Bio-Functional Nanomaterials for Enhanced Lung Cancer Therapy: The Synergistic Roles of Vitamins D and K
Abstract
1. Introduction
2. Targeted Nanomaterials in Cancer Therapy: Principles and Applications
2.1. Fundamental Principles of Nanomedicine in Oncology
2.2. Types of Targeted Nanomaterials for Lung Cancer Drug Delivery: Advantages and Disadvantages
2.2.1. Liposomes
2.2.2. Polymeric Nanoparticles
2.2.3. Inorganic Nanoparticles
2.2.4. Micelles
3. Vitamin D: Anti-Cancer Mechanisms and Clinical Challenges
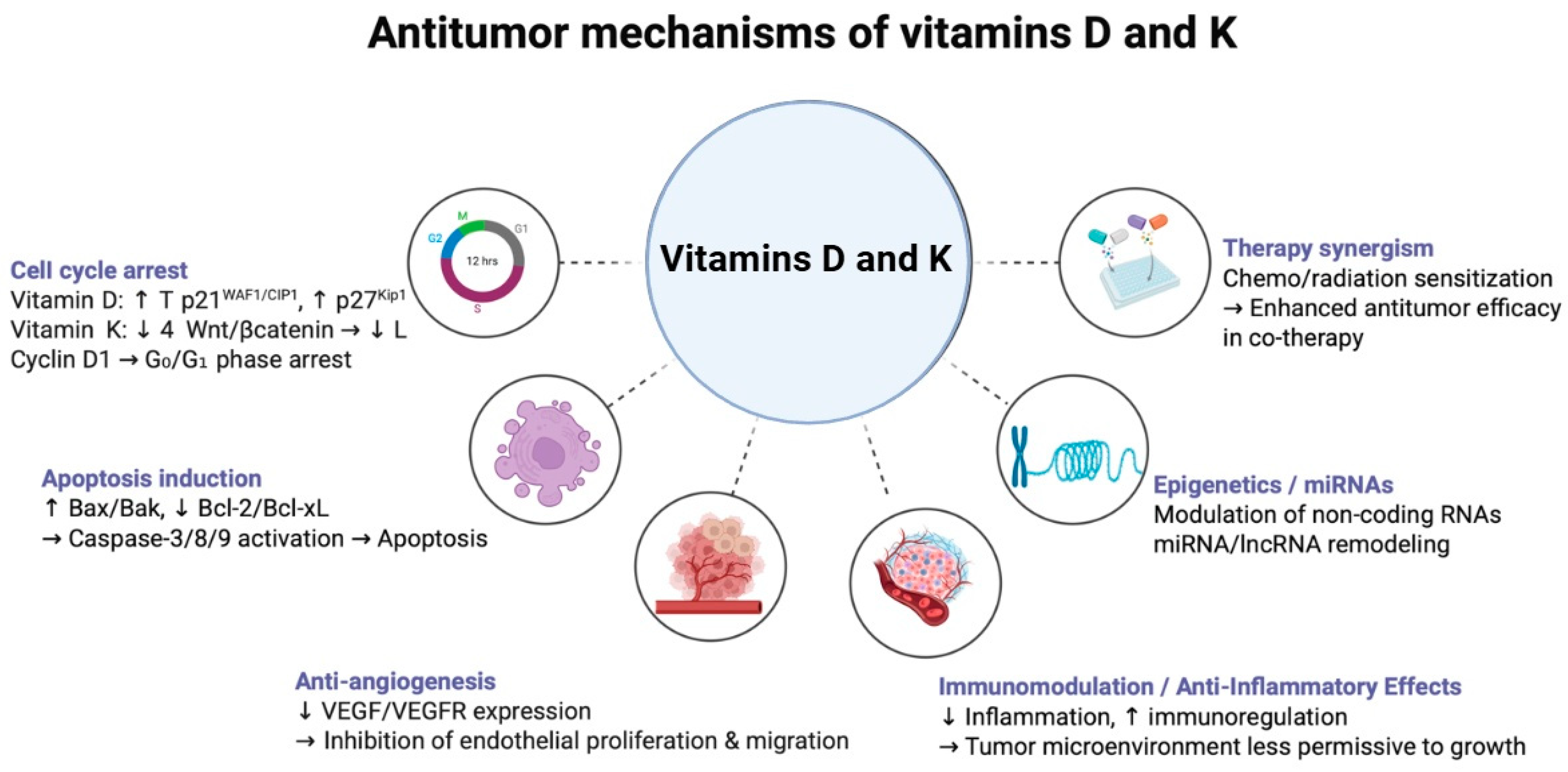
3.1. Anti-Cancer Mechanisms of Vitamin D
3.2. The Vitamin D Puzzle in Lung Cancer Patients
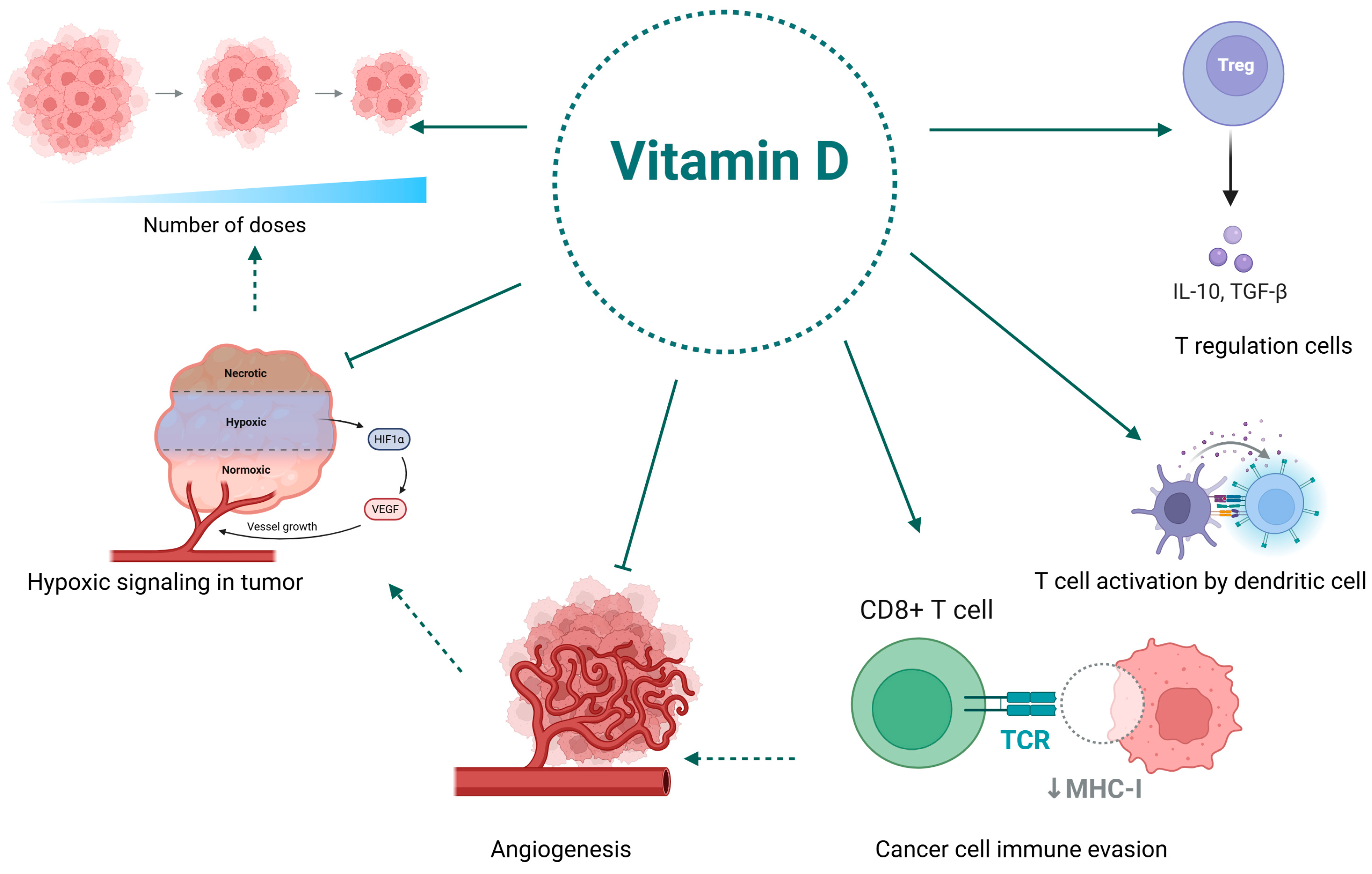
3.3. The Role of Vitamin D in Reprogramming the Immunosuppressive Tumor Microenvironment for Enhanced Immunotherapy
3.4. Clinical Limitations of Vitamin D
4. Vitamin K: Anti-Cancer Mechanisms and Clinical Challenges
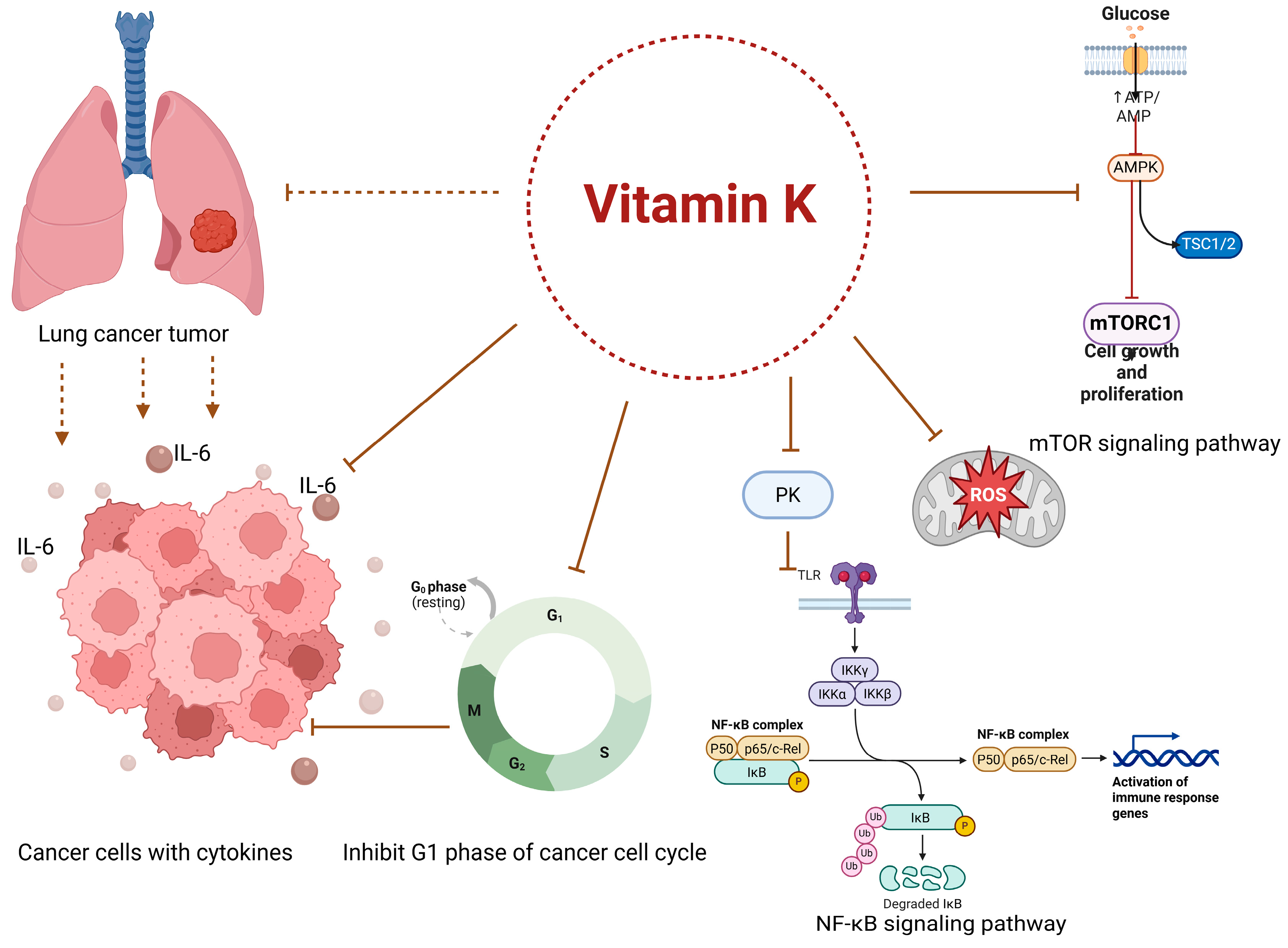
4.1. Anti-Cancer Mechanisms of Vitamin K
4.2. The Relationship Between Vitamin K Status and Lung Cancer
4.3. The Tumor Microenvironment, Inflammation, and the Role of Vitamin K in Cancer Progression
4.4. Clinical Limitations of Vitamin K
5. Nanomaterial-Enabled Delivery of Vitamins D and K
5.1. Overcoming Bioavailability and Stability Issues
5.2. Achieving Targeted Accumulation and Reduced Off-Target Effects
5.3. Synergistic Therapeutic Potential of Co-Delivery
6. Challenges and Future Perspectives in Clinical Translation
6.1. Translational Challenges
6.2. Clinical Translation and Implementation
6.3. Future Research Directions
6.3.1. Development of Responsive Nanocarriers
6.3.2. Multifunctional and Theranostic Platforms
6.3.3. Personalized Nanomedicine Using Biomarkers and Organoids
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zappa, C.; Mousa, S.A. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Che, X.; Li, X. Vitamin D and Lung Cancer Risk: A Comprehensive Review and Meta-Analysis. Cell Physiol. Biochem. 2015, 36, 299–305. [Google Scholar] [CrossRef]
- Henn, M.; Martin-Gorgojo, V.; Martin-Moreno, J.M. Vitamin D in Cancer Prevention: Gaps in Current Knowledge and Room for Hope. Nutrients 2022, 14, 4512. [Google Scholar] [CrossRef]
- Ramasamy, I. Vitamin D Metabolism and Guidelines for Vitamin D Supplementation. Clin. Biochem. Rev. 2020, 41, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.; Bak, M.J.; Narvaez, C.J. New Insights on Vitamin K Biology with Relevance to Cancer. Trends Mol. Med. 2022, 28, 864–881. [Google Scholar] [CrossRef]
- Munteanu, C.; Mârza, S.M.; Papuc, I. The Immunomodulatory Effects of Vitamins in Cancer. Front. Immunol. 2024, 15, 1464329. [Google Scholar] [CrossRef]
- Li, C.; Lei, S.; Ding, L.; Xu, Y.; Wu, X.; Wang, H.; Zhang, Z.; Gao, T.; Zhang, Y.; Li, L. Global Burden and Trends of Lung Cancer Incidence and Mortality. Chin. Med. J. 2023, 136, 1583–1590. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and Limitations in Traditional Anti-Cancer Therapies: A Comprehensive Review of Surgery, Chemotherapy, Radiation Therapy, and Hormonal Therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Lin, Y.; Mai, Z.; Zheng, Y.; Zheng, J.; Zhou, Z.; Zhao, X.; Cui, L. Targeting Cancer with Precision: Strategical Insights into TCR-Engineered T Cell Therapies. Theranostics 2025, 15, 300–323. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, D.; Mukhin, D.; Ovcharenko, G. Alternative Cancer Therapeutics: Unpatentable Compounds and Their Potential in Oncology. Pharmaceutics 2024, 16, 1237. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing Nanotechnology to Improve Targeted Cancer Treatment: Overcoming Hurdles in Its Clinical Implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Kurul, F.; Turkmen, H.; Cetin, A.E.; Topkaya, S.N. Nanomedicine: How Nanomaterials Are Transforming Drug Delivery, Bio-Imaging, and Diagnosis. Next Nanotechnol. 2025, 7, 100129. [Google Scholar] [CrossRef]
- Islam, S.; Ahmed, M.M.S.; Islam, M.A.; Hossain, N.; Chowdhury, M.A. Advances in Nanoparticles in Targeted Drug Delivery—A Review. Results Surf. Interfaces 2025, 19, 100529. [Google Scholar] [CrossRef]
- Manzari-Tavakoli, A.; Babajani, A.; Tavakoli, M.M.; Safaeinejad, F.; Jafari, A. Integrating Natural Compounds and Nanoparticle-based Drug Delivery Systems: A Novel Strategy for Enhanced Efficacy and Selectivity in Cancer Therapy. Cancer Med. 2024, 13, e7010. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Ahmed Jum’AH, D.A.; Attallah, Z.S.; Jallad, M.S.; Al Kury, L.T.; Hadi, R.W.; Mahmod, A.I. Role of Vitamins A, C, D, E in Cancer Prevention and Therapy: Therapeutic Potentials and Mechanisms of Action. Front. Nutr. 2024, 10, 1281879. [Google Scholar] [CrossRef] [PubMed]
- Sugandhi, V.V.; Pangeni, R.; Vora, L.K.; Poudel, S.; Nangare, S.; Jagwani, S.; Gadhave, D.; Qin, C.; Pandya, A.; Shah, P.; et al. Pharmacokinetics of Vitamin Dosage Forms: A Complete Overview. Food Sci. Nutr. 2023, 12, 48–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer Nanomedicine: From Targeted Delivery to Combination Therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef]
- Sabit, H.; Pawlik, T.M.; Radwan, F.; Abdel-Hakeem, M.; Abdel-Ghany, S.; Wadan, A.-H.S.; Elzawahri, M.; El-Hashash, A.; Arneth, B. Precision Nanomedicine: Navigating the Tumor Microenvironment for Enhanced Cancer Immunotherapy and Targeted Drug Delivery. Mol. Cancer 2025, 24, 160. [Google Scholar] [CrossRef]
- Islam, W.; Niidome, T.; Sawa, T. Enhanced Permeability and Retention Effect as a Ubiquitous and Epoch-Making Phenomenon for the Selective Drug Targeting of Solid Tumors. J. Pers. Med. 2022, 12, 1964. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, D.G.; Ghosh, D.; Das, A. Recent Developments in Nanocarriers for Cancer Chemotherapy. OpenNano 2022, 8, 100080. [Google Scholar] [CrossRef]
- Bajracharya, R.; Song, J.G.; Patil, B.R.; Lee, S.H.; Noh, H.-M.; Kim, D.-H.; Kim, G.-L.; Seo, S.-H.; Park, J.-W.; Jeong, S.H.; et al. Functional Ligands for Improving Anticancer Drug Therapy: Current Status and Applications to Drug Delivery Systems. Drug Deliv. 2022, 29, 1959–1970. [Google Scholar] [CrossRef]
- Toporkiewicz, M.; Meissner, J.; Matusewicz, L.; Czogalla, A.; Sikorski, A.F. Toward a Magic or Imaginary Bullet? Ligands for Drug Targeting to Cancer Cells: Principles, Hopes, and Challenges. Int. J. Nanomed. 2015, 10, 1399–1414. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer Nanotechnology: The Impact of Passive and Active Targeting in the Era of Modern Cancer Biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An Overview of Properties, Biomedical Applications and Obstacles to Clinical Translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Hajebi, S.; Chamanara, M.; Nasiri, S.S.; Ghasri, M.; Mouraki, A.; Heidari, R.; Nourmohammadi, A. Advances in Stimuli-Responsive Gold Nanorods for Drug-Delivery and Targeted Therapy Systems. Biomed. Pharmacother. 2024, 180, 117493. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Valenzuela Oses, J.K.; Rabasco-Álvarez, A.M.; González-Rodríguez, M.L.; García, M.C. Innovations in Cancer Therapy: Endogenous Stimuli-Responsive Liposomes as Advanced Nanocarriers. Pharmaceutics 2025, 17, 245. [Google Scholar] [CrossRef]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in Cancer Theragnostic and Drug Delivery: A Comprehensive Review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef]
- Belge Bilgin, G.; Bilgin, C.; Burkett, B.J.; Orme, J.J.; Childs, D.S.; Thorpe, M.P.; Halfdanarson, T.R.; Johnson, G.B.; Kendi, A.T.; Sartor, O. Theranostics and Artificial Intelligence: New Frontiers in Personalized Medicine. Theranostics 2024, 14, 2367–2378. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene Therapy in Cancer Treatment: Why Go Nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef]
- Javid, H.; Oryani, M.A.; Rezagholinejad, N.; Esparham, A.; Tajaldini, M.; Karimi-Shahri, M. RGD Peptide in Cancer Targeting: Benefits, Challenges, Solutions, and Possible Integrin–RGD Interactions. Cancer Med. 2024, 13, e6800. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nano-Drug Delivery: Is the Enhanced Permeability and Retention (EPR) Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jon, S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Li, W.; Li, Y.; Yin, S. Nanoparticle-Based Drug Delivery Systems Targeting Cancer Cell Surfaces. RSC Adv. 2023, 13, 21365–21382. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Sathuvan, M.; Narayanan, K.; Hong, H.; Vivek, R.; Zhong, S.; Cheong, K.-L.; Yoon, J.; Song, G.; Kang, H.; Thangam, R. Serial Stimuli-Responsive Theranostic Nanomaterials for Cancer Therapy and Imaging. Coord. Chem. Rev. 2025, 542, 216897. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Li, Y.; Wu, Y.; Tu, J.; Chen, Q.; Sun, C. Engineering Strategies of Sequential Drug Delivery Systems for Combination Tumor Immunotherapy. Acta Pharm. Sin. B 2025, 15, 3951–3977. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart Drug Delivery Systems for Precise Cancer Therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- Shah, S.; D’Souza, G.G.M. Modeling Tumor Microenvironment Complexity In Vitro: Spheroids as Physiologically Relevant Tumor Models and Strategies for Their Analysis. Cells 2025, 14, 732. [Google Scholar] [CrossRef]
- Ho, D.; Quake, S.R.; McCabe, E.R.B.; Chng, W.J.; Chow, E.K.; Ding, X.; Gelb, B.D.; Ginsburg, G.S.; Hassenstab, J.; Ho, C.-M.; et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol. 2020, 38, 497–518. [Google Scholar] [CrossRef]
- Puccetti, M.; Pariano, M.; Schoubben, A.; Giovagnoli, S.; Ricci, M. Biologics, Theranostics, and Personalized Medicine in Drug Delivery Systems. Pharmacol. Res. 2024, 201, 107086. [Google Scholar] [CrossRef]
- Basumallik, N.; Agarwal, M. Small Cell Lung Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Sarwar, M.; Thorat, N.D. Targeting the Tumor Microenvironment: Potential Strategy for Cancer Therapeutics. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166746. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Ajith, S.; Almomani, F.; Elhissi, A.; Husseini, G.A. Nanoparticle-Based Materials in Anticancer Drug Delivery: Current and Future Prospects. Heliyon 2023, 9, e21227. [Google Scholar] [CrossRef]
- Lan, H.; Jamil, M.; Ke, G.; Dong, N. The Role of Nanoparticles and Nanomaterials in Cancer Diagnosis and Treatment: A Comprehensive Review. Am. J. Cancer Res. 2023, 13, 5751–5784. [Google Scholar] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Ghadami, S.; Dellinger, K. The Lipid Composition of Extracellular Vesicles: Applications in Diagnostics and Therapeutic Delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A Review on Phospholipids and Their Main Applications in Drug Delivery Systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, S.; Liu, X.; Wang, W.; Zhu, Z.; Chen, L. Innovations in Breaking Barriers: Liposomes as Near-Perfect Drug Carriers in Ischemic Stroke Therapy. Int. J. Nanomed. 2024, 19, 3715–3735. [Google Scholar] [CrossRef]
- Ghosh, R.; De, M. Liposome-Based Antibacterial Delivery: An Emergent Approach to Combat Bacterial Infections. ACS Omega 2023, 8, 35442–35451. [Google Scholar] [CrossRef]
- Santhanakrishnan, K.R.; Koilpillai, J.; Narayanasamy, D. PEGylation in Pharmaceutical Development: Current Status and Emerging Trends in Macromolecular and Immunotherapeutic Drugs. Cureus 2024, 16, e66669. [Google Scholar] [CrossRef]
- Ishida, T.; Atobe, K.; Wang, X.; Kiwada, H. Accelerated Blood Clearance of PEGylated Liposomes upon Repeated Injections: Effect of Doxorubicin-Encapsulation and High-Dose First Injection. J. Control. Release 2006, 115, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.S.; Fernandes, R.S.; Cavalcante, C.H.; da Costa César, I.; Leite, E.A.; Lopes, S.C.A.; Ferretti, A.; Rubello, D.; Townsend, D.M.; de Oliveira, M.C.; et al. Influence of PEG Coating on the Biodistribution and Tumor Accumulation of pH-Sensitive Liposomes. Drug Deliv. Transl. Res. 2019, 9, 123–130. [Google Scholar] [CrossRef]
- Master, A.M.; Gupta, A.S. EGF Receptor-Targeted Nanocarriers for Enhanced Cancer Treatment. Nanomedicine 2012, 7, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Boukherroub, R.; Sanyal, A.; Szunerits, S. Treatment of Lung Diseases via Nanoparticles and Nanorobots: Are These Viable Alternatives to Overcome Current Treatments? Mater. Today Bio. 2025, 31, 101616. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Su, H.-C.; Raj, E.N.; Liu, K.-K.; Chang, C.-J.; Hsu, T.-C.; Cheng, P.-Y.; Wang, R.-H.; Lai, Y.-H.; Chen, C.-H.; et al. Targeting EGFR and Monitoring Tumorigenesis of Human Lung Cancer Cells In Vitro and In Vivo Using Nanodiamond-Conjugated Specific EGFR Antibody. Pharmaceutics 2022, 15, 111. [Google Scholar] [CrossRef]
- Chen, J.; Hu, S.; Sun, M.; Shi, J.; Zhang, H.; Yu, H.; Yang, Z. Recent Advances and Clinical Translation of Liposomal Delivery Systems in Cancer Therapy. Eur. J. Pharm. Sci. 2024, 193, 106688. [Google Scholar] [CrossRef]
- Agrawal, S.S.; Baliga, V.; Londhe, V.Y. Liposomal Formulations: A Recent Update. Pharmaceutics 2025, 17, 36. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Basak, S.; Das, T.K. Liposome-Based Drug Delivery Systems: From Laboratory Research to Industrial Production—Instruments and Challenges. ChemEngineering 2025, 9, 56. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Eltaib, L. Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication. Polymers 2025, 17, 833. [Google Scholar] [CrossRef] [PubMed]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, J.; Wu, L. Application of Nanomaterials in Precision Treatment of Lung Cancer. iScience 2024, 28, 111704. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Targeted Delivery of Drugs and Genes Using Polymer Nanocarriers for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 9118. [Google Scholar] [CrossRef] [PubMed]
- Borandeh, S.; van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric Drug Delivery Systems by Additive Manufacturing. Adv. Drug Deliv. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef]
- Qiu, S.; Zhu, F.; Tong, L. Application of Targeted Drug Delivery by Cell Membrane-Based Biomimetic Nanoparticles for Inflammatory Diseases and Cancers. Eur. J. Med. Res. 2024, 29, 523. [Google Scholar] [CrossRef]
- Adeniji, T.M.; Haroon, N.; Stine, K.J. Applications of Nanomaterial Coatings in Solid-Phase Microextraction (SPME). Processes 2025, 13, 244. [Google Scholar] [CrossRef]
- Alhalili, Z. Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules 2023, 28, 3086. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Zou, F.; Gu, J.; Deng, S.; Cao, Y.; Cai, K. The Role of Inorganic Nanomaterials in Overcoming Challenges in Colorectal Cancer Diagnosis and Therapy. Pharmaceutics 2025, 17, 409. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sheikh, A.; Akhtar, M.; Ghazwani, M.; Hani, U.; Sahebkar, A.; Kesharwani, P. Understanding Gold Nanoparticles and Their Attributes in Ovarian Cancer Therapy. Mol. Cancer 2025, 24, 88. [Google Scholar] [CrossRef]
- AlQurashi, D.M.; AlQurashi, T.F.; Alam, R.I.; Shaikh, S.; Tarkistani, M.A.M. Advanced Nanoparticles in Combating Antibiotic Resistance: Current Innovations and Future Directions. J. Nanotheranostics 2025, 6, 9. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in Nanomaterial-Based Targeted Drug Delivery Systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef] [PubMed]
- Ewii, U.E.; Attama, A.A.; Olorunsola, E.O.; Onugwu, A.L.; Nwakpa, F.U.; Anyiam, C.; Chijioke, C.; Ogbulie, T. Nanoparticles for Drug Delivery: Insight into in Vitro and in Vivo Drug Release from Nanomedicines. Nano TransMed 2025, 4, 100083. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B. Polymeric Micellar Systems—A Special Emphasis on “Smart” Drug Delivery. Pharmaceutics 2023, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Figueiras, A.; Domingues, C.; Jarak, I.; Santos, A.I.; Parra, A.; Pais, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Kabanov, A.; Cabral, H.; et al. New Advances in Biomedical Application of Polymeric Micelles. Pharmaceutics 2022, 14, 1700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Atluri, K.; Tiwari, A.K.; Babu, R.J. Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine. Pharmaceuticals 2023, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Wilner, S.E.; Sparks, S.E.; Cowburn, D.; Girvin, M.E.; Levy, M. Controlling Lipid Micelle Stability Using Oligonucleotide Headgroups. J. Am. Chem. Soc. 2015, 137, 2171–2174. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to Improve Micelle Stability for Drug Delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric Micelles and Alternative Nanonized Delivery Vehicles for Poorly Soluble Drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Production, Metabolism, and Mechanism of Action. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Bilezikian, J.P.; Formenti, A.M.; Adler, R.A.; Binkley, N.; Bouillon, R.; Lazaretti-Castro, M.; Marcocci, C.; Napoli, N.; Rizzoli, R.; Giustina, A. Vitamin D: Dosing, Levels, Form, and Route of Administration: Does One Approach Fit All? Rev. Endocr. Metab. Disord. 2021, 22, 1201–1218. [Google Scholar] [CrossRef]
- El-Sharkawy, A.; Malki, A. Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules 2020, 25, 3219. [Google Scholar] [CrossRef]
- Srinivasan, M.; Parwani, A.V.; Hershberger, P.A.; Lenzner, D.E.; Weissfeld, J.L. Nuclear Vitamin D Receptor Expression Is Associated with Improved Survival in Non-Small Cell Lung Cancer. J. Steroid Biochem. Mol. Biol. 2011, 123, 30–36. [Google Scholar] [CrossRef]
- Campbell, M.J.; Trump, D.L. Vitamin D Receptor Signaling and Cancer. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1009–1038. [Google Scholar] [CrossRef]
- Díaz, L.; Díaz-Muñoz, M.; García-Gaytán, A.C.; Méndez, I. Mechanistic Effects of Calcitriol in Cancer Biology. Nutrients 2015, 7, 5020–5050. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, M.I.; Habib, S.; et al. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The Role of BCL-2 Family Proteins in Regulating Apoptosis and Cancer Therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fang, Y.; Ma, Y.; Wang, F.; Wang, Y.; Jia, J.; Yang, Y.; Sun, W.; Zhou, Q.; Li, Z. Angiogenesis and Targeted Therapy in the Tumour Microenvironment: From Basic to Clinical Practice. Clin. Transl. Med. 2025, 15, e70313. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell Mol. Life Sci. 2019, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Vanoirbeek, E.; Krishnan, A.V.; Eelen, I.G.; Verlinden, L.; Bouillon, R.; Feldman, D.; Verstuyf, A. The Anti-Cancer and Anti-Inflammatory Actions of 1,25(OH)2D3. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Trump, D.L.; Johnson, C.S.; Feldman, D. The Role of Vitamin D in Cancer Prevention and Treatment. Endocrinol. Metab. Clin. N. Am. 2010, 39, 401–418. [Google Scholar] [CrossRef]
- Yun, X.; Qin, H.; Du, B.; Peng, Y.; Liu, Y.; Yang, B. Inhibitory Effect and Mechanism of Hirsuteine on NCI-H1299 Lung Cancer Cell Lines. Oncol. Lett. 2023, 25, 202. [Google Scholar] [CrossRef]
- Trump, D.L. Calcitriol and Cancer Therapy: A Missed Opportunity. Bone Rep. 2018, 9, 110–119. [Google Scholar] [CrossRef]
- Verma, A.K.; Naseeb, M.A.; Basaqr, R.O.; Albajri, E.A.; Khan, M.I.; Dev, K.; Beg, M.M.A. Cell-Free SLC30A10 Messenger Ribonucleic Acid (mRNA) Expression and Their Association with Vitamin-D Level among Non-Small Cell Lung Cancer (NSCLC) Patients. J. Cancer Res. Ther. 2023, 19, S764. [Google Scholar] [CrossRef]
- de Groot, P.; Munden, R.F. Lung Cancer Epidemiology, Risk Factors, and Prevention. Radiol. Clin. 2012, 50, 863–876. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, H.; Liu, K.; Wang, Y.; Liu, Q.; Sun, T.; Chen, S.; Ren, L. Smoking Behavior and Circulating Vitamin D Levels in Adults: A Meta-analysis. Food Sci. Nutr. 2021, 9, 5820–5832. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Bhoora, S.; Pillay, T.S.; Punchoo, R. Induction of Cell Death and Regulation of Autocrine Vitamin D Metabolism in Cervical Cancer by Physiological and GI20 Doses of 25-Hydroxycholecalciferol. Int. J. Mol. Sci. 2025, 26, 4008. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Lung Carcinogenesis by Tobacco Smoke. Int. J. Cancer 2012, 131, 2724–2732. [Google Scholar] [CrossRef]
- Matsunawa, M.; Amano, Y.; Endo, K.; Uno, S.; Sakaki, T.; Yamada, S.; Makishima, M. The Aryl Hydrocarbon Receptor Activator Benzo[a]Pyrene Enhances Vitamin D3 Catabolism in Macrophages. Toxicol. Sci. 2009, 109, 50–58. [Google Scholar] [CrossRef]
- Shiratsuchi, H.; Wang, Z.; Chen, G.; Ray, P.; Lin, J.; Zhang, Z.; Zhao, L.; Beer, D.; Ray, D.; Ramnath, N. Oncogenic Potential of CYP24A1 in Lung Adenocarcinoma. J. Thorac. Oncol. 2017, 12, 269–280. [Google Scholar] [CrossRef]
- Liu, H.; Yang, W.; Jiang, J. Targeting Tumor Metabolism to Augment CD8+ T Cell Anti-Tumor Immunity. J. Pharm. Anal. 2025, 15, 101150. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ‘T Cell Exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef]
- Dolina, J.S.; Van Braeckel-Budimir, N.; Thomas, G.D.; Salek-Ardakani, S. CD8+ T Cell Exhaustion in Cancer. Front. Immunol. 2021, 12, 715234. [Google Scholar] [CrossRef]
- Li, P.; Zhu, X.; Cao, G.; Wu, R.; Li, K.; Yuan, W.; Chen, B.; Sun, G.; Xia, X.; Zhang, H.; et al. 1α,25(OH)2D3 Reverses Exhaustion and Enhances Antitumor Immunity of Human Cytotoxic T Cells. J. Immunother. Cancer 2022, 10, e003477. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Yoshikawa, S.; Morikawa, S.; Ikeda, Y.; Taniguchi, K.; Sawamura, H.; Asai, T.; Matsuda, S. Potential Tactics with Vitamin D and Certain Phytochemicals for Enhancing the Effectiveness of Immune-Checkpoint Blockade Therapies. Explor. Target. Antitumor Ther. 2023, 4, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xu, F.; Dai, D.; Xiong, A.; Sun, R.; Ling, Y.; Qiu, L.; Wang, R.; Ding, Y.; Lin, M.; et al. VDR Is a Potential Prognostic Biomarker and Positively Correlated with Immune Infiltration: A Comprehensive Pan-Cancer Analysis with Experimental Verification. Biosci. Rep. 2024, 44, BSR20231845. [Google Scholar] [CrossRef] [PubMed]
- Mempel, T.R.; Lill, J.K.; Altenburger, L.M. How Chemokines Organize the Tumour Microenvironment. Nat. Rev. Cancer 2024, 24, 28–50. [Google Scholar] [CrossRef]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory Actions of Vitamin D in Various Immune-Related Disorders: A Comprehensive Review. Front. Immunol. 2023, 14, 950465. [Google Scholar] [CrossRef]
- Argano, C.; Torres, A.; Orlando, V.; Cangialosi, V.; Maggio, D.; Pollicino, C.; Corrao, S. Molecular Insight into the Role of Vitamin D in Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2025, 26, 4798. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Zhong, W.; Zhao, J.; Liu, X.; Gao, X.; Chen, M.; Wang, M. Vitamin D and Immune Checkpoint Inhibitors in Lung Cancer: A Synergistic Approach to Enhancing Treatment Efficacy. Int. J. Mol. Sci. 2025, 26, 4511. [Google Scholar] [CrossRef] [PubMed]
- Abu El Maaty, M.A.; Wölfl, S. Effects of 1,25(OH)2D3 on Cancer Cells and Potential Applications in Combination with Established and Putative Anti-Cancer Agents. Nutrients 2017, 9, 87. [Google Scholar] [CrossRef]
- Bennin, D.; Hartery, S.A.; Kirby, B.J.; Maekawa, A.S.; St-Arnaud, R.; Kovacs, C.S. Loss of 24-Hydroxylated Catabolism Increases Calcitriol and Fibroblast Growth Factor 23 and Alters Calcium and Phosphate Metabolism in Fetal Mice. JBMR Plus 2024, 8, ziae012. [Google Scholar] [CrossRef]
- Aberger, S.; Schreiber, N.; Pilz, S.; Eller, K.; Rosenkranz, A.R.; Kirsch, A.H. Targeting Calcitriol Metabolism in Acute Vitamin D Toxicity—A Comprehensive Review and Clinical Insight. Int. J. Mol. Sci. 2024, 25, 10003. [Google Scholar] [CrossRef]
- Muindi, J.R.; Peng, Y.; Potter, D.M.; Hershberger, P.A.; Tauch, J.S.; Capozzoli, M.J.; Egorin, M.J.; Johnson, C.S.; Trump, D.L. Pharmacokinetics of High-Dose Oral Calcitriol: Results from a Phase 1 Trial of Calcitriol and Paclitaxel. Clin. Pharmacol. Ther. 2002, 72, 648–659. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, T.; O’Connor, C.; Barlow, J.W.; Walsh, J.; Scalabrino, G.; Xu, F.; Sheridan, H. The Biological Responses of Vitamin K2: A Comprehensive Review. Food Sci. Nutr. 2023, 11, 1634–1656. [Google Scholar] [CrossRef]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a Diet Supplement with Impact in Human Health: Current Evidence in Age-Related Diseases. Nutrients 2020, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.-K.; Stafford, D.W. Structural and Functional Insights into Enzymes of the Vitamin K Cycle. J. Thromb. Haemost. 2016, 14, 236–247. [Google Scholar] [CrossRef]
- Mladěnka, P.; Macáková, K.; Kujovská Krčmová, L.; Javorská, L.; Mrštná, K.; Carazo, A.; Protti, M.; Remião, F.; Nováková, L. Vitamin K—Sources, Physiological Role, Kinetics, Deficiency, Detection, Therapeutic Use, and Toxicity. Nutr. Rev. 2021, 80, 677–698. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ma, P.; Kong, L.; Wang, X.; Wang, Y.; Jiang, L. Vitamin K2 Inhibits Hepatocellular Carcinoma Cell Proliferation by Binding to 17β-Hydroxysteroid Dehydrogenase 4. Front. Oncol. 2021, 11, 757603. [Google Scholar] [CrossRef] [PubMed]
- Xv, F.; Chen, J.; Duan, L.; Li, S. Research Progress on the Anticancer Effects of Vitamin K2. Oncol. Lett. 2018, 15, 8926–8934. [Google Scholar] [CrossRef]
- Halma, M.T.J.; Tuszynski, J.A.; Marik, P.E. Cancer Metabolism as a Therapeutic Target and Review of Interventions. Nutrients 2023, 15, 4245. [Google Scholar] [CrossRef]
- Yoshida, T.; Miyazawa, K.; Kasuga, I.; Yokoyama, T.; Minemura, K.; Ustumi, K.; Aoshima, M.; Ohyashiki, K. Apoptosis Induction of Vitamin K2 in Lung Carcinoma Cell Lines: The Possibility of Vitamin K2 Therapy for Lung Cancer. Int. J. Oncol. 2003, 23, 627–632. [Google Scholar] [CrossRef]
- Nimptsch, K.; Rohrmann, S.; Kaaks, R.; Linseisen, J. Dietary Vitamin K Intake in Relation to Cancer Incidence and Mortality: Results from the Heidelberg Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg). Am. J. Clin. Nutr. 2010, 91, 1348–1358. [Google Scholar] [CrossRef]
- Hoyt, M.; Reger, M.; Marley, A.; Fan, H.; Liu, Z.; Zhang, J. Vitamin K Intake and Prostate Cancer Risk in the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening Trial. Am. J. Clin. Nutr. 2019, 109, 392–401. [Google Scholar] [CrossRef]
- Juanola-Falgarona, M.; Salas-Salvadó, J.; Estruch, R.; Portillo, M.P.; Casas, R.; Miranda, J.; Martínez-González, M.A.; Bulló, M. Association between Dietary Phylloquinone Intake and Peripheral Metabolic Risk Markers Related to Insulin Resistance and Diabetes in Elderly Subjects at High Cardiovascular Risk. Cardiovasc. Diabetol. 2013, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Spek, C.A.; Arruda, V.R. The Protein C Pathway in Cancer Metastasis. Thromb. Res. 2012, 129 (Suppl. S1), S80–S84. [Google Scholar] [CrossRef]
- van Sluis, G.L.; Büller, H.R.; Spek, C.A. The Role of Activated Protein C in Cancer Progression. Thromb. Res. 2010, 125 (Suppl. S2), S138–S142. [Google Scholar] [CrossRef]
- Okano, T.; Shimomura, Y.; Yamane, M.; Suhara, Y.; Kamao, M.; Sugiura, M.; Nakagawa, K. Conversion of Phylloquinone (Vitamin K1) into Menaquinone-4 (Vitamin K2) in Mice: Two Possible Routes for Menaquinone-4 Accumulation in Cerebra of Mice. J. Biol. Chem. 2008, 283, 11270–11279. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. Vitamins K1 and K2: The Emerging Group of Vitamins Required for Human Health. J. Nutr. Metab. 2017, 2017, 6254836. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Matsuhashi, S.; Hamajima, H.; Iwane, S.; Takahashi, H.; Eguchi, Y.; Mizuta, T.; Fujimoto, K.; Kuroda, S.; Ozaki, I. The Role of PKC Isoforms in the Inhibition of NF-κB Activation by Vitamin K2 in Human Hepatocellular Carcinoma Cells. J. Nutr. Biochem. 2012, 23, 1668–1675. [Google Scholar] [CrossRef]
- Wellington, K.W.; Hlatshwayo, V.; Kolesnikova, N.I.; Saha, S.T.; Kaur, M.; Motadi, L.R. Anticancer Activities of Vitamin K3 Analogues. Investig. New Drugs 2020, 38, 378–391. [Google Scholar] [CrossRef]
- Wu, F.Y.; Liao, W.C.; Chang, H.M. Comparison of Antitumor Activity of Vitamins K1, K2 and K3 on Human Tumor Cells by Two (MTT and SRB) Cell Viability Assays. Life Sci. 1993, 52, 1797–1804. [Google Scholar] [CrossRef]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, H.H.W.; Vervoort, L.M.T.; Schurgers, L.J.; Shearer, M.J. Menadione Is a Metabolite of Oral Vitamin K. Br. J. Nutr. 2006, 95, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Eshak, E.S.; Arafa, A.; Tamakoshi, A.; Iso, H. Vitamin K Intake and Risk of Lung Cancer: The Japan Collaborative Cohort Study. J. Epidemiol. 2023, 33, 536–542. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Fane, M.; Weeraratna, A.T. Normal Aging and Its Role in Cancer Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037341. [Google Scholar] [CrossRef]
- López-Otín, C.; Pietrocola, F.; Roiz-Valle, D.; Galluzzi, L.; Kroemer, G. Meta-Hallmarks of Aging and Cancer. Cell Metab. 2023, 35, 12–35. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the Association of Chronic Inflammation and Cancer: Insights and Implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Samykutty, A.; Shetty, A.V.; Dakshinamoorthy, G.; Kalyanasundaram, R.; Zheng, G.; Chen, A.; Bosland, M.C.; Kajdacsy-Balla, A.; Gnanasekar, M. Vitamin K2, a Naturally Occurring Menaquinone, Exerts Therapeutic Effects on Both Hormone-Dependent and Hormone-Independent Prostate Cancer Cells. Evid. Based Complement. Altern. Med. 2013, 2013, 287358. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical Causes of Cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef]
- Joyce, D.E.; Grinnell, B.W. Recombinant Human Activated Protein C Attenuates the Inflammatory Response in Endothelium and Monocytes by Modulating Nuclear Factor-kappaB. Crit. Care Med. 2002, 30, S288–S293. [Google Scholar] [CrossRef]
- Aaseth, J.O.; Finnes, T.E.; Askim, M.; Alexander, J. The Importance of Vitamin K and the Combination of Vitamins K and D for Calcium Metabolism and Bone Health: A Review. Nutrients 2024, 16, 2420. [Google Scholar] [CrossRef]
- van Ballegooijen, A.J.; Pilz, S.; Tomaschitz, A.; Grübler, M.R.; Verheyen, N. The Synergistic Interplay between Vitamins D and K for Bone and Cardiovascular Health: A Narrative Review. Int. J. Endocrinol. 2017, 2017, 7454376. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Q.; Yang, M.; Liu, D.; Hou, X.; Tang, L.; Wang, X.; Lyu, Y.; Chen, X.; Liu, K.; et al. Current Trends in Drug Metabolism and Pharmacokinetics. Acta Pharm. Sin. B 2019, 9, 1113–1144. [Google Scholar] [CrossRef]
- Hao, Z.; Jin, D.-Y.; Stafford, D.W.; Tie, J.-K. Vitamin K-Dependent Carboxylation of Coagulation Factors: Insights from a Cell-Based Functional Study. Haematologica 2020, 105, 2164–2173. [Google Scholar] [CrossRef]
- Wu, S.; Chen, X.; Jin, D.-Y.; Stafford, D.W.; Pedersen, L.G.; Tie, J.-K. Warfarin and Vitamin K Epoxide Reductase: A Molecular Accounting for Observed Inhibition. Blood 2018, 132, 647–657. [Google Scholar] [CrossRef]
- Vega, A.J.; Smith, C.; Matejowsky, H.G.; Thornhill, K.J.; Borne, G.E.; Mosieri, C.N.; Shekoohi, S.; Cornett, E.M.; Kaye, A.D. Warfarin and Antibiotics: Drug Interactions and Clinical Considerations. Life 2023, 13, 1661. [Google Scholar] [CrossRef]
- Preeti; Sambhakar, S.; Saharan, R.; Narwal, S.; Malik, R.; Gahlot, V.; Khalid, A.; Najmi, A.; Zoghebi, K.; Halawi, M.A.; et al. Exploring LIPIDs for Their Potential to Improves Bioavailability of Lipophilic Drugs Candidates: A Review. Saudi Pharm. J. 2023, 31, 101870. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Miah, M.S.; Chy, M.W.R.; Ahmed, T.; Suchi, M.; Muhtady, M.A.; Ahmad, S.N.U.; Hossain, M.A. Emerging Trends in Nanotechnologies for Vitamin Delivery: Innovation and Future Prospects. Nano Trends 2025, 10, 100126. [Google Scholar] [CrossRef]
- Chen, A.; Stecker, E.; Warden, B.A. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J. Am. Heart Assoc. 2020, 9, e017559. [Google Scholar] [CrossRef]
- Cross, B.; Turner, R.M.; Zhang, J.E.; Pirmohamed, M. Being Precise with Anticoagulation to Reduce Adverse Drug Reactions: Are We There Yet? Pharmacogenom. J. 2024, 24, 7. [Google Scholar] [CrossRef]
- Xue, X.; Qu, H.; Li, Y. Stimuli-Responsive Crosslinked Nanomedicine for Cancer Treatment. Exploration 2022, 2, 20210134. [Google Scholar] [CrossRef]
- Rehman, M.; Tahir, N.; Sohail, M.F.; Qadri, M.U.; Duarte, S.O.D.; Brandão, P.; Esteves, T.; Javed, I.; Fonte, P. Lipid-Based Nanoformulations for Drug Delivery: An Ongoing Perspective. Pharmaceutics 2024, 16, 1376. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Attimarad, M.; Nair, A.B. Nanosuspension Innovations: Expanding Horizons in Drug Delivery Techniques. Pharmaceutics 2025, 17, 136. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yuhong, J.; Xin, P.; Han, J.L.; Du, Y.; Yu, X.; Zhu, R.; Zhang, M.; Chen, W.; et al. Advances in Nanotechnology for Enhancing the Solubility and Bioavailability of Poorly Soluble Drugs. Drug Des. Devel. Ther. 2024, 18, 1469–1495. [Google Scholar] [CrossRef]
- Mauricio, M.D.; Guerra-Ojeda, S.; Marchio, P.; Valles, S.L.; Aldasoro, M.; Escribano-Lopez, I.; Herance, J.R.; Rocha, M.; Vila, J.M.; Victor, V.M. Nanoparticles in Medicine: A Focus on Vascular Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, 6231482. [Google Scholar] [CrossRef]
- Hassan, A.A.A.; Ramadan, E.; Kristó, K.; Regdon, G.; Sovány, T. Lipid-Polymer Hybrid Nanoparticles as a Smart Drug Delivery System for Peptide/Protein Delivery. Pharmaceutics 2025, 17, 797. [Google Scholar] [CrossRef] [PubMed]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, J.; Yang, X. Deformable Nanocarriers for Enhanced Drug Delivery and Cancer Therapy. Exploration 2024, 4, 20230037. [Google Scholar] [CrossRef] [PubMed]
- Ly, P.-D.; Ly, K.-N.; Phan, H.-L.; Nguyen, H.H.T.; Duong, V.-A.; Nguyen, H.V. Recent Advances in Surface Decoration of Nanoparticles in Drug Delivery. Front. Nanotechnol. 2024, 6, 1456939. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, Y.; Shen, Y.; Zhou, Z. Surface Engineering of Nanoparticles for Precision Medicine. Precis. Med. Eng. 2025, 2, 100037. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef]
- Yan, Y.; Kulsoom; Sun, Y.; Li, Y.; Wang, Z.; Xue, L.; Wang, F. Advancing Cancer Therapy: Nanomaterial-Based Encapsulation Strategies for Enhanced Delivery and Efficacy of Curcumin. Mater. Today Bio. 2025, 33, 101963. [Google Scholar] [CrossRef]
- Prajapati, A.; Rangra, S.; Patil, R.; Desai, N.; Jyothi, V.G.S.S.; Salave, S.; Amate, P.; Benival, D.; Kommineni, N. Receptor-Targeted Nanomedicine for Cancer Therapy. Receptors 2024, 3, 323–361. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing Factors and Strategies of Enhancing Nanoparticles into Tumors in Vivo. Acta Pharm. Sin. B 2021, 11, 2265–2285. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in Cancer Therapy. Sig. Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D Toxicity—A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef]
- Venturini, J.; Chakraborty, A.; Baysal, M.A.; Tsimberidou, A.M. Developments in Nanotechnology Approaches for the Treatment of Solid Tumors. Exp. Hematol. Oncol. 2025, 14, 76. [Google Scholar] [CrossRef]
- Sharma, A.; Verwilst, P.; Li, M.; Ma, D.; Singh, N.; Yoo, J.; Kim, Y.; Yang, Y.; Zhu, J.-H.; Huang, H.; et al. Theranostic Fluorescent Probes. Chem. Rev. 2024, 124, 2699–2804. [Google Scholar] [CrossRef]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal Growth Factor Receptor (EGFR) in Lung Cancer: An Overview and Update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar]
- Thomas, B.J.; Guldenpfennig, C.; Guan, Y.; Winkler, C.; Beecher, M.; Beedy, M.; Berendzen, A.F.; Ma, L.; Daniels, M.A.; Burke, D.H.; et al. Targeting Lung Cancer with Clinically Relevant EGFR Mutations Using Anti-EGFR RNA Aptamer. Mol. Ther. Nucleic. Acids 2023, 34, 102046. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, T.; Liu, X.; Fang, X.; Mo, Y.; Xie, N.; Nie, G.; Zhang, B.; Fan, X. Smart Nanoplatforms Responding to the Tumor Microenvironment for Precise Drug Delivery in Cancer Therapy. Int. J. Nanomed. 2024, 19, 6253–6277. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lane, L.A.; Nie, S. Stimuli-Responsive Nanoparticles for Targeting the Tumor Microenvironment. J. Control. Release 2015, 219, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Guo, F.; Du, Y.; Wang, Y.; Wang, M.; Wang, L.; Yu, N.; Luo, S.; Wu, F.; Yang, G. Targeted Drug Delivery Systems for Matrix Metalloproteinase-Responsive Anoparticles in Tumor Cells: A Review. Int. J. Biol. Macromol. 2024, 257, 128658. [Google Scholar] [CrossRef]
- Karimi, M.; Zangabad, P.S.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Asl, H.G.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery Of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef]
- Guzmán-Sastoque, P.; Rodríguez, C.F.; Monsalve, M.C.; Castellanos, S.; Manrique-Moreno, A.; Reyes, L.H.; Cruz, J.C. Nanotheranostics Revolutionizing Gene Therapy: Emerging Applications in Gene Delivery Enhancement. J. Nanotheranostics 2025, 6, 10. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Li, Z.; Wan, D.; Pan, J. Targeted Drug Delivery Strategies for the Treatment of Hepatocellular Carcinoma. Molecules 2024, 29, 4405. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Kalafateli, M.; Geramoutsos, G.; Triantos, C. Recent Advances in the Use of Vitamin D Organic Nanocarriers for Drug Delivery. Biomolecules 2024, 14, 1090. [Google Scholar] [CrossRef]
- Fleet, J.C.; DeSmet, M.; Johnson, R.; Li, Y. Vitamin D and Cancer: A Review of Molecular Mechanisms. Biochem. J. 2012, 441, 61–76. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Karwowski, B.T. Vitamin K Contribution to DNA Damage—Advantage or Disadvantage? A Human Health Response. Nutrients 2022, 14, 4219. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Rosenberg, P.A. Vitamin K Prevents Oxidative Cell Death by Inhibiting Activation of 12-Lipoxygenase in Developing Oligodendrocytes. J. Neurosci. Res. 2009, 87, 1997–2005. [Google Scholar] [CrossRef]
- Minich, D.M.; Henning, M.; Darley, C.; Fahoum, M.; Schuler, C.B.; Frame, J. Is Melatonin the “Next Vitamin D”?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients 2022, 14, 3934. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current Advance of Nanotechnology in Diagnosis and Treatment for Malignant Tumors. Sig. Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef]
- Walweel, N.; Aydin, O. Enhancing Therapeutic Efficacy in Cancer Treatment: Integrating Nanomedicine with Autophagy Inhibition Strategies. ACS Omega 2024, 9, 27832–27852. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Malhotra, J.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Emerging Therapeutic Strategies to Overcome Drug Resistance in Cancer Cells. Cancers 2024, 16, 2478. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, Y.; Kim, J.; Cho, H.; Kim, K. Overcoming Cancer Drug Resistance with Nanoparticle Strategies for Key Protein Inhibition. Molecules 2024, 29, 3994. [Google Scholar] [CrossRef]
- Moukayed, M.; Grant, W.B. Molecular Link between Vitamin D and Cancer Prevention. Nutrients 2013, 5, 3993–4021. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Awajan, D.; Hamed, R.A.; Azzam, A.O.; Mahmod, A.I.; AL-Yasari, I.H. Combination Anticancer Therapies Using Selected Phytochemicals. Molecules 2022, 27, 5452. [Google Scholar] [CrossRef]
- Boța, M.; Vlaia, L.; Jîjie, A.-R.; Marcovici, I.; Crişan, F.; Oancea, C.; Dehelean, C.A.; Mateescu, T.; Moacă, E.-A. Exploring Synergistic Interactions between Natural Compounds and Conventional Chemotherapeutic Drugs in Preclinical Models of Lung Cancer. Pharmaceuticals 2024, 17, 598. [Google Scholar] [CrossRef]
- Crintea, A.; Dutu, A.G.; Samasca, G.; Florian, I.A.; Lupan, I.; Craciun, A.M. The Nanosystems Involved in Treating Lung Cancer. Life 2021, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Crintea, A.; Dutu, A.G.; Sovrea, A.; Constantin, A.-M.; Samasca, G.; Masalar, A.L.; Ifju, B.; Linga, E.; Neamti, L.; Tranca, R.A.; et al. Nanocarriers for Drug Delivery: An Overview with Emphasis on Vitamin D and K Transportation. Nanomaterials 2022, 12, 1376. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2022, 10, 1367–1401. [Google Scholar] [CrossRef]
- Pacheco, C.; Baião, A.; Ding, T.; Cui, W.; Sarmento, B. Recent Advances in Long-Acting Drug Delivery Systems for Anticancer Drug. Adv. Drug Deliv. Rev. 2023, 194, 114724. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Jumeaux, C.; Gupta, B.; Stevens, M.M. Peptide and Protein Nanoparticle Conjugates: Versatile Platforms for Biomedical Applications. Chem. Soc. Rev. 2018, 47, 3574–3620. [Google Scholar] [CrossRef]
- Patel, V.; Lalani, R.; Vhora, I.; Bardoliwala, D.; Patel, A.; Ghosh, S.; Misra, A. Co-Delivery of Cisplatin and siRNA through Hybrid Nanocarrier Platform for Masking Resistance to Chemotherapy in Lung Cancer. Drug Deliv. Transl. Res. 2021, 11, 2052–2071. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.; Siddiqui, J.A.; Lakshmanan, I.; Ganti, A.K.; Salgia, R.; Jain, M.; Batra, S.K.; Nasser, M.W. RNA-Based Therapies: A Cog in the Wheel of Lung Cancer Defense. Mol. Cancer 2021, 20, 54. [Google Scholar] [CrossRef]
- Song, W.; Musetti, S.N.; Huang, L. Nanomaterials for Cancer Immunotherapy. Biomaterials 2017, 148, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Balkhi, S.; Zuccolotto, G.; Di Spirito, A.; Rosato, A.; Mortara, L. CAR-NK Cell Therapy: Promise and Challenges in Solid Tumors. Front. Immunol. 2025, 16, 1574742. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.U.; Gandhi, S.M.; Swarn, S.; Lal, B.; Prajapati, B.G.; Khondee, S.; Mangmool, S.; Singh, S.; Chittasupho, C. Polymeric Nanoparticles for Targeted Lung Cancer Treatment: Review and Perspectives. Pharmaceutics 2025, 17, 1091. [Google Scholar] [CrossRef]
- Xu, F.; Tian, Y.; Huang, Y.; Zhang, L.-L.; Guo, Z.-Z.; Huang, J.-J.; Lin, T.-Y. EGFR Inhibitors Sensitize Non–Small Cell Lung Cancer Cells to TRAIL-Induced Apoptosis. Chin. J. Cancer 2011, 30, 701–711. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, C.; Li, Y.; Chen, Z.-S.; Zhang, L. Advancing Cancer Treatment: Innovative Materials in PDT and Diagnostic Integration. Int. J. Nanomed. 2025, 20, 7037–7060. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.M.; Ali, R.; Abd Elaziz, N.A.; Habib, H.; Abbas, F.M.; Yassin, M.T.; Maniah, K.; Abdelaziz, R. Nanotechnology in Oncology: Advances in Biosynthesis, Drug Delivery, and Theranostics. Discov. Oncol. 2025, 16, 1172. [Google Scholar] [CrossRef]
- Zhang, X.; Chan, H.W.; Shao, Z.; Wang, Q.; Chow, S.; Chow, S.F. Navigating Translational Research in Nanomedicine: A Strategic Guide to Formulation and Manufacturing. Int. J. Pharm. 2025, 671, 125202. [Google Scholar] [CrossRef]
- El-Tanani, M.; Satyam, S.M.; Rabbani, S.A.; El-Tanani, Y.; Aljabali, A.A.A.; Al Faouri, I.; Rehman, A. Revolutionizing Drug Delivery: The Impact of Advanced Materials Science and Technology on Precision Medicine. Pharmaceutics 2025, 17, 375. [Google Scholar] [CrossRef]
- Costa, C.; Padrela, L. Progress on Drug Nanoparticle Manufacturing: Exploring the Adaptability of Batch Bottom-up Approaches to Continuous Manufacturing. J. Drug Deliv. Sci. Technol. 2025, 111, 107120. [Google Scholar] [CrossRef]
- Bi, Y.; Xie, S.; Li, Z.; Dong, S.; Teng, L. Precise Nanoscale Fabrication Technologies, the “Last Mile” of Medicinal Development. Acta Pharm. Sin. B 2025, 15, 2372–2401. [Google Scholar] [CrossRef]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Amaechi, E.C.; Elendu, I.D.; Omeludike, J.C.; Omeludike, E.K.; Onubogu, N.C.; Ogelle, E.C.; Meduoye, O.O.M.; et al. Essential Information about Nanotechnology in Cardiology. Ann. Med. Surg. 2025, 87, 748–779. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.; Huang, R. Nanoparticles-induced Potential Toxicity on Human Health: Applications, Toxicity Mechanisms, and Evaluation Models. MedComm (2020) 2023, 4, e327. [Google Scholar] [CrossRef]
- Prieložná, J.; Mikušová, V.; Mikuš, P. Advances in the Delivery of Anticancer Drugs by Nanoparticles and Chitosan-Based Nanoparticles. Int. J. Pharm. X 2024, 8, 100281. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Chen, Q.; Riviere, J.E.; Lin, Z. Pharmacokinetics and Tumor Delivery of Nanoparticles. J. Drug Deliv. Sci. Technol. 2023, 83, 104404. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Franzè, S.; Condorelli, F.; Minghetti, P.; Caliceti, P. Feeding Next-Generation Nanomedicines to Europe: Regulatory and Quality Challenges. Adv. Healthc. Mater. 2023, 12, 2301956. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.I.; Villacis-Aguirre, C.A.; López-Aguilar, K.V.; Santiago Padilla, L.; Altamirano, C.; Toledo, J.R.; Santiago Vispo, N. The Hitchhiker’s Guide to Human Therapeutic Nanoparticle Development. Pharmaceutics 2022, 14, 247. [Google Scholar] [CrossRef]
- Elzein, B. Nano Revolution: “Tiny Tech, Big Impact: How Nanotechnology Is Driving SDGs Progress”. Heliyon 2024, 10, e31393. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.M.; Hassan, A.I. Synthesis and Characterization of Nanomaterials for Application in Cost-Effective Electrochemical Devices. Sustainability 2023, 15, 10891. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef]
- Sharmile, N.; Chowdhury, R.R.; Desai, S. A Comprehensive Review of Quality Control and Reliability Research in Micro–Nano Technology. Technologies 2025, 13, 94. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current Hurdles to the Translation of Nanomedicines from Bench to the Clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.; Liu, H.; Zhang, X.; Deng, X. Carrier-Free Nanomedicines: Mechanisms of Formation and Biomedical Applications. Giant 2024, 18, 100256. [Google Scholar] [CrossRef]
- Kellar, A.; Egan, C.; Morris, D. Preclinical Murine Models for Lung Cancer: Clinical Trial Applications. Biomed. Res. Int. 2015, 2015, 621324. [Google Scholar] [CrossRef]
- Yu, J.-Z.; Kiss, Z.; Ma, W.; Liang, R.; Li, T. Preclinical Models for Functional Precision Lung Cancer Research. Cancers 2025, 17, 22. [Google Scholar] [CrossRef]
- Shen, J.; Swift, B.; Mamelok, R.; Pine, S.; Sinclair, J.; Attar, M. Design and Conduct Considerations for First-in-Human Trials. Clin. Transl. Sci. 2019, 12, 6–19. [Google Scholar] [CrossRef]
- Crintea, A.; Drugan, C.; Constantin, A.-M.; Lupan, I.; Fekete, Z.; Silaghi, C.N.; Crăciun, A.M. Assessment of Specific Tumoral Markers, Inflammatory Status, and Vitamin D Metabolism before and after the First Chemotherapy Cycle in Patients with Lung Cancer. Biology 2022, 11, 1033. [Google Scholar] [CrossRef]
- Johnson, J.R.; Martini, R.N.; Yuan, Y.-C.; Woods-Burnham, L.; Walker, M.; Ortiz-Hernandez, G.L.; Kobeissy, F.; Galloway, D.; Gaddy, A.; Oguejiofor, C.; et al. 1,25-Dihydroxyvitamin D3 Suppresses Prognostic Survival Biomarkers Associated with Cell Cycle and Actin Organization in a Non-Malignant African American Prostate Cell Line. Biology 2024, 13, 346. [Google Scholar] [CrossRef]
- Ziegler, A.; Koch, A.; Krockenberger, K.; Großhennig, A. Personalized Medicine Using DNA Biomarkers: A Review. Hum Genet 2012, 131, 1627–1638. [Google Scholar] [CrossRef]
- Ramón y Cajal, S.; Sesé, M.; Capdevila, C.; Aasen, T.; De Mattos-Arruda, L.; Diaz-Cano, S.J.; Hernández-Losa, J.; Castellví, J. Clinical Implications of Intratumor Heterogeneity: Challenges and Opportunities. J. Mol. Med. 2020, 98, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Liao, J.; Ma, Y.; Sarwar, M.T.; Yang, H. Advances in Targeted Therapy for Tumor with Nanocarriers: A Review. Mater. Today Bio. 2025, 31, 101583. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Almadani, I.F.; Almadani, M.F.; AlSawaftah, N.; Abuwatfa, W.H.; Husseini, G.A. Nanocarriers Responsive to Light—A Review. Micro 2024, 4, 827–844. [Google Scholar] [CrossRef]
- Wang, X.; Xuan, Z.; Zhu, X.; Sun, H.; Li, J.; Xie, Z. Near-Infrared Photoresponsive Drug Delivery Nanosystems for Cancer Photo-Chemotherapy. J. Nanobiotechnology 2020, 18, 108. [Google Scholar] [CrossRef]
- Gong, J.; Shi, T.; Liu, J.; Pei, Z.; Liu, J.; Ren, X.; Li, F.; Qiu, F. Dual-Drug Codelivery Nanosystems: An Emerging Approach for Overcoming Cancer Multidrug Resistance. Biomed. Pharmacother. 2023, 161, 114505. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Li, W.; Huang, J.; Yang, G.; Wang, Y. Multifunctional Nanoplatforms Co-Delivering Combinatorial Dual-Drug for Eliminating Cancer Multidrug Resistance. Theranostics 2021, 11, 6334–6354. [Google Scholar] [CrossRef]
- Anitha, K.; Chenchula, S.; Surendran, V.; Shvetank, B.; Ravula, P.; Milan, R.; Chikatipalli, R.; Padmavathi, R. Advancing Cancer Theranostics through Biomimetics: A Comprehensive Review. Heliyon 2024, 10, e27692. [Google Scholar] [CrossRef] [PubMed]
- Samathoti, P.; Kumarachari, R.K.; Bukke, S.P.N.; Rajasekhar, E.S.K.; Jaiswal, A.A.; Eftekhari, Z. The Role of Nanomedicine and Artificial Intelligence in Cancer Health Care: Individual Applications and Emerging Integrations—A Narrative Review. Discov. Oncol. 2025, 16, 697. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, J.; Sun, X.; Gao, H. Targeted Nanomedicines Remodeling Immunosuppressive Tumor Microenvironment for Enhanced Cancer Immunotherapy. Acta Pharm. Sin. B 2022, 12, 4327–4347. [Google Scholar] [CrossRef] [PubMed]
| Principle | Description | Advantages | Disadvantages |
|---|---|---|---|
| Passive targeting | Nanoparticles accumulate preferentially in solid tumors due to leaky tumor vasculature and impaired lymphatic drainage. The size of nanoparticles (20–200 nm) is key [12,35]. | Simplifies targeting, as no specific surface modification is required. Leads to higher drug concentrations in the tumor, reducing systemic toxicity [36]. | Heterogeneity of EPR effect across tumors and patients. It can be influenced by tumor type, size, and location. Some tumors may have less leaky vasculature, limiting efficacy [37]. |
| Active targeting | Nanomaterials are surface-modified with specific ligands that bind selectively to receptors overexpressed on cancer cells or tumor microenvironment components. Ligand-receptor binding often triggers internalization [12,38]. | Enhances specificity and cellular uptake into cancer cells, leading to higher intracellular drug concentrations. Can overcome limitations of heterogeneous EPR. Enables personalized therapy based on biomarkers [37,39]. | Potential for off-target binding to healthy cells expressing the target receptor at lower levels. It can be challenging to maintain ligand stability in vivo. May face immune responses against targeting ligands [12,24]. |
| Controlled and stimuli-responsive release | Drug release kinetics are precisely controlled, ranging from sustained release to liberation triggered by specific endogenous cues (pH, enzymes, hypoxia, redox potential) or exogenous cues (light, magnetic fields, ultrasound) in the tumor microenvironment [40,41]. | Optimizes drug concentration at the tumor site for longer durations. Minimizes premature drug release and systemic side effects. Allows for on-demand drug release [12,42]. | Complexity in designing and synthesizing responsive materials. Potential for incomplete release. Variability in tumor microenvironment conditions can affect the predictability of release [43]. |
| Multimodal therapy and diagnostics | Nanomaterials are engineered to incorporate multiple therapeutic agents (combination therapy) and/or diagnostic imaging agents, enabling simultaneous treatment and real-time monitoring [13]. | Offers comprehensive treatment strategies by combining therapies. Enables real-time visualization of drug delivery and therapeutic response. Facilitates personalized medicine and dynamic treatment adjustments [44,45]. | Increased complexity in design, synthesis, and characterization. Potential for interaction between different functionalities. Higher regulatory hurdles due to dual diagnostic and therapeutic roles [13]. |
| Limitations | ||
|---|---|---|
| Vitamin D | Shared | Vitamin K |
| - Heterogeneous outcomes in supplementation studies, some showing no significant benefits despite correcting the deficiency. - Excessive supplementation may cause toxicity (hypercalcemia and kidney dysfunction). - Uncertainty in optimal dosing for prevention vs. treatment. | - Poor bioavailability due to lipophilic nature. - Rapid metabolism and short half-life, frequent intake required. - Variability in individual responses and lack of consensus on optimal dosing and treatment duration. - Limited long-term clinical trial evidence for efficacy. | - Evidence for benefits beyond coagulation (e.g., bone, cardiovascular, cancer) is inconsistent. - Forms K1 vs. K2 vary in bioavailability and effects, complicating therapeutic use. - Risk of interactions with anticoagulant drugs (e.g., warfarin), restricting use in some patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crintea, A.; Munteanu, C.; Ilyés, T.; Silaghi, C.N.; Crăciun, A.M. Bio-Functional Nanomaterials for Enhanced Lung Cancer Therapy: The Synergistic Roles of Vitamins D and K. J. Funct. Biomater. 2025, 16, 352. https://doi.org/10.3390/jfb16090352
Crintea A, Munteanu C, Ilyés T, Silaghi CN, Crăciun AM. Bio-Functional Nanomaterials for Enhanced Lung Cancer Therapy: The Synergistic Roles of Vitamins D and K. Journal of Functional Biomaterials. 2025; 16(9):352. https://doi.org/10.3390/jfb16090352
Chicago/Turabian StyleCrintea, Andreea, Camelia Munteanu, Tamás Ilyés, Ciprian N. Silaghi, and Alexandra M. Crăciun. 2025. "Bio-Functional Nanomaterials for Enhanced Lung Cancer Therapy: The Synergistic Roles of Vitamins D and K" Journal of Functional Biomaterials 16, no. 9: 352. https://doi.org/10.3390/jfb16090352
APA StyleCrintea, A., Munteanu, C., Ilyés, T., Silaghi, C. N., & Crăciun, A. M. (2025). Bio-Functional Nanomaterials for Enhanced Lung Cancer Therapy: The Synergistic Roles of Vitamins D and K. Journal of Functional Biomaterials, 16(9), 352. https://doi.org/10.3390/jfb16090352








