Scholar] [CrossRef]
- Mansouri, A.; Hachem, L.D.; Mansouri, S.; Nassiri, F.; Laperriere, N.J.; Xia, D.; Lindeman, N.I.; Wen, P.Y.; Chakravarti, A.; Mehta, M.P.; et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: Refining the approach based on emerging evidence and current challenges. Neuro-Oncol. 2019, 21, 167–178. [Google Scholar] [CrossRef]
- Yoo, B.; Greninger, P.; Stein, G.T.; Egan, R.K.; McClanaghan, J.; Moore, A.; Benes, C.H.; Medarova, Z. Potent and selective effect of the mir-10b inhibitor MN-anti-mir10b in human cancer cells of diverse primary disease origin. PLoS ONE 2018, 13, e0201046. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neurooncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef]

| Parameter | Oligo | Iron |
|---|---|---|
| kα (h−1) | 0.145 ± 0.004 | 0.144 ± 0.005 |
| t1/2,α (h) | 4.8 ± 0.13 | 4.8 ± 0.16 |
| kβ (h−1) | 0.033 ± 0.001 | 0.031 ± 0.001 |
| t1/2,β (h) | 21.0 ± 0.64 | 22.2 ± 0.72 |
| A (ng/mL) | 328,041 ± 14,500 | 449,462 ± 19,000 |
| B (ng/mL) | 218,694 ± 11,000 | 299,641 ± 14,500 |
| AUC0–∞ (ng·h/mL) | 8,889,443 ± 275,000 | 12,789,145 ± 350,000 |
| CL (mL/h/kg) | 3.37 ± 0.10 | 2.35 ± 0.06 |
| Vd (mL/kg) | 102 ± 3.2 | 75.8 ± 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kim, B.D.; Chen, M.; Mondal, S.K.; Kenyon, E.; Mallett, C.L.; deCarvalho, A.; Medarova, Z.; Moore, A. Nanotherapy Targeting miR-10b Improves Survival in Orthotopic Glioblastoma Models. J. Funct. Biomater. 2026, 17, 15. https://doi.org/10.3390/jfb17010015
Kim BD, Chen M, Mondal SK, Kenyon E, Mallett CL, deCarvalho A, Medarova Z, Moore A. Nanotherapy Targeting miR-10b Improves Survival in Orthotopic Glioblastoma Models. Journal of Functional Biomaterials. 2026; 17(1):15. https://doi.org/10.3390/jfb17010015
Chicago/Turabian StyleKim, Bryan D., Ming Chen, Sujan K. Mondal, Elizabeth Kenyon, Christiane L. Mallett, Ana deCarvalho, Zdravka Medarova, and Anna Moore. 2026. "Nanotherapy Targeting miR-10b Improves Survival in Orthotopic Glioblastoma Models" Journal of Functional Biomaterials 17, no. 1: 15. https://doi.org/10.3390/jfb17010015
APA StyleKim, B. D., Chen, M., Mondal, S. K., Kenyon, E., Mallett, C. L., deCarvalho, A., Medarova, Z., & Moore, A. (2026). Nanotherapy Targeting miR-10b Improves Survival in Orthotopic Glioblastoma Models. Journal of Functional Biomaterials, 17(1), 15. https://doi.org/10.3390/jfb17010015







