Modified Polysaccharides: Potential Biomaterials for Bioprinting
Abstract
1. Introduction
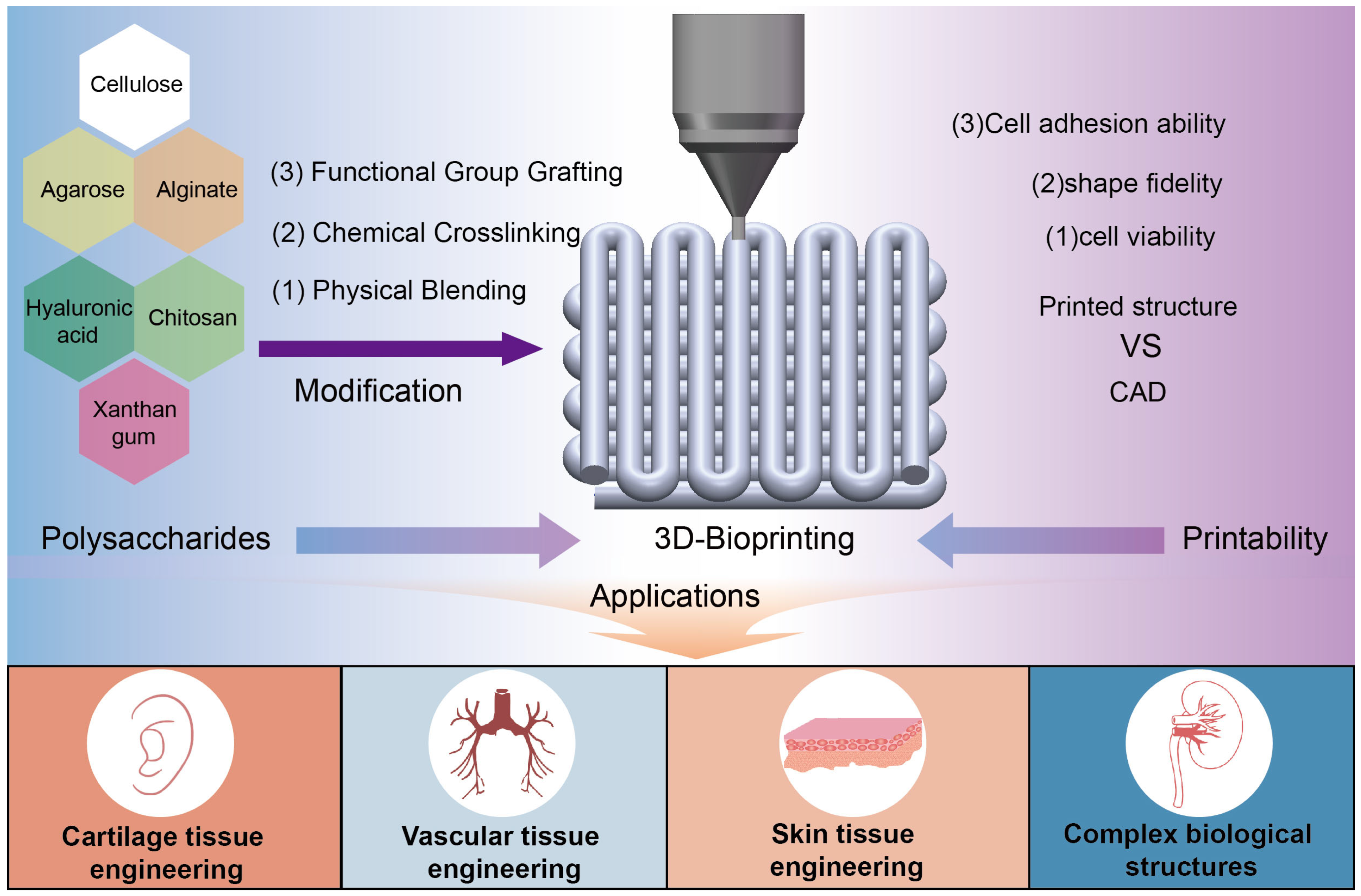
2. Classification and Modification of Polysaccharides
2.1. Plant Polysaccharides
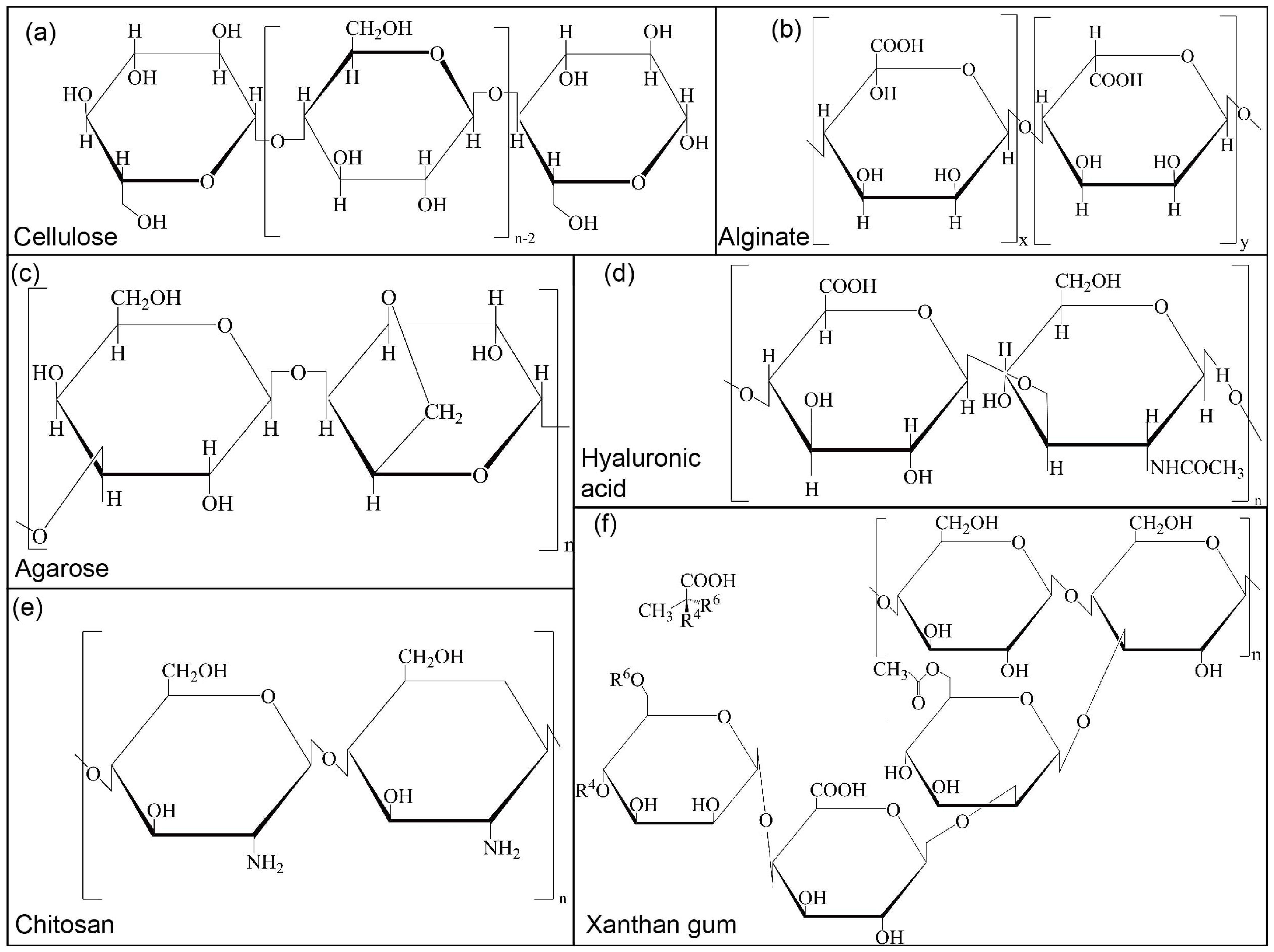
2.1.1. Cellulose
2.1.2. Alginate
2.1.3. Agarose
2.2. Animal Polysaccharides
2.2.1. Hyaluronic Acid
2.2.2. Chitosan
2.3. Microbial Polysaccharide (Xanthan Gum)
3. 3D Bioprinting Using Modified Polysaccharides
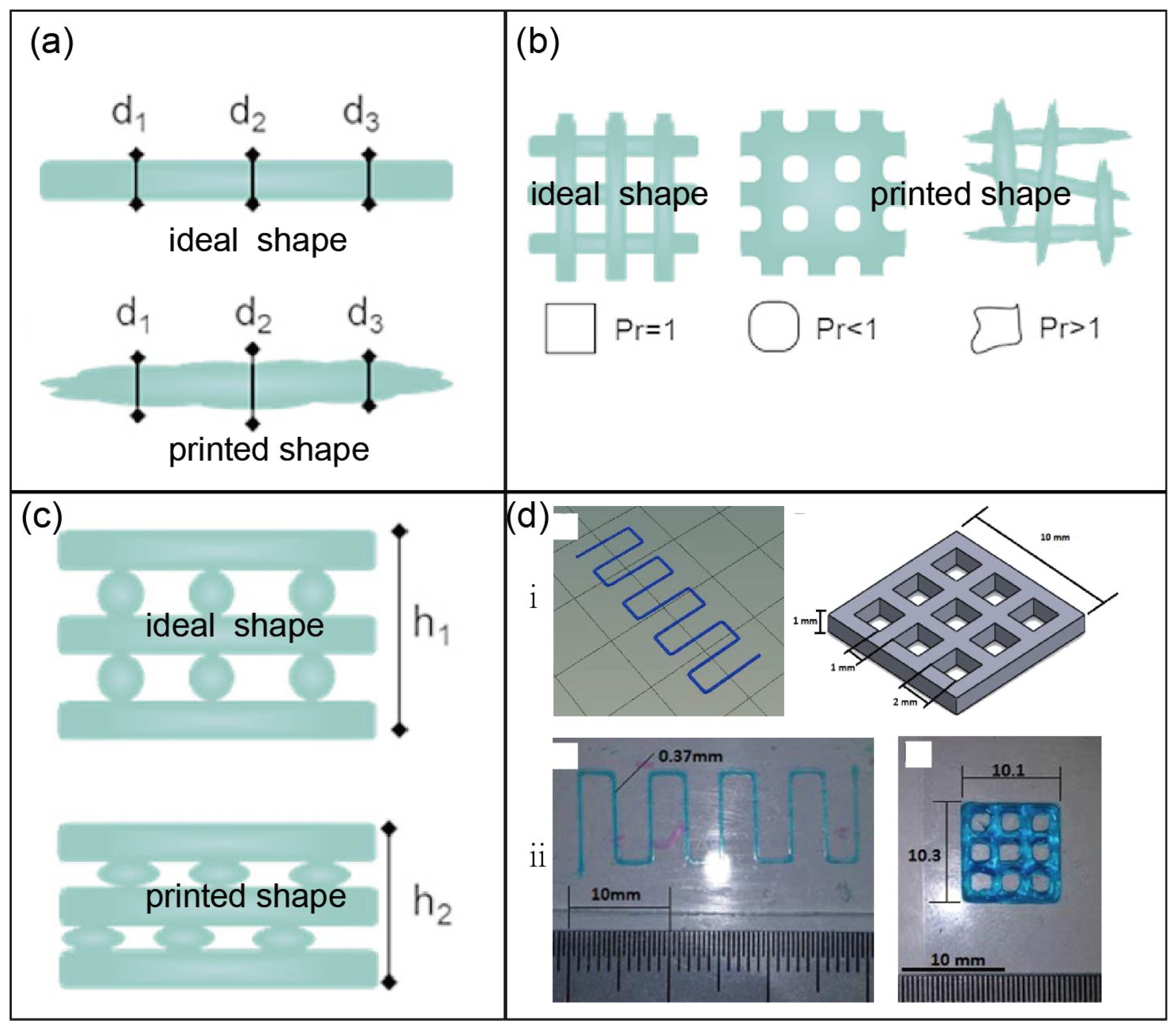
3.1. Factors Affecting the Printing Performance of Modified Polysaccharides
3.2. Printability Assessment of Modified Polysaccharides
4. Application of Modified Polysaccharides in Tissue Engineering
4.1. Cartilage Tissue Engineering
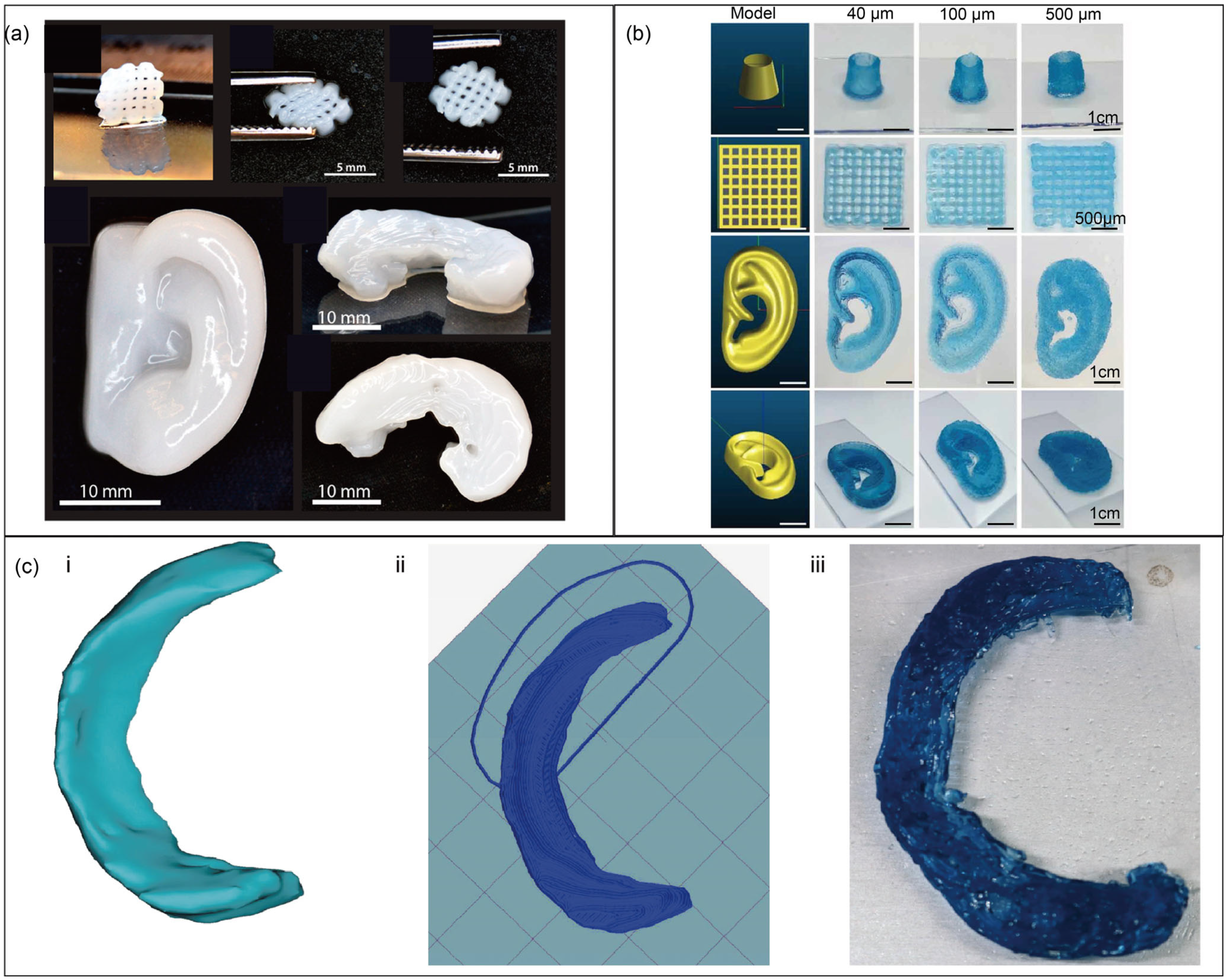
4.2. Vascular Tissue Engineering
4.3. Skin Tissue Engineering
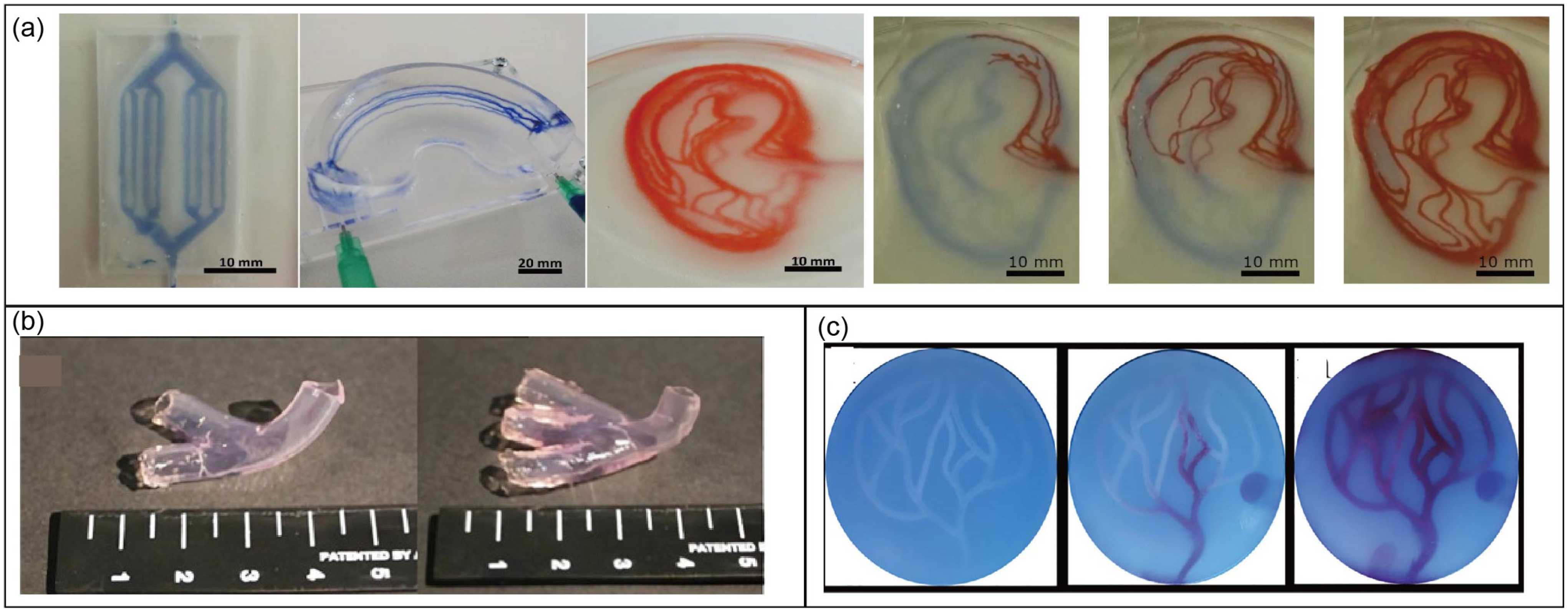
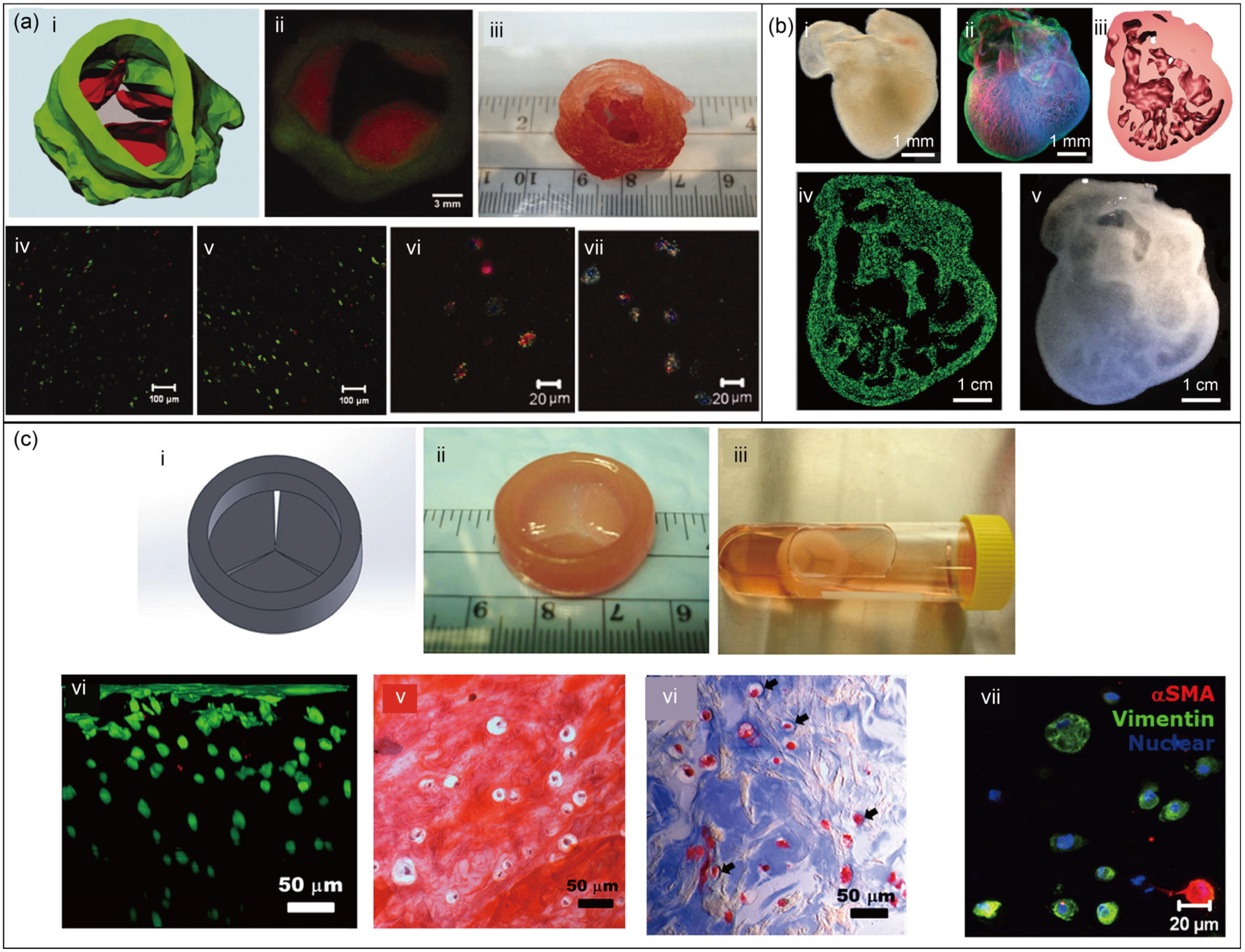
4.4. Complex Biological Structures
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riccardo, L.; Oksana, D.; Carlos, E.G.-M.; Bruce, E.K.; Riccardo, R.; Jacob, S.; Kristi, S.A.; Shaochen, C.; Marcy, Z.-W.; Yu, S.Z. Light-based vat-polymerization bioprinting. Nat. Rev. Methods Primers 2023, 3, 46. [Google Scholar] [CrossRef]
- Daly, A.C.; Prendergast, M.E.; Hughes, A.J.; Burdick, J.A. Bioprinting for the Biologist. Cell 2021, 184, 18–32. [Google Scholar] [CrossRef]
- Zhu, J.; He, Y.; Wang, Y.; Cai, L.-H. Voxelated bioprinting of modular double-network bio-ink droplets. Nat. Commun. 2024, 15, 5902. [Google Scholar] [CrossRef] [PubMed]
- Urciuolo, A.; Giobbe, G.G.; Dong, Y.; Michielin, F.; Brandolino, L.; Magnussen, M.; Gagliano, O.; Selmin, G.; Scattolini, V.; Raffa, P.; et al. Hydrogel-in-hydrogel live bioprinting for guidance and control of organoids and organotypic cultures. Nat. Commun. 2023, 14, 3128. [Google Scholar] [CrossRef] [PubMed]
- Mahendiran, B.; Muthusamy, S.; Sampath, S.; Jaisankar, S.N.; Popat, K.C.; Selvakumar, R.; Krishnakumar, G.S. Recent trends in natural polysaccharide based bioinks for multiscale 3D printing in tissue regeneration: A review. Int. J. Biol. Macromol. 2021, 183, 564–588. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Song, J.; Zhu, G.; Li, X.; Liu, L.; Shi, X.; Wang, Y. Periosteum tissue engineering—A review. Biomater. Sci. 2016, 4, 1554–1561. [Google Scholar] [CrossRef]
- Bhamare, N.; Tardalkar, K.; Khadilkar, A.; Parulekar, P.; Joshi, M.G. Tissue engineering of human ear pinna. Cell Tissue Bank. 2022, 23, 441–457. [Google Scholar] [CrossRef]
- Moon, K.H.; Ko, I.K.; Yoo, J.J.; Atala, A. Kidney diseases and tissue engineering. Methods 2016, 99, 112–119. [Google Scholar] [CrossRef]
- Brody, H. Regenerative medicine. Nature 2016, 540, S49. [Google Scholar] [CrossRef]
- Nii, T.; Katayama, Y. Biomaterial-Assisted Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8657. [Google Scholar] [CrossRef]
- Radeke, C.; Pons, R.; Mihajlovic, M.; Knudsen, J.R.; Butdayev, S.; Kempen, P.J.; Segeritz, C.P.; Andresen, T.L.; Pehmoller, C.K.; Jensen, T.E.; et al. Transparent and Cell-Guiding Cellulose Nanofiber 3D Printing Bioinks. ACS Appl. Mater. Interfaces 2023, 15, 2564–2577. [Google Scholar] [CrossRef]
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.W.; Eglin, D.; D’Este, M. Hyaluronic acid as a bioink for extrusion-based 3D printing. Biofabrication 2020, 12, 032001. [Google Scholar] [CrossRef]
- Li, C.; Zheng, Z.; Jia, J.; Zhang, W.; Qin, L.; Zhang, W.; Lai, Y. Preparation and characterization of photocurable composite extracellular matrix-methacrylated hyaluronic acid bioink. J. Mater. Chem. B 2022, 10, 4242–4253. [Google Scholar] [CrossRef]
- Chakraborty, J.; Mu, X.; Pramanick, A.; Kaplan, D.L.; Ghosh, S. Recent advances in bioprinting using silk protein-based bioinks. Biomaterials 2022, 287, 121672. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, J.; Liu, M.; Wang, H.; Li, C.; Rodriguez, M.J.; Li, G.; Wang, X.; Kaplan, D.L. 3D Bioprinting of Self-Standing Silk-Based Bioink. Adv. Healthc. Mater. 2018, 7, 1701026. [Google Scholar] [CrossRef]
- Chester, D.; Theetharappan, P.; Ngobili, T.; Daniele, M.; Brown, A.C. Ultrasonic Microplotting of Microgel Bioinks. ACS Appl. Mater. Interfaces 2020, 12, 47309–47319. [Google Scholar] [CrossRef] [PubMed]
- Tchobanian, A.; Van Oosterwyck, H.; Fardim, P. Polysaccharides for tissue engineering: Current landscape and future prospects. Carbohydr. Polym. 2019, 205, 601–625. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Grinstaff, M.W. Chemical synthesis of polysaccharides and polysaccharide mimetics. Prog. Polym. Sci. 2017, 74, 78–116. [Google Scholar] [CrossRef]
- DeVree, B.T.; Steiner, L.M.; Głazowska, S.; Ruhnow, F.; Herburger, K.; Persson, S.; Mravec, J. Current and future advances in fluorescence-based visualization of plant cell wall components and cell wall biosynthetic machineries. Biotechnol. Biofuels 2021, 14, 78. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Sigroha, S.; Khatkar, A. Chitosan—A Naturally Derived Antioxidant Polymer with Diverse Applications. Curr. Org. Chem. 2017, 21, 333–341. [Google Scholar] [CrossRef]
- Simó, G.; Fernández-Fernández, E.; Vila-Crespo, J.; Ruipérez, V.; Rodríguez-Nogales, J.M. Research progress in coating techniques of alginate gel polymer for cell encapsulation. Carbohydr. Polym. 2017, 170, 1–14. [Google Scholar] [CrossRef]
- Gu, Y.; Cheong, K.L.; Du, H. Modification and comparison of three Gracilaria spp. agarose with methylation for promotion of its gelling properties. Chem. Cent. J. 2017, 11, 104. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mehta, M.; Satija, S.; Aljabali, A.A.; Tambuwala, M.M.; Anand, K.; Sharma, N.; Dureja, H.; Jha, N.K.; et al. Current-status and applications of polysaccharides in drug delivery systems. Colloid Interface Sci. Commun. 2021, 42, 100418. [Google Scholar] [CrossRef]
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D.A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2021, 27, 604–626. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, M.; Wang, M.; Hu, Q.; Liu, J.; Duan, Y.; Liu, B. Direct Synthesis of Photosensitizable Bacterial Cellulose as Engineered Living Material for Skin Wound Repair. Adv. Mater. 2022, 34, 2109010. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenko, V.; Karabulut, E.; Pernevik, E.; Enoksson, P.; Gatenholm, P. Tailor-made conductive inks from cellulose nanofibrils for 3D printing of neural guidelines. Carbohydr. Polym. 2018, 189, 22–30. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G. Potential and Limitations of Nanocelluloses as Components in Biocomposite Inks for Three-Dimensional Bioprinting and for Biomedical Devices. Biomacromolecules 2018, 19, 701–711. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Processing and characterization of natural cellulose fibers/thermoset polymer composites. Carbohydr. Polym. 2014, 109, 102–117. [Google Scholar] [CrossRef]
- Piras, C.C.; Fernandez-Prieto, S.; De Borggraeve, W.M. Nanocellulosic materials as bioinks for 3D bioprinting. Biomater. Sci. 2017, 5, 1988–1992. [Google Scholar] [CrossRef]
- Torres-Rendon, J.G.; Kopf, M.; Gehlen, D.; Blaeser, A.; Fischer, H.; De Laporte, L.; Walther, A. Cellulose Nanofibril Hydrogel Tubes as Sacrificial Templates for Freestanding Tubular Cell Constructs. Biomacromolecules 2016, 17, 905–913. [Google Scholar] [CrossRef]
- Abraham, E.; Weber, D.E.; Sharon, S.; Lapidot, S.; Shoseyov, O. Multifunctional Cellulosic Scaffolds from Modified Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2017, 9, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications. Carbohydr. Polym. 2021, 256, 117561. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.L.; Soliman, A.A.F.; Ali, E.A.; Abou-Zeid, N.Y.; Nada, A.A. Hydrogel bioink based on clickable cellulose derivatives: Synthesis, characterization and In Vitro assessment. Int. J. Biol. Macromol. 2020, 163, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, Z.; Ma, L.; Yin, J.; Gao, Z.; Shen, L.; Yang, H.; Cui, Z.; Ye, H.; Zhou, H. A versatile embedding medium for freeform bioprinting with multi-crosslinking methods. Biofabrication 2022, 14, 035022. [Google Scholar] [CrossRef]
- Khoshnood, N.; Shahrezaee, M.H.; Shahrezaee, M.; Zamanian, A. Three-dimensional bioprinting of tragacanth/hydroxyapaptite modified alginate bioinks for bone tissue engineering with tunable printability and bioactivity. J. Appl. Polym. Sci. 2022, 139, e52833. [Google Scholar] [CrossRef]
- Ooi, H.W.; Mota, C.; Ten Cate, A.T.; Calore, A.; Moroni, L.; Baker, M.B. Thiol-Ene Alginate Hydrogels as Versatile Bioinks for Bioprinting. Biomacromolecules 2018, 19, 3390–3400. [Google Scholar] [CrossRef]
- Barcelo, X.; Eichholz, K.F.; Garcia, O.; Kelly, D.J. Tuning the Degradation Rate of Alginate-Based Bioinks for Bioprinting Functional Cartilage Tissue. Biomedicines 2022, 10, 1621. [Google Scholar] [CrossRef]
- Piras, C.C.; Smith, D.K. Multicomponent polysaccharide alginate-based bioinks. J. Mater. Chem. B 2020, 8, 8171–8188. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, M.; He, H.; Liang, Q.; Hu, C.; Zeng, Z.; Cheng, D.; Wang, G.; Chen, D.; Pan, H.; et al. 3D Printing of Mechanically Stable Calcium-Free Alginate-Based Scaffolds with Tunable Surface Charge to Enable Cell Adhesion and Facile Biofunctionalization. Adv. Funct. Mater. 2019, 29, 1808439. [Google Scholar] [CrossRef]
- Gu, Y.; Schwarz, B.; Forget, A.; Barbero, A.; Martin, I.; Shastri, V.P. Advanced Bioink for 3D Bioprinting of Complex Free-Standing Structures with High Stiffness. Bioengineering 2020, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Nadernezhad, A.; Caliskan, O.S.; Topuz, F.; Afghah, F.; Erman, B.; Koc, B. Nanocomposite Bioinks Based on Agarose and 2D Nanosilicates with Tunable Flow Properties and Bioactivity for 3D Bioprinting. ACS Appl. Bio Mater. J. 2019, 2, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Zhang, M.; Zeng, Q.; Pan, W.; Huang, Y.; Qian, Y.; Dong, W.; Qi, X.; Shen, J. Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioact. Mater. 2021, 6, 579–588. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Goversen, B.; de Ruijter, M.; Abbadessa, A.; Melchels, F.P.W.; Oner, F.C.; Dhert, W.J.A.; Vermonden, T.; Alblas, J. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef]
- Antich, C.; de Vicente, J.; Jimenez, G.; Chocarro, C.; Carrillo, E.; Montanez, E.; Galvez-Martin, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Hossain Rakin, R.; Kumar, H.; Rajeev, A.; Natale, G.; Menard, F.; Li, I.T.S.; Kim, K. Tunable metacrylated hyaluronic acid-based hybrid bioinks for stereolithography 3D bioprinting. Biofabrication 2021, 13, 044109. [Google Scholar] [CrossRef]
- Zhou, K.; Feng, M.; Mao, H.; Gu, Z. Photoclick polysaccharide-based bioinks with an extended biofabrication window for 3D embedded bioprinting. Biomater. Sci. 2022, 10, 4479–4491. [Google Scholar] [CrossRef]
- Coskun, S.; Akbulut, S.O.; Sarikaya, B.; Cakmak, S.; Gumusderelioglu, M. Formulation of chitosan and chitosan-nanoHAp bioinks and investigation of printability with optimized bioprinting parameters. Int. J. Biol. Macromol. 2022, 222, 1453–1464. [Google Scholar] [CrossRef]
- He, Y.; Wang, F.; Wang, X.; Zhang, J.; Wang, D.; Huang, X. A photocurable hybrid chitosan/acrylamide bioink for DLP based 3D bioprinting. Mater. Des. 2021, 202, 109588. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.; Huang, X.; Hang, R.; Zhang, X.; Wang, Y.; Yao, X. DLP printing photocurable chitosan to build bio-constructs for tissue engineering. Carbohydr. Polym. 2020, 235, 115970. [Google Scholar] [CrossRef]
- Demirtas, T.T.; Irmak, G.; Gumusderelioglu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, M.; Chen, Z.; Zhang, T.; Huang, J.; Dai, J.; Zhang, Z. 3D bioprinted neural tissue constructs for spinal cord injury repair. Biomaterials 2021, 272, 120771. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi Nagahara, M.H.; Caiado Decarli, M.; Inforçatti Neto, P.; da Silva, J.V.L.; Moraes, Â.M. Crosslinked alginate-xanthan gum blends as effective hydrogels for 3D bioprinting of biological tissues. J. Appl. Polym. Sci. 2022, 139, e52612. [Google Scholar] [CrossRef]
- Shapira, A.; Noor, N.; Oved, H.; Dvir, T. Transparent support media for high resolution 3D printing of volumetric cell-containing ECM structures. Biomed. Mater. 2020, 15, 045018. [Google Scholar] [CrossRef]
- Lim, W.; Shin, S.Y.; Cha, J.M.; Bae, H. Optimization of Polysaccharide Hydrocolloid for the Development of Bioink with High Printability/Biocompatibility for Coextrusion 3D Bioprinting. Polymers 2021, 13, 1773. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Lin, Q.; Liu, H.; Shen, C.; Nan, K.; Chen, H. In Vitro and In Vivo evaluation of xanthan gum-succinic anhydride hydrogels for the ionic strength-sensitive release of antibacterial agents. J. Mater. Chem. B 2016, 4, 1853–1861. [Google Scholar] [CrossRef]
- Luo, W.; Song, Z.; Wang, Z.; Wang, Z.; Li, Z.; Wang, C.; Liu, H.; Liu, Q.; Wang, J. Printability Optimization of Gelatin-Alginate Bioinks by Cellulose Nanofiber Modification for Potential Meniscus Bioprinting. J. Nanomater. 2020, 2020, 863428. [Google Scholar] [CrossRef]
- Das, R.; Lee, C.P.; Prakash, A.; Hashimoto, M.; Fernandez, J.G. Geometrical control of degradation and cell delivery in 3D printed nanocellulose hydrogels. Mater. Today Commun. 2022, 30, 103023. [Google Scholar] [CrossRef]
- Mu, H.; Wang, Y.; Wei, H.; Lu, H.; Feng, Z.; Yu, H.; Xing, Y.; Wang, H. Collagen peptide modified carboxymethyl cellulose as both antioxidant drug and carrier for drug delivery against retinal ischaemia/reperfusion injury. J. Cell. Mol. Med. 2018, 22, 5008–5019. [Google Scholar] [CrossRef]
- Yang, J.; He, H.; Li, D.; Zhang, Q.; Xu, L.; Ruan, C. Advanced strategies in the application of gelatin-based bioink for extrusion bioprinting. Bio-Des. Manuf. 2023, 6, 586–608. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, S.; Liu, Y.; Guo, F.; Miao, Q.; Huang, H. A composite hydrogel scaffold based on collagen and carboxymethyl chitosan for cartilage regeneration through one-step chemical crosslinking. Int. J. Biol. Macromol. 2023, 226, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Yalman, V.; Laçin, N.T. Development of humic acid and alginate-based wound dressing and evaluation on inflammation. Mater. Technol. 2019, 34, 705–717. [Google Scholar] [CrossRef]
- Wong, C.-C.; Lu, C.-X.; Cho, E.-C.; Lee, P.-W.; Chi, N.-W.; Lin, P.-Y.; Jheng, P.-R.; Chen, H.-L.; Mansel, B.W.; Chen, Y.-M.; et al. Calcium peroxide aids tyramine-alginate gel to crosslink with tyrosinase for efficient cartilage repair. Int. J. Biol. Macromol. 2022, 208, 299–313. [Google Scholar] [CrossRef]
- Rosiak, P.; Latanska, I.; Paul, P.; Sujka, W.; Kolesinska, B. Modification of Alginates to Modulate Their Physic-Chemical Properties and Obtain Biomaterials with Different Functional Properties. Molecules 2021, 26, 7264. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of Partially Oxidized Alginate and Its Potential Application for Tissue Engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Hurtado, A.; Aljabali, A.A.A.; Mishra, V.; Tambuwala, M.M.; Serrano-Aroca, Á. Alginate: Enhancement Strategies for Advanced Applications. Int. J. Mol. Sci. 2022, 23, 4486. [Google Scholar] [CrossRef]
- Dalheim, M.O.; Vanacker, J.; Najmi, M.A.; Aachmann, F.L.; Strand, B.L.; Christensen, B.E. Efficient functionalization of alginate biomaterials. Biomaterials 2016, 80, 146–156. [Google Scholar] [CrossRef]
- Ramos, P.E.; Silva, P.; Alario, M.M.; Pastrana, L.M.; Teixeira, J.A.; Cerqueira, M.A.; Vicente, A.A. Effect of alginate molecular weight and M/G ratio in beads properties foreseeing the protection of probiotics. Food Hydrocoll. 2018, 77, 8–16. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking Strategies for 3D Bioprinting of Polymeric Hydrogels. Small 2020, 16, 2002931. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, S.J.; Chung, S.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Cell-laden 3D bioprinting hydrogel matrix depending on different compositions for soft tissue engineering: Characterization and evaluation. Mater. Sci. Eng. C 2017, 71, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Stanisci, A.; Aarstad, O.A.; Tondervik, A.; Sletta, H.; Dypas, L.B.; Skjak-Braek, G.; Aachmann, F.L. Overall size of mannuronan C5-Epimerases influences their ability to epimerize modified alginates and alginate gels. Carbohydr. Polym. 2018, 180, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, A.A.H.; Elsayed, N.H.; Monier, M. Development and characterization of photo-responsive cinnamoly modified alginate. Carbohydr. Polym. 2021, 260, 117771. [Google Scholar] [CrossRef]
- Im, S.; Choe, G.; Seok, J.M.; Yeo, S.J.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. An osteogenic bioink composed of alginate, cellulose nanofibrils, and polydopamine nanoparticles for 3D bioprinting and bone tissue engineering. Int. J. Biol. Macromol. 2022, 205, 520–529. [Google Scholar] [CrossRef]
- Habib, A.; Khoda, B. Development of clay based novel hybrid bio-ink for 3D bio-printing process. J. Manuf. Process. 2019, 38, 76–87. [Google Scholar] [CrossRef]
- Yao, B.; Hu, T.; Cui, X.; Song, W.; Fu, X.; Huang, S. Enzymatically degradable alginate/gelatin bioink promotes cellular behavior and degradation In Vitro and In Vivo. Biofabrication 2019, 11, 045020. [Google Scholar] [CrossRef]
- Zhu, Y.; Stark, C.J.; Madira, S.; Ethiraj, S.; Venkatesh, A.; Anilkumar, S.; Jung, J.; Lee, S.; Wu, C.A.; Walsh, S.K.; et al. Three-Dimensional Bioprinting with Alginate by Freeform Reversible Embedding of Suspended Hydrogels with Tunable Physical Properties and Cell Proliferation. Bioengineering 2022, 9, 807. [Google Scholar] [CrossRef]
- Singh, Y.P.; Bhardwaj, N.; Mandal, B.B. Potential of Agarose/Silk Fibroin Blended Hydrogel for In Vitro Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 21236–21249. [Google Scholar] [CrossRef]
- Ionescu, A.-M.; Alaminos, M.; Cardona, J.d.l.C.; García-López Durán, J.d.D.; González-Andrades, M.; Ghinea, R.; Campos, A.; Hita, E.; Pérez, M.d.M. Investigating a novel nanostructured fibrin–agarose biomaterial for human cornea tissue engineering: Rheological properties. J. Mech. Behav. Biomed. Mater. 2011, 4, 1963–1973. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Z.; Cheng, D.; Mao, X. Agarose degradation for utilization: Enzymes, pathways, metabolic engineering methods and products. Biotechnol. Adv. 2020, 45, 107641. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Tian, X.; Luo, S.; Yuan, D.; Xu, S.; Yang, L.; Ma, M.; Ye, C. Agarose composite hydrogel and PVA sacrificial materials for bioprinting large-scale, personalized face-like with nutrient networks. Carbohydr. Polym. 2021, 269, 118222. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Marcial, G.R.; Zeng, A.Y.; Osuna, C.; Dennis, J.; Garcia, J.M.; O’Connell, G.D. Agarose-Based Hydrogels as Suitable Bioprinting Materials for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 3610–3616. [Google Scholar] [CrossRef] [PubMed]
- Forget, A.; Derme, T.; Mitterberger, D.; Heiny, M.; Sweeney, C.; Mudili, L.; Dargaville, T.R.; Shastri, V.P. Architecture-inspired paradigm for 3D bioprinting of vessel-like structures using extrudable carboxylated agarose hydrogels. Emergent Mater. 2019, 2, 233–243. [Google Scholar] [CrossRef]
- Su, Y.; Chu, B.; Gao, Y.; Wu, C.; Zhang, L.; Chen, P.; Wang, X.; Tang, S. Modification of agarose with carboxylation and grafting dopamine for promotion of its cell-adhesiveness. Carbohydr. Polym. 2013, 92, 2245–2251. [Google Scholar] [CrossRef]
- Lee, S.-w.; Kim, J.; Do, M.; Namkoong, E.; Lee, H.; Ryu, J.H.; Park, K. Developmental role of hyaluronic acid and its application in salivary gland tissue engineering. Acta Biomater. 2020, 115, 275–287. [Google Scholar] [CrossRef]
- Singh, D.; Wang, S.-B.; Xia, T.; Tainsh, L.; Ghiassi-Nejad, M.; Xu, T.; Peng, S.; Adelman, R.A.; Rizzolo, L.J. A biodegradable scaffold enhances differentiation of embryonic stem cells into a thick sheet of retinal cells. Biomaterials 2018, 154, 158–168. [Google Scholar] [CrossRef]
- Grandoch, M.; Bollyky, P.L.; Fischer, J.W. Hyaluronan: A Master Switch Between Vascular Homeostasis and Inflammation. Circ. Res. 2018, 122, 1341–1343. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.J.; Kumar, S. Hyaluronic Acid: Incorporating the Bio into the Material. ACS Biomater. Sci. Eng. 2019, 5, 3753–3765. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.M.; Sanders, M.E.; Kiyotake, E.A.; Detamore, M.S. Independent control of molecular weight, concentration, and stiffness of hyaluronic acid hydrogels. Biomed. Mater. 2022, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Krishnan, N.; Mokhtari, H.; Oommen, O.P.; Varghese, O.P. Fine-tuning Dynamic Cross–linking for Enhanced 3D Bioprinting of Hyaluronic Acid Hydrogels. Adv. Funct. Mater. 2024, 34, 2307040. [Google Scholar] [CrossRef]
- Gamarra, A.; Missagia, B.; Urpí, L.; Morató, J.; Muñoz-Guerra, S. Ionic coupling of hyaluronic acid with ethyl N-lauroyl l-arginate (LAE): Structure, properties and biocide activity of complexes. Carbohydr. Polym. 2018, 197, 109–116. [Google Scholar] [CrossRef]
- Fan, F.; Su, B.; Kolodychak, A.; Ekwueme, E.; Alderfer, L.; Saha, S.; Webber, M.J.; Hanjaya-Putra, D. Hyaluronic Acid Hydrogels with Phototunable Supramolecular Cross-Linking for Spatially Controlled Lymphatic Tube Formation. ACS Appl. Mater. Interfaces 2023, 15, 58181–58195. [Google Scholar] [CrossRef]
- Kotla, N.G.; Bonam, S.R.; Rasala, S.; Wankar, J.; Bohara, R.A.; Bayry, J.; Rochev, Y.; Pandit, A. Recent advances and prospects of hyaluronan as a multifunctional therapeutic system. J. Control. Release 2021, 336, 598–620. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Dhivya, S.; Selvamurugan, N. Chitosan based nanofibers in bone tissue engineering. Int. J. Biol. Macromol. 2017, 104, 1372–1382. [Google Scholar] [CrossRef]
- Chesnutt, B.M.; Yuan, Y.; Buddington, K.; Haggard, W.O.; Bumgardner, J.D. Composite Chitosan/Nano-Hydroxyapatite Scaffolds Induce Osteocalcin Production by Osteoblasts In Vitro and Support Bone Formation In Vivo. Tissue Eng. Part A 2009, 15, 2571–2579. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemes, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Wu, P.; Xi, X.; Li, R.; Sun, G. Engineering Polysaccharides for Tissue Repair and Regeneration. Macromol. Biosci. 2021, 21, e2100141. [Google Scholar] [CrossRef]
- Levengood, S.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Ji, J.; Wang, L.; Yu, H.; Chen, Y.; Zhao, Y.; Zhang, H.; Amer, W.A.; Sun, Y.; Huang, L.; Saleem, M. Chemical Modifications of Chitosan and Its Applications. Polym.-Plast. Technol. Eng. 2014, 53, 1494–1505. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Ali, A.; Sheikh, J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018, 116, 849–862. [Google Scholar] [CrossRef]
- Rezaei, F.S.; Sharifianjazi, F.; Esmaeilkhanian, A.; Salehi, E. Chitosan films and scaffolds for regenerative medicine applications: A review. Carbohydr. Polym. 2021, 273, 118631. [Google Scholar] [CrossRef] [PubMed]
- Klaypradit, W.; Huang, Y.-W. Fish oil encapsulation with chitosan using ultrasonic atomizer. LWT-Food Sci. Technol. 2008, 41, 1133–1139. [Google Scholar] [CrossRef]
- Choo, C.K.; Kong, X.Y.; Goh, T.L.; Ngoh, G.C.; Horri, B.A.; Salamatinia, B. Chitosan/halloysite beads fabricated by ultrasonic-assisted extrusion-dripping and a case study application for copper ion removal. Carbohydr. Polym. 2016, 138, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chang, P.R.; Chen, M.; Wu, Q. Chitosan colloidal suspension composed of mechanically disassembled nanofibers. J. Colloid Interface Sci. 2011, 354, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Brysch, C.N.; Wold, E.; Patterson, M.; Ordoñez Olivares, R.; Eberth, J.F.; Robles Hernandez, F.C. Chitosan and chitosan composites reinforced with carbon nanostructures. J. Alloys Compd. 2014, 615, S515–S521. [Google Scholar] [CrossRef]
- Yue, W.; He, R.; Yao, P.; Wei, Y. Ultraviolet radiation-induced accelerated degradation of chitosan by ozone treatment. Carbohydr. Polym. 2009, 77, 639–642. [Google Scholar] [CrossRef]
- Kurita, K.; Ikeda, H.; Yoshida, Y.; Shimojoh, M.; Harata, M. Chemoselective Protection of the Amino Groups of Chitosan by Controlled Phthaloylation: Facile Preparation of a Precursor Useful for Chemical Modifications. Biomacromolecules 2002, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejska, M.; Jankowska, K.; Klak, M.; Wszola, M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials 2021, 11, 3019. [Google Scholar] [CrossRef]
- Yang, J.; Xie, Q.; Zhu, J.; Zou, C.; Chen, L.; Du, Y.; Li, D. Preparation and In Vitro antioxidant activities of 6-amino-6-deoxychitosan and its sulfonated derivatives. Biopolymers 2015, 103, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, A.; Kathiresan, K.; Nayak, L. Preparation, characterization and antibacterial activity of chitosan and phosphorylated chitosan from cuttlebone of Sepia kobiensis (Hoyle, 1885). Biotechnol. Rep. 2016, 9, 25–30. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef]
- Aberg, C.M.; Chen, T.; Olumide, A.; Raghavan, S.R.; Payne, G.F. Enzymatic Grafting of Peptides from Casein Hydrolysate to Chitosan. Potential for Value-Added Byproducts from Food-Processing Wastes. J. Agric. Food Chem. 2004, 52, 788–793. [Google Scholar] [CrossRef]
- Wang, D.; Lv, P.; Zhang, L.; Yang, S.; Gao, Y. Structural and Functional Characterization of Laccase-Induced β-Lactoglobulin-Ferulic Acid-Chitosan Ternary Conjugates. J. Agric. Food Chem. 2019, 67, 12054–12060. [Google Scholar] [CrossRef] [PubMed]
- Hajikhani, M.; Khanghahi, M.M.; Shahrousvand, M.; Mohammadi-Rovshandeh, J.; Babaei, A.; Khademi, S.M.H. Intelligent superabsorbents based on a xanthan gum/poly (acrylic acid) semi-interpenetrating polymer network for application in drug delivery systems. Int. J. Biol. Macromol. 2019, 139, 509–520. [Google Scholar] [CrossRef]
- Piola, B.; Sabbatini, M.; Gino, S.; Invernizzi, M.; Reno, F. 3D Bioprinting of Gelatin-Xanthan Gum Composite Hydrogels for Growth of Human Skin Cells. Int. J. Mol. Sci. 2022, 23, 539. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- Zhong, L.; Oostrom, M.; Truex, M.J.; Vermeul, V.R.; Szecsody, J.E. Rheological behavior of xanthan gum solution related to shear thinning fluid delivery for subsurface remediation. J. Hazard. Mater. 2013, 244–245, 160–170. [Google Scholar] [CrossRef]
- Choppe, E.; Puaud, F.; Nicolai, T.; Benyahia, L. Rheology of xanthan solutions as a function of temperature, concentration and ionic strength. Carbohydr. Polym. 2010, 82, 1228–1235. [Google Scholar] [CrossRef]
- Arimura, T.; Omagari, Y.; Yamamoto, K.; Kadokawa, J.-i. Chemoenzymatic synthesis and hydrogelation of amylose-grafted xanthan gums. Int. J. Biol. Macromol. 2011, 49, 498–503. [Google Scholar] [CrossRef]
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Masoodi, F.A. Advances in xanthan gum production, modifications and its applications. Biocatal. Agric. Biotechnol. 2022, 42, 102328. [Google Scholar] [CrossRef]
- Pinto, E.P.; Furlan, L.; Vendruscolo, C.T. Chemical deacetylation natural xanthan (Jungbunzlauer®). Polímeros 2011, 21, 47–52. [Google Scholar] [CrossRef]
- Jena, S.R.; Dalei, G.; Das, S.; Nayak, J.; Pradhan, M.; Samanta, L. Harnessing the potential of dialdehyde alginate-xanthan gum hydrogels as niche bioscaffolds for tissue engineering. Int. J. Biol. Macromol. 2022, 207, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Hayrabolulu, H.; Demeter, M.; Cutrubinis, M.; Şen, M. Radiation synthesis and characterization of xanthan gum hydrogels. Radiat. Phys. Chem. 2021, 188, 109613. [Google Scholar] [CrossRef]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Kort-Mascort, J.; Kinsella, J.M. Extrusion bioprinting of soft materials: An emerging technique for biological model fabrication. Appl. Phys. Rev. 2019, 6, 011310. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, H.; Jiang, J.; Zhao, C.; Zhang, J.; Chen, P.; Lin, X.; Fan, S. Desktop-Stereolithography 3D Printing of a Polyporous Extracellular Matrix Bioink for Bone Defect Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 589094. [Google Scholar] [CrossRef] [PubMed]
- Compaan, A.M.; Christensen, K.; Huang, Y. Inkjet Bioprinting of 3D Silk Fibroin Cellular Constructs Using Sacrificial Alginate. ACS Biomater. Sci. Eng. 2017, 3, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef]
- Lian, L.; Zhou, C.; Tang, G.; Xie, M.; Wang, Z.; Luo, Z.; Japo, J.; Wang, D.; Zhou, J.; Wang, M.; et al. Uniaxial and Coaxial Vertical Embedded Extrusion Bioprinting. Adv. Healthc. Mater. 2022, 11, 2102411. [Google Scholar] [CrossRef]
- Tembadamani, S.; Mohan, T.S.; Thrivikraman, G.; Muthuvijayan, V.; Barman, S.R. Engineering Polysaccharide Biomaterials: Modifications and Crosslinking Strategies for Soft Tissue Bioprinting. Macromol. Rapid Commun. 2025, in press. e00236. [Google Scholar] [CrossRef]
- Vajda, J.; Vihar, B.; Ćurić, L.Č.; Maver, U.; Vesenjak, M.; Dubrovski, P.D.; Milojević, M. Sr2+ vs. Ca2+ as post-processing ionic crosslinkers: Implications for 3D bioprinting of polysaccharide hydrogels in tissue engineering. J. Mater. Res. Technol. 2023, 23, 1805–1820. [Google Scholar] [CrossRef]
- Nagaraja, K.; Dhokare, P.; Bhattacharyya, A.; Noh, I. Recent advances in 3D bioprinting of polysaccharide-based bioinks for fabrication of bioengineered tissues. Mol. Syst. Des. Eng. 2024, 9, 977–999. [Google Scholar] [CrossRef]
- Kaith, A.; Jain, N.; Kaul, S.; Nagaich, U. Polysaccharide-infused bio-fabrication: Advancements in 3D bioprinting for tissue engineering and bone regeneration. Mater. Today Commun. 2024, 40, 109429. [Google Scholar] [CrossRef]
- Lin, S.; Li, B.; Yang, L.; Zhai, Y.; Wang, X.; Wang, C. New method for reducing viscosity and shear stress in hydrogel 3D printing via multidimension vibration. Comput. Methods Biomech. Biomed. Engin 2022, 25, 1796–1811. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Mao, S.; Sun, W.; Yao, R. The influence of printing parameters on cell survival rate and printability in microextrusion-based 3D cell printing technology. Biofabrication 2015, 7, 045002. [Google Scholar] [CrossRef] [PubMed]
- Kiyotake, E.A.; Douglas, A.W.; Thomas, E.E.; Nimmo, S.L.; Detamore, M.S. Development and quantitative characterization of the precursor rheology of hyaluronic acid hydrogels for bioprinting. Acta Biomater. 2019, 95, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.T.; Basu, A.; Saha, A.; Nelson, A. Chemical modification and printability of shear-thinning hydrogel inks for direct-write 3D printing. Polymer 2018, 152, 42–50. [Google Scholar] [CrossRef]
- Rawal, P.; Tripathi, D.M.; Ramakrishna, S.; Kaur, S. Prospects for 3D bioprinting of organoids. Bio-Des. Manuf. 2021, 4, 627–640. [Google Scholar] [CrossRef]
- Distler, T.; Solisito, A.A.; Schneidereit, D.; Friedrich, O.; Detsch, R.; Boccaccini, A.R. 3D printed oxidized alginate-gelatin bioink provides guidance for C2C12 muscle precursor cell orientation and differentiation via shear stress during bioprinting. Biofabrication 2020, 12, 045005. [Google Scholar] [CrossRef]
- Nair, K.; Gandhi, M.; Khalil, S.; Yan, K.C.; Marcolongo, M.; Barbee, K.; Sun, W. Characterization of cell viability during bioprinting processes. Biotechnol. J. 2009, 4, 1168–1177. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Emmermacher, J.; Spura, D.; Cziommer, J.; Kilian, D.; Wollborn, T.; Fritsching, U.; Steingroewer, J.; Walther, T.; Gelinsky, M.; Lode, A. Engineering considerations on extrusion-based bioprinting: Interactions of material behavior, mechanical forces and cells in the printing needle. Biofabrication 2020, 12, 025022. [Google Scholar] [CrossRef]
- Adhikari, J.; Roy, A.; Das, A.; Ghosh, M.; Thomas, S.; Sinha, A.; Kim, J.; Saha, P. Effects of Processing Parameters of 3D Bioprinting on the Cellular Activity of Bioinks. Macromol. Biosci. 2021, 21, e2000179. [Google Scholar] [CrossRef]
- Ribeiro, A.; Blokzijl, M.M.; Levato, R.; Visser, C.W.; Castilho, M.; Hennink, W.E.; Vermonden, T.; Malda, J. Assessing bioink shape fidelity to aid material development in 3D bioprinting. Biofabrication 2017, 10, 014102. [Google Scholar] [CrossRef]
- Sotorrío, G.; Alonso, J.; Olsson, N.O.E.; Tenorio, J.A. Printability of materials for extrusion 3D printing technologies: A review of material requirements and testin. Mater. De Construcción 2021, 71, 344. [Google Scholar] [CrossRef]
- Gillispie, G.; Prim, P.; Copus, J.; Fisher, J.; Mikos, A.G.; Yoo, J.J.; Atala, A.; Lee, S.J. Assessment methodologies for extrusion-based bioink printability. Biofabrication 2020, 12, 022003. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, X.; Sun, Y.; Gao, Q.; Fu, J.; Cai, X.; He, Y. Printability during projection-based 3D bioprinting. Bioact. Mater. 2022, 11, 254–267. [Google Scholar] [CrossRef]
- Fu, Z.; Naghieh, S.; Xu, C.; Wang, C.; Sun, W.; Chen, X. Printability in extrusion bioprinting. Biofabrication 2021, 13. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.E.; Luo, L.; Zhou, Y.; Si, P. Iterative feedback bio-printing-derived cell-laden hydrogel scaffolds with optimal geometrical fidelity and cellular controllability. Sci. Rep. 2018, 8, 2802. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Pr, A.K.; Seol, Y.J.; Atala, A.; Yoo, J.J.; Lee, S.J. Optimization of gelatin-alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication 2018, 10, 034106. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jiang, T.; Kinsella, J.M.; Shang, J.; Luo, Z. Assessing roughness of extrusion printed soft materials using a semi-quantitative method. Mater. Lett. 2021, 303, 130480. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Hazur, J.; Detsch, R.; Karakaya, E.; Kaschta, J.; Tessmar, J.; Schneidereit, D.; Friedrich, O.; Schubert, D.W.; Boccaccini, A.R. Improving alginate printability for biofabrication: Establishment of a universal and homogeneous pre-crosslinking technique. Biofabrication 2020, 12, 045004. [Google Scholar] [CrossRef]
- Nelson, C.; Tuladhar, S.; Launen, L.; Habib, A. 3D Bio-Printability of Hybrid Pre-Crosslinked Hydrogels. Int. J. Mol. Sci. 2021, 22, 13481. [Google Scholar] [CrossRef]
- Giuseppe, M.D.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Kozusko, S.D.; Riccio, C.; Goulart, M.; Bumgardner, J.; Jing, X.L.; Konofaos, P. Chitosan as a Bone Scaffold Biomaterial. J. Craniofacial Surg. 2018, 29, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martinez Avila, H.; Hagg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Flegeau, K.; Puiggali-Jou, A.; Zenobi-Wong, M. Cartilage tissue engineering by extrusion bioprinting utilizing porous hyaluronic acid microgel bioinks. Biofabrication 2022, 14, 3. [Google Scholar] [CrossRef]
- Kreimendahl, F.; Kopf, M.; Thiebes, A.L.; Duarte Campos, D.F.; Blaeser, A.; Schmitz-Rode, T.; Apel, C.; Jockenhoevel, S.; Fischer, H. Three-Dimensional Printing and Angiogenesis: Tailored Agarose-Type I Collagen Blends Comprise Three-Dimensional Printability and Angiogenesis Potential for Tissue-Engineered Substitutes. Tissue Eng. Part C Methods 2017, 23, 604–615. [Google Scholar] [CrossRef]
- Mendes, A.C.; Baran, E.T.; Pereira, R.C.; Azevedo, H.S.; Reis, R.L. Encapsulation and survival of a chondrocyte cell line within xanthan gum derivative. Macromol. Biosci. 2012, 12, 350–359. [Google Scholar] [CrossRef]
- Schwarz, S.; Kuth, S.; Distler, T.; Gogele, C.; Stolzel, K.; Detsch, R.; Boccaccini, A.R.; Schulze-Tanzil, G. 3D printing and characterization of human nasoseptal chondrocytes laden dual crosslinked oxidized alginate-gelatin hydrogels for cartilage repair approaches. Mater. Sci. Eng. C 2020, 116, 111189. [Google Scholar] [CrossRef]
- Stumberger, G.; Vihar, B. Freeform Perfusable Microfluidics Embedded in Hydrogel Matrices. Materials 2018, 11, 2529. [Google Scholar] [CrossRef]
- Antunes, M.; Bonani, W.; Reis, R.L.; Migliaresi, C.; Ferreira, H.; Motta, A.; Neves, N.M. Development of alginate-based hydrogels for blood vessel engineering. Mater. Sci. Eng. C 2021, 134, 112588. [Google Scholar] [CrossRef]
- Dogan, L.; Scheuring, R.; Wagner, N.; Ueda, Y.; Schmidt, S.; Wörsdörfer, P.; Groll, J.; Ergün, S. Human iPSC-derived mesodermal progenitor cells preserve their vasculogenesis potential after extrusion and form hierarchically organized blood vessels. Biofabrication 2021, 13, 045028. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Faccendini, A.; Del Favero, E.; Di Cola, E.; Icaro Cornaglia, A.; Boselli, C.; Luxbacher, T.; et al. Chitosan/glycosaminoglycan scaffolds for skin reparation. Carbohydr. Polym. 2019, 220, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Somasekharan, L.T.; Raju, R.; Kumar, S.; Geevarghese, R.; Nair, R.P.; Kasoju, N.; Bhatt, A. Biofabrication of skin tissue constructs using alginate, gelatin and diethylaminoethyl cellulose bioink. Int. J. Biol. Macromol. 2021, 189, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D Bioprinting of heterogeneous aortic valve conduits with alginate_gelatin hydrogels. J. Biomed. Mater. Res. Part A 2012, 101, 1255–1264. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Duan, B.; Kapetanovic, E.; Hockaday, L.A.; Butcher, J.T. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014, 10, 1836–1846. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Lameirinhas, N.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. A Guide to Polysaccharide-Based Hydrogel Bioinks for 3D Bioprinting Applications. Int. J. Mol. Sci. 2022, 23, 6564. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczesna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Chen, T.; Chen, X. A review of the antibacterial activity and mechanisms of plant polysaccharides. Trends Food Sci. Technol. 2022, 123, 264–280. [Google Scholar] [CrossRef]
- Jo, Y.; Hwang, D.G.; Kim, M.; Yong, U.; Jang, J. Bioprinting-assisted tissue assembly to generate organ substitutes at scale. Trends Biotechnol. 2023, 41, 93–105. [Google Scholar] [CrossRef]
- An, J.; Chua, C.K.; Mironov, V. Application of Machine Learning in 3D Bioprinting: Focus on Development of Big Data and Digital Twin. Int. J. Bioprinting 2024, 7, 342. [Google Scholar] [CrossRef]
- Shokrani, H.; Shokrani, A.; Seidi, F.; Kucińska-Lipka, J.; Makurat-Kasprolewicz, B.; Saeb, M.R.; Ramakrishna, S. Artificial intelligence for biomedical engineering of polysaccharides: A short overview. Curr. Opin. Biomed. Eng. 2023, 27, 100463. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, J.; Kim, H.; Ritchie, R.O.; Gu, G.X. Advancing programmable metamaterials through machine learning-driven buckling strength optimization. Curr. Opin. Solid State Mater. Sci. 2024, 31, 101161. [Google Scholar] [CrossRef]
| Polysaccharides | Substrate Material | Method or Substance of Modification | Target Substance | Applications | Characteristics | Refs. |
|---|---|---|---|---|---|---|
| Plant polysaccharides | Cellulose | Mechanical shearing | Nanofibrillated cellulose | 3D bioprinting and the automated fabrication of complex tissue-mimicking constructs | Accessibility, biocompatibility, and shear-thinning properties | [11] |
| Nanometer granulation | Cellulose nanocrystals | Biobased cellulosic scaffold material | Hydrophobicity, oleophilicity, and lipophilicity | [27] | ||
| Alkalization/mercerisation and etherification reactions | Carboxymethyl cellulose | Bioinks for printing bioconstructs | Good viscosity modifier, shear alignment, and shape memory property | [28] | ||
| The open-ring reaction of 1-azido-2,3-epoxypropane (AEP) with hydroxyethyl cellulose | Azido-hydroxy-ethyl cellulose | A novel bioink for bone tissue engineering | Biocompatibility, biodegradability, and printability | [29] | ||
| Hydrophobic modification and hydroxypropyl methylation | Hydroxypropyl methyl cellulose | Bioinks for freeform writing of the millimetric complex tubular structures | Tunable rheological properties, good stability, and compatibility with additives without strong hydrophilic groups | [30] | ||
| Alginate | Tragacanth/hydroxyapatite | Tragacanth/hydroxyapatite modified alginate bioinks | Repair of significant bone tissue defects | Improving compressive strength, viscosity, printability properties, resolution, and shape fidelity | [31] | |
| Norbornene functionalising | Modular alginate-based bioinks | Construction of complex multi-ink geometries | High cell survivability, stable 3D constructs | [32] | ||
| Oxidised alginate | Degradable alginate-based bioinks | Bioprinting functional cartilage tissue | Rapidly degrade, excellent shape fidelity | [33] | ||
| Molecular weights, concentration, and viscosities | —— | Porous bioprinted constructs for bone tissue engineering | Good biocompatibility and tailorable performance | [34] | ||
| ε-polylysine (ε-PL) | ε-polylysine (ε-PL)-modified Alginate-based bioinks (Alg/ε-PL) | Alginate-based scaffolds For the precise and individualised therapy of tissue defects | Excellent self-supporting stability, mechanical stability | [35] | ||
| Agarose | Carboxylated agarose | —— | Bioink for Bioprinting of free-standing structures with high Stiffness | Printing high-aspect ratio objects possessing anatomically relevant curvature and architecture | [36] | |
| 2D nanosilicate additives | Nanocomposite agarose | Strong shear-thinning bioinks for extrusion 3D bioprinting applications | Tunable flow properties and bioactivity | [37] | ||
| Polydopamine | Agarose-polydopamine | Hydrogel scaffolds for skin wound healing | Good cell adhesion, biodegradability, and biocompatibility | [38] | ||
| Animal polysaccharides | Hyaluronic Acid | Methacrylation of high-molecular-weight hyaluronic acid | Methacrylated hyaluronic acid | Scaffold materials for application in 3D-printed, tissue-engineered bone substitutes | Good primary cell survival and excellent spontaneous osteogenic differentiation in vitro | [39] |
| Alginate | A new bioink for cartilage tissue 3D bioprinting | Highly viable and functional bioprinted 3D hybrid structures for Articular cartilage regeneration | Printability, gelling abilities, stiffness, and good degradability | [40] | ||
| Gelatin methacryloyl (GelMA), methacrylated hyaluronic acid (MAHA) | Tunable MAHA-GelMA (metacrylated hyaluronic acid-based hybrid bioinks) | Stereolithographic (SLA) 3D bioprinting | Excellent mechanical strength, printability, and cell-adhesive nature | [41] | ||
| Norbornene functional groups (Nor) and cysteamine hydrochloride (Cys) | Hiol-norbornene photoclick polysaccharide-based bioink | Bioprinting a liver model in vitro | Increased viscoelastic properties, reduced ROS (reactive oxygen species) accumulation, and superior shape fidelity | [42] | ||
| Chitosan | Nanohydroxyapatite (nhap) | Chitosan-nanohap bioinks | 3D cellular structures and bone tissue engineering applications | High resolution, shape fidelity, and high printability index | [43] | |
| Acrylamide (AM), chitosan modified with methacryloyl groups (CHIMA) | CHIMA/AM | A favourable bioink for the DLP-based 3D printing in the field of tissue engineering and regenerative medicine | Enhanced compression strength, improved elasticity, and favourable biocompatibility | [44] | ||
| Grafting chitosan molecular chains with methacryloyl groups | A photocurable chitosan bioink (CHI-MA) | A potential bioink for the DLP and other photocuring-based 3D printing technologies | High resolution, high fidelity, and good biocompatibility | [45] | ||
| Nanostructured bone-like hydroxyapatite(HA) | Chitosan-HA hydrogels | 3D bioprinting of tissue constructs | Enabling good mechanical support after printing, providing highly active cell platforms | [46] | ||
| Hyaluronic acid derivatives and Matrigel. | NSC(neural stem cell)-laden scaffold | A neural tissue scaffold | Fast gelation and spontaneous covalent crosslinking capability | [47] | ||
| Microbial polysaccharide | Xanthan gum(XG) | Alginate, strontium ions | Crosslinked alginate-xanthan gum blend | Simple cellularized structures and microtissue models to complex organ bioprinting | Noncytotoxic, shear-thinning, and easily sterilizable | [48] |
| Calcium-alginate nanoparticles | Alginate-XG hybrid medium | A promising support medium for 3D printing of tissues and organs | Allowing long-term, high resolution, and accurate printing of bio-structures with a high degree of anatomical complexity | [49] | ||
| Carboxymethyl cellulose | Utilising extrusion-based 3D bioprinting | Tunability regarding pore size and mechanical strength optimisation | [50] | |||
| Succinic anhydride | Succinic anhydride (SA)-modified xanthan (XG–SA) derivatives | Promising drug delivery materials for antibacterial applications | Higher storage (G’) and loss (G’) modulus | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Yang, Y.; Lin, Z.; Hong, Y.; Luo, Z. Modified Polysaccharides: Potential Biomaterials for Bioprinting. J. Funct. Biomater. 2025, 16, 338. https://doi.org/10.3390/jfb16090338
Jiang T, Yang Y, Lin Z, Hong Y, Luo Z. Modified Polysaccharides: Potential Biomaterials for Bioprinting. Journal of Functional Biomaterials. 2025; 16(9):338. https://doi.org/10.3390/jfb16090338
Chicago/Turabian StyleJiang, Tao, Yun Yang, Zening Lin, Yang Hong, and Zirong Luo. 2025. "Modified Polysaccharides: Potential Biomaterials for Bioprinting" Journal of Functional Biomaterials 16, no. 9: 338. https://doi.org/10.3390/jfb16090338
APA StyleJiang, T., Yang, Y., Lin, Z., Hong, Y., & Luo, Z. (2025). Modified Polysaccharides: Potential Biomaterials for Bioprinting. Journal of Functional Biomaterials, 16(9), 338. https://doi.org/10.3390/jfb16090338








